Highlights
-
•
Supercooling of reproductive shoots provides frost protection.
-
•
Supercooling is maintained by histological ice barriers at the pedicel base.
-
•
Ice barriers are active at all reproductive stages (bud, anthesis, and fruit).
Keywords: Biological ice nucleation; Ice propagation; Supercooling; Infrared video thermography, Frost injury, Alpine ecology
Abstract
Over-wintering reproductive buds of many woody plants survive frost by supercooling. The bud tissues are isolated from acropetally advancing ice by the presence of ice barriers that restrict ice growth. Plants living in alpine environments also face the risk of ice formation in summer months. Little knowledge exists, how reproductive structures of woody alpine plants are protected from frost injury during episodic summer frosts. In order to address this question, frost resistance of three common dwarf shrubs, Calluna vulgaris, Empetrum hermaphroditum and Loiseleuria procumbens was measured and ice formation and propagation were monitored in twigs bearing reproductive shoots during various stages of reproductive development (bud, anthesis, and fruit) throughout the alpine summer. Results indicated that, in the investigated species, ice barriers were present at all reproductive stages, isolating the reproductive shoots from ice advancing from the subtending vegetative shoot. Additionally, in the reproductive stems ice nucleating agents that are active at warm, sub-zero temperatures, were absent. The ice barriers were 100% effective, with the exception of L. procumbens, where in 13% of the total observations, the ice barrier failed. The ice barriers were localized at the base of the pedicel, at the anatomical junction of the vegetative and reproductive shoot. There, structural aspects of the tissue impede or prevent ice from advancing from the frozen stem into the pedicel of the reproductive shoot. Under the experimental conditions used in this study, ice nucleation initially occurred in the stem of the vegetative shoot at species-specific mean temperatures in the range of −4.7 to −5.8 °C. Reproductive shoots, however, remained supercooled and ice free down to a range of −7.2 to −18.2 °C or even below −22 °C, the lowest temperature applied in the study. This level of supercooling is sufficient to prevent freezing of reproductive structures at the lowest air temperature occurring at the altitude of the upper distribution boundary of the natural habitat of the investigated species which is between −8 and −10 °C in summer. Frost resistance assays indicated that reproductive shoots are much less frost resistant than vegetative stems, and in contrast to vegetative shoots, are not ice tolerant. Supercooling of reproductive shoots in alpine, woody plant species is an effective mechanism that protects developing offspring from potential frost damage resulting from episodic summer freezing events.
1. Introduction
Freezing temperatures can occur in alpine environments throughout the whole growing season (Körner, 2003) and frost severity and frequency increase significantly with gains in elevation (Taschler and Neuner, 2004; Larcher and Wagner, 2009). While air temperature minima in the timberline ecotone do not fall below −6.0 °C during the summer months, the situation changes dramatically at higher elevations. Around the elevation limit of closed vegetation in the European Alps, air temperature can drop down to −14.0 °C (measured in the alpine-nival ecotone at 2850 m a.s.l.; Ladinig et al., 2013; Neuner and Buchner, 2012). Additionally, the frequency of frost events increases significantly with increasing elevation. Night frosts were recorded on 20% of 174 snow free days at 1950 m, 69% of 74 snow free days at 3450 m (plant canopy temperature records; Larcher and Wagner, 2009), and daily above 4000 m a.s.l. (Körner, 2011). Hence, plants living in alpine environments may regularly face the risk of ice formation in their tissues, even during the period of active growth.
Vegetative parts of alpine plants typically exhibit some degree of ice tolerance (Taschler and Neuner, 2004), however, ice formation is the immediate cause of frost damage to reproductive organs in most alpine species. Previous studies have demonstrated that the reproductive organs of alpine species, with the exception of Ranunculus glacialis, are not ice tolerant (Hacker et al., 2011; Ladinig et al., 2013). The stigma and style are the most freezing sensitive reproductive tissues (Neuner et al., 2013). Hence, the supercooling of reproductive organs as a means to avoid ice formation must be considered as an ecologically significant adaption for safeguarding reproductive success in woody alpine plants.
In some alpine plant species, particularly cushion plants, freezing sensitive reproductive shoots (Ladinig et al., 2013; Neuner et al., 2013) are isolated from ice intrusion by thermal gradients (Hacker et al., 2011). In order for a thermal ice barrier to be active, an adjoining plant part needs to be warmer than 0 °C allowing attached plant parts to remain supercooled for a period of time as temperatures decrease. This temperature differential is often evident in below-ground shoots and buds, and woody stems, but can also be present in species with a densely packed above-ground plant stature, as found in the cushion growth form (Wisniewski et al., 2014).
Structural ice barriers, in addition to thermal gradients, may also protect bud meristems from ice intrusion. The presence of structural barriers have been mainly reported in reproductive over-wintering buds of woody plants (Rhododendron species: Ishikawa and Sakai, 1981, Prunus and Forsythia: Ashworth and Davis, 1984; Ashworth et al., 1992; Cornus officinalis: Ishikawa and Sakai, 1985; Ribes nigrum: Stone et al., 1993; Juniperus virginiana: George et al., 1982) and in meristems of vegetative conifer buds (reviewed in Sakai and Larcher, 1987; Zwiazek et al., 2001). In the few seasonal studies on supercooling capacity of reproductive buds and shoots (Quamme, 1978; Graham and Mullin, 1976; Andrews and Proebsting, 1987), ice barriers were found to persist only until flower bud expansion in spring (Burke et al., 1976). No barriers to the spread of ice were observed during and after bloom in peach (Anderson and Smith, 1989) and apple flowers (Pramsohler and Neuner, 2013).
There is little evidence that structural barriers to ice intrusion are active during anthesis or when fruits are present. Individual flowers in the complex inflorescence of black currant (Ribes nigrum) can freeze independently and survive by supercooling down to temperatures of about −5 °C (Carter et al., 1999, 2001). Since dormant black currant buds survive by deep supercooling (Stone et al., 1993), ice barriers may remain active (Carter et al., 1999). Ripening fruits in cranberry (Vaccinium macrocarpon) were able to supercool which was attributed to the presence of internal barriers to ice propagation from the stem or pedicel and the lack of extrinsic nucleation from the fruit surface (Workmaster et al., 1999). Alpine woody plant species face an imminent risk of ice formation throughout the whole summer, yet little knowledge exists about the freezing response of these alpine species during different reproductive stages. Freezing response may have a strong impact on reproductive success since reproductive organs have been found to be freezing sensitive, i.e. have little ice tolerance (Neuner et al., 2013; Ladinig et al., 2013). Initial results for Rhododendron ferrugineum, obtained by infrared differential thermal analysis (IDTA; Hacker and Neuner, 2007, 2008; Hacker et al., 2008), indicated that ice can propagate nearly unhindered into flowers during anthesis but not into individual florets of winter buds (Neuner and Hacker, 2012). These initial observations (Neuner and Hacker, 2012), and the findings reported for cranberry and black currant, suggest that ice barriers may be active in some woody species during anthesis and subsequent stages of fruit development.
Generally, little is known about ice barriers that influence the rates or avenues of ice propagation in plants (Wisniewski et al., 2009). In the present study, detailed studies of freezing response in three commonly distributed alpine dwarf shrub species was investigated in order to determine whether ice barriers exist between vegetative and reproductive shoots and to what extent reproductive tissues are protected from ice intrusion and frost damage in summer months when plants are actively growing and reproducing. Ice propagation was visualized using IDTA. This method of analysis was selected so the exact localization of the tissue responsible for blocking ice propagation into reproductive shoots could be determined. Ice nucleation temperatures were recorded and the level of frost resistance was assessed for reproductive and vegetative shoots in response to simulated frost events. Observations of freezing response were conducted on reproductive structures during the entire reproductive process, from flower bud to mature fruit, in order to determine if structural barriers to ice propagation were continually present.
2. Materials and methods
2.1. Plant material and collection site
Investigations were carried out on Calluna vulgaris (L.) HULL., Empetrum nigrum subsp. hermaphroditum (HAGERUP.) BÖCHER, and Loiseleuria procumbens (L.) DESV. All three species are evergreen, woody dwarf shrubs that are commonly distributed in the alpine zone of the Central European Alps. Their characteristics are summarized in Table 1. The upper distribution boundary of all three species is at elevations between 2500 and 2800 m.
Table 1.
Characteristics of the investigated evergreen woody alpine dwarf shrubs.
| Plant species | Vertical distribution (m a.s.l.) in the European Alpsa | Flowering time | Habitat characteristics |
|
|---|---|---|---|---|
| Exposition | Snow cover in winter | |||
| Calluna vulgaris | 700–2500 collin – alpine | July–August | South exposed slopes | Snow cover not needed |
| Empetrum hermaphroditum | 1700–2600 subalpine – alpine | April–May | North exposed slopes | Chionophyte – snow cover absolutely necessary |
| Loiseleuria procumbens | 1800–2800 subalpine – alpine | May–June | Wind exposed ridges | Snow cover not needed |
Vertical distribution according to Landolt (1992).
Plant samples were collected approximately 6 km south of Innsbruck, Austria on the summit of Mt. Patscherkofel (2248 m) which is 200–500 m below the elevation limit of the species. At this site, all three species grow in close vicinity to each other, despite different habitat preferences. C. vulgaris was sampled on the south exposed slope of the summit (47°12′29.81″ N, 11°27′42.37″ E), while L. procumbens and E. hermaphroditum were collected on the north exposed slope (47°12′33.42″ N, 11°27′44.93″ E). Samples were taken randomly from at least 10 individuals and a total of 30 twigs per species were collected at each sampling. Single twigs (12 cm) were detached with a pruning shear, placed on wetted paper towels, and stored during the transport to the laboratory inside sealable plastic bags in a cooler box. The total time for transport of the samples to the laboratory environment was approximately 1.5 h. Plant material was stored at 4 °C in a dark cold room and used in experiments within 7 days after collection.
The presence of ice barriers, patterns of ice propagation, and frost resistance were evaluated at three different stages of reproductive development (b = unopened floral buds, a = anthesis, and f = mature fruit) throughout the growing period. Flower buds of C. vulgaris form in spring, and flowering time is usually midsummer, while fruits ripen in autumn. E. hermaphroditum and L. procumbens have over-wintering reproductive buds, are early-flowering, and have fruits that develop in summer. Since the species differ with respect to the timing of reproductive development, the stage of development and sampling design for each species is provided in Table 2.
Table 2.
Stage of reproductive development of the investigated species at the time of sampling.
| Reproductive stage | Definition of reproductive stages (sampling periods are indicated in brackets) |
||
|---|---|---|---|
| C. vulgaris | E. hermaphroditum | L. procumbens | |
| Bud stage | Corolla visible until shortly before flower opening (13.–31.8.12, 6.–20.8.13) | Overwintering bud before bud burst (24.9.–10.10.12, 9.10.13) | Budburst until shortly before flower opening (12.6.–13.6.12, 17.10.12, 28.5.–11.6.13, 8.10.13) |
| Anthesis | (22.8.–5.9.12, 27.8.–3.9.13) | (29.5.13) | (18.–20.6.12) |
| Fruit stage | Early stages till fruit and seed maturation (12.9.–11.10.12, 17.9.–7.10.13) | Mature fruits (19.9.13) | Early stages till fruit maturation (28.6.–8.8.12, 11.7.–18.9.13) |
2.2. Freezing treatment
Freezing of the plant samples was conducted in computer-controlled commercial chest freezers (Hacker and Neuner, 2007). The freezing compartments of these freezers have a volume of 141.9 L (43 cm × 50 cm × 66 cm). The freezing chambers used in this study allow for the programming of user defined cooling protocols which enable linear cooling and thawing rates. Temperature set points and the duration at each temperature can be freely set and designed to maintain temperature oscillations at a minimum (<0.2 K). Consistent with standard practices for bioclimatology (Leuzinger et al., 2010), temperatures are presented in °C and temperature differences in Kelvin (K).
2.3. Detection, localization and quantification of ice barriers
Ice barriers were detected by IDTA (Hacker and Neuner, 2007, 2008; Hacker et al., 2008; Neuner and Kuprian, 2014). A ThermaCAM S60 (FLIR Systems, Danderyd, Sweden) was used. The camera was equipped with close-up lenses (LW64/150; LW34/80) to achieve a spatial resolution of 200 μm and 106 μm, respectively. Infrared images were recorded at a measurement interval of 100 ms. Further analysis of the infrared images was performed with ThermaCAM Researcher (FLIR Systems, Danderyd, Sweden) software.
Two to four sample twigs were fixed together on a specially designed sample holder plate (20 cm × 20 cm) using double sided glue tape (4 cm width) to bring all plant parts, i.e. shoots, leaves and reproductive shoots (in the present study the term reproductive shoot refers to a single flower or fruit attached to a pedicel), into the focus plane of the infrared camera. The actual measurement area of the infrared camera equipped with the close-up lens (LW64/150) was approximately 3.7 cm × 4.6 cm. After a digital image of the prepared sample twigs was recorded, the sample holder plate was placed inside the cooling compartment of a chest freezer on a laboratory lifting plate. The infrared camera, contained inside a special housing (Neuner and Kuprian, 2014), was then placed upside down into the chest freezer and the twig samples were subsequently brought into focus. Identical freezing protocols were used during each IDTA recording. Once the chest freezers and the samples within had equilibrated at 2 °C, temperatures were lowered at 3 K/h down to a minimum temperature of −22 °C.
For the quantification of the functionality of ice barriers between vegetative and reproductive shoots in the various stages of reproductive development, ice nucleation temperatures for each reproductive shoot visible in the frame of the infrared camera were compared to that of the supporting vegetative shoot. Ice barriers were assumed to be present if the ice nucleation temperature in the vegetative shoot was significantly warmer (P < 0.05) than in the reproductive shoot.
2.4. Determination of frost resistance
The freezing resistance of vegetative and reproductive shoots of the investigated species was assessed by exposing samples to a range of freezing temperatures. At least four twigs, bearing numerous reproductive shoots, were randomly selected from the sample pool obtained from 10 individuals per sampling date. Twig samples were placed on wetted paper towels inside sealed plastic bags and the freezing protocol was initiated as soon as the samples had equilibrated at 2 °C. Cooling proceeded at a rate of 2 K/h down to a target temperature and samples were then held at that temperature for 4 h. Thawing took place at a rate of 2 K/h till samples had warmed to 5 °C. The highest target temperature was −2 °C and the lowest was one that would ensure killing of the twig samples. Intermediate target temperatures were usually at 2 K intervals down to −14 °C, and if necessary an additional 4 K below −14 °C.
After the freezing test and subsequent thawing, samples remained on wetted paper towels inside plastic bags, at 22 °C and 40 μmol photons m−2 s−1 for 3–4 days to allow symptoms of freezing injury to develop. Visual assessment of injury was difficult to determine due to naturally occurring, non-lethal discoloration of the vegetative shoots. Therefore, viability of the vegetative shoots was determined on 10 randomly selected measurement spots on the stem of each sample by chlorophyll fluorescence, using a MINI PAM (Walz, Effeltrich, Germany). Viability of reproductive shoots was visually determined (n = 12–91 shoots per exposure temperature, depending on the species). Symptoms of injury could clearly be seen as a distinct brown discoloration of the reproductive tissues. Reproductive shoots were either undamaged (0%) or frost killed (100%). According to Neuner and Buchner (1999), results obtained by different viability assays are generally similar when plants are actively growing. Percent freezing injury for each exposure temperature was obtained using both viability assays.
Percent freezing injury of vegetative and reproductive shoots obtained from each of 10 viability scores was individually plotted against the exposure temperature, and a logistic function (Boltzmann function) was fitted to the data using OriginPro 7G SR4 (OriginLab Corporation, Northampton, MA, USA) software. From the parameters of the logistic function an LT50 (temperature resulting in 50% freeze damage) was determined. Mean values and SD of these LT50 values were calculated for vegetative and reproductive shoots at each sampling date.
2.5. Statistical analyses
The significance of differences between mean values of ice nucleation temperatures of vegetative versus reproductive shoots at each reproductive stage and between each species was tested by a t-test (R Core Team, 2013, version 3.0.0). For each species, statistical differences in the mean ice nucleation temperature and frost resistance among the different reproductive stages in vegetative and reproductive shoots was tested by ANOVA followed by the Bonferroni post hoc test after a positive Levene test. In all comparisons in which homoscedasticity was not found, the Mann–Whitney-U-Test was used as a non-parametric post hoc test (PASW Statistics 18, version 18.0.2., Chicago, SPSS Inc.). Three-way ANOVA was applied to test for effects of shoot type (reproductive, vegetative), stage (bud, anthesis, fruit) and date as fixed factors on ice nucleation temperature and frost resistance. For all statistical analyses, the critical level of significance was α = 0.05.
3. Results
3.1. Localization of ice barriers
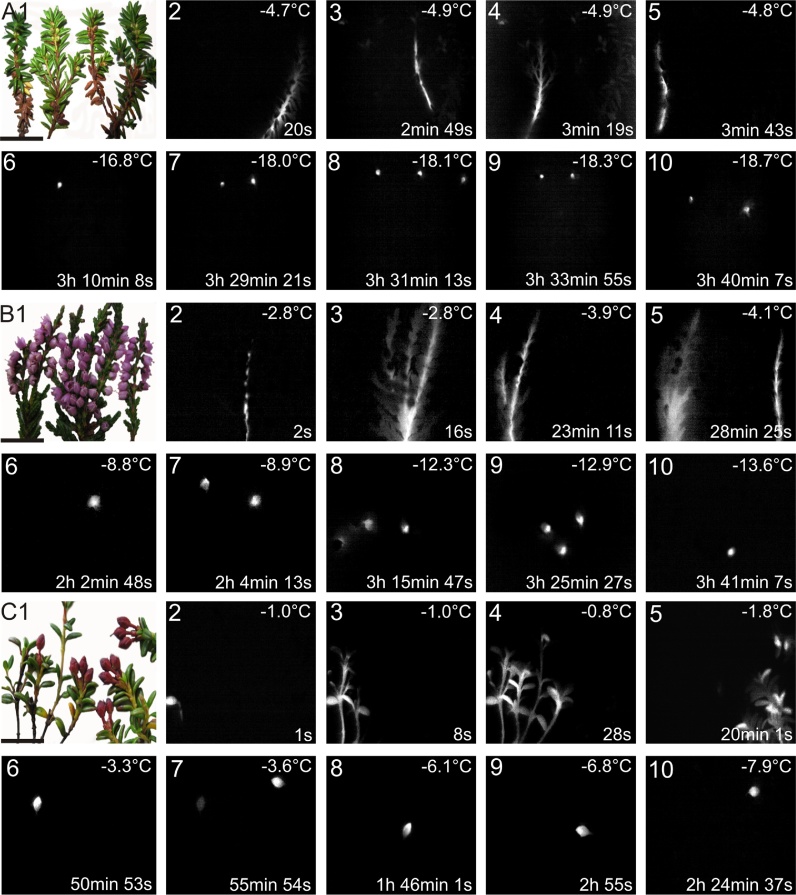
All observations, in all species, consistently indicated that ice formed initially in the vegetative stem and moved unhindered into the evergreen leaves but did not immediately enter the pedicels. These observations indicated the presence of ice barriers between vegetative stems and reproductive shoots that inhibited the spread of ice into reproductive tissues. These ice barriers prevailed throughout all reproductive developmental stages, from unopened buds, to anthesis, and through late stages of fruit development. The ice barriers, as visualized by sequences of IDTA images, are shown in floral buds of E. hermaphroditum (Fig. 1A), during anthesis in C. vulgaris (Fig. 1B), and when fruits are present in L. procumbens (Fig. 1C). While, in all observed instances, the ice barrier prevented immediate ice propagation from vegetative shoots into reproductive shoots of C. vulgaris and E. hermaphroditum, the barrier was only effective in 87.2% of the observations made in L. procumbens (Table 3).
Fig. 1.
Representative images of the investigated species prior to and during the freezing treatment (horizontal black bar = 1.0 cm). (A1) Digital image of twigs of E. hermaphroditum with attached reproductive buds, (B1) C. vulgaris bearing flowers and (C1) L. procumbens with attached fruits. Ice nucleation, initiated in the vegetative stems, occurred at −4.7 °C, −2.8 °C and −1.0 °C in E. hermaphroditum, C. vulgaris and L. procumbens, respectively. Freezing events are visualized as brightened regions in the image, while unfrozen areas appear black. The IDTA image sequences show unblocked ice propagation in the vegetative shoot of each species (2–5). After a time lapse and at significantly lower temperatures single buds, flowers, or fruits froze independently from the vegetative shoot and from each other (6–10). Actual temperatures are indicated in the top right corner of each image. The time span (in hours, minutes and seconds) after initial ice nucleation in the vegetative shoot is indicated at the bottom right corner of each image.
Table 3.
Quantification of observations on the prevention of ice propagation from frozen vegetative shoots into attached reproductive shoots in the investigated species at the different stages of reproductive development: b, bud stage; a, anthesis; f, fruiting stage.
| Plant species | Reproductive stage | Total number of observed shoots | Percentage of reproductive shoots with an active ice barrier | Percentage of reproductive shoots remaining supercooled to −22 °C |
|---|---|---|---|---|
| C. vulgaris | b | 197 | 100 | 4.1 |
| a | 197 | 100 | 6.6 | |
| f | 216 | 100 | 9.7 | |
| E. hermaphroditum | b | 42 | 100 | 11.9 |
| a | 25 | 100 | 20.0 | |
| f | 3 | 100 | 0 | |
| L. procumbens | b | 152 | 88.2 | 17.1 |
| a | 46 | 82.6 | 19.6 | |
| f | 115 | 87.8 | 22.6 | |
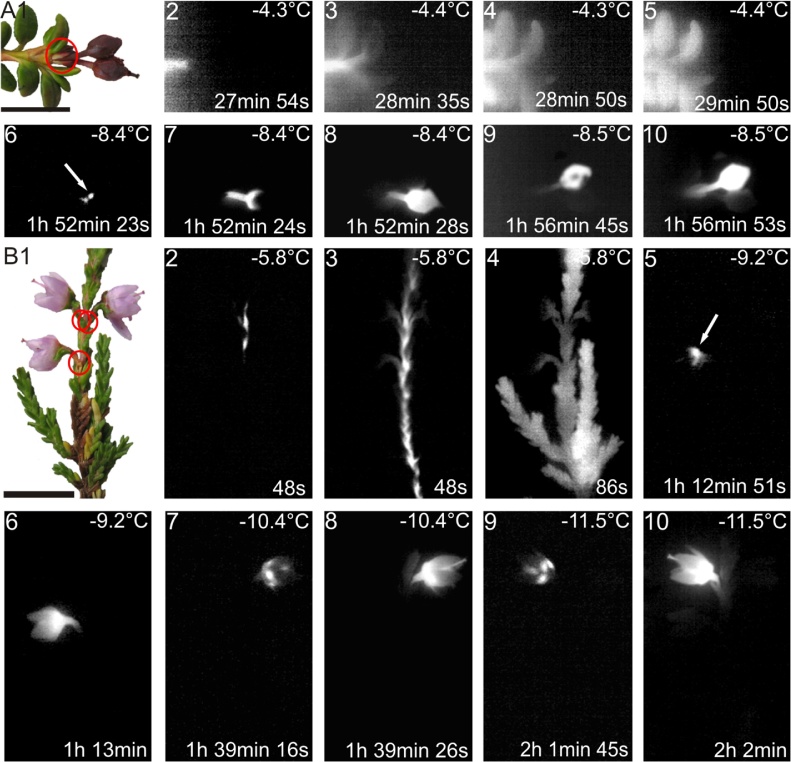
The use of a high resolution close up lens (106 μm) made the localization of the ice barrier possible when performing IDTA (Fig. 2). In all species, the ice barrier appeared to be located in tissues at the base of the pedicel. This is where the ice propagation stopped. In L. procumbens (Fig. 2A) and in C. vulgaris (Fig. 2B), each reproductive shoot froze independently, resulting in lethal freeze damage to the respective reproductive shoot. Ice nucleation, when it occurred, took place in the calyx region in both fruits of L. procumbens and flowers of C. vulgaris.
Fig. 2.
Representative images of L. procumbens and C. vulgaris prior to and during the freezing treatment (horizontal black bar = 1.0 cm). (A1) Digital image of twigs of L. procumbens with attached fruits, and (B1) C. vulgaris shoots bearing flowers. Initial ice nucleation in the vegetative stem occurred at −3.1 °C and −5.8 °C in L. procumbens and C. vulgaris, respectively. Freezing events are visualized as brightened regions in the image, while unfrozen areas appear black. IDTA image sequences show ice nucleation in the vegetative shoot and subsequent unhindered ice propagation throughout the whole vegetative shoot in each species (A2-5, B2-4). Ice nucleation was initiated in the region of the calyx (white arrows) of reproductive shoots (A6: L. procumbens fruit; B5: C. vulgaris flower). IDTA images indicate that the ice barrier is localized at the base of the pedicel (A7, A10, B6 and B10). Red circles in the digital images (A1 and B1) indicate the location of the structural ice barrier. Temperatures are indicated in the top right corner of each image. The time span (in hours, minutes and seconds) after ice nucleation in the vegetative shoot is indicated in the bottom right corner of each image.
3.2. Ice nucleation temperatures in vegetative versus reproductive shoots
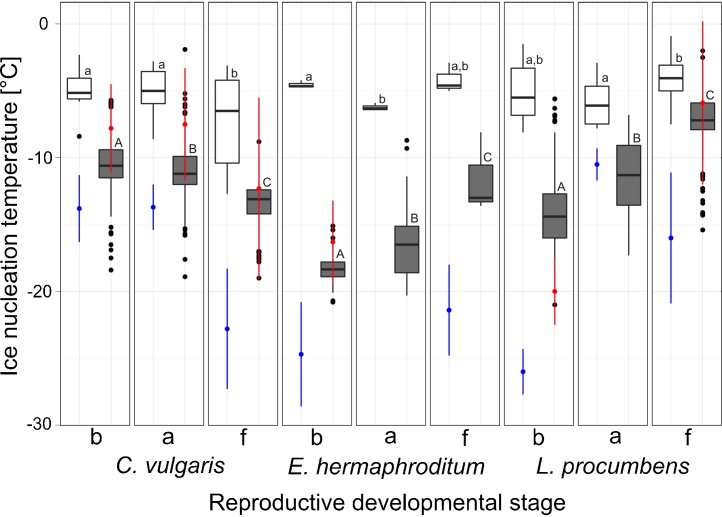
Ice formed in vegetative shoots, under the experimental conditions, at a mean temperature of −4.7 °C, −5.0 °C and −5.8 °C in L. procumbens, E. hermaphroditum and C. vulgaris, respectively (Fig. 3). Ice nucleation in the reproductive shoots occurred independently of ice initiation in vegetative stems and occurred over a broader temperature range than in vegetative shoots. In reproductive shoots, ice nucleation occurred at a mean temperature between −7.2 °C and −18.4 °C. This range was significantly lower than the mean freezing temperatures observed in vegetative shoots (−4.0 to −7.3 °C). Despite the ultimate freezing temperature of −22 °C, in several cases, one or several reproductive shoots remained in the supercooled state and did not show any signs of freezing within the course of the experiment. More specifically, the percent of reproductive shoots that maintained supercooling below −22 °C in reproductive shoots, across all developmental stages, was up to 9.7% in C. vulgaris, up to 20.0% in E. hermaphroditum, and up to 22.6% in L. procumbens (Table 3).
Fig. 3.
Ice nucleation temperatures (°C) in vegetative (white bars) and reproductive (grey bars) shoots during different stages of reproductive development, from bud (b), to anthesis (a), and fruiting (f), in C. vulgaris, E. hermaphroditum and L. prcumbens are shown in comparison to mean values ±SD of frost resistance of vegetative (blue) versus reproductive (red) shoots. The box plots indicate the median (=second quartile; line inside the box) and extend from the first to the third quartile. The whiskers show at maximum the 1.5 fold interquartile range. Outliers are shown as black dots. Within a single species, significant differences in ice nucleation temperatures at different reproductive stages in reproductive shoots and vegetative shoots are indicated by capital and lower case letters, respectively.
Mean ice nucleation temperatures in vegetative shoots showed little, but in some cases still significant, variation as reproductive development proceeded (1.8–2.4 K; Fig. 3). In contrast, ice nucleation temperatures in reproductive shoots changed significantly, by between 3.0 and 6.9 K, during the course of reproductive development. While supercooling capacity decreased significantly from the bud to the fruit stage in L. procumbens and in E. hermaphroditum, it increased in C. vulgaris in the fruit stage but was similar in both the bud stage and during anthesis.
3.3. Frost resistance of vegetative versus reproductive shoots
Vegetative shoots had a greater level of frost resistance than reproductive shoots (Fig. 3). In vegetative shoots, freeze injury was observed at a mean of 13.5 K (4.7–20.9 K) below the mean temperature at which ice nucleation had occurred. This indicates that vegetative shoots of the investigated species were freeze tolerant (cold hardy) during the period of active growth. In contrast, the mean temperature of freeze injury of reproductive shoots was very similar to the mean temperature of ice nucleation. Mean frost resistance (LT50) was an average of 3.3 K (1.1–6.6 K) lower than the mean ice nucleation temperature. The difference between vegetative and reproductive shoots is evident when frost resistance is plotted against ice nucleation temperature (Fig. 4). Frost resistance and ice nucleation temperature are linearly correlated in reproductive shoots but are not correlated in vegetative shoots (Fig. 4). The plotted data indicate that a 1 K increase in supercooling would result in a 1 K higher frost resistance in reproductive shoots. While frost resistance of vegetative shoots changed significantly through the course of the sampling period, only minor changes in ice nucleation temperatures were observed. Therefore, it appears that freeze damage was largely independent of ice nucleation temperature.
Fig. 4.
Correlation between frost resistance (LT50; °C) and ice nucleation temperature in vegetative (○) and reproductive (●) shoots measured during the bud stage, anthesis, and fruiting in the three investigated woody alpine species: C. vulgaris, E. hermaphroditum and L. procumbens.
The level of frost resistance in reproductive shoots changed significantly during reproductive development and was dependent on the timing of reproductive development (Supplement 1). Frost resistance was relatively low during fruiting in species that fruited in summer, whereas in C. vulgaris, which develops fruits in autumn, frost resistance increased significantly in the fruiting stage (Fig. 3).
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.envexpbot.2014.01.011.
Results of a three-way fixed factor analysis of variance (GLM) of the effects of ‘type of shoot’ (vegetative and reproductive), ‘stage’ (bud, anthesis, fruit) and ‘date’ on ice nucleation temperature and frost resistance (LT50) determined for Calluna vulgaris, Empetrum hermaphroditum and Loiseleuria procumbens. Significant effects are given in bold. Abbreviations: df, degrees of freedom; F, variance ratio; p, error probability; n.d., not determinable.
4. Discussion
Reproductive shoots of the investigated alpine dwarf shrubs exhibited freeze injury as soon as ice formed in their tissue whereas vegetative shoots were ice tolerant. These results corroborate similar findings reported on several alpine plant species including dwarf shrubs (Hacker et al., 2011; Ladinig et al., 2013; Neuner et al., 2013). These reports indicated that in reproductive shoots, ice formation was only tolerated at early bud stages and somewhat during fruiting, whereas vegetative shoots were freezing tolerant throughout reproductive development. Only the reproductive organs of Ranunculus glacialis remained freezing tolerant during all stages of development. Hence, freeze avoidance by supercooling must be considered as an important factor to assure reproductive success in the alpine environment.
In the investigated species, IDTA images indicated that the ice barrier was located in tissues at the base of the pedicel. Data indicate that this barrier must be active in preventing ice propagation into the reproductive shoots throughout the whole reproductive period, i.e. from bud to fruit, in order to achieve reproductive success for these species. Additionally, in the reproductive stems ice nucleating agents that are active at warm, sub-zero temperatures, must have been absent. To the best of our knowledge this is the first detailed study which demonstrates that reproductive shoots are protected by an ice barrier and supercool throughout reproductive development. Earlier observations on black currant during anthesis (Carter et al., 1999, 2001) and in cranberry fruits (Workmaster et al., 1999) suggested that this might be possible, but these systems were only studied at one stage of reproductive development.
Thermal ice barriers have been described in alpine cushion plants with a densely packed shoot system (Hacker et al., 2011). For a thermal ice barrier to be relevant and effective, adjoining plant parts need to be warmer than 0 °C. This temperature differential can inhibit ice propagation allowing supercooling to occur in other plant parts. Thermal ice barriers may be established in many alpine species by burial of shoots and woody stems in the soil, as well as the dense canopy structure of cushion plants. In contrast, the loosely arranged shoots of woody dwarf shrubs, do not permit them to act as thermal insulation and thus as barriers to ice propagation. Structural ice barriers, however, may provide effective protection to freezing sensitive reproductive tissues. Unfortunately, these structural barriers do not appear to be generally active in woody alpine dwarf shrubs. For example, structural ice barriers found in over-wintering reproductive buds of Rhododendron ferrugineum, disappear in spring prior to anthesis (Neuner and Hacker, 2012).
Ice nucleation in reproductive shoots of the investigated species occurred on average at a mean temperature between −7 °C and −22 °C (the lowest freezing temperature applied in the IDTA experiments). Summer night temperatures can be expected to drop down to between −8 and −10 °C at the upper distribution limits (2500–2800 m) of the investigated species in the European Alps (Taschler and Neuner, 2004). This suggests that the reproductive shoots of the investigated species must be safely protected, in some manner, from potential freezing injury. This is not the case in R. ferrugineum which lacks ice barriers and can regularly suffer from frost damage to their reproductive shoots in summer (Larcher and Wagner, 2004).
Ice nucleation temperatures in reproductive shoots typically occurred over a broad range of freezing temperatures (more than 14 K in some cases). Similar to the results of the current study, supercooling of black currant flowers was also reported to occur over a range of freezing temperatures (Carter et al., 2001). However, the range was much narrower and the supercooling capacity of the reproductive shoots was much less than in the alpine dwarf shrub species investigated in the current study. Ice nucleation in flowers of black currant was reported to occur between −1.6 and −5.5 °C (Carter et al., 2001), and in another study, cranberry fruits were reported to supercool down to −6 and −7 °C for extended periods of time (Workmaster et al., 1999). These are temperatures close to the ice nucleation temperatures obtained in vegetative shoots of the dwarf shrubs investigated in the present study, and not comparable with ice nucleation temperatures measured in reproductive shoots which had the capacity to supercool to temperatures below −22 °C.
The ice barrier in the investigated species was localized in tissues at the base of the pedicel, which is the anatomical junction to the vegetative stem. Barriers to the movement of ice in black currant also appeared to exist at certain anatomical junctions within the plant, notably where the peduncle of an inflorescence was attached to the stem and where a flower pedicel joined a peduncle (Carter et al., 1999). The capacity of cranberry fruits to supercool appears to be due to barriers that either prevent ice propagation from the vegetative shoot into the pedicel or from extrinsic nucleation events originating on the fruit surface (Workmaster et al., 1999). Ice nucleation in fruit was observed to be initiated only at the calyx (distal) end of fruit. No fruit still attached to the parent plant was nucleated at their pedicel (proximal) end and ice was never clearly seen to propagate along the pedicel (Workmaster et al., 1999). The report indicated that stomata in the remnant area of the nectary may provide avenues for extrinsic ice nucleation in the area of the calyx.
Little information has been reported regarding the structural properties of tissues that prevent ice propagation into reproductive shoots during anthesis or fruiting. It was reported that the vascular connection to the ripe fruit of cranberries is greatly reduced and that in ripe field-grown cranberries the pedicel turns brown, dries up, and decreases in diameter (Workmaster et al., 1999). Anatomical junctions are also discussed in this respect in black currant (Carter et al., 1999, 2001). The breakdown of an ice barrier present in overwintering reproductive buds of angiosperms occurs in the spring once xylem continuity between the floral bud and the subtending stem is established. Once xylem continuity is established, ice readily propagates from the stem into the reproductive bud via the vascular system (Prunus persica: Ashworth 1984, Forsythia: Ashworth et al., 1992, Prunus species: Kader and Proebsting, 1992). Outside of a direct role for the presence of mature vascular tissue which acts as a conduit for the spread of ice, the determining factors involved in the prevention, formation and spread of ice into buds remain to be elucidated (Wisniewski and Davis, 1995). For the species investigated here, the anatomical peculiarities at the base of the pedicel, whose diameter appears extraordinarily narrow in all investigated species, still remain to be investigated in more detail. The properties of the tissue forming a barrier to ice propagation may be similar to the properties reported for over-wintering buds of other woody plants. For instance, the anatomical features of the ice barrier in Prunus persica may involve the presence of provascular strands rather than mature xylem and a zone of small cells at the flower base with thick cell walls, devoid of vacuoles and intercellular spaces (Quamme et al., 1995). The walls of cells in the tissue immediately subtending floral primordial in peach were reported to be very thick and rich in highly esterified pectins (Wisniewski and Davis, 1995). In Vitis, the ice barrier between the stem axis and the bud also contains tightly packed cells with narrow intercellular spaces (Jones et al., 2000).
Ice nucleation temperatures and the frost resistance of reproductive shoots changed significantly during reproductive development. Such changes have only been reported in cranberry fruits where ripe fruits typically supercooled to colder temperatures and for longer durations than unripe fruits (Workmaster et al., 1999). This was attributed to be a result of a reduction in the functional vascular connection during fruit ripening. The underlying mechanism of how the supercooling capacity of the reproductive shoots of the investigated species is altered during the course of reproductive development is not understood. Notably, the weakest supercooling capacity occurred at midsummer indicating a seasonal effect rather than an effect of the stage of reproductive development (Supplement 1). The species that fruit in summer exhibited decreased frost resistance during fruiting. In contrast, freezing resistance significantly increased in C. vulgaris, which develops fruits in autumn. Still, changes in the durability of the barriers to ice movement during seasonal development may be the basis of genotypic differences in frost resistance of reproductive shoots in certain species and potential targets for breeding as has been suggested for black currant (Carter et al., 2001). The ability of a plant to segregate ice into specific areas of its tissue where it will not do any harm may be as critical to survival as the ability of its cells to withstand freeze dehydration (Gusta and Wisniewski, 2012). Hence, ice barriers observed in alpine dwarf shrubs may not only be of ecological significance but may also serve as a model for frost survival in other species.
Acknowledgements
This research was funded by the Austrian Science Fund (FWF): P23681-B16 and P20010-B16. We thank Ramona Miller for technical assistance, Benjamin Dietre for graphical help with Fig. 3, anonymous reviewers for their comments and the Patscherkofelbahn for free transportation to the study site by cable-car.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Edith Kuprian, Email: Edith.kuprian@uibk.ac.at.
Verónica F. Briceño, Email: Veronica.briceno@anu.edu.au.
Johanna Wagner, Email: Johanna.wagner@uibk.ac.at.
Gilbert Neuner, Email: Gilbert.neuner@uibk.ac.at.
References
- Anderson J.A., Smith M.W. Ice propagation in peach shoots and flowers. HortScience. 1989;24:450–452. [Google Scholar]
- Andrews P.K., Proebsting E.L. Effects of temperature on the deep supercooling characteristics of dormant de-acclimating sweet cherry flower buds. J. Am. Soc. Hort. Sci. 1987;112(2):334–340. [Google Scholar]
- Ashworth E.N., Davis G.A. Ice nucleation within peach trees. J. Am. Soc. Hort. Sci. 1984;109(2):198–201. [Google Scholar]
- Ashworth E.N., Willard T.J., Malone S.R. The relationship between vascular differentiation and the distribution of ice within Forsythia flower buds. Plant Cell Environ. 1992;15:607–612. [Google Scholar]
- Burke M.J., Gusta L.V., Quamme H.A., Weiser C.J., Li P.H. Freezing and injury in plants. Annu. Rev. Plant Physiol. 1976;27:507–528. [Google Scholar]
- Carter J., Brennan R., Wisniewski M. Low-temperature tolerance of black currant flowers. HortScience. 1999;34(5):855–859. [Google Scholar]
- Carter J., Brennan R., Wisniewski M. Patterns of ice formation and movement in black currant. HortScience. 2001;36(6):855–859. [Google Scholar]
- George M.F., Becwar M.F., Burke M.J. Freezing avoidance by deep undercooling of tissue water in winter-hardy plants. Cryobiology. 1982;19:628–639. doi: 10.1016/0011-2240(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Graham P.R., Mullin R. A study of flower bud hardiness in azalea. J. Am. Soc. Hort. Sci. 1976;101:7–10. [Google Scholar]
- Gusta L.V., Wisniewski M. Understanding plant cold hardiness: an opinion. Physiol. Plant. 2012;147:4–14. doi: 10.1111/j.1399-3054.2012.01611.x. [DOI] [PubMed] [Google Scholar]
- Hacker J., Neuner G. Ice propagation in plants visualized at the tissue level by infrared differential thermal analysis (IDTA) Tree Physiol. 2007;27:1661–1670. doi: 10.1093/treephys/27.12.1661. [DOI] [PubMed] [Google Scholar]
- Hacker J., Neuner G. Ice propagation in dehardened alpine plant species studied by infrared differential thermal analysis (IDTA) Arc. Antarc. Alp. Res. 2008;40(4):660–670. [Google Scholar]
- Hacker J., Spindelböck J., Neuner G. Mesophyll freezing and effects of freeze dehydration visualized by simultaneous measurement of IDTA and differential imaging chlorophyll fluorescence. Plant Cell Environ. 2008;31:1725–1733. doi: 10.1111/j.1365-3040.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- Hacker J., Ladinig U., Wagner J., Neuner G. Inflorescences of alpine cushion plants freeze autonomously and may survive subzero temperatures by supercooling. Plant Sci. 2011;180:149–156. doi: 10.1016/j.plantsci.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Sakai A. Freezing avoidance mechanisms by supercooling in some Rhododendron flower buds with reference to water relations. Plant Cell Physiol. 1981;22(6):953–967. [Google Scholar]
- Ishikawa M., Sakai A. Extraorgan freezing in wintering flower buds of Cornus officinalis Sieb. et Zucc. Plant Cell Environ. 1985;8(5):333–338. [Google Scholar]
- Jones K.S., McKersie B.D., Paroschy J. Prevention of ice propagation by permeability barriers in bud axes of Vitis vinifera. Can. J. Bot. 2000;78:3–9. [Google Scholar]
- Kader S.A., Proebsting E.L. Freezing behaviour of Prunus, subgenus padus, flower buds. J. Am. Soc. Hort. Sci. 1992;117(6):955–960. [Google Scholar]
- Körner Ch. Springer; Berlin: 2003. Alpine Plant Life. 349 pp. [Google Scholar]
- Körner Ch. Coldest places on earth with angiosperm plant life. Alp. Bot. 2011;121:11–22. [Google Scholar]
- Ladinig U., Hacker J., Neuner G., Wagner J. How endangered is sexual reproduction of high-mountain plants by summer frosts? – Frost resistance, frequency of frost events and risk assessment. Oecologia. 2013;171:743–760. doi: 10.1007/s00442-012-2581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt E. Gustav Fischer; Stuttgart: 1992. Unsere Alpenflora. [Google Scholar]
- Larcher W., Wagner J. Lebensweise der Alpenrosen in ihrer Umwelt: 70 Jahre ökophysiologische Forschung in Innsbruck. Ber. Naturwiss. -Med. Ver. Innsbruck. 2004;91:251–291. [Google Scholar]
- Larcher W., Wagner J. High mountain bioclimate: temperatures near the ground recorded from the timberline to the nival zone in the Central Alps. Contrib. Nat. Hist. 2009;12:857–874. [Google Scholar]
- Leuzinger S., Vogt R., Körner C. Tree surface temperature in an urban environment. Agr. For. Meteorol. 2010;150:56–62. [Google Scholar]
- Neuner G., Buchner O. Assessment of foliar frost damage: a comparison of in vivo chlorophyll fluorescence with other viability tests. J. Appl. Bot. 1999;73:50–54. [Google Scholar]
- Neuner G., Buchner O. Dynamic of tissue heat tolerance and thermotolerance of PS II in alpine plants. In: Lütz C., editor. Plants in Alpine Regions: Cell Physiology of Adaptation and Survival Strategies. Springer; Wien: 2012. pp. 61–74. [Google Scholar]
- Neuner G., Hacker J. Ice formation and propagation in alpine plants. In: Lütz C., editor. Plants in Alpine Regions: Cell Physiology of Adaptation and Survival Strategies. Springer; Wien: 2012. pp. 163–174. [Google Scholar]
- Neuner G., Erler A., Hacker J., Ladinig U., Wagner J. Frost resistance of reproductive tissues in various reproductive stages of high alpine plant species. Physiol. Plant. 2013;147:88–100. doi: 10.1111/j.1399-3054.2012.01616.x. [DOI] [PubMed] [Google Scholar]
- Neuner G., Kuprian E. Infrared thermal analysis of plant freezing processes. In: Hincha D., Zuther E., editors. Methods in Molecular Biology: Plant Cold Acclimation. Springer; Berlin: 2014. [DOI] [PubMed] [Google Scholar]
- Pramsohler M., Neuner G. Dehydration and osmotic adjustment in apple stem tissue during winter related to frost resistance of buds. Tree Physiol. 2013;33(8):807–816. doi: 10.1093/treephys/tpt057. [DOI] [PubMed] [Google Scholar]
- Quamme H.A. Mechanism of supercooling in overwintering peach flower buds. J. Am. Soc. Hort. Sci. 1978;103(1):57–61. [Google Scholar]
- Quamme H.A., Su W.A., Veto L.J. Anatomical features facilitating supercooling of the flower within the dormant peach flower bud. J. Am. Soc. Hort. Sci. 1995;120:814–822. [Google Scholar]
- Sakai A., Larcher W. Frost survival of plants. Responses and adaptation to freezing stress. In: Billings W.D., Golley F., Lange O.L., Olson J.S., Remmert H., editors. Vol. 62. Springer; Berlin/Heidelberg/New York/London/Paris/Tokyo: 1987. (Ecological Studies). 321 pp. [Google Scholar]
- Stone W., Idle D.B., Brennan R.M. Freezing events within overwintering buds of black currant (Ribes nigrum L.) Ann. Bot. 1993;72:613–617. [Google Scholar]
- Taschler D., Neuner G. Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell Environ. 2004;27(6):737–746. [Google Scholar]
- Wisniewski M., Davis G. Immunogold localization of pectins and glycoproteins in tissues of peach with reference to deep supercooling. Trees. 1995;9(5):253–260. [Google Scholar]
- Wisniewski M.E., Gusta L.V., Fuller M.P., Karlson D. Ice nucleation, propagation and deep supercooling: the lost tribes of freezing studies. In: Gusta L.V., Wisniewski M.E., Tanino K.K., editors. Plant Cold Hardiness: From the Laboratory to the Field. CAB International; Cambridge: 2009. pp. 1–11. [Google Scholar]
- Wisniewski M., Gusta L., Neuner G. Adaptive mechanisms of freeze avoidance in plants. A brief update. Env. Exp. Bot. 2014;99:133–140. [Google Scholar]
- Workmaster B.A.A., Palta J.P., Wisniewski M. Ice nucleation and propagation in cranberry uprights and fruit using infrared video thermography. J. Am. Soc. Hort. Sci. 1999;124(6):619–625. [Google Scholar]
- Zwiazek J.J.R.S., Croser C., Hansen J., Beck E. Biochemical and biophysical changes in relation to cold hardiness. In: Bigras F.J., Colombo S.J., editors. Conifer Cold Hardiness. Kluwer Academic Publisher; Dordrecht/Boston/London: 2001. pp. 165–186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of a three-way fixed factor analysis of variance (GLM) of the effects of ‘type of shoot’ (vegetative and reproductive), ‘stage’ (bud, anthesis, fruit) and ‘date’ on ice nucleation temperature and frost resistance (LT50) determined for Calluna vulgaris, Empetrum hermaphroditum and Loiseleuria procumbens. Significant effects are given in bold. Abbreviations: df, degrees of freedom; F, variance ratio; p, error probability; n.d., not determinable.