Abstract
Prostate cancer is the second most common cancer in men, with 1.1 million new cases worldwide reported by the World Health Organization in one recent year. Transrectal ultrasound (TRUS)-guided biopsy has been used for the diagnosis of prostate cancer for over 2 decades, but the technique is usually blind to cancer location. Moreover, the false negative rate of TRUS biopsy has been reported to be as high as 47%. Multiparametric magnetic resonance imaging (mp-MRI) includes T1- and T2-weighted imaging as well as dynamic contrast-enhanced (DCE) and diffusion-weighted imaging (DWI). mp-MRI is a major advance in the imaging of prostate cancer, enabling targeted biopsy of suspicious lesions. Evolving targeted biopsy techniques—including direct in-bore biopsy, cognitive fusion and software-based MRI-ultrasound (MRI-US) fusion—have led to a several-fold improvement in cancer detection compared to the earlier method. Importantly, the detection of clinically significant cancers has been greatly facilitated by targeting, compared to systematic biopsy alone. Targeted biopsy via MRI-US fusion may dramatically alter the way prostate cancer is diagnosed and managed.
Keywords: fusion biopsy, magnetic resonance imaging (MRI), prostate biopsy, prostate cancer, targeted biopsy, ultrasound
INTRODUCTION
Prostate cancer is the second most common cancer in men, with 1.1 million new cases worldwide reported by the World Health Organization in 2012.1 Biopsy for the diagnosis of prostate cancer, first described nearly a century ago,2 is in a period of rapid evolution today. This paper describes advances in multiparametric magnetic resonance imaging (mp-MRI) for diagnosis of prostate cancer and the opportunities afforded for tumor detection and targeted biopsy. In-bore, cognitive and software-based MRI-US fusion techniques are discussed, and future directions in clinical research are highlighted.
Transrectal ultrasound (TRUS)-guided systematic sextant biopsy
In 1989, Hodge et al.3,4 published landmark studies describing TRUS-guided systematic sextant biopsy of the prostate. They also advocated US-guided biopsies of any hypoechoic lesions not captured by systematic sampling. This work provided a major improvement in cancer detection over the previously used technique of digitally-guided prostate biopsy, and over the next decade, the sextant biopsy became accepted as standard of care.5
The 1980s also bore witness to the introduction of prostate-specific antigen (PSA), which received approval by the Food and Drug Administration (FDA) of the United States for monitoring of prostate cancer progression in 1986 and for screening in asymptomatic men in 1994.6 Widespread adoption of PSA screening in Western countries has resulted in the downward risk migration of prostate cancer.5 In the United States, by the early 2000s, only 16% of prostate cancer diagnoses were classified as high risk.7
The classic hypoechoic lesion, corresponding to a palpable nodule, has now given way to the current era where TRUS-guided prostate biopsy is a largely blind endeavor. By 2000, 25% of patients were diagnosed with cancer within 1 year of an initial negative biopsy, highlighting a need for improved biopsy strategy.8 Nevertheless, the method of TRUS-guided biopsy remained the standard for diagnosis of prostate cancer.
Evolution of modern TRUS biopsy
Efforts to improve the cancer detection rate of the original sextant biopsy have given rise to extended biopsy schemes, with attention to lateral peripheral zone and anterior apex sampling. Extended biopsy schemes have been found to detect cancers missed by the traditional sextant biopsy.9,10,11,12,13 The current recommendation is for a standard 12-core biopsy.14,15 Still, the false negative rate for TRUS biopsy remains high, with initial biopsies missing cancer in 21%–47% of patients.16,17
Patients for whom clinical suspicion for cancer remains high, despite negative biopsy, represent a clinical dilemma and are often subjected to repeated biopsies with diminishing returns. Thirty-eight percent of Medicare patients undergo repeat biopsy within 5 years.18 Cancer detection declines with each subsequent biopsy. In large cohort studies, Keetch et al.19 reported cancer detection rates of 19%, 8% and 7% on first, second and third repeat TRUS biopsy results, respectively; and Djavan et al.20 similarly reported declining detection rates of 10%, 5% and 4% with subsequent biopsies.
Saturation biopsy schemes, employing 24–45 cores taken under anesthesia, may not detect more significant cancers than standard TRUS biopsy but do incur increased morbidity, particularly urinary retention.21,22,23 Other authors have described transperineal template biopsy, which is purported to uniformly sample the prostate with improved detection of anterior and apical tumors, but this procedure requires general anesthesia and substantial biopsy-related morbidity.17,24,25
Furthermore, saturation biopsy strategies may lead to detection of many clinically insignificant cancers. Zaytoun et al.26 reported 40% of cancers detected on transrectal saturation biopsy were clinically insignificant. Taira et al.17 reported 44% of cancers detected using transperineal biopsy were Gleason grade 6, and Pinkstaff et al.25 reported 55% Gleason 6 or less. The ‘vanishing’ prostate cancer phenomenon27 may be an extreme example of detection of clinically insignificant cancers. Thus, a more rational strategy than simply increasing numbers of systematic biopsy cores may be directed targeting of tumors radiologically identified prior to biopsy. Multiparametric MRI provides this opportunity.
MULTIPARAMETRIC MRI OF THE PROSTATE
In 1990, a large, multicenter trial comparing performance of MRI and TRUS found that neither modality was highly accurate in predicting pathologic staging following radical prostatectomy for clinically localized disease.28 However, in the more than two intervening decades, an approach for mp-MRI of the prostate has evolved, combining information from various sequences including T1- and T2-weighted, dynamic contrast-enhanced (DCE), diffusion-weighted imaging (DWI) and MR spectroscopy. These advances have greatly improved tumor detection and staging in the hands of experienced radiologists.29,30
T2-weighted images allow for excellent spatial resolution and delineation of prostate anatomy. Prostate cancer lesions may be visualized as low intensity lesions on T2, and tumor-to-muscle signal intensity ratio on has been shown to correlate with increasing Gleason grade.31 The sensitivity for cancer detection using T2 alone varies widely in the literature, from 60% to 96%; given disparate methodology and definitions of significant disease.32
Post-biopsy hemorrhage can confound interpretation of T2-weighted images, with low intensity artifact that must be correlated with high intensity changes on T1-weighted imaging. Hemorrhage may take months to resolve, and capsular irregularity may persist indefinitely.33 Most cancers arise in the peripheral zone; in the transition zone, heterogeneous signal from benign prostatic hyperplasia poses additional diagnostic challenge. Homogeneous low signal intensity, lenticular shape and invasion of the anterior fibromuscular stroma have been found useful in identification of cancers in the transition zone.34
To overcome these difficulties and assist in tumor detection, additional imaging sequences are used that take advantage of microvasculature/perfusion and cellular density differences between tumor and surrounding tissue. DCE examines enhancement patterns and pharmacokinetic properties of the prostate following contrast administration.35,36,37 In DWI, increased cellular density is reflected in a decreased apparent diffusion coefficient (ADC). The quantifiable nature of ADC makes it a useful research and clinical tool, and it has been shown to correlate with density of prostate cancer in prostatectomy specimens as well as increased Gleason and D’Amico risk scores.38,39,40
Minimal requirements for a diagnostic mp-MRI protocol should include T1, T2, DCE and DWI; neither the use of an endorectal coil nor MR spectroscopy appears to be requisite for diagnostic purposes. An endorectal coil may further improve anatomic detail to improve local staging accuracy, but is not required for the purposes of tumor detection.41,42 MR spectroscopy identifies chemical signatures owing to different metabolic rates between tumor and benign tissue; this may add specificity to lesion characterization, although a multi-institution study failed to show improvement of MRI specificity by addition of spectroscopy, possibly due to varying study quality and experience between centers.43
Reporting systems of mp-MRI vary widely. For example, investigators at the University of California, Los Angeles (UCLA) use a 5-point Likert-type scale that has been previously described.44 The establishment of a standardized, validated reporting scheme is imperative for cross-study interpretation of mp-MRI accuracy.45 The European Society of Urogenital Radiology (ESUR) recently published guidelines in an attempt to standardize acquisition and reporting of prostate MRI.46 Protocols for tumor detection and tumor staging were recommended, as well as a structured reporting system called PI-RADS (Prostate Imaging-Reporting and Data System), following the precedent set by breast cancer imaging. Initial studies demonstrate inter-reader agreement using this system.47 The American College of Radiology is in the process of establishing its own guidelines.
MRI-GUIDED TARGETED BIOPSY
Given the advances in prostate imaging, a promising role has emerged for biopsy modalities that target lesions identified by mp-MRI. Suspicious lesions can be targeted either by in-bore MRI-guided biopsy or US fusion-guided biopsy, either by cognitive or software-based fusion devices.
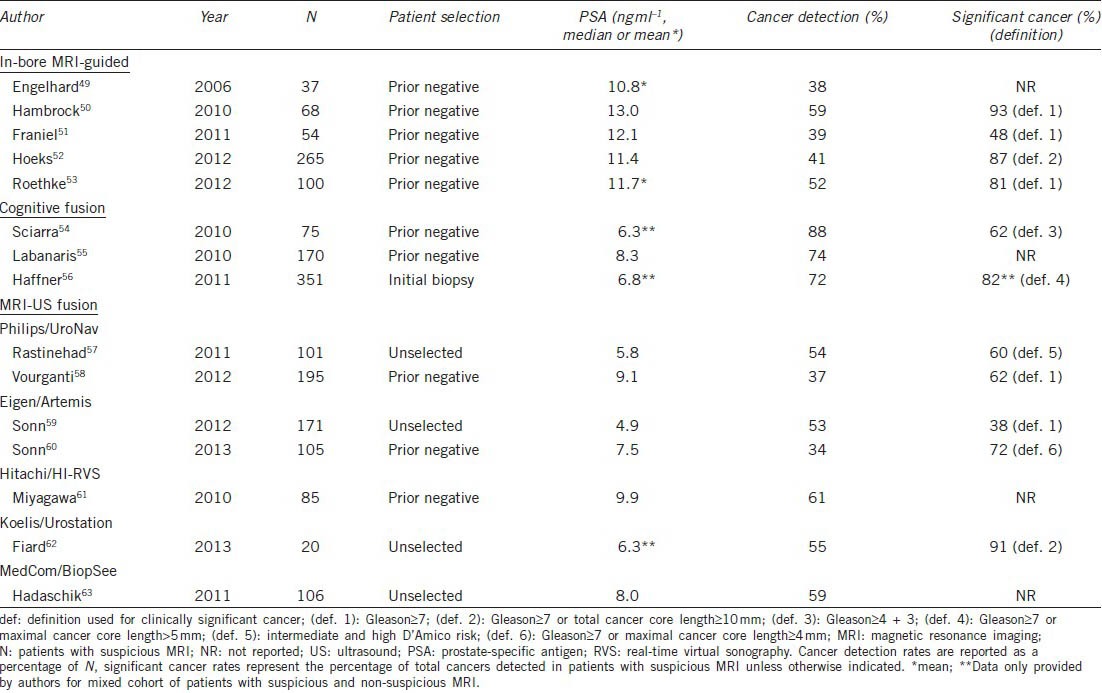
A recent comprehensive systematic review of targeted biopsy using MRI-derived targets by Moore et al.48 examined the results of all available studies, totaling 50 unique reports from 16 unique patient populations. Although studies demonstrated considerable heterogeneity in study design, the authors found an overall cancer detection rate of 66%, with targeted biopsy representing a more efficient approach than standard biopsy. Using targeted biopsy, a mean of 3.8 cores was required to make a diagnosis of cancer versus 12 cores required when using systematic cores; additionally, a targeted approach helped avoid the diagnosis of insignificant cancer.48 Some of the largest studies, separated by method of performing targeted biopsy, are summarized below and in Table 1.
Table 1.
Selected studies employing the three MRI-targeted methods of prostate biopsy
In-bore MRI-guided biopsy
MRI-derived targets may be biopsied directly in the bore of an MRI scanner, and several groups have published results with this technique.49,50,51,52,53,64 Direct in-bore biopsy requires a pre-procedure MRI scan to identify possible lesions in addition to rescanning at the time of biopsy with T2-weighted imaging. Specialized MR-compatible equipment is used, and anesthesia ranges from sedation to general anesthesia. Because of the lengthy time involved to obtain each biopsy core, only targeted cores (no systematic cores) are commonly taken with in-bore biopsy protocols.
Engelhard et al.49 first used 1.5 T imaging and detected cancer using an in-bore technique in 38% of 37 patients with prior negative TRUS biopsy. Franiel et al.51 demonstrated cancer in 39% of 54 patients. Roethke et al.53 reported a 52% cancer detection rate in 100 patients, of which 81% were considered clinically significant cancers.
Hambrock et al.50 used 3 T imaging and reported a cancer detection rate of 59% in 68 patients with at least two negative prior TRUS biopsies, 93% of which were considered clinically significant. The same group of researchers from Nijmegen, the Netherlands, reported additional data from 265 patients with at least one prior negative TRUS biopsy, demonstrating a cancer detection of rate of 41%, with 87% of those cancers considered clinically significant. They also reported cancer detection in eight of 10 patients undergoing repeat MRI-guided biopsy after a previously negative in-bore biopsy.52
The above cancer detection rates using MRI-targeting appear quite meaningful, since the patients had all undergone previous negative biopsy, and repeat conventional biopsy has been shown to entail much lower detection rates.19,20
Cognitive fusion biopsy
‘Cognitive fusion’ is a term given to freehand targeting of MRI-derived targets by the clinician using a standard TRUS biopsy probe. No additional equipment is required aside from the clinician's knowledge of the location of the target on MRI and familiarity with prostate anatomy on ultrasonography.
In a large 555 patient study, Haffner et al.56 performed mp-MRI prior to biopsy in an initial biopsy population. These patients then underwent systematic biopsy, and those with an abnormal MRI also had additional cognitive fusion targeted biopsies performed. The authors reported improved detection accuracy of cognitive fusion targeted biopsy over systematic biopsy alone for clinically significant cancer (P < 0.001). The overall cancer detection rate was 54%, 82% of which were clinically significant. There was a higher rate of 72% for cancer detection in the 351 patients with an abnormal MRI, all of whom had targeted biopsy.56
In men with prior negative biopsies, Sciarra et al.54 randomized 180 to repeat standard TRUS biopsy (n = 90) versus DCE-MRI with spectroscopy and TRUS biopsy (n = 90) with cognitive fusion biopsy of any targets seen. There was a significant increase in cancer detection found in the group that underwent MRI with cognitive fusion biopsy over the group that underwent repeat standard TRUS biopsy alone (46% vs 24%, P = 0.01). Those men whose second standard TRUS biopsy was still negative were then offered MRI and cognitive fusion biopsy. Of all 75 men with an abnormal MRI who underwent cognitive fusion biopsy, the authors reported a cancer detection rate of 88%, the majority being clinically significant.54
Labanaris et al.55 studied 260 consecutive patients with prior negative biopsy, finding MRI abnormalities in 65% of cases (n = 170). Extended 18-core TRUS biopsies were obtained for both cohorts, with additional cognitively targeted cores in those with a suspicious MRI. Cancer detection rate was 56% for targeted cores versus 18% for extended systematic cores; overall cancer detection for the suspicious MRI cohort was 74% vs 19% in those with non-suspicious MRI.55
Taken together, these studies and others,48 show promise for cognitive targeting of suspicious lesions. The success of this technique, however, depends largely on technical skill of the operator, and although precise details regarding MRI lesion size are lacking within these reports, larger lesions are presumably more easily targeted; whereas, smaller lesions may be missed without the assistance of software fusion. Moreover, tracking of biopsy sites is not possible with the cognitive fusion technique.
Software-based MRI-US fusion biopsy
Several systems have been developed that allow the software-based fusion of a preacquired MRI-derived target with real-time TRUS imaging, which may represent an advance over cognitive fusion. The appeal of MRI-US fusion lies in the ability to precisely target and perform biopsies in the outpatient setting, under local anesthesia, with the familiarity of traditional TRUS. Unlike with in-bore MRI-guided biopsies, both systematic and targeted biopsies may be obtained quickly within the same session. Further operational details regarding the various commercially available devices using software fusion have been previously documented and are summarized in Table 2.65
Table 2.
MRI/ultrasound fusion devices approved by US FDA
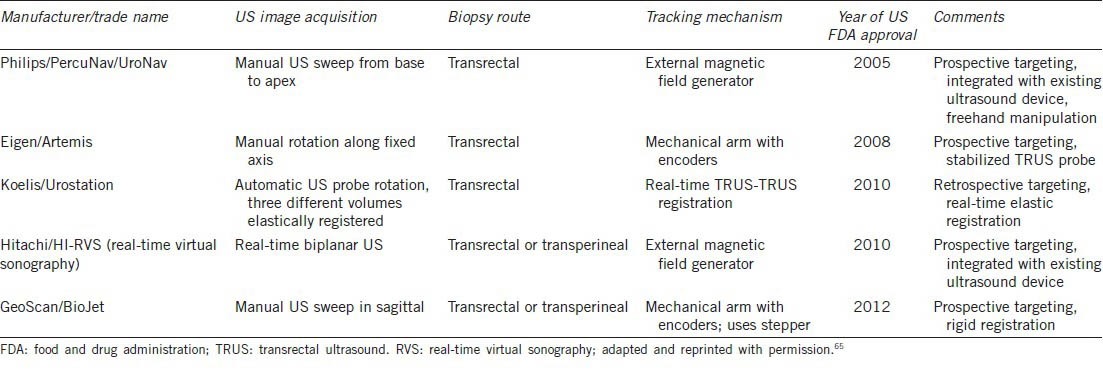
UroNav device
The Philips/PercuNav system (Philips Electronics, Amsterdam, the Netherlands), currently Philips Invivo/UroNav (Figure 1), was developed in collaboration with the National Cancer Institute/National Institutes of Health (NIH) of the US. This system uses an electromagnetic field generator for co-registration of imaging and allows for prospective targeting and freehand manipulation using a standard TRUS probe. Pinto et al.66 reported findings from their first 101 consecutive patients, finding a 90% cancer detection rate in patients with highly suspicious MRI findings and higher per-core detection rates when comparing targeted versus systematic biopsies at each level of MRI suspicion (54% versus 30% for the highly suspicious MRI group).66 Rastinehad et al.57 reported statistically significant correlation between MRI level of suspicion (low, moderate and high) and D’Amico risk stratification (P < 0.01) in this same patient population. The overall cancer detection rate for targeted biopsy was 54%. More recent work from the NIH group reporting results from a prior negative biopsy cohort of 195 men revealed a 37% cancer detection rate, 11% of which were Gleason grade 8 or greater. Of these high grade cancers, 52% were missed on systematic biopsy, all of which were diagnosed on targeted cores.58
Figure 1.
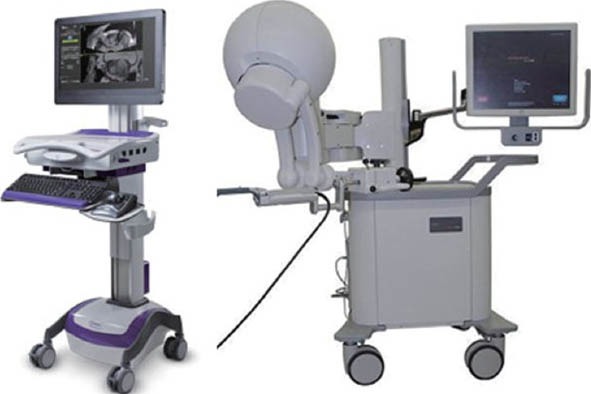
UroNav from Philips Invivo (left) and Artemis from Eigen (right), are two of the FDA-approved image fusion devices (Table 2). FDA: food and drug administration.
Artemis device
The Artemis device (Eigen, Grass Valley, California, U.S.A.) (Figure 1) has been used extensively for targeted biopsy at UCLA. This device uses a robotic mechanical arm with tracking encoders, which allows for tracking of the targeted and systematic core locations for later re-biopsy.44 Sonn et al.59 reported a cancer detection rate of 53% among 171 initial patients, and a per-core detection rate of 21% targeted versus 7% systematic biopsy (P = 0.001). Consistent with the NIH findings, cancer detection was correlated with MRI suspicion level, with 94% cancer detection within the highest suspicion group.59 The review by Marks et al.65 contains updated data from 360 consecutive patients and similar cancer detection rates of 19% in targeted versus 9% in systematic cores. A study of the cohort of 105 patients with prior negative biopsy from UCLA revealed cancer in 34% of men, of whom 72% had clinically significant disease. Significant cancer was more often diagnosed by targeted (91%) than systematic (54%) biopsies.60
Other MRI-US fusion devices
Other devices have had relatively less clinical experience documented in the literature.
The Hitachi/HI-RVS (Real-time Virtual Sonography) system (Hitachi Medical Corporation, Tokyo, Japan) as well uses an electromagnetic field generator and allows for prospective targeting. Miyagawa et al.61 reported cancer detection in 61% of 85 patients, with 32% of targeted cores versus 9% systematic cores positive for cancer, a statistically significant difference (P < 0.01).
The Koelis/Urostation system (Koelis, La Tronche, France) uses elastic, real-time TRUS-TRUS registration but with retrospective targeting, where confirmation of needle position is made by scanning after the biopsy is taken. Fiard et al.62 reported a targeted biopsy cancer detection rate of 55% in a small study of 20 patients with a suspicious MRI and 41% targeted versus 8% systematic biopsy cores positive for cancer (P = 0.017).
Hadaschik et al. used the MedCom/BiopSee (MedCom, Darmstadt, Germany) system to perform transperineal biopsies with TRUS visualization, reporting a cancer detection rate of 59% of 106 patients. Operationally, this device is similar to the GeoScan/BioJet device, FDA approved in the US. Targeted cores had a cancer detection rate of 25% versus 9% of systematic biopsies.63
CASE STUDIES OF EVASIVE PROSTATE TUMORS
The patient cases below, of an apical and an anterior lesion, illustrate the clinical utility of targeted prostate biopsy and the value of mp-MRI in diagnosis.
Apical prostate tumor
Wright and Ellis demonstrated that tumors are commonly missed at the apex, particularly at the anterior apex, where 17% of tumors evade detection using standard TRUS biopsy.67 Biopsy of the prostate apex is known to be more painful for patients given its proximity to the rectal pain fibers, which may lead urologists to avoid this area to minimize pain.68 Nevertheless, cancer detection at the apex is imperative, as Moussa et al.69 demonstrated the highest cancer detection rates at the apex relative to other zones in the prostate (74%, n = 181). Nix et al.70 described the utility of MRI-US fusion biopsy in this scenario. Additionally, Haffner et al.71 reported a more than twofold risk of extraprostatic extension of tumor at the apex versus base (47% vs 19%, n = 188).
The case example is that of a 68-year-old man, who presented with lower urinary tract symptoms and a PSA level of 12.6 ng ml−1 with prior negative biopsies and a PCA3 (prostate cancer gene 3) score of 45 (Figure 2). Digital rectal examination revealed a large, smooth prostate. A single 1.8 cm posterior apical lesion was demonstrated on mp-MRI, with image grade 5 suspicion by UCLA criteria44 as well as possible extraprostatic extension and seminal vesicle involvement.
Figure 2.
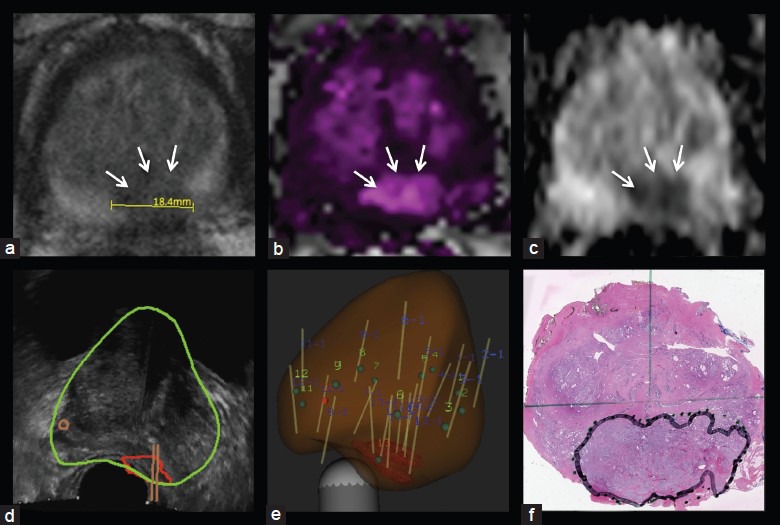
Apical tumor in 86 ml prostate. Axial view of 1.8 cm lesion on T2-weighted imaging (a), dynamic contrast-enhanced imaging (b), diffusion-weighted imaging (c), transrectal ultrasound with segmented prostate and region of interest and biopsy trajectories (d), reconstructed 3D view of prostate, showing region of interest and biopsy tracks (e), whole mount pathology specimen with tumor outlined (f). Note that MRI size of tumor underestimates actual size of tumor, a consistent finding in most studies. MRI: magnetic resonance imaging.
He underwent targeted biopsy using MRI-US fusion, revealing seven of seven targeted biopsy cores positive for cancer, Gleason grade 4 + 5 = 9. No systematic cores were positive for cancer. Seminal vesicle involvement was confirmed at prostatectomy, which revealed Gleason 4 + 5 pT3bN1Mx disease.
Anterior prostate tumor
Anterior prostate cancers account for 15%–21% of prostate cancers72,73,74,75 but are challenging to sample by TRUS biopsy, more often requiring multiple biopsies to diagnose.72 Lawrentschuk et al.76 have coined the term ‘prostate evasive anterior tumor syndrome’ to describe this subset of patients, who have nonpalpable anterior predominant tumors, a rising PSA with multiple negative TRUS biopsies, and may require MRI and targeted biopsy for diagnosis. Ouzzane et al.75 found that anterior prostate cancers identified on MRI were missed by 12-core TRUS systematic biopsy in 46% of cases (n = 45), all subsequently identified by cognitive targeted biopsy. Targeted biopsy improved cancer detection rate, volume and Gleason grading.
Patients with anterior tumors have been shown to have relatively high rates of positive surgical margins at surgery, although it is unclear if they also demonstrate more aggressive pathologic features. Lawrentschuk et al.76 reported a positive surgical margin rate of 54% (n = 13), and a third of patients had biochemical recurrence at 1 year. Koppie et al.73 also found anterior tumors to have a higher positive surgical margin rate than posterior tumors (12% versus 7%, n = 1290, P = 0.01) as well as larger tumor volume. However, anterior tumors were lower Gleason grade and had a lower extraprostatic extension rate.73 Al-Ahmadie et al.74 reported similar positive margin rates (14% for anterior tumors of peripheral zone origin and 13% for anterior tumors of transitional zone origin, n = 197).
The diagnostic difficulty posed by an anterior tumor is illustrated by a 70-year-old man with a rising PSA despite multiple negative TRUS biopsies (Figure 3). His PSA increased from 2.2 to 8.9 ng ml−1 over the course of 8 years. Digital rectal examination revealed a 30-g prostate without nodules. The patient ultimately underwent a 32-core saturation biopsy, which revealed only focal high-grade prostatic intraepithelial neoplasia. Persistently rising PSA prompted yet another 32-core saturation biopsy 2 years later, which returned entirely benign. PCA3 testing was also sought and his score was elevated to 50, again concerning for malignancy. He was then referred for targeted biopsy. An anterior central gland lesion measuring 2.3 cm was visualized by mp-MRI, image grade 5 suspicion by UCLA grading criteria.44 Two small image grade 4 targets were also identified in the central gland. On MRI-US fusion-targeted biopsy, the most suspicious lesion was positive in four of five targeted cores (Gleason grade 4 + 3 in 80%, 3 + 4 in 60% and two cores of 3 + 3 in 5% of core length). The systematic cores and targeted cores from the lesions of lesser suspicion were all negative. He subsequently elected to undergo surgery, and final pathology revealed Gleason grade 3 + 4 pT3aN0Mx disease, with a positive surgical margin and established extraprostatic extension.
Figure 3.
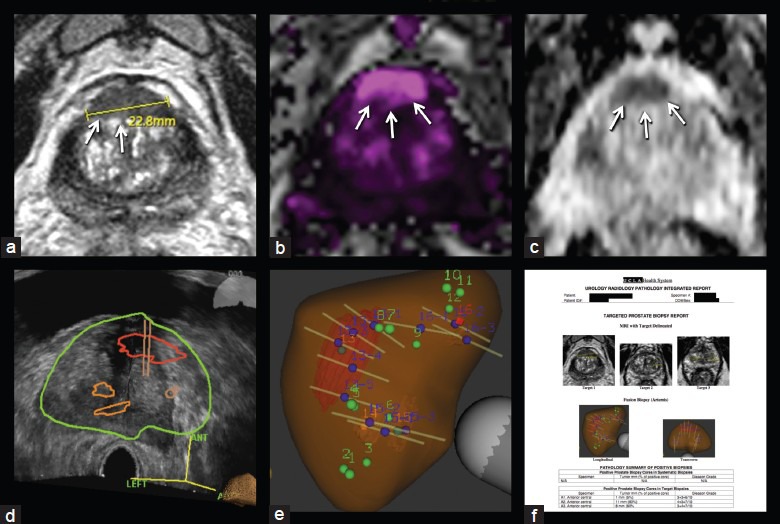
Anterior tumor in 38 ml prostate. Axial view of 2.3 cm lesion on T2-weighted imaging (a), dynamic contrast-enhanced imaging (b), diffusion-weighted imaging (c), transrectal ultrasound with segmented prostate and regions of interest and biopsy trajectories (d), reconstructed 3D view of prostate showing regions of interest and biopsy tracks (e), example of targeted biopsy report—incorporating key aspects of urology, radiology and pathology components—in use at UCLA (f). Smaller targets were negative for cancer. Anterior tumors may be missed by conventional biopsy; in this case, two prior ‘saturation’ biopsies had failed to detect the tumor, a Gleason 7 cancer. UCLA: University of California, Los Angeles.
FUTURE DIRECTIONS
The urologist's dual goals of maximizing early detection of prostate cancer and minimizing overtreatment underscore a need to reexamine the current diagnostic pathway. Technologic advancements in mp-MRI of the prostate and targeted biopsy have been shown to increase cancer detection and may ultimately improve patient care within the construct of a revised diagnostic pathway, although long-term data examining oncologic outcomes are currently lacking.
Targeted biopsy of MRI-derived lesions is clearly limited by the radiologist's ability to identify suspicious targets. While the studies discussed above indicate increasing cancer yield with increasing level of suspicion on MRI, definitions and interpretation of MRI vary from study to study. Standardization of MRI imaging protocols and reporting schemes is an important priority. Additionally, training resources and certification programs for radiologists seeking to achieve expertise in prostate MRI need to be established. Computer-aided diagnostic tools to assist the radiologist are the subject of ongoing investigation.77,78,79
Studies correlating histopathology with MRI findings are needed to refine and improve imaging diagnosis,37 particularly within the central gland,80 and studies incorporating targeted biopsy data are currently lacking. While such studies aim to characterize the sensitivity and specificity of MRI, the true false negative rate is unknown, as whole mount histopathologic correlation for these studies is available only after prostatectomy for known malignancy.
While most studies on targeted biopsy have focused on the population of men with prior negative biopsy, the role of mp-MRI and targeted biopsy in other settings is continuing to evolve. Active surveillance has been shown to be cost-effective over radical therapy for low risk prostate cancer and is being increasingly utilized.81 MRI and targeted biopsy are also being incorporated as part of active surveillance protocols, and the cost-effectiveness impact of the new method is unknown.82,83 Finally, while increasing number of men may seek a targeted biopsy in lieu of a standard initial TRUS biopsy, its role in the initial biopsy setting has been less studied.56,84
Additional work is warranted to examine the cost-effectiveness of these emerging technologies. A recent report from the National Health Services of the UK indicates that mp-MRI may be a cost-effective approach over TRUS biopsy in certain circumstances if shown to have ‘high sensitivity for detecting moderate/high-risk cancer, while negating patients with no cancer/low-risk disease to undergo biopsy’.85
CONCLUSIONS
TRUS-guided biopsy has been the standard of care in the diagnosis of prostate cancer since its introduction in the late 1980s. Multiparametric MRI, which can add functional diffusion, perfusion and metabolic information to traditional T1 and T2 sequences, represents an important advance that allows for visualization of suspicious lesions. MRI-guided biopsy—either by the direct in-bore method, by cognitive fusion or by using a fusion device—has resulted in a several-fold improvement in cancer detection rates. Additionally, significant cancers can be detected more often by targeted than systematic biopsy, a development with important implications for avoiding overdiagnosis and overtreatment of indolent cancers.
AUTHOR CONTRIBUTIONS
All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
Work of the authors is supported in part by Award Number R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National institute of Health. Additional support was provided by the Beckman Coulter Foundation, the Jean Perkins Foundation and the Steven C. Gordon Family Foundation.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, et al. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 11 Feb 2014]. GLOBOCAN 2012 v1. 0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Available from: http://globocan.iarc.fr . [Google Scholar]
- 2.Silletti JP, Gordon GJ, Bueno R, Jaklitsch M, Loughlin KR. Prostate biopsy: past, present, and future. Urology. 2007;69:413–6. doi: 10.1016/j.urology.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 3.Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70. doi: 10.1016/s0022-5347(17)38663-9. [DOI] [PubMed] [Google Scholar]
- 4.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–5. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 5.Orozco R, O’Dowd G, Kunnel B, Miller MC, Veltri RW. Observations on pathology trends in 62,537 prostate biopsies obtained from urology private practices in the United States. Urology. 1998;51:186–95. doi: 10.1016/s0090-4295(97)00620-1. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis G, Rittenhouse HG, Mikolajczyk SD, Shamel LB, Semjonow A. Twenty years of PSA: from Prostate Antigen to Tumor Marker. Rev Urol. 2007;9:113–23. [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–51. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 8.O’Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553–9. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 9.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199–202. [PubMed] [Google Scholar]
- 10.Chon CH, Lai FC, McNeal JE, Presti JC. Use of extended systematic sampling in patients with a prior negative prostate needle biopsy. J Urol. 2002;167:2457–60. [PubMed] [Google Scholar]
- 11.Presti JC, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–6. [PubMed] [Google Scholar]
- 12.Presti JC, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003;169:125–9. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 13.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–7. [PubMed] [Google Scholar]
- 14.Presti JC. Prostate biopsy strategies. Nature clinical practice. Urology. 2007;4:505–11. doi: 10.1038/ncpuro0887. [DOI] [PubMed] [Google Scholar]
- 15.Presti JC. Repeat prostate biopsy-when, where, and how. Urol Oncol. 2009;27:312–4. doi: 10.1016/j.urolonc.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Singh H, Canto EI, Shariat SF, Kadmon D, Miles BJ, et al. Predictors of prostate cancer after initial negative systematic 12 core biopsy. J Urol. 2004;171:1850–4. doi: 10.1097/01.ju.0000119667.86071.e7. [DOI] [PubMed] [Google Scholar]
- 17.Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71–7. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch HG, Fisher ES, Gottlieb DJ, Barry MJ. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395–400. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 19.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–4. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 20.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679–83. [PubMed] [Google Scholar]
- 21.Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2001;166:86–91. [PubMed] [Google Scholar]
- 22.Ashley RA, Inman BA, Routh JC, Mynderse LA, Gettman MT, et al. Reassessing the diagnostic yield of saturation biopsy of the prostate. Eur Urol. 2008;53:976–83. doi: 10.1016/j.eururo.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Fleshner N, Klotz L. Role of “saturation biopsy” in the detection of prostate cancer among difficult diagnostic cases. Urology. 2002;60:93–7. doi: 10.1016/s0090-4295(02)01625-4. [DOI] [PubMed] [Google Scholar]
- 24.Furuno T, Demura T, Kaneta T, Gotoda H, Muraoka S, et al. Difference of cancer core distribution between first and repeat biopsy: in patients diagnosed by extensive transperineal ultrasound guided template prostate biopsy. Prostate. 2004;58:76–81. doi: 10.1002/pros.10298. [DOI] [PubMed] [Google Scholar]
- 25.Pinkstaff DM, Igel TC, Petrou SP, Broderick GA, Wehle MJ, et al. Systematic transperineal ultrasound-guided template biopsy of the prostate: three-year experience. Urology. 2005;65:735–9. doi: 10.1016/j.urology.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 26.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850–4. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 27.Loeb S, Schaeffer EM, Epstein JI. The Vanishing Prostate Cancer Phenomenon. Urology. 2010;76:605–7. doi: 10.1016/j.urology.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Rifkin MD, Zerhouni EA, Gatsonis CA, Quint LE, Paushter DM, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med. 1990;323:621–6. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 29.Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 30.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, et al. Prostate Cancer: value of multiparametric MR imaging at 3 t for detection-histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Mazaheri Y, Zhang J, Ishill NM, Kuroiwa K, et al. Assessment of biologic aggressiveness of prostate cancer: correlation of MR signal intensity with gleason grade after radical prostatectomy. Radiology. 2007;246:168–76. doi: 10.1148/radiol.2461070057. [DOI] [PubMed] [Google Scholar]
- 32.Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–75. doi: 10.1016/j.eururo.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Qayyum A, Coakley FV, Lu Y, Olpin JD, Wu L, et al. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenology. 2004;183:1079–83. doi: 10.2214/ajr.183.4.1831079. [DOI] [PubMed] [Google Scholar]
- 34.Akin O, Sala E, Moskowitz CS, Kuroiwa K, Ishill NM, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology. 2006;239:784–92. doi: 10.1148/radiol.2392050949. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull LW, Buckley DL, Turnbull LS, Liney GP, Knowles AJ. Differentiation of prostatic carcinoma and benign prostatic hyperplasia: correlation between dynamic Gd-DTPA-enhanced MR imaging and histopathology. J Magn Reson Imaging. 1999;9:311–6. doi: 10.1002/(sici)1522-2586(199902)9:2<311::aid-jmri24>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Schlemmer HP, Merkle J, Grobholz R, Jaeger T, Michel MS, et al. Can pre-operative contrast-enhanced dynamic MR imaging for prostate cancer predict microvessel density in prostatectomy specimens? Eur Radiol. 2004;14:309–17. doi: 10.1007/s00330-003-2025-2. [DOI] [PubMed] [Google Scholar]
- 37.Puech P, Potiron E, Lemaitre L, Leroy X, Haber GP, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74:1094–9. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 38.Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009;103:883–8. doi: 10.1111/j.1464-410X.2008.08130.x. [DOI] [PubMed] [Google Scholar]
- 39.Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and gleason grade in peripheral zone prostate cancer. Radiology. 2011:453–61. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 41.Fütterer JJ, Engelbrecht MR, Jager GJ, Hartman RP, King BF, et al. Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol. 2007;17:1055–65. doi: 10.1007/s00330-006-0418-8. [DOI] [PubMed] [Google Scholar]
- 42.Park BK, Kim B, Kim CK, Lee HM, Kwon GY. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007;31:534–8. doi: 10.1097/01.rct.0000250108.85799.e1. [DOI] [PubMed] [Google Scholar]
- 43.Scheenen TW, Klomp DW, Röll SA, Fütterer JJ, Barentsz JO, et al. Fast acquisition-weighted three-dimensional proton MR spectroscopic imaging of the human prostate. Magn Reson Med. 2004;52:80–8. doi: 10.1002/mrm.20103. [DOI] [PubMed] [Google Scholar]
- 44.Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–42. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37:48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- 46.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schimmöller L, Quentin M, Arsov C, Lanzman RS, Hiester A, et al. Inter-reader agreement of the ESUR score for prostate MRI using in-bore MRI-guided biopsies as the reference standard. Eur Radiol. 2013;23:3185–90. doi: 10.1007/s00330-013-2922-y. [DOI] [PubMed] [Google Scholar]
- 48.Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125–40. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, et al. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–43. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 50.Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–8. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, et al. Areas suspicious for prostate cancer: mr-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–72. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 52.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, de Kaa CA, et al. Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012:1–8. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 53.Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–8. doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 54.Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010;16:1875–83. doi: 10.1158/1078-0432.CCR-09-2195. [DOI] [PubMed] [Google Scholar]
- 55.Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010;13:65–70. doi: 10.1038/pcan.2009.41. [DOI] [PubMed] [Google Scholar]
- 56.Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Intl. 2011;108:E171–8. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 57.Rastinehad AR, Baccala J, Angelo A, Chung PH, Proano JM, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815–20. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, et al. multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012;188:2152–7. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2012;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.025. doi: 10.1016/j.eururo.2013.03.025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyagawa T, Ishikawa S, Kimura T, Suetomi T, Tsutsumi M, et al. Real-time virtual sonography for navigation during targeted prostate biopsy using magnetic resonance imaging data. Int J Urol. 2010;17:855–60. doi: 10.1111/j.1442-2042.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- 62.Fiard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, et al. Targeted MRI-guided prostate biopsies for the detection of prostate cancer: initial Clinical Experience With Real-time 3-Dimensional Transrectal Ultrasound Guidance and Magnetic Resonance/Transrectal Ultrasound Image Fusion. Urology. 2013:1372–8. doi: 10.1016/j.urology.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Hadaschik BA, Kuru TH, Tulea C, Rieker P, Popeneciu IV, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186:2214–20. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 64.Pondman KM, Futterer JJ, ten Haken B, Schultze Kool LJ, Witjes JA, et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol. 2008;54:517–27. doi: 10.1016/j.eururo.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinto PA, Chung PH, Rastinehad AR, Baccala J, Angelo A, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–5. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright JL, Ellis WJ. Improved prostate cancer detection with anterior apical prostate biopsies. Urol Oncol. 2006;24:492–5. doi: 10.1016/j.urolonc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Jones JS, Zippe CD. Rectal sensation test helps avoid pain of apical prostate biopsy. J Urol. 2003;170:2316–8. doi: 10.1097/01.ju.0000095792.42718.83. [DOI] [PubMed] [Google Scholar]
- 69.Moussa AS, Meshref A, Schoenfield L, Masoud A, Abdel-Rahman S, et al. Importance of additional extreme anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034–9. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Nix JW, Turkbey B, Hoang A, Volkin D, Yerram N, et al. Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int. 2012;110:E694–700. doi: 10.1111/j.1464-410X.2012.11503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haffner J, Potiron E, Bouyé S, Puech P, Leroy X, et al. Peripheral zone prostate cancers: location and intraprostatic patterns of spread at histopathology. Prostate. 2009;69:276–82. doi: 10.1002/pros.20881. [DOI] [PubMed] [Google Scholar]
- 72.Bott SR, Young MP, Kellett MJ, Parkinson MC. Anterior prostate cancer: is it more difficult to diagnose? BJU Int. 2002;89:886–9. doi: 10.1046/j.1464-410x.2002.02796.x. [DOI] [PubMed] [Google Scholar]
- 73.Koppie TM, Bianco FJ, Jr, Kuroiwa K, Reuter VE, Guillonneau B, et al. The clinical features of anterior prostate cancers. BJU Int. 2006;98:1167–71. doi: 10.1111/j.1464-410X.2006.06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Ahmadie HA, Tickoo SK, Olgac S, Gopalan A, Scardino PT, et al. Anterior-predominant prostatic tumors: zone of origin and pathologic outcomes at radical prostatectomy. Am J Surg Pathol. 2008;32:229–35. doi: 10.1097/PAS.0b013e31812f7b27. [DOI] [PubMed] [Google Scholar]
- 75.Ouzzane A, Puech P, Lemaitre L, Leroy X, Nevoux P, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78:1356–62. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 76.Lawrentschuk N, Haider MA, Daljeet N, Evans A, Toi A, et al. ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU Intl. 2010;105:1231–6. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 77.Tiwari P, Viswanath S, Kurhanewicz J, Sridhar A, Madabhushi A. Multimodal wavelet embedding representation for data combination (MaWERiC): integrating magnetic resonance imaging and spectroscopy for prostate cancer detection. NMR Biomed. 2012;25:607–19. doi: 10.1002/nbm.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vos PC, Barentsz JO, Karssemeijer N, Huisman HJ. Automatic computer-aided detection of prostate cancer based on multiparametric magnetic resonance image analysis. Phys Med Biol. 2012;57:1527–42. doi: 10.1088/0031-9155/57/6/1527. [DOI] [PubMed] [Google Scholar]
- 79.Shah V, Turkbey B, Mani H, Pang Y, Pohida T, et al. Decision support system for localizing prostate cancer based on multiparametric magnetic resonance imaging. Med Phys. 2012;39:4093–103. doi: 10.1118/1.4722753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viswanath SE, Bloch NB, Chappelow JC, Toth R, Rofsky NM, et al. Central gland and peripheral zone prostate tumors have significantly different quantitative imaging signatures on 3 Tesla endorectal, in vivo T2-weighted MR imagery. J Magn Reson Imaging. 2012;36:213–24. doi: 10.1002/jmri.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dall’Era MA. The economics of active surveillance for prostate cancer. Curr Opin Urol. 2013;23:278–82. doi: 10.1097/MOU.0b013e32835f4b6b. [DOI] [PubMed] [Google Scholar]
- 82.Turkbey B, Mani H, Aras O, Ho J, Hoang A, et al. Prostate Cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144–52. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mullins JK, Bonekamp D, Landis P, Begum H, Partin AW, et al. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013;111:1037–45. doi: 10.1111/j.1464-410X.2012.11641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park BK, Park JW, Park SY, Kim CK, Lee HM, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011;197:W876–81. doi: 10.2214/AJR.11.6829. [DOI] [PubMed] [Google Scholar]
- 85.Mowatt G, Scotland G, Boachie C, Cruickshank M, Ford JA, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol Assess (Winchester, England) 2013;17:1–281. doi: 10.3310/hta17200. vii.xix. [DOI] [PMC free article] [PubMed] [Google Scholar]


