Abstract
Intermittent androgen deprivation therapy (IADT) is an alternative to continuous androgen deprivation therapy (ADT) in prostate cancer patients with nonmetastatic disease. ADT is associated with numerous side effects such as hot flashes, sexual dysfunction, anemia, fatigue, loss of muscle mass, osteoporosis, metabolic syndrome and premature cardiovascular disease. IADT was developed with the intention of improving the quality of life and to delay progression of prostate cancer to castration resistance. The benefits of slightly improved quality of life by IADT compared to ADT were demonstrated in multiple clinical trials. IADT was noted to be noninferior to ADT in patients with biochemical recurrence of prostate cancer but in studies performed in patients with metastatic prostate cancer, the results were inconclusive. Our recent studies suggested that the administration of 5 alpha-reductase inhibitors during the off-cycle of IADT can significantly prolong the survival of mice bearing androgen-sensitive prostate tumors when off-cycle duration was short. This review discusses the survival benefit of 5 alpha-reductase inhibition in IADT in animal models and the potential translation of this finding into clinic.
Keywords: 5 alpha-reductase inhibitor, androgen-responsive genes, intermittent androgen deprivation therapy (IADT), prostate cancer, testosterone
INTRODUCTION
In 2013, there will be an estimated 238 590 new patients with prostate cancer and 29 720 deaths related to prostate cancer in the United States.1 Thus, prostate cancer is the most frequently diagnosed and second leading cause of cancer deaths in males in the United States.1 Worldwide 914 000 new cases of prostate cancer were diagnosed in 2008. Even though three-quarters of new patients (659 000) were detected in developed countries, the number of deaths from prostate cancer was similar between developed (136 000) and developing regions (122 000).2 Thus, there is an increasing need to improve current prostate cancer treatments available in the developing world. Although the prostate cancer incidence rate in China and other Asian countries is much lower than in Western countries, both incidence and death rate have been rising rapidly in recent years in China.3 As a result, prostate cancer research is becoming increasingly important in China.
In their seminal study, published in 1941, Dr. Charles Huggins and Dr. Clarence Hodges4,5 investigated the role of androgens in prostate cancer. They observed that patients with metastatic prostate cancer developed remarkable improvements in clinical symptoms and circulating acid phosphatases (a surrogate biomarker for bony metastatic disease) in response to orchiectomy (surgical castration) or diethylstilbestrol (chemical castration).4,5 Nearly 3 decades later in 1971, Andrew Schally and Roger Guillemin6,7 discovered the structure of luteinizing hormone releasing hormone (LHRH) in separate laboratories. The Leuprolide Study compared leuprolide (1 mg daily subcutaneously) to diethylsilbesterol (3 mg oral daily) in patients with metastatic hormone-naïve prostate adenocarcinoma. In this study, leuprolide (an LHRH analogue) was noted to have similar efficacy, but significant improvement in painful gynecomastia, nausea, vomiting, edema and thromboembolism compared to diethylsilbesterol.8 ADT using LHRH agonists has remained the mainstay of treatment and multiple options including long-acting depot preparations and LHRH antagonists have been developed to achieve chemical castration defined as a testosterone level of less than 50 ng dl−1.9 Given the ease of use and the option to stop, LHRH agonists have largely replaced surgical orchiectomy for the treatment of metastatic prostate cancer.
In patients with metastatic prostate adenocarcinoma, treatment with ADT is palliative and most patients eventually develop castrate resistance defined as disease progression in spite of a total testosterone level of < 1.7 nmol l−1. Newly developed therapies for castrate resistant metastatic prostate cancer include hormonal agents like enzalutamide and abiraterone, immunotherapy with sipuleucel-T, cytotoxic chemotherapy with cabazitaxel and novel radioimmunotherapy using Radium-223. These approaches can prolong survival of patients with castrate resistant prostate cancer by a few months to a few years.10,11,12,13,14,15,16,17 Therefore, it is highly desirable to delay the progression of prostate cancer to castration resistance. ADT is also known to cause a wide array of side-effects, including hot flashes, gynecomastia, loss of libido, erectile dysfunction, metabolic syndrome, weight gain and potential to develop muscle and bone loss.9 In an effort to reduce the side effects in an endocrine therapy setting, Klotz and colleagues18 treated 20 symptomatic patients with advanced prostate cancer using intermittent endocrine therapy using diethylstilbestrol until clinical improvement was demonstrated and withheld therapy until symptoms recurred. They observed adequate palliation of symptoms with improvement in sexual side-effects using intermittent endocrine therapy.18 Independently, Bruchovsky and colleagues19,20,21,22 proposed the mechanism of intermittent androgen deprivation therapy (IADT); intermittent recovery of androgen levels during IADT could restore or enhance the apoptotic potential of prostate cancer cells, thereby delaying the progression to castration resistance. Using the Shionogi mammary androgen-dependent tumor model, Bruchovsky and colleagues22 showed intermittent exposure to testosterone slowed down the progression of Shionogi tumor to castration resistance. There have been multiple Phase II and III trials since the initial development of IADT.23,24 These clinical studies have clarified the benefits of IADT in quality of life. Patients on IADT exhibited fewer hot flashes, improved sexual function and fewer problems with bone health. Three thousand and forty men (n = 3040) with metastatic, hormone-sensitive prostate adenocarcinoma were randomized to receive ADT or IADT. Of the 1535 evaluable patients, 770 were randomly assigned to receive ADT and 765 patients to receive IADT with a median follow-up of 9.8 years. Median survival after randomization was 5.8 years for ADT arm compared to 5.1 years for the IADT arm with statistically significant improvement in erectile dysfunction and mental health in the IADT arm at 3 months follow-up but not thereafter. There was a 10% relative increase in the risk of death in the IADT arm, compared to continuous ADT; however, a 20% increase relative risk of death in the IADT arm over the ADT arm could not be ruled out with 90% confidence. Thus, the final results were inconclusive and continuous ADT remains the standard of care in patients with metastatic hormone naïve prostate cancer.25 Interestingly, after randomization to continuous ADT or IADT it took nearly 5 years for the survival curves to separate which highlights the significance of long term follow-up in these patients.
In contrast, for patients with biochemical recurrence after primary or salvage radiotherapy for localized prostate cancer, IADT was noninferior to ADT (median overall survival 8.8 vs 9.1 years, respectively). IADT was associated with statistically significant improvements in hot flashes, sexual desire and urinary symptoms compared to ADT in patients with biochemical recurrence.26 Further improvement of IADT through basic and translational research could have significant implications in prostate cancer treatment. Understanding the mechanisms of androgen activity in the prostate will provide guidance to potentially improve IADT.
Androgens: a double-edged sword
Androgens play an important role in prostate growth, development and homeostasis.27 In animal studies, androgen deprivation by castration leads to dramatic prostate regression via apoptosis.28,29,30 On the other hand, androgen replacement stimulates rapid proliferation and differentiation of a regressed prostate until it reaches normal size.27 Androgen action in a regressed prostate is very different from that in a full-grown prostate because androgens do not stimulate proliferation in a full-grown prostate (Table 1). During the regrowth of a regressed prostate, androgens induce proliferation transiently and induce and maintain differentiation.
Table 1.
Response of regressed prostate and full-grown prostate to androgen manipulation
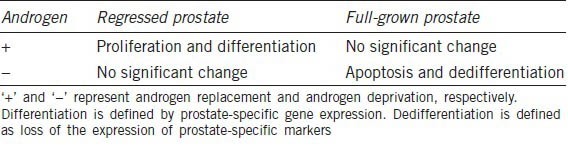
Androgen action in prostate tumor cells exhibits some similarities with that in the normal prostate because many of the androgen-responsive genes expressed in the normal prostate, such as prostate-specific antigen (PSA), remain responsive to androgens in prostate cancer cells.31,32 Androgens induce prostate cancer cell proliferation while stimulating differentiation, which is marked by the expression of PSA. While androgen-stimulated proliferation of prostate cancer cells is not desirable, androgen-stimulated differentiation of prostate cancer cells is likely beneficial to patients. Androgen-induced differentiation of prostate cancer cells is likely associated with increased apoptotic potential.33 Novel approaches capable of specifically suppressing androgen-induced proliferation but not differentiation in prostate cancer cells may potentially inhibit prostate tumor growth and progression.
Differential actions of testosterone versus dihydrotestosterone (DHT)
Testosterone and DHT are the two major biologically active androgens in animals.34,35,36,37 Testosterone is synthesized in the testes and then transported to target organs via blood circulation. Testosterone can be converted to DHT in target organs such as the prostate by 5 alpha-reductase.38 DHT is more potent than testosterone in activating promoters containing androgen-responsive elements in cell-based transfection assays39,40 and the conversion of testosterone to DHT is necessary for normal prostate development because 5 alpha-reductase inactivation prevents normal prostate development.41,42 It was thought that the conversion was merely an amplification step for androgen action. However, this seems to be an oversimplification, as suggested by recent findings from our lab and other investigators.43,44,45,46
An unexpected finding in our previous study was that testosterone was more potent than DHT in induction of a subset of androgen-responsive genes during regrowth of regressed prostate.44 Type II 5 alpha-reductase inhibitor, finasteride enhanced the expression of growth-inhibitory androgen-responsive genes during testosterone-stimulated regrowth of the regressed prostate and not in the full-grown prostate. This observation seemed counterintuitive because DHT is more potent than testosterone in activating the androgen receptor. The exact mechanism by which testosterone is more potent than DHT in inducing the expression of androgen-responsive genes in the regressed prostate is under evaluation. This phenomenon was only observed in the regressed prostate, but not in the full-grown prostate.44 Blocking testosterone conversion to DHT by finasteride inhibited the expression of androgen-responsive genes in the full-grown prostate. The above observations suggest that the mechanisms of androgen action in the regressed prostate are different from that in the intact prostate.
The elevated expression of androgen-responsive genes by finasteride was associated with inhibition of prostate regrowth upon testosterone replacement.44 We were originally puzzled by this observation. However, this puzzle is partly resolved by functional studies of various androgen-responsive genes including EAF2/U19, calreticulin and adrenomedullin that demonstrated their inhibition of prostate cancer cell proliferation in vitro.47,48,49,50,51 Among these growth-suppressive androgen-responsive genes, EAF2/U19 is particularly potent. Transfected expression of EAF2/U19 induced apoptosis in cultured cells and suppressed xenograft tumor growth in nude mice.48 Furthermore, EAF2/U19 knockout mice had increased prostate epithelial cell proliferation and developed high-grade prostatic intraepithelial neoplasia, which further supports a tumor suppressive role of EAF2/U19.47
The effect of blocking testosterone to DHT conversion in IADT off-cycle
Androgen-sensitive tumors may mimic androgen-responsiveness of the normal prostate. Based on this assumption, we predicted that blocking testosterone to DHT conversion should also enhance the induction of androgen-responsive genes by testosterone during regrowth of regressed prostate tumors. Indeed, our studies demonstrated that finasteride enhanced the expression of androgen-responsive genes, particularly the tumor suppressive androgen-responsive gene EAF2/U19 during the initial regrowth of the regressed prostate tumor in the LNCaP xenograft tumor model.52 The enhancement of EAF2/U19 expression by finasteride was approximately two-fold and statistically significant. Similar results were observed when a dual (Type I and II) 5-alpha reductase inhibitor, dutasteride was used.52 The increased expression of EAF2/U19 expression by finasteride or dutasteride was only observed during the initial regrowth of LNCaP tumor, and no enhancement of EAF2/U19 mRNA level by finasteride or dutasteride was observed at day 14 after the testosterone replacement in castrated animals.53
The elevated expression of tumor suppressive genes such as EAF2/U19 during the initial tumor regrowth by 5 alpha-reductase inhibitors can be potentially translated into the improvement of IADT. IADT involves tumor regression and regrowth, which provides an opportunity for applying 5 alpha-reductase inhibitors during tumor regrowth in the off-cycle. Our objective was to test whether administration of 5 alpha-reductase inhibitors during the off-cycle can improve the efficacy of IADT leading to survival benefits in the animal models. We tested this hypothesis in LNCaP xenograft tumors because LNCaP is a widely used androgen-sensitive prostate tumor model.54 In our studies, LNCaP cells were subcutaneously injected into nude mice. When the tumor volume reached ~0.5 ml, animals were castrated and randomized to four different groups: (i) continuous androgen deprivation (ADT), (ii) ADT plus finasteride (ADT + F), (iii) IADT (testosterone implantation after 10–14 days) and (iv) IADT plus finasteride (IADT + F) (Figure 1).55 Testosterone implantation in groups 3 and 4 mimics the intermittent recovery of testicular function during the ‘off-cycle’ of IADT. After one cycle of treatment, mice treated with IADT + F displayed significantly less tumor growth as compared to the other treatment group. Most importantly, mice treated with IADT + F had the longest survival and the difference was statistically significant.55 One key question is whether the prolonged survival of animals by 5 alpha-reductase inhibitor during the off-cycle of IADT can be translated into clinical improvement in human patients.
Figure 1.
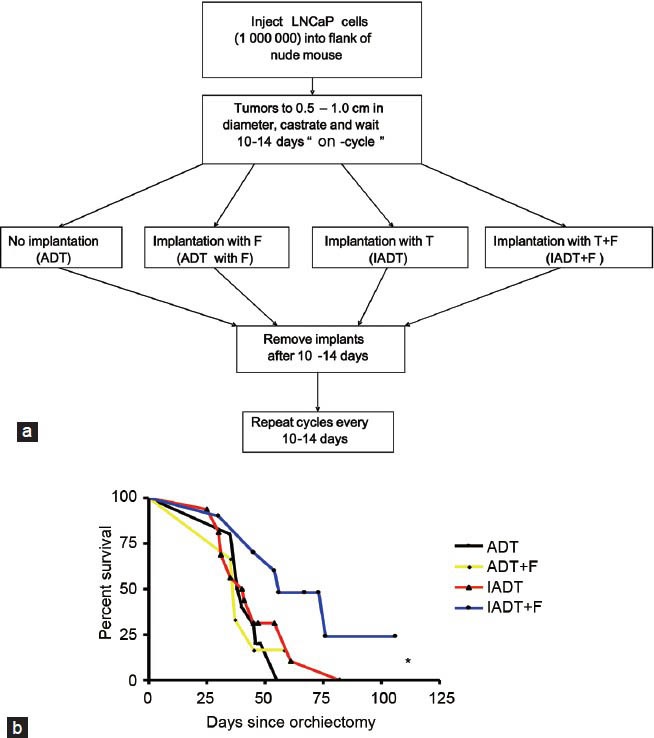
(a) Treatment strategy of LNCaP xenograft tumor in nude mice. After the tumor establishment, animals were castrated and tumors are allowed to regress for 10–14 days, which was considered as the ‘on-cycle’ of androgen ablation during intermittent androgen deprivation (IADT). The animals were then implanted with no pellet, testosterone (T) pellet, finasteride (F) pellet or both T and F pellets for 14 days or longer. Testosterone implantation mimics intermittent recovery of testicular function during the ‘off-cycle’ of IADT. Controls were continuous androgen deprivation (ADT) in the absence or presence of finasteride. (b) Kaplan-Meier survival curve of indicated treatment groups. Euthanasia was performed if tumor diameter > 2.0 cm, tumor ulceration or tumor-related morbidity. *P < 0.05. From Eggener et al.55
We recognized in the above preclinical animal model, the off-cycle duration in IADT was fixed, which is different from the real world scenario. For prostate cancer patients on IADT, the switch from off-cycle to on-cycle is determined based on the serum PSA value, which is determined by the tumor volume and PSA expression level within the tumor. To mimic IADT in a clinical setting, we conducted IADT in the animal model using a predetermined tumor volume as the trigger for switch from the off-cycle to on-cycle.56 Because 5 alpha-reductase inhibitor could reduce tumor growth rate, the off-cycle duration was prolonged in the presence of finasteride. In the LNCaP model, we observed a two-fold prolongation of off-cycle duration. However, the 5 alpha-reductase inhibition did not result in a survival benefit to animals on IADT when off-cycle duration was not fixed and prolongation was allowed.56
A retrospective analysis of clinical trials showed that finasteride treatment during the off-cycle in IADT prolonged off-cycle duration (31 vs 15 months) when compared to the control IADT arm without finasteride.57 In the clinical setting, the switch from off-cycle to on-cycle was determined based on serum-based PSA value. The two-fold increase in off-cycle duration by finasteride in patients on IADT is likely due to the possibility that 5 alpha-reductase inhibitor could reduce tumor growth and also can inhibit the expression of PSA. However, the two-fold increase in off-cycle duration by finasteride administration did not translate into survival benefit for patients on IADT.57 The above clinical trial results are similar to our preclinical study of IADT using a predetermined tumor volume as a trigger for switching off-cycle to on-cycle, which showed that finasteride also led to a two-fold increase in off-cycle duration without survival benefits for the animals.56 The finasteride effect on IADT in human patients and the animal model were very similar when off-cycle prolongation was allowed. One temptation is to speculate that the finasteride effect on IADT between human patients and the animal model should also be very similar when off-cycle was fixed and not prolonged. If this is the case, 5 alpha-reductase inhibitor should also prolong the survival of prostate cancer patients on IADT when the off-cycle duration is not prolonged. A randomized clinical trial can address this important question.
We were intrigued by the observation that 5 alpha-reductase inhibition plus IADT resulted in survival benefits only when the off-cycle duration was fixed and prolongation was not permitted.55,56 To explore the mechanism underlying this interesting and clinically relevant phenomenon, we compared the growth curve of testosterone-stimulated regrowth of regressed prostate tumors in the absence or presence of 5 alpha-reductase inhibitors. We observed that finasteride or dutasteride caused an initial inhibition of regrowth and then the tumors resume growth at the same rate as that of the control group as measured by tumor volume (Figure 2). The initial regrowth inhibition by 5 alpha-reductase inhibitor was confirmed by reduced Ki-67 staining and BrdU incorporation assay.53 Both assays showed a statistically significant inhibition (~10-fold) of proliferation index by 5 alpha-reductase inhibitors during the initial phase of regrowth, but not after prolonged exposure to testosterone. A control experiment showed that finasteride or dutasteride inhibition of testosterone-induced initial regrowth of prostate xenograft tumor was not inhibited by letrozole, a potent inhibitor of aromatase, an enzyme that converts testosterone to estradiol. Thus, the inhibition of initial regrowth by 5 alpha-reductase inhibitors was unlikely mediated through the conversion of testosterone to estrogen when testosterone conversion to DHT was blocked.
Figure 2.
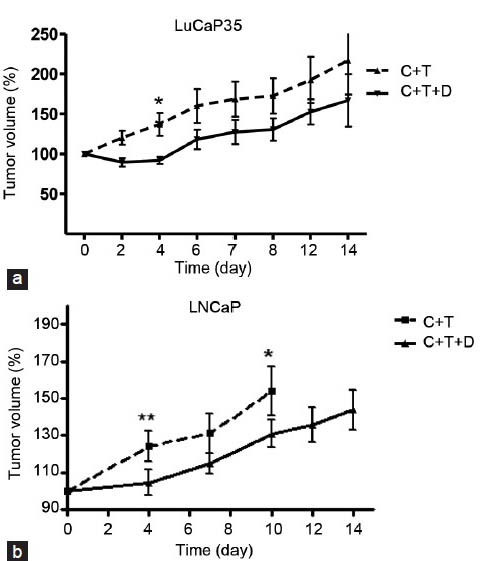
Response of LuCaP35 and LNCaP xenograft tumors to 5 alpha-reductase inhibitors—dutasteride and finasteride. (a) Effect of dutasteride on LuCaP35 tumor volume. Tumor volume in castrated animals with testosterone replacement (C + T) and animals treated with dutasteride (C + T + D) were determined at the indicated time points. (b) Effect of finasteride on LNCaP tumor volume. Tumor volume in castrated animals with testosterone replacement (C + T) and in castrated animals with testosterone replacement along with finasteride (C + T + F) at indicated time points were plotted. Tumor volume (%) was determined as the percentage of the tumor volume at the time of implantation of T, D and/or F pellets. *P < 0.05 and **P < 0.01, respectively. From Masoodi et al.53
Inhibition of the initial regrowth of prostate cancer by 5 alpha-reductase inhibitor during the off-cycle also occurred in patients. In a Phase II clinical trial led by Dr. Daniel Shevrin,58 dutasteride significantly inhibited Ki-67 index during the initial phase of regrowth of regressed prostate cancer. It is important to point out that dutasteride did not inhibit Ki67 index in human prostate cancers naïve to androgen manipulation.59 According to our knowledge, this is the first demonstration of dutasteride inhibition of proliferation in prostate cancer, which occurred only in the initial phase of tumor regrowth during the off-cycles. IADT created a window of opportunity, which is the initial phase of the off-cycle, for finasteride or dutasteride to inhibit prostate tumor regrowth.
Based on our studies, we propose that testosterone and DHT can function very differently during the initial phase regrowth of regressed prostate tumor (Figure 3a). DHT stimulates proliferation and differentiation in the regressed prostate tumor. In contrast, testosterone can stimulate differentiation, as marked by PSA expression, but not proliferation during the initial phase of prostate tumor regrowth. Once prostate cancer cells have differentiated, they may respond to testosterone by undergoing proliferation. This model provides a potential mechanism to explain the phenomenon that the survival benefit was lost when off-cycle prolongation was allowed in the preclinical animal model. The prolonged stimulation of testosterone in the presence of finasteride or dutasteride can also effectively stimulate prostate cancer proliferation (Figure 3b). In contrast, when off-cycle prolongation is not permitted, testosterone can stimulate prostate cancer cell differentiation with minimal proliferation during the initial tumor regrowth (Figure 3c), which may be the underlying mechanism for finasteride or dutasteride to prolong the survival when the off-cycle duration is short in IADT.
Figure 3.
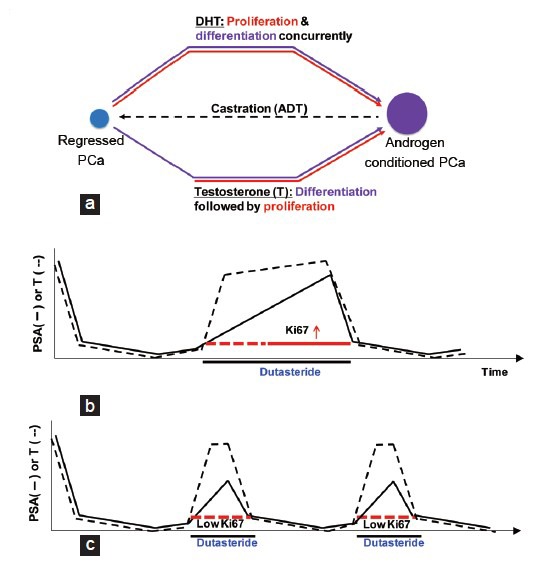
(a) Model for differential effect of testosterone and DHT on prostate tumor regrowth. Differentiation is defined as the expression of prostate-specific markers such as prostate-specific antigen (PSA). Proliferation was measured by Ki-67 staining or BrdU incorporation. (b) The effect of dutasteride on intermittent androgen deprivation therapy (IADT) when off-cycle prolongation was allowed. (c) The effect of dutasteride on IADT when off-cycle prolongation was not allowed. In panels (b) and (c), dotted red lines indicate low Ki-67 index as compared to the solid red line. The expected serum testosterone (T) and PSA levels are indicated in the diagram. Androgen deprivation occurs in the on-cycle (On) and testicular function is allowed to recover in the off-cycle (Off)
FUTURE PERSPECTIVE
Our basic and translational research led to a testable hypothesis that 5 alpha-reductase inhibition during the off-cycle of IADT can prolong the survival of patients when the off-cycle duration is short. Since we observed a significant inhibition of Ki-67 index during the initial phase of off-cycle by dutasteride in prostate cancer patients in a Phase II clinical study, the observation of Ki-67 inhibition by finasteride or dutasteride during the initial off-cycle in the animal model is clinically relevant. A challenge of using finasteride or dutasteride in patients on IADT is to establish a new trigger for the switch from the off-cycle to the on-cycle in IADT because using serum PSA value as a trigger can result in a two fold increase in the off-cycle duration, which is not desirable. We suggest that a predetermined serum testosterone level could be used as a trigger for switching from the off-cycle to the on-cycle. However, testosterone levels may vary from patient-to-patient, which could be an issue, as some patients have particularly low testosterone levels and/or slow recovery of testosterone. Theoretically, endogenous and exogenous testosterone are identical, and a brief period of exogenous testosterone administration together with dutasteride could be utilized to stimulate prostate cancer cell differentiation without stimulating proliferation in the off-cycle. Since the effects of exogenous testosterone administration in prostate cancer patients are not well studied and can potentially have deleterious effects, clinical trials will be required to determine the potential benefits and risks of exogenous testosterone treatment. Obviously, we will need to learn more about the molecular mechanisms underlying testosterone versus DHT action in IADT before we can explore the use of exogenous testosterone.
Newer therapeutic agents like enzalutamide and abiraterone are being developed for use very early in the disease. One possibility may be to also incorporate enzalutamide or abiraterone in IADT, with enzalutamide or abiraterone being used in the on-cycle and dutasteride in the off-cycle. If finasteride or dutasteride can improve the efficacy of IADT, the impact will be very significant because of longer survival; improved quality of life; and reduced cost due to less usage of LHRH agonists, enzalutamide and/or abiraterone. We hope this review article can stimulate further discussion on the potential improvement of IADT by 5 alpha-reductase inhibition and eventually lead to a clinical trial to determine if finasteride or dutasteride can prolong the survival of prostate cancer patients on IADT when short off-cycle durations are used.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors would like to thank the organizers of the 7th Shanghai-PCF Joint Symposium. The research was supported in part by National Institutes of Health Grants 1 P50 CA90386 and 5 R37 DK51193.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Ren SC, Chen R, Sun YH. Prostate cancer research in China. Asian J Androl. 2013;15:350–3. doi: 10.1038/aja.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer.I. The effects of castration of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. [Google Scholar]
- 5.Huggins C, Stevens RJ, Hodges CV. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23. [Google Scholar]
- 6.Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43:393–9. doi: 10.1016/0006-291x(71)90766-2. [DOI] [PubMed] [Google Scholar]
- 7.Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, et al. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44:205–10. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- 8.The Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med. 1984;311:1281–6. doi: 10.1056/NEJM198411153112004. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski JM, Grayhack JT. Carcinoma of the Prostate. In: Gillenwater J.Y, Grayhack JT, Howards S.S, Duckett J.W, editors. Adult and Pediatric Urology. 2nd. Chicago: Mosby Year Book; 1991. pp. 1277–393. [Google Scholar]
- 10.Courtney KD, Taplin ME. The evolving paradigm of second-line hormonal therapy options for castration-resistant prostate cancer. Curr Opin Oncol. 2012;24:272–7. doi: 10.1097/CCO.0b013e328351059d. [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lorenzo G, Ferro M, Buonerba C. Sipuleucel-T (Provenge®) for castration-resistant prostate cancer. BJU Int. 2012;110:E99–104. doi: 10.1111/j.1464-410X.2011.10790.x. [DOI] [PubMed] [Google Scholar]
- 13.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 18.Klotz LH, Herr HW, Morse MJ, Whitmore WF., Jr Intermittent endocrine therapy for advanced prostate cancer. Cancer. 1986;58:2546–50. doi: 10.1002/1097-0142(19861201)58:11<2546::aid-cncr2820581131>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, et al. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–90. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Bruchovsky N, Klotz LH, Sadar M, Crook JM, Hoffart D, et al. Intermittent androgen suppression for prostate cancer: canadian Prospective Trial and related observations. Mol Urol. 2000;4:191–9. [PubMed] [Google Scholar]
- 21.Bruchovsky N, Snoek R, Rennie PS, Akakura K, Goldenberg LS, et al. Control of tumor progression by maintenance of apoptosis. Prostate Suppl. 1996;6:13–21. [PubMed] [Google Scholar]
- 22.Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, To M, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–82. [PubMed] [Google Scholar]
- 23.Klotz L, Toren P. Androgen deprivation therapy in advanced prostate cancer: is intermittent therapy the new standard of care? Curr Oncol. 2012;19:S13–21. doi: 10.3747/co.19.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz L. Intermittent versus continuous androgen deprivation therapy in advanced prostate cancer. Curr Urol Rep. 2013;14:159–67. doi: 10.1007/s11934-013-0325-x. [DOI] [PubMed] [Google Scholar]
- 25.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–25. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruchovsky N, Lesser B, Van Doorn E, Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/s0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- 28.English HF, Kyprianou N, Isaacs JT. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. Prostate. 1989;15:233–50. doi: 10.1002/pros.2990150304. [DOI] [PubMed] [Google Scholar]
- 29.Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–62. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- 30.Colombel M, Olsson CA, Ng PY, Buttyan R. Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res. 1992;52:4313–9. [PubMed] [Google Scholar]
- 31.Montgomery BT, Young CY, Bilhartz DL, Andrews PE, Prescott JL, et al. Hormonal regulation of prostate-specific antigen (PSA) glycoprotein in the human prostatic adenocarcinoma cell line, LNCaP. Prostate. 1992;21:63–73. doi: 10.1002/pros.2990210107. [DOI] [PubMed] [Google Scholar]
- 32.Schuur ER, Henderson GA, Kmetec LA, Miller JD, Lamparski HG, et al. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–51. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- 33.Gleave M, Bruchovsky N, Goldenberg SL, Rennie P. Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Eur Urol. 1998;34:37–41. doi: 10.1159/000052297. [DOI] [PubMed] [Google Scholar]
- 34.Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–21. [PubMed] [Google Scholar]
- 35.George F, Wilson J. Sex Determination and Differentiation. In: Knobil E, Neill J, editors. The Physiology of Reproduction. 2nd ed. Vol. 1. New York: Raven Press Ltd; 1994. pp. 3–28. [Google Scholar]
- 36.Liao S, Liang T, Fang S, Castaneda E, Shao TC. Steroid structure and androgenic activity. Specificities involved in the receptor binding and nuclear retention of various androgens. J Biol Chem. 1973;248:6154–62. [PubMed] [Google Scholar]
- 37.Wilson EM, French FS. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976;251:5620–9. [PubMed] [Google Scholar]
- 38.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 39.Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 40.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–72. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins EP, Andersson S, Imperato-McGinley J, Wilson JD, Russell DW. Genetic and pharmacological evidence for more than one human steroid 5 alpha-reductase. J Clin Invest. 1992;89:293–300. doi: 10.1172/JCI115574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh PC, Madden JD, Harrod MJ, Goldstein JL, MacDonald PC, et al. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974;291:944–9. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- 43.Avila DM, Fuqua SA, George FW, McPhaul MJ. Identification of genes expressed in the rat prostate that are modulated differently by castration and Finasteride treatment. J Endocrinol. 1998;159:403–11. doi: 10.1677/joe.0.1590403. [DOI] [PubMed] [Google Scholar]
- 44.Dadras SS, Cai X, Abasolo I, Wang Z. Inhibition of 5alpha-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr. 2001;9:183–94. doi: 10.3727/000000001783992551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George FW, Russell DW, Wilson JD. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci U S A. 1991;88:8044–7. doi: 10.1073/pnas.88.18.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin TM, Chang C. Cloning and characterization of TDD5, an androgen target gene that is differentially repressed by testosterone and dihydrotestosterone. Proc Natl Acad Sci U S A. 1997;94:4988–993. doi: 10.1073/pnas.94.10.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, et al. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene. 2008;27:1536–44. doi: 10.1038/sj.onc.1210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, et al. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63:4698–704. [PubMed] [Google Scholar]
- 49.Alur M, Nguyen MM, Eggener SE, Jiang F, Dadras SS, et al. Suppressive roles of calreticulin in prostate cancer growth and metastasis. Am J Pathol. 2009;175:882–90. doi: 10.2353/ajpath.2009.080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abasolo I, Wang Z, Montuenga LM, Calvo A. Adrenomedullin inhibits prostate cancer cell proliferation through a cAMP-independent autocrine mechanism. Biochem Biophys Res Commun. 2004;322:878–86. doi: 10.1016/j.bbrc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Abasolo I, Yang L, Haleem R, Xiao W, Pio R, et al. Overexpression of adrenomedullin gene markedly inhibits proliferation of PC3 prostate cancer cells in vitro and in vivo. Mol Cell Endocrinol. 2003;199:179–87. doi: 10.1016/s0303-7207(02)00229-0. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S, Wang Y, Ramos-Garcia R, Shevrin D, Nelson JB, et al. Inhibition of 5alpha-reductase enhances testosterone-induced expression of U19/Eaf2 tumor suppressor during the regrowth of LNCaP xenograft tumor in nude mice. Prostate. 2010;70:1575–85. doi: 10.1002/pros.21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masoodi KZ, Ramos Garcia R, Pascal LE, Wang Y, Ma HM, et al. 5α-reductase inhibition suppresses testosterone-induced initial regrowth of regressed xenograft prostate tumors in animal models. Endocrinology. 2013;154:2296–307. doi: 10.1210/en.2012-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–18. [PubMed] [Google Scholar]
- 55.Eggener SE, Stern JA, Jain PM, Oram S, Ai J, et al. Enhancement of intermittent androgen ablation by “off-cycle” maintenance with finasteride in LNCaP prostate cancer xenograft model. Prostate. 2006;66:495–502. doi: 10.1002/pros.20297. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Gupta S, Hua V, Ramos-Garcia R, Shevrin D, et al. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate. 2010;70:147–54. doi: 10.1002/pros.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scholz MC, Jennrich RI, Strum SB, Johnson HJ, Guess BW, et al. Intermittent use of testosterone inactivating pharmaceuticals using finasteride prolongs the time off period. J Urol. 2006;175:1673–8. doi: 10.1016/S0022-5347(05)00975-4. [DOI] [PubMed] [Google Scholar]
- 58.Shevrin DH, MacVicar GR, Kuzel T, Stadler WM, Jovanovic B, et al. Effect of dutasteride on tumor proliferation during the regrowth phase of intermittent androgen ablation therapy in men with advanced prostate cancer. ASCO 2013 Genitourinary Cancers Symposium. J Clin Oncol. 2013;(suppl 6) abstr 21. [Google Scholar]
- 59.Gleave M, Qian J, Andreou C, Pommerville P, Chin J, et al. The effects of the dual 5alpha-reductase inhibitor dutasteride on localized prostate cancer--results from a 4-month pre-radical prostatectomy study. Prostate. 2006;66:1674–85. doi: 10.1002/pros.20499. [DOI] [PubMed] [Google Scholar]


