Abstract
Prostate cancer is a leading cause of cancer death in men. Despite recent advances in our understanding and treatment of advanced disease, no systemic therapy is curative and new therapies are needed. Targeting angiogenesis is an attractive therapeutic strategy, as angiogenic pathways are upregulated in prostate tumors similar to other malignancies due to imbalance of pro- and anti-angiogenic factors secreted by tumor, endothelial and stromal cells and increased neovasculature.1 Vascular endothelial growth factor (VEGF) is the most well-characterized pro-angiogenenic factor, with several small molecule inhibitors (sunitinib, sorafenib, pazopanib, axitinib, others), antibodies (bevacizumab) and other drugs that target the VEGF pathway approved and/or in development for the treatment of a wide range of tumor types.
Based on encouraging preclinical data and early phase clinical studies, several drugs with anti-angiogenic properties have proceeded to phase 3 evaluation for the treatment of men with castration resistant prostate cancer (CRPC) (Table 1). Unfortunately, no phase 3 study to date has succeeded in demonstrating a survival benefit in large randomized studies. In the most recently reported study by Michaelson et al.2 873 patients with docetaxel-pretreated metastatic CRPC were randomized to either the small molecule inhibitor sunitinib plus prednisone or prednisone alone. Sunitinib is a tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, along with platelet-derived growth factor receptors, c-kit, and RET kinases amongst others, and has significant clinical activity leading to approval in several tumor types.3,4,5 Although, there was a significant improvement in progression free survival (PFS) in the sunitinib/prednisone arm (5.6 months vs 4.1 months, hazard ratio (HR) 0.725, P < 0.001), the study did not meet its primary endpoint of overall survival (OS) benefit. The trial was prematurely terminated based on a second interim analysis that determined no significant difference in the median OS between the treatment and placebo arms (13.1 months (95% confidence interval (CI), 12.0–14.1 months) vs 11.8 months (95% CI, 10.8–14.2 months)) and HR of 0.914 (95% CI, 0.762–1.097; P = 0.168; stratified log-rank test). For those with measurable disease, response rate was modest but statistically improved with sunitinib plus prednisone compared to prednisone alone (6% vs 2%, P = 0.04) and there was no difference in proportion of patients with stable disease greater than 3 months between the two arms (26% vs 30%, respectively). This lack of benefit occurred at a cost of greater toxicity in the sunitinib arm.
Table 1.
Summary of Phase 3 Clinical Trials evaluating the efficacy of anti-angiogenic therapies for the treatment of patients with metastatic CRPC
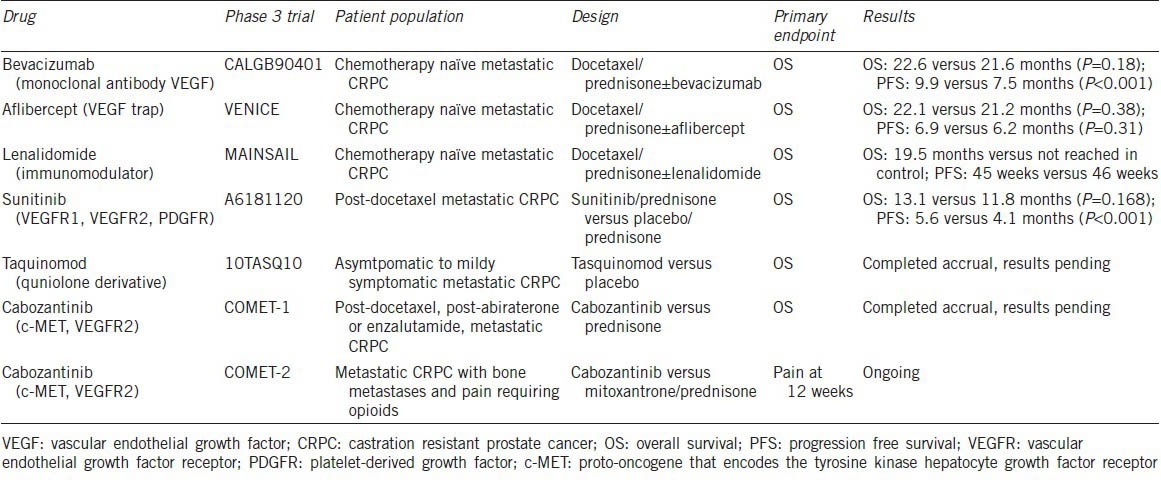
This study is yet another example in prostate cancer in which promising results of phase 2 trials6,7,8 did not translate into a phase 3 survival benefit. In the phase 2 sunitinib studies, a reasonable amount of prostate-specific antigen (PSA) declines were seen for a non-androgen receptor-targeted therapy and radiologic responses were seen even in the absence of PSA decline. However, as seen with other tumor types, improvement in PFS with anti-angiogenic drugs in phase 3 studies did not lead to OS benefit. Whether this is class effect (also seen in the adjuvant settings) and the underlying mechanisms remain unclear.
In the phase 3 sunitinib study, there were frequent dose reductions and early discontinuations in the treatment arm, perhaps due to a heavily pretreated, elderly patient population when compared to other sunitinib populations. Closer clinical monitoring and aggressive supportive care for early toxic effects might have mitigated these events and may have helped to prolong the median treatment duration of only 98 days, which may have ultimately influenced lack of benefit in median OS. A similar plausible explanation for lower than expected activity was seen with the combination of aflibercept and docetaxel which led to a significantly smaller number of men receiving at least 4 cycles of drug compared with the placebo and docetaxel, diluting any efficacy of the drug. Another possibility is that there may be a subset of clinical responders embedded into these studies and retrospective analysis of tissue and blood samples may lead to greater understanding of subgroups particularly vulnerable to anti-angiogenic drugs and assist in the design of future clinical trials.
These negative data combined with prior negative studies of several other anti-angiogenic therapies (Table 1) does dampen initial enthusiasm regarding the role of VEGF targeted therapies for the treatment of advanced prostate cancer. However, newer anti-angiogenics such as cabozantinib (a small molecule that targets VEGFR, mesenchymal–epithelial transition and other downstream effectors) and tasquinimod (a quinolone derivative with direct and indirect anti-angiogenic effects) have shown promise in phase 2 studies9,10 and phase 3 studies of each have recently completed accrual. Understanding how these newer drugs target angiogenesis and potentially therapeutic off-target effects will be key in understanding response and resistance. With several targeted drugs with varied mechanisms of action either approved or in development for the treatment of men with CRPC, appropriate patient selection and identification of biomarkers of response will be key in developing personalized treatment strategies.
REFERENCES
- 1.Antonarakis ES, Carducci MA. Targeting angiogenesis for the treatment of prostate cancer. Expert Opin Ther Targets. 2012;16:365–76. doi: 10.1517/14728222.2012.668887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelson MD, Oudard S, Ou YC, Sengeløv L, Saad F, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 3.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 4.Rini BI. Sunitinib. Expert Opin Pharmacother. 2007;8:2359–69. doi: 10.1517/14656566.8.14.2359. [DOI] [PubMed] [Google Scholar]
- 5.Kim DW, Jo YS, Jung HS, Chung HK, Song JH, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroidoncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91:4070–6. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 6.Dror Michaelson M, Regan MM, Oh WK, Kaufman DS, Olivier K, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20:913–20. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonpavde G, Periman PO, Bernold D, Weckstein D, Fleming MT, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21:319–24. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 8.Oudard SM, Caty M, Rolland F. An open-label phase II study of treatment with sunitinib in patients suffering from metastatic castrated refractory prostate cancer (CRPC) after progression with docetaxel based regimen. Ann Oncol. 2010;21 abstr 4718. [Google Scholar]
- 9.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–9. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pili R, Häggman M, Stadler WM, Gingrich JR, Assikis VJ, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29:4022–8. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]


