Abstract
Erectile dysfunction (ED) is a frequent complication of obesity. The aim of this review is to critically analyze the framework of obesity and ED, dissecting the connections between the two pathological entities. Current clinical evidence shows that obesity, and in particular central obesity, is associated with both arteriogenic ED and reduced testosterone (T) levels. It is conceivable that obesity-associated hypogonadism and increased cardiovascular risk might partially justify the higher prevalence of ED in overweight and obese individuals. Conversely, the psychological disturbances related to obesity do not seem to play a major role in the pathogenesis of obesity-related ED. However, both clinical and preclinical data show that the association between ED and visceral fat accumulation is independent from known obesity-associated comorbidities. Therefore, how visceral fat could impair penile microcirculation still remains unknown. This point is particularly relevant since central obesity in ED subjects categorizes individuals at high cardiovascular risk, especially in the youngest ones. The presence of ED in obese subjects might help healthcare professionals in convincing them to initiate a virtuous cycle, where the correction of sexual dysfunction will be the reward for improved lifestyle behavior. Unsatisfying sexual activity represents a meaningful, straightforward motivation for consulting healthcare professionals, who, in turn, should take advantage of the opportunity to encourage obese patients to treat, besides ED, the underlying unfavorable conditions, thus not only restoring erectile function, but also overall health.
Keywords: androgens, erectile dysfunction, impotence, vasculogenic
INTRODUCTION AND DEFINITION
The World Health Organization defines overweight and obesity as an abnormal or excessive fat accumulation that may impair overall health. The usual definition of obesity is based on body mass index (BMI), a simple index of weight-per-height (a person's weight in kilograms divided by the square of his/her height in meters, kg m−2) (http://www.who.int/topics/obesity/en/). In Western countries, people are considered obese when their BMI exceeds 30 kg m−2 and overweight when it is below this value, but equal to or higher than 25 kg m−2. Since Asian populations have a higher percentage of body fat-in comparison with people of the same age, sex and BMI of different ethnicities-World Health Organization has suggested different categories, with BMI between 23 and 27.5 kg m−2 indicating overweight and higher than 27.5 kg m−2 indicating obesity.1 Overweight and obesity have become an epidemic problem, on the rise not only in Western societies, but also in developing countries. Obesity causes concern because it constitutes a worldwide, major health problem and is a risk factor for major adverse cardiovascular events (MACE), type 2 diabetes mellitus, certain types of cancer, sleep apnea, osteoarthritis and most of all, excess mortality.2,3 In fact, together with smoking habits, obesity is considered one of the most common preventable, and therefore treatable causes of premature death. World Health Organization recognizes the need for other indicators beyond BMI to better define the risk of obesity-related morbidities (http://whqlibdoc.who.int/publications/2011/9789241501491_eng.pdf). Body weight, which is expressed by BMI, is made up of the sum of fat and lean mass. Therefore, although there is a correlation between BMI and fat mass, individuals with expanded lean mass (e.g. some athletes) can have a high BMI without being obese. On the other hand, in subjects with reduced muscle mass (e.g. the elderly), excess body fat can occur within the normal BMI range; this is the so-called ‘sarcopenic obesity’, which is associated with a particularly relevant burden of obesity-related complications.4 In addition, the distribution of fat is another relevant factor which is not computed in BMI. In fact, BMI does not take into account the accumulation of visceral fat that characterizes the most morbigenous form of obesity: abdominal or central obesity. Alternative measures that reflect abdominal adiposity, such as waist circumference (WC), waist-hip ratio and waist-height ratio, have been suggested as being superior to BMI in predicting cardiovascular disease (CVD) risk.5 The superiority of WC-based indices over BMI in the prediction of CVD and diabetes is remarkable, considering that measurement of WC has a lower reproducibility than that of body weight. This is based largely on the rationale that increased visceral adipose tissue (VAT) is associated with a range of metabolic abnormalities, including decreased glucose tolerance, reduced insulin sensitivity and adverse lipid profiles, which are all risk factors for type 2 diabetes mellitus and CVD.6,7 It has also been suggested that abdominal subcutaneous fat could be metabolically more ‘active’ (and therefore more associated with complications) than peripheral subcutaneous fat.8,9 Two action levels of WC were determined in Western countries to identify people whose health risks were increasing (action level 1: men >94 cm, women >80 cm) or high (action level 2: men >102 cm, women >88 cm).10 Action levels based on WC measurements were shown to be a valuable, simple method for alerting people with an increased risk of CVD, who might benefit from weight management.10 People at action level 1 are 1.5 times to twice as likely to have one or more major CV risk factors; people with WC above action level 2 are 2.5 to 4.5 times as likely to have one or more major CV risk factors.
EPIDEMIOLOGY OF OBESITY IN ITALY
According to data released in 2008 by the Organization for Economic Co-operation and Development (OECD), in Italy about 1 in 10 people were obese, a figure significantly lower than the OECD average of one in six (http://www.oecd.org/els/health-systems/obesityandtheeconomicsofpreventionfitnotfat-italykeyfacts.htm). In fact, OECD estimates that, globally, 15.5% of the European adult population in 2008 was obese, according to BMI (BMI ≥30 kg m−2). A recently published analysis from five surveys conducted annually between 2006 and 2010, involving a representative sample of almost 15,000 Italians, reported an overall prevalence of obesity of 9% (8.5% men and 9.4% women).9 According to that study, in Italy, almost one in two men and one in three women were overweight (39.8% and 24.4% with 25 < BMI < 30 kg m−2, respectively). OECD projections indicate that overweight rates will increase by a further 5% within 10 years. The Gallus study also indicated that the BMI trend over calendar years was rather stable in men and even decreasing in women.11 It should be considered that those figures were based on self-reported height and weight, and that self-reporting overestimates height and underestimates weight. Actual figures could therefore be higher. In contrast, Italy detected one of the highest rates in the OECD of childhood obesity, because one in three children are overweight. Women with poor education in Italy are three times more likely to be overweight than more educated women. Disparities are smaller in men, but still higher than in many other OECD countries. Poorly educated men are 1.3 times more likely to be overweight than more educated ones (http://www.oecd.org/els/health-systems/obesityandtheeconomicsofpreventionfitnotfat-italykeyfacts.htm).
All the treatments of obesity are aimed at identifying and modifying eating, activity and thinking behaviors that contribute to the patient's weight problem. Essentially the frontline treatment is a recommendation to eat less, exercise more and adopt a healthier lifestyle. In fact, several studies have demonstrated that, in individuals at risk, intensive lifestyle intervention, along with nutritional counseling and physical activity, is able to reduce weight and insulin resistance, preventing the progression of obesity to other diseases, such as overt diabetes.12,13,14,15 Lifestyle intervention is therefore the usual first-line approach for all patients. Unfortunately, diet and behavioral therapies often ultimately fail. The majority of obese patients are not willing to undergo a lifestyle change program; of those who enroll in such a program, the majority fail; of those who succeed, the majority regain the weight lost within a few years.16 In fact, from 60% to 86% of weight lost is regained after 3 years and 75%–121% after 5 years.16 Generally speaking, humans, like other animals, tend to develop behaviors which produce pleasure and to avoid those which induce discomfort. Eating is more pleasant than abstaining from food, unless some other form of reward is provided. We strongly believe that promising a better sex life to obese subjects will help them in overcoming barriers to lifestyle change, improving adherence to diet and physical exercise, at least in male individuals. Unsatisfying sexual activity, because of impotence, represents, in fact, a meaningful, straightforward motivation for consulting healthcare professionals, who, in turn, should take advantage of the opportunity to encourage obese patients to treat, besides erectile dysfunction (ED), the underlying unfavorable condition; thus not only restoring erectile function, but also overall health.7 Hence, the aim of this review is to depict the framework of obesity and ED, dissecting the connections between the two pathological entities and their possible clinical implications for both ED and obese subjects.
VISCERAL OBESITY, ED AND LOW TESTOSTERONE ARE COMORBID
In both the cross-sectional Massachusetts Male Aging Study and Health Professionals Follow-up Study cohorts it was observed that obesity doubles the risk of having ED, even after adjusting for lifestyle confounders.17,18 Similar figures were derived from European studies.19,20,21,22,23 The close association between the two conditions was further strengthened by observations derived from longitudinal studies. In fact, having obesity at study entry was a significant predictor of incident ED at follow-up in both US and European populations.24
The European Male Ageing Study is a multicenter, population-based, prospective study of ageing in Europe.20,25 Noninstitutionalized men aged 40–79 years were recruited from municipal or population registers in eight centers: Florence (Italy); Leuven (Belgium); Łódź (Poland); Malmö (Sweden); Manchester (UK); Santiago de Compostela (Spain); Szeged (Hungary) and Tartu (Estonia). In the European Male Ageing Study study, compared with the reference group of lean European individuals (BMI <30 kg m−2 and WC <102 cm), men with a BMI > 30 kg m−2 and/or WC >102 cm were, in fact, twice as likely to have ED.22 As for other complications of obesity, WC was superior to BMI in predicting ED,22 confirming the results from two previous studies.26,27
In the European Male Ageing Study survey, Florence was the center with the lowest reporting of overall obesity (17.1% of the study cohort).20 Nonetheless, even in this ‘less-obese’ cohort, there is a significant, stepwise, association between severity of ED and obesity, when evaluated with WC measurement (r = 0.166, P = 0.001, Figure 1a); whereas, for BMI, only a nonsignificant trend was observed (r = 0.09, P = 0.067, Figure 1b). In this population, as in others;28 both WC and BMI were associated with an alteration in circulating testosterone (T) levels (r = −0.328 and r = −0.308, respectively, both P < 0.0001, n = 415). Figure 1c shows the negative, stepwise relationship between increasing grades of WC and total testosterone (TT) (mass spectrometry-analyzed). The association between decreased TT and accumulation of visceral fat is well-known and has been extensively described in recent reviews, also by our group.6,7,29,30 Essentially, it is a bidirectional association, with hypogonadism facilitating the accumulation of abdominal adiposity28,29,30,31,32,33,34,35 and weight loss resulting in a substantial rise in T levels.36
Figure 1.
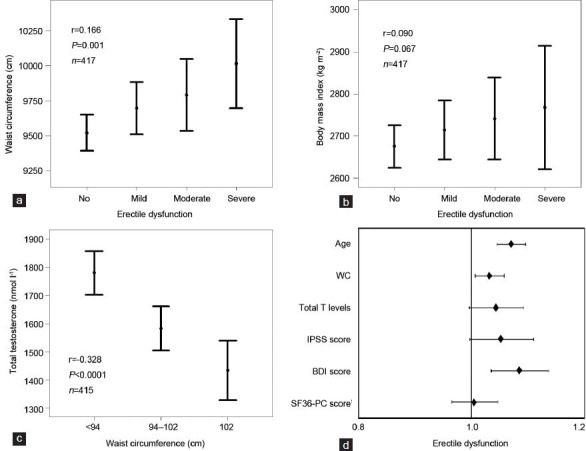
Relationship between erectile dysfunction (ED) severity and waist circumference (WC; a) or body mass index (b). (c) Relationship between total testosterone (TT; mass spectrometry measured) levels and WC classes.10(d) Smoking adjusted risk for any form of ED (expressed as a dummy variable yes/ no) as a function of several other putative determinants of penile erection including total T levels, low urinary tract symptoms as expressed by International Prostate Symptom Score (IPSS), depressive symptoms as detected by Beck Depression Inventory (BDI) score and quality of life as measured by Short Form 36 (SF-36) health survey physical component (PC). Data (unpublished) are derived from the European Male Aging Study (EMAS) when only the Florentine Centre was considered (n = 433 mean age 60.1 ± 10.9 years).20 The presence of ED was investigated with question #10 (You are: 1) Always able to keep an erection which would be good enough for sexual intercourse (no ED); 2) Usually able to get and keep an erection which would be good enough for sexual intercourse (mild ED); 3) Sometimes able to get and keep an erection which would be good enough for sexual intercourse (moderate ED); 4) Never able to get and keep an erection which would be good enough for sexual intercourse (severe ED) of the EMAS sexual function questionnaire (SFQ).25 Note that WC was considered as a continuous variable in all the analyses.
It is conceivable that obesity-associated hypogonadism might partially justify the higher prevalence of ED in overweight and obese individuals.37 However, the relationship between ED severity and waist reported in Figure 1a, retains significance in an ordinal logistic model, even after adjusting for TT and other putative determinants of penile erection, such as smoking habit, Short Form 36 Health Survey physical composite score, total Beck Depression Inventory (BDI) score and International Prostate Symptom Score (Wald = 4.196, P < 0.05). Figure 1d shows the hazard ratio (HR) of WC, and of the aforementioned covariates, for having any form of ED (expressed as dummy variable: yes/no), as derived from a binary logistic model. For each increase of 1 cm of WC there is a 3% increase in the risk of having ED. In this analysis, even after the adjustment for smoking habit, other significant, waist-independent modulators of ED risk were age and BDI score. Although a worse BDI total score was positively associated with WC (age-adjusted r = 0.103, P = 0.034, n = 426), we found that an increased WC was a BDI-independent risk factor for having any form of ED (Figure 1d). These results suggest that, in the general population from Florence, the accumulation of visceral fat has a detrimental effect on erectile function, independently from other organic or even psychological risk factors.
To further verify this hypothesis, we analyzed the association between WC and ED in a population from the same geographical area, but complaining of sexual dysfunction, and therefore consulting our Andrology Clinic (University of Florence, Florence, Italy), a cohort extensively described in previous studies.38,39,40,41,42,43,44 Data on ED were collected using the Structured Interview on Erectile Dysfunction (SIEDY)45 and its appendix A, which scores ED severity.46 Data on the short version of the International Index of Erectile Function (IIEF-5)47 were available for a subset of almost 600 individuals, the characteristics of which do not differ from the rest of the sample (not shown; see also ref. 46). Figure 2 shows the relationship between increasing ED severity (depicted as tertiles of the SIEDY validated index of ED severity46 and WC in this population (SIEDY ED score: r = 0.103, n = 2425 and IIEF-5 score: r = −0.173, n = 527; both P < 0.0001). The associations retained significance in an ordinal logistic model, even after adjusting for TT, smoking habit and age (Figure 2). In a similar ordinal model, we also explored possible associations between increasing WC and other aspects of male sexuality, such as reduced spontaneous erection, hypoactive sexual desire and premature ejaculation, measured as previously reported.42,48,49,50 Both increasing severity of reduced spontaneous erection and loss of libido were positively associated with increased WC (Wald = 30.151, n = 3433, P < 0.0001 and Wald = 8.622, n = 3456, P < 0.005; respectively), while premature ejaculation showed an opposite trend, being less prevalent in abdominal obese individuals (Wald = 4.457, n = 3416, P < 0.05).
Figure 2.
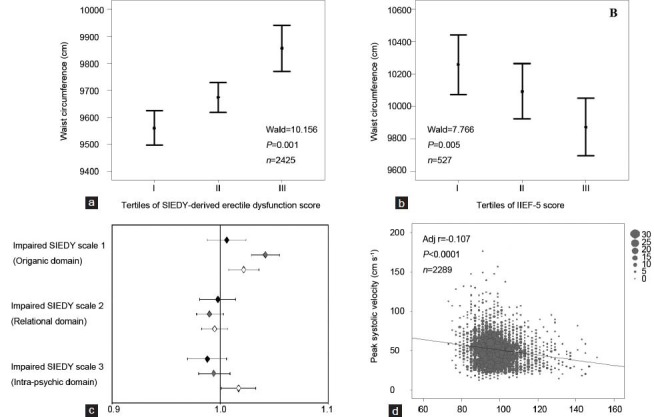
Relationship between waist circumference (WC) and erectile dysfunction (ED) severity as expressed as tertiles of the SIEDY validated index of ED severity (a)42 or short version of the International Index of Erectile function (b; IIEF-5).47 (c) age, total testosterone (TT) and smoking habit adjusted contribution of organic, marital or psychological factors as derived from SIEDY scales43,45,46 in obesity-associated ED. Data are reported as a function of age tertiles (black, gray and open diamonds for I: 17–45, II: 46–59 and III: 60–88 years; age tertiles, respectively). (d) relationship between WC and prostaglandin E1 (PGE1)-stimulated (dynamic) peak systolic velocity (D-PSV). Data (unpublished) are derived from a nonselected series of men (mean age = 51.3 ± 13.3 years) attending our Sexual Medicine and Andrology Clinic for sexual dysfunction between 2000 and 2013. The age-TT-smoking habit correlation coefficients for the indicated observations are also reported in a, b and d. Note that WC was considered as a continuous variable in all the analyses.
The contribution of organic, marital or psychological factors to obesity-associated ED in subjects complaining of sexual dysfunction was analyzed using SIEDY Scales. SIEDY is a multidomain, structured interview able to quantify in three separate scales the relevance of body, couple and mind disturbances in the pathogenesis of ED.42 Pathological cores for Scale 1 (organic component),45 Scale 2 (relational component)43 and Scale 3 (intrapsychic component)46 were iteratively introduced in a binary logistic model along with WC and other possible confounders (age, TT and smoking) as covariates. The analyses were stratified according to age tertiles and are reported in Figure 2c. In the old- and middle-aged subjects, an increased WC significantly contributed to the organic component of ED, while relational factors did not play any significant role. A mild, but significant, contribution of psychological disturbances were apparent in older individuals, but not in the youngest ones. Present data confirm the previous finding23 that the psychological disturbances related to obesity do not seem to play a major role in the pathogenesis of obesity-related ED.
Based on those data, hypogonadism appears to be a concurrent factor, but not the only alteration underlying obesity-associated ED. In addition, the role of T supplementation in ED subjects is a matter of debate.51 Even the T combination with phosphodiesterase type 5 inhibitors as a possible treatment for phosphodiesterase type 5 inhibitor-resistant ED men has been questioned.51 Recently, Spitzer et al.52 did not document any additional effect on ED subjects of T supplementation to sildenafil. However, in the Spitzer trial, T supplementation was initiated after a sildenafil alone run-in period leading to increased T levels up to the normal range (about 12.0 nmol l−1 (345 ng dl−1)).53
Hence, after the run-in period, ED subjects were, on average, not hypogonadal anymore. Failure of T supplementation in eugonadal men is well-documented.51 In addition, while hypogonadism can be the main cause of ED in younger patients, it is generally only one causative element of multifactorial ED in older ones.51
Considering that obesity (and in particular visceral adiposity) is associated with an increased risk of CVD and related metabolic abnormalities (see below), it is conceivable that vascular damage induced by excess fat could contribute to the pathogenesis of ED. Figure 2d, shows the negative impact of increasing WC on prostaglandin E1-stimulated (dynamic) maximal peak systolic velocity (D-PSV), as measured on the cavernous arteries of the same cohort by penile color Doppler ultrasound. In a multivariate linear regression model, after adjusting for the aforementioned confounders, the negative relationship between WC and D-PSV was confirmed (adjusted r = −0.107, P < 0.0001).
TREATING OBESITY RESTORES ERECTILE FUNCTION
Esposito et al.54 was the first to describe a positive effect of lifestyle modifications on erectile function. In a randomized, single-blind trial of 110 obese men-without known CV risk factors, but with ED-erectile function was greatly improved by strict dietary and physical activity recommendations for 2 years. Accordingly 17/55 men in the active arm recovered from ED and only 3/55 in the control (generic recommendation) group. Improvement in IIEF score was paralleled by a statistical decrease in inflammatory markers. Similar results were, later on, replicated in other cohorts of ED subjects with overweight/obesity,55 metabolic syndrome56 or type 2 diabetes mellitus.57 One of the main limitations of all these studies is the lack of measurements of T levels during the course or at the end of the trials. In fact, it is well-known that T increases as a function of lifestyle modifications and weight loss.36 Even weight loss induced by bariatric surgery was able to improve erectile function and, interestingly, IIEF score increase was accompanied by an increase in T levels.58 Accordingly, a recently published meta-analysis of all the controlled studies on lifestyle intervention on ED in obesity suggests a significant effect of lifestyle intervention of IIEF score.59
EXPERIMENTAL MODELS OF VISCERAL OBESITY
Epidemiological data imply a direct association between WC and ED in both general and symptomatic (sexual dysfunction) populations. Although WC is an accepted proxy of visceral obesity, often used for epidemiological studies, it does not represent a true measure of visceral fat content in the abdomen. The only reliable measures of visceral fat mass in humans (i.e. magnetic resonance imaging and computed tomography scans) are complex and have never been used in large-scale studies assessing relationships between adiposity and male sexual function. We recently described two experimental, nongenomic protocols to increase visceral adiposity in a rodent, the rabbit, which shows the unique advantage of a mechanism of penile erection very similar to that of humans.60 We obtained a substantial increase in visceral adiposity (directly measured by abdominal inspection) with two distinct strategies: (i) by treating with a longlasting gonadotropin-releasing hormone analog (triptorelin pomoate)61 to induce hypogonadotropic hypogonadism or (ii) by feeding the animals a cholesterol-enriched high fat diet.60 The latter experimental protocol was also associated with hypogonadotropic hypogonadism60 and with a cluster of CV and metabolic abnormalities, closely reflecting the human condition of metabolic syndrome (hypertension, dyslipidemia, glucose intolerance and increased visceral adiposity). Interestingly, we previously showed that with both experimental protocols there was an impairment in erectile response, as demonstrated by a decreased acetylcholine (Ach)-induced relaxation of phenylephrine precontracted corpora cavernosa (CC) strips (Figure 3a). Pharmacological treatments with T60,63 or with the selective farnesoid X receptor agonist obeticholic acid,64 restored Ach responsiveness and decreased visceral adiposity (Figure 3b).
Figure 3.
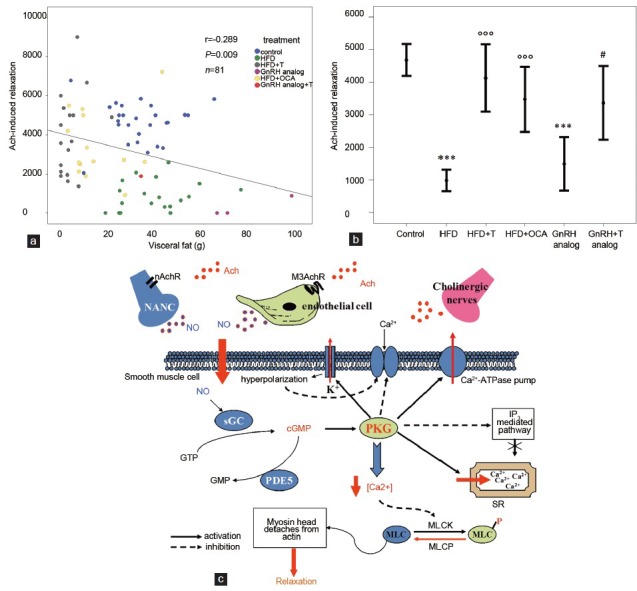
Effect of visceral fat accumulation in rabbit experimental models of increased visceral adiposity on maximal (3 μmol l− 1) acetylcholine (Ach)-induced relaxation in corpora cavernosa (CC) strips. (a) The dot plot represents the relationship between visceral fat accumulation (g) and maximal responsiveness to Ach in the following experimental groups: control (blue circles), high fat diet (HFD, green circles), HFD + chronic testosterone (T) dosing (gray circles),57 HFD + chronic obeticholic acid (OCA) dosing (yellow circles),60,65 chronic gonadotropin-releasing hormone (GnRH) analog (triptorelin pamoate) with (red circles) or without T (purple circles)58 and regular diet (blue circles).57 (b) Mean Ach-induced (3 μmol l− 1) relaxation of corpora cavernosa strips of rabbits according to different experimental groups. ***P < 0.0001 vs control; °°°P < 0.0001 vs HFD; #P < 0.05 vs GnRH analog. (c) Schematic representation of putative mechanisms of Ach-induced CC relaxation. Ach: acetylcholine; ATPase: adenosine triphosphatase; cGMP: cyclic guanosine monophosphate; GMP: guanosine monophosphate; GTP: guanosine triphosphate; IP3: inositol trisphosphate; MLC: myosin light chain; MLCK: myosin light-chain kinase; MLCP: myosin light-chain phosphatase; M3AchR: M3 muscarinic receptors; nAchR: nicotinic receptors; NANC: non-adrenergic, non-cholinergic nerves; NO: nitric oxide; PDE5: phosphodiesterase 5; PKG: cGMP-dependent protein kinase; sGC: soluble guanylate cyclase; SR: sarcoplasmic reticulum.
Ach responsiveness in CC is an important parameter that reflects endothelial integrity and recapitulates the chain of events leading to physiological erection (see the schematic cartoon in (Figure 3c). In fact, penile tissues in animals and humans receive a rich cholinergic innervation.65 In the late 1970s, Dr Robert Furchgott observed, in a systemic vascular bed, that Ach released a substance that produced relaxation, but only when the endothelium was intact. This observation led to his receiving a Nobel prize.62 Initially, Furchgott called this substance endothelium-derived relaxing factor, but by the mid-1980s, he and others identified this substance as being nitric oxide (NO).62 Today, we know that Ach released from parasympathetic terminations acts on M3 muscarinic receptors, on vascular endothelium and on nicotinic receptors located on non-adrenergic, non-cholinergic nerves;66 promoting the synthesis and release of NO, which finally leads to the relaxation of arterial and trabecular smooth muscle in the CC67 (Figure 3b). NO is produced as the enzymatic byproduct of molecular oxygen and L-arginine, under the control of nitric oxide synthase. Interestingly, maximal Ach responsiveness (3 μmol l−1) was substantially down-regulated by conditions associated with visceral fat accumulation, including gonadotropin-releasing hormone analog administration or feeding an HFD, and reverted by supplementation with T60 and/or the farnesoid X receptor agonist, obeticholic acid.64,68 Figure 3a shows the close association between visceral fat mass and maximal Ach responsiveness in the CC of rabbits from the aforementioned experimental groups. In these rabbits, CC responsiveness to Ach was also negatively associated with other metabolic parameters, glycemia (r = −0.321, P = 0.002, n = 90), cholesterol and triglyceride levels (r = −0.376, P < 0.0001, n = 93 and r = −0.339, P = 0.001, n = 90, respectively), glutamic oxaloacetic transaminase (r = −0.292, P = 0.014, n = 70) and blood pressure (r = −0.325, P = 0.01, n = 62), while it was positively related to circulating T (r = 0.472, P < 0.0001, n = 97). However, the association depicted in Figure 3a retained statistical significance even after adjusting for all the aforementioned parameters in a multivariate analysis (adjusted r = −0,505, P < 0.005), suggesting a direct link between visceral adiposity and the Ach-driven erectile process. We also investigated which genes expressed by VAT were more closely associated with an impaired Ach-induced relaxation in CC, by analyzing its association with a library of almost 50 genes involved in different steps of VAT metabolism. When the area under the (relaxant) curve of Ach was considered, we found only a significant, negative association between Ach area under the curve and the gene expression in visceral adipose tissue of a Wnt antagonist, Dickkopf 1, an androgen-regulated protein secreted by human preadipocytes, promoting adipogenesis (r = −0.415, n = 28, P = 0.02). The association retained significance even after adjusting for T levels (adjusted r = −0.401, P < 0.05). No other relationships reached statistical significance (not shown). Similar results were obtained when maximal responsiveness to Ach in CC was analyzed (not shown). These data, taken together, imply that visceral fat accumulation has a detrimental effect on the erectile process, apparently not explained by any of the known (measured) metabolic or hormonal VAT-associated abnormalities in the peripheral blood or within VAT homogenates. Therefore, how visceral fat could signal to the penile microcirculation, impairing Ach-mediated relaxation, is still unknown. However, two main hypotheses can be drawn. The first is that some factors (or a syndromic constellation of factors) not taken into consideration in our analysis are mediating the association between VAT and ED, the second one is that we omitted from the analysis some other important elements, such as, for instance, the liver, that, reasonably, can act as a bridge between visceral fat accumulation and the erectile process. It is widely accepted that visceral obesity is characterized by a state of a low-grade, chronic inflammation that might involve not only visceral fat but also other tissues that directly communicate with VAT, such as the liver. It has been hypothesized, but never proven, that inflammatory cytokines, (e.g. tumor necrosis factor-α or interleukin-6) derived from increased visceral fat, or the liver, might have deleterious effects on penile erection.69
OBESITY, ED AND CV RISK
It is widely accepted that CVD predicts the incidence of ED, largely because both conditions share the same CV risk factors.70 It has also been hypothesized that ED may be a marker of further CV events.70 According to the 3rd Princeton Consensus Recommendations, a man with organic ED should be considered at increased CVD risk until recommended checks suggest otherwise.70 In particular, Montorsi et al.71 demonstrated that ED, on average, occurs 3 years earlier than a subsequent CV disease, hypothesizing that the smaller penile arteries reach critical narrowing, with insufficient blood flow earlier than larger vessels (‘the artery size hypothesis’). A few years later, Thompson et al.72 in the Prostate Cancer Prevention Trial, reported that incident ED was associated with a HR of 1.25 for subsequent CV events, during a 9-year follow-up. Hence, detecting arteriogenic ED might help clinicians in preventing further CV diseases.7 Our data obtained in a large series of subjects consulting for ED, confirmed that, among patients with ED, those with a more severe problem have a 75% increased risk of forthcoming MACE.73 We also demonstrated, in the same population of ED subjects, that D-PSV lower than 25 cm s−1-as assessed by penile color Doppler ultrasound, the gold standard in studying arteriogenic ED was associated with a doubled risk of forthcoming MACE;73 but we also found that PSV in the flaccid state can provide additional information on penile and overall CV status.44,73,74,75,76,77 Flaccid penile acceleration is a dimension that reflects both PSV and the time elapsed to reach it. It essentially represents the slope of the tangent of the systolic rise waveform. In a recent study on the aforementioned population of ED subjects, we demonstrated the superiority of flaccid penile acceleration, over other penile color Doppler ultrasound parameters, in predicting MACE.76 Hence, in our experience, reported severe ED and measures of arteriogenic ED are precious indicators of an unfavorable CV profile and of forthcoming MACE, in particular in young subjects and in those at a ‘lower risk’. It is important to note that in the general population, the majority of CV events occur in subjects who would be classified as ‘lower risk’ when using conventional parameters.78,79 Searching for new parameters capable of identifying this ‘residual CV risk’ is clinically relevant.80,81 Our recent data76 suggest that flaccid penile acceleration may be considered a new surrogate marker of arterial stiffness in men with ED, predicting CV events even in young and at ‘lower-risk’ ED subjects. Interestingly, in the Olmsted study, a population-based survey on more than 2200 men where sexual function was assessed biennially from 1996 to 2004, ED had relatively little impact on the development of incident cardiac events in the oldest men, but it resulted in a nearly 50-fold increase in the 10-year incidence of heart disease in men aged 40–49 years.82 Many other epidemiologic studies have investigated the link between ED and risk of CVD, and most found a positive association. In 2010 Guo et al.83 provided the first meta-analysis investigating the relationship between ED and CVD. From seven cohort studies they found an HR of 1.47 for CVD events in ED subjects compared to healthy individuals.83 Similar results were reported by Dong et al.84 in a larger analysis of 12 prospective cohort studies involving 36 744 participants. The overall combined HR for men with ED, compared with the reference group, were 1.48 for CVD, 1.46 for coronary heart disease, 1.35 for stroke and 1.19 for all-cause mortality,84 respectively. Interestingly, more recently, Vlachopoulos et al.85 extending the evaluation to 92 757 participants, with a mean follow-up of 6.1 years, reported that the CV risk of ED individuals is particularly high at younger ages and at intermediate baseline CV risk. In addition, the same authors showed that the risk conferred by ED on CV events was of a magnitude similar to that of the risk conferred by events with established risk predictors, such as hypertension and dyslipidemia.85 Hence, current evidence emphasizes the importance of an early diagnosis of ED and of the meticulous CV investigation that is required in specific groups of ED patients. In particular, the higher CV risk in younger patients and in those with low-intermediate baseline CV risk compared to ED patients with high CV risk is particularly important.76 In fact, ED could be a particularly useful CV predictor in young patients in whom the CV engine may underestimate risk.
From the evidence provided so far, it is clear that the presence of central obesity in subjects complaining of ED, and particularly in young individuals with low-to-moderate CV risk, should alert the healthcare clinician that such people deserve more intensive lifestyle therapy to delay progression to a higher CV risk category, eventually preventing forthcoming MACE. However, the impact of obesity in ED subjects on the risk of incident CVD has been endorsed only by few studies,3,86 and the role of central obesity has never been explored. We now originally report associations between WC and potential CV risk factors in a large series of subjects seeking medical care for ED at our unit (see above). We confirmed that also different WC grades, as previously reported for BMI classes,3,23 are associated with an increasing severity of hyperglycemia, insulin resistance and the prevalence of diabetes mellitus (Figure 4a–c) as well as dyslipidemia (Figure 4d–f). Similarly, pulse pressure, mean blood pressure and prevalence of hypertension were all closely related to WC increment (Figure 5a–c). Accordingly, patients with higher WC reported a higher frequency of associated morbidities as detected by Chronic Disease Score (a validated index of associated-comorbidities87 (see also Figure 5d), a higher predicted CV risk as detected by Progetto Cuore risk engine (Figure 5e) and a higher organic domain of ED, as detected by SIEDY scale 1 score (Figure 5f, panel).
Figure 4.
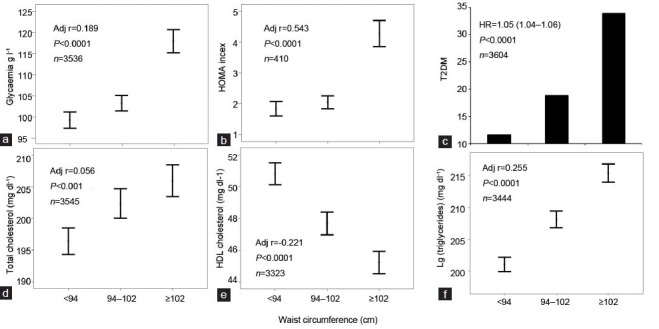
Relationships between waist circumference (WC) and glycemia (panel a), Homeostasis Model Assessment (HOMA) index (b), type 2 diabetes mellitus (T2DM) (c), total cholesterol (d), high density lipoprotein (HDL) cholesterol (e) and triglyceride levels (f). Data (unpublished) are derived from a consecutive, nonselected series of men (mean age = 51.3 ± 13.3 years) attending our Sexual Medicine and Andrology Clinic for sexual dysfunction between 2000 and 2013. The age-adjusted correlation coefficients for the indicated observations are also reported in each panel. Note that WC was considered as a continuous variable in all the analyses.
Figure 5.
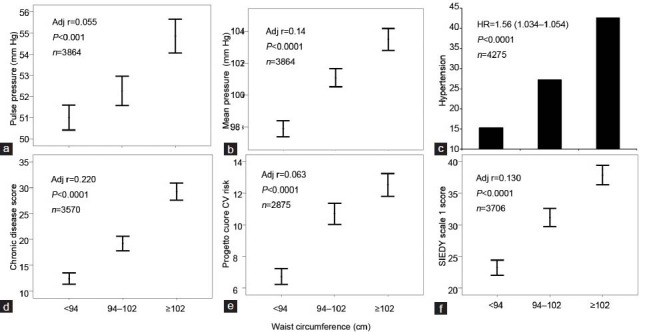
Relationships between waist circumference (WC) and pulse pressure (a), mean blood pressure (b), hypertension (c), chronic disease score (a validated Index of associated morbidities; (d),87 cardiovascular (CV) risk (as assessed by Progetto Cuore risk engine; (e) and organic domain of ED (as assessed by SIEDY Scale 1 score; (f),45 Data (unpublished) are derived from a consecutive, nonselected series of men (mean age = 51.3 ± 13.3 years) attending our Sexual Medicine and Andrology Clinic for sexual dysfunction between 2000 and 2013. The age-adjusted correlation coefficients for the indicated observations are also reported in each panel. Note that WC was considered as a continuous variable in all the analyses.
From the evidence provided in Figures 4 and 5, it can be derived that ED subjects with central obesity are a category of high-risk individuals for incident CV events. Accordingly, when WC was applied as a possible predictor of MACE in a sample of 1687 patients attending our unit, with a mean follow up of 4.3 ± 2.6 years,87,88,89,90,91 we observed an unadjusted association between WC classes and increased MACE (Figure 6a). However, in a Cox regression model, after adjusting for lifestyle and Chronic Disease Score confounders, only a severe WC increment (>102 cm) still maintained an independent, significant association with MACE (Figure 6b). Figure 6c, shows that, for each centimeter increment of WC increase, there is a 2% higher HR for MACE, as derived from a Cox model adjusted for the aforementioned confounders. When the same population was divided according to the median age (55 years), a WC-associated increased HR was observed only in the youngest cohort (HR = 1.043 (1.007–1.080), P = 0.02), which was further augmented to an almost 6% increased risk for each centimeter of WC, when TT was introduced as a further covariate (HR = 1.058 (1.017–1.102), P = 0.006). These data indicate that in subjects with ED, in particular in the youngest ones, central obesity is an independent risk factor for incident CVD.
Figure 6.
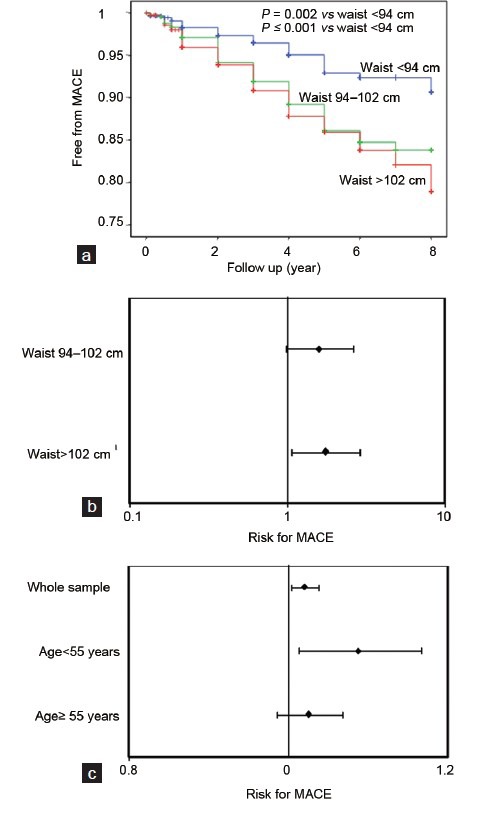
Risk of incident major adverse cardiovascular events (MACE), as derived from Kaplan–Meier curves (a), according to waist circumference (WC) classes. (b) Age, lifestyle (smoking and alcohol habits) and associated morbidity-adjusted risk for MACE, according to WC thresholds, as derived from Cox regression analysis in the whole sample. (c) Age, lifestyle (smoking and alcohol habits) and associated morbidity-adjusted risk for MACE, according to each centimeter increment in WC as derived from Cox regression analysis in the whole population or according to the median age of the cohort (55 years). Data are derived from a consecutive nonselected series of 1687 patients attending our unit, with a mean follow-up of 4.3 ± 2.6 years.84,85,86,87,88
CONCLUSIONS
Obesity, and in particular central obesity, is associated with both ED and low T. However, the association between ED and increased WC is apparently independent from obesity-associated comorbidities and hypogonadism. In fact, in two distinct cohorts from Florence, Italy, reflecting a general and a symptomatic (ED) population, the association between ED and central obesity was confirmed even after adjusting for total T and known obesity-associated comorbidities and was more dependent on organic than psychological and relational problems. Results derived from experimental models of central obesity in rabbits are similar. In fact, the strong association between visceral adiposity and impaired Ach-induced relaxation in CC was not explained by any metabolic or hormonal alteration considered, even when gene expression in VAT was examined as a function of Ach responsiveness within the penis. Hence, the organic factor (s) linking visceral fat accumulation and impaired erectile function deserves further studies. This point is relevant because an increased WC in ED subjects categorizes individuals at high CV risk, especially in the youngest ones. Few studies have addressed the effect of lifestyle changes on obesity-associated ED, but all report that decreasing weight and increasing physical exercise result in improved erectile function and reverse hypogonadism. Identifying young, obese, ED individuals as those at ‘residual risk’ of incident vascular events might open up new possibilities of lifestyle or pharmacotherapeutic or even bariatric interventions. The ‘residual risk’ of incident vascular events or the progression of established vascular damage might be eventually slowed or even halted by some form of current, evidence-based, recommended care. However, it is difficult to communicate and to convince young obese people that lifestyle changes are needed for modifying their risk profile. We previously developed the concept that ‘impotent patients are lucky’;7 we now want to extend it to obesity-associated ED. Unsatisfying sexual activity, because of impotence, represents a meaningful, straightforward motivation for consulting healthcare professionals, who can screen for the presence of unfavorable associated conditions, such as obesity. The presence of ED in an obese subject might help healthcare professionals in convincing them to initiate a virtuous cycle, where the correction of sexual dysfunction will be the reward for an improved lifestyle for the obese, ED individual and a lower risk of MACE will be the ultimate goal for both the obese subject and the entire community. Clinicians are often led to classify young subjects with ED as ‘psychogenic’, and therefore treat them with symptomatic medicaments, underestimating the important aspects of their general health, even those that could be specifically corrected, such as obesity. The data presented in this review should prompt physicians to reclassify obese ED subjects as ‘high-risk’ individuals, even if they are young, or better, because they are young. Obesity in childhood is dramatically increasing, even in a ‘virtuous’, Mediterranean-diet based country like Italy. Tomorrow we will face a large wave of obesity in young people and very likely, of associated impotence. We now claim that, also for obese individuals, being impotent could be recomputed as a resource,7 rather than a further sanitary complication. Eating less, exercising more and adopting a healthier lifestyle will be easier if it is associated with a restored, satisfactory sex life! A combination of lifestyle changes, phosphodiesterase type 5 inhibitors and T supplementation must be considered as a new armamentarium in obesity.92
AUTHOR CONTRIBUTIONS
GC, GR, SF and LV contributed to the data collection. LV and EM contributed to the intellectual revision of the manuscript. RG helped to draft the manuscript. GC and MM conceived of the study, participated in its design and coordination, performed the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; 2009. Global health risks: mortality and burden of disease attributable to selected major risks. [Google Scholar]
- 3.Corona G, Monami M, Boddi V, Balzi D, Melani C, et al. In obesity a further cardiovascular risk factor in patients with erectile dysfunction. J Sex Med. 2010;7:2538–46. doi: 10.1111/j.1743-6109.2010.01839.x. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–95. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl. 2009;32:587–98. doi: 10.1111/j.1365-2605.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Forti G, Maggi M. Why can patients with erectile dysfunction be considered lucky. The association with testosterone deficiency and metabolic syndrome? Aging Male. 2008;11:193–9. doi: 10.1080/13685530802468497. [DOI] [PubMed] [Google Scholar]
- 8.Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, et al. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997;5:93–9. doi: 10.1002/j.1550-8528.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith SR, Lovejoy JC, Greenway F, Ryan D, de Jonge L, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 10.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallus S, Odone A, Lugo A, Bosetti C, Colombo P, et al. Overweight and obesity prevalence and determinants in Italy: an update to 2010. Eur J Nutr. 2013;52:677–85. doi: 10.1007/s00394-012-0372-y. [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2005;28:2780–6. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 15.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray GA. Uses and misuses of the new pharmacotherapy of obesity. Ann Med. 1999;31:1–3. doi: 10.3109/07853899909019257. [DOI] [PubMed] [Google Scholar]
- 17.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 18.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, et al. A prospective study of risk factors for erectile dysfunction. J Urol. 2006;176:217–21. doi: 10.1016/S0022-5347(06)00589-1. [DOI] [PubMed] [Google Scholar]
- 19.Andersen I, Heitmann BL, Wagner G. Obesity and sexual dysfunction in younger Danish men. J Sex Med. 2008;5:2053–60. doi: 10.1111/j.1743-6109.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 20.Corona G, Lee DM, Forti G, O’Connor DB, Maggi M, et al. EMAS Study Group. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS) J Sex Med. 2010;7:1362–80. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 21.Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J Am Geriatr Soc. 2001;49:436–42. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- 22.Han TS, Tajar A, O’Neill TW, Jiang M, Bartfai G, et al. EMAS group. Impaired quality of life and sexual function in overweight and obese men: the European Male Ageing Study. Eur J Endocrinol. 2011;164:1003–11. doi: 10.1530/EJE-10-1129. [DOI] [PubMed] [Google Scholar]
- 23.Corona G, Mannucci E, Fisher AD, Lotti F, Petrone L, et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5:2454–63. doi: 10.1111/j.1743-6109.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 24.Larsen SH, Wagner G, Heitmann BL. Sexual function and obesity. Int J Obes (Lond) 2007;31:1189–98. doi: 10.1038/sj.ijo.0803604. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor DB, Corona G, Forti G, Tajar A, Lee DM, et al. Assessment of sexual health in aging men in Europe: development and validation of the European Male Ageing Study sexual function questionnaire. J Sex Med. 2008;5:1374–85. doi: 10.1111/j.1743-6109.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 26.Janiszewski PM, Janssen I, Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med. 2009;6:1990–8. doi: 10.1111/j.1743-6109.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 27.Riedner CE, Rhoden EL, Ribeiro EP, Fuchs SC. Central obesity is an independent predictor of erectile dysfunction in older men. J Urol. 2006;176:1519–23. doi: 10.1016/j.juro.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, et al. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–67. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- 29.Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. How to recognize late-onset hypogonadism in men with sexual dysfunction. Asian J Androl. 2012;14:251–9. doi: 10.1038/aja.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012;8:131–43. doi: 10.2174/157339912799424573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25:337–53. doi: 10.1016/j.beem.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34(6 Pt 1):528–40. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 33.Corona G, Mannucci E, Forti G, Maggi M. Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes? Int J Androl. 2009;32:431–41. doi: 10.1111/j.1365-2605.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 34.Corona G, Rastrelli G, Forti G, Maggi M. Update in testosterone therapy for men. J Sex Med. 2011;8:639–54. doi: 10.1111/j.1743-6109.2010.02200.x. [DOI] [PubMed] [Google Scholar]
- 35.Corona G, Mannucci E, Petrone L, Schulman C, Balercia G, et al. A comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. J Sex Med. 2007;4:789–96. doi: 10.1111/j.1743-6109.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 36.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 37.Corona G, Mannucci E, Ricca V, Lotti F, Boddi V, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl. 2009;32:720–8. doi: 10.1111/j.1365-2605.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 38.Corona G, Mannucci E, Lotti F, Fisher AD, Bandini E, et al. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med. 2009;6:285–93. doi: 10.1111/j.1743-6109.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 39.Corona G, Petrone L, Mannucci E, Magini A, Lotti F, et al. Assessment of the relational factor in male patients consulting for sexual dysfunction: the concept of couple sexual dysfunction. J Androl. 2006;27:795–801. doi: 10.2164/jandrol.106.000638. [DOI] [PubMed] [Google Scholar]
- 40.Corona G, Mannucci E, Lotti F, Boddi V, Jannini EA, et al. Impairment of couple relationship in male patients with sexual dysfunction is associated with overt hypogonadism. J Sex Med. 2009;6:2591–600. doi: 10.1111/j.1743-6109.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 41.Corona G, Mannucci E, Petrone L, Ricca V, Balercia G, et al. Psycho-biological correlates of free-floating anxiety symptoms in male patients with sexual dysfunctions. J Androl. 2006;27:86–93. doi: 10.2164/jandrol.05070. [DOI] [PubMed] [Google Scholar]
- 42.Corona G, Rastrelli G, Ricca V, Jannini EA, Vignozzi L, et al. Risk factors associated with primary and secondary reduced libido in male patients with sexual dysfunction. J Sex Med. 2013;10:1074–89. doi: 10.1111/jsm.12043. [DOI] [PubMed] [Google Scholar]
- 43.Boddi V, Corona G, Fisher AD, Mannucci E, Ricca V, et al. “It takes two to tango”: the relational domain in a cohort of subjects with erectile dysfunction (ED) J Sex Med. 2012;9:3126–36. doi: 10.1111/j.1743-6109.2012.02948.x. [DOI] [PubMed] [Google Scholar]
- 44.Corona G, Fagioli G, Mannucci E, Romeo A, Rossi M, et al. Penile doppler ultrasound in patients with erectile dysfunction (ED): role of peak systolic velocity measured in the flaccid state in predicting arteriogenic ED and silent coronary artery disease. J Sex Med. 2008;5:2623–34. doi: 10.1111/j.1743-6109.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 45.Petrone L, Mannucci E, Corona G, Bartolini M, Forti G, et al. Structured interview on erectile dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res. 2003;15:210–20. doi: 10.1038/sj.ijir.3901006. [DOI] [PubMed] [Google Scholar]
- 46.Corona G, Ricca V, Bandini E, Rastrelli G, Casale H, et al. SIEDY scale 3, a new instrument to detect psychological component in subjects with erectile dysfunction. J Sex Med. 2012;9:2017–26. doi: 10.1111/j.1743-6109.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 48.Corona G, Rastrelli G, Balercia G, Sforza A, Forti G, et al. Perceived reduced sleep-related erections in subjects with erectile dysfunction: psychobiological correlates. J Sex Med. 2011;8:1780–8. doi: 10.1111/j.1743-6109.2011.02241.x. [DOI] [PubMed] [Google Scholar]
- 49.Corona G, Jannini EA, Vignozzi L, Rastrelli G, Maggi M. The hormonal control of ejaculation. Nat Rev Urol. 2012;9:508–19. doi: 10.1038/nrurol.2012.147. [DOI] [PubMed] [Google Scholar]
- 50.Corona G, Jannini EA, Lotti F, Boddi V, De Vita G, et al. Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Androl. 2011;34:41–8. doi: 10.1111/j.1365-2605.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- 51.Isidori AM, Buvat J, Corona G, Goldstein I, Jannini EA, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment—a systematic review. Eur Urol. 2014;65:99–112. doi: 10.1016/j.eururo.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 52.Spitzer M, Basaria S, Travison TG, Bhasin S. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013;158:570–1. doi: 10.7326/0003-4819-158-7-201304020-00019. [DOI] [PubMed] [Google Scholar]
- 53.Aversa A, Jannini EA, Maggi M, Lenzi A. Effects of testosterone replacement on response to sildenafil citrate. Ann Intern Med. 2013;158:569–70. doi: 10.7326/0003-4819-158-7-201304020-00018. [DOI] [PubMed] [Google Scholar]
- 54.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 55.Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, et al. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med. 2009;6:243–50. doi: 10.1111/j.1743-6109.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 56.Esposito K, Ciotola M, Giugliano F, De Sio M, Giugliano G, et al. Mediterranean diet improves erectile function in subjects with the metabolic syndrome. Int J Impot Res. 2006;18:405–10. doi: 10.1038/sj.ijir.3901447. [DOI] [PubMed] [Google Scholar]
- 57.Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7:156–65. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reis LO, Favaro WJ, Barreiro GC, de Oliveira LC, Chaim EA, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33:736–44. doi: 10.1111/j.1365-2605.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 59.Gupta BP, Murad MH, Clifton MM, Prokop L, Nehra A, et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171:1797–803. doi: 10.1001/archinternmed.2011.440. [DOI] [PubMed] [Google Scholar]
- 60.Filippi S, Vignozzi L, Morelli A, Chavalmane AK, Sarchielli E, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–88. doi: 10.1111/j.1743-6109.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 61.Filippi S, Luconi M, Granchi S, Vignozzi L, Bettuzzi S, et al. Estrogens, but not androgens, regulate expression and functional activity of oxytocin receptor in rabbit epididymis. Endocrinology. 2002;143:4271–80. doi: 10.1210/en.2002-220384. [DOI] [PubMed] [Google Scholar]
- 62.Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–8. [PubMed] [Google Scholar]
- 63.Filippi S, Luconi M, Granchi S, Natali A, Tozzi P, et al. Endothelium-dependency of yohimbine-induced corpus cavernosum relaxation. Int J Impot Res. 2002;14:295–307. doi: 10.1038/sj.ijir.3900890. [DOI] [PubMed] [Google Scholar]
- 64.Vignozzi L, Morelli A, Filippi S, Comeglio P, Chavalmane AK, et al. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011;8:57–77. doi: 10.1111/j.1743-6109.2010.02073.x. [DOI] [PubMed] [Google Scholar]
- 65.Andersson KE. Neurophysiology/pharmacology of erection. Int J Impot Res. 2001;13(Suppl 3):S8–17. doi: 10.1038/sj.ijir.3900718. [DOI] [PubMed] [Google Scholar]
- 66.Bozkurt NB, Vural IM, Sarioglu Y, Pekiner C. Nicotine potentiates the nitrergic relaxation responses of rabbit corpus cavernosum tissue via nicotinic acetylcholine receptors. Eur J Pharmacol. 2007;558:172–8. doi: 10.1016/j.ejphar.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 67.Maggi M, Filippi S, Ledda F, Magini A, Forti G. Erectile dysfunction: from biochemical pharmacology to advances in medical therapy. Eur J Endocrinol. 2000;143:143–54. doi: 10.1530/eje.0.1430143. [DOI] [PubMed] [Google Scholar]
- 68.Maneschi E, Vignozzi L, Morelli A, Mello T, Filippi S, et al. FXR activation normalizes insulin sensitivity in visceral preadipocytes of a rabbit model of MetS. J Endocrinol. 2013;218:215–31. doi: 10.1530/JOE-13-0109. [DOI] [PubMed] [Google Scholar]
- 69.Traish AM, Feeley RJ, Guay A. Mechanisms of obesity and related pathologies: androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J. 2009;276:5755–67. doi: 10.1111/j.1742-4658.2009.07305.x. [DOI] [PubMed] [Google Scholar]
- 70.Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766–78. doi: 10.1016/j.mayocp.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, et al. Association between erectile dysfunction and coronary artery disease: Matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–31. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 73.Corona G, Monami M, Boddi V, Cameron-Smith M, Lotti F, et al. Male sexuality and cardiovascular risk. A cohort study in patients with erectile dysfunction. J Sex Med. 2010;7:1918–27. doi: 10.1111/j.1743-6109.2010.01744.x. [DOI] [PubMed] [Google Scholar]
- 74.Mancini M, Bartolini M, Maggi M, Innocenti P, Villari N, et al. Duplex ultrasound evaluation of cavernosal peak systolic velocity and waveform acceleration in the penile flaccid state: clinical significance in the assessment of the arterial supply in patients with erectile dysfunction. Int J Androl. 2000;23:199–204. doi: 10.1046/j.1365-2605.2000.00227.x. [DOI] [PubMed] [Google Scholar]
- 75.Roy C, Saussine C, Tuchmann C, Castel E, Lang H, et al. Duplex Doppler sonography of the flaccid penis: potential role in the evaluation of impotence. J Clin Ultrasound. 2000;28:290–4. doi: 10.1002/1097-0096(200007/08)28:6<290::aid-jcu4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 76.Rastrelli G, Corona G, Lotti F, Aversa A, Bartolini M, et al. Flaccid penile acceleration as a marker of cardiovascular risk in men without classical risk factors. J Sex Med. 2014;11:173–86. doi: 10.1111/jsm.12342. [DOI] [PubMed] [Google Scholar]
- 77.Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MC, et al. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: the Edinburgh Artery Study. Circulation. 2004;110:3075–80. doi: 10.1161/01.CIR.0000143102.38256.DE. [DOI] [PubMed] [Google Scholar]
- 78.Zannad F, De Backer G, Graham I, Lorenz M, Mancia G, et al. ESC Working Group on CardioVascular Pharmacology and Drug Therapy. Risk stratification in cardiovascular disease primary prevention-scoring systems, novel markers, and imaging techniques. Fundam Clin Pharmacol. 2012;26:163–74. doi: 10.1111/j.1472-8206.2011.01023.x. [DOI] [PubMed] [Google Scholar]
- 79.Corona G, Rastrelli G, Silverii A, Monami M, Sforza A, et al. The identification of prediabetes condition with ARIC algorithm predicts long-term CV events in patients with erectile dysfunction. J Sex Med. 2013;10:1114–23. doi: 10.1111/jsm.12066. [DOI] [PubMed] [Google Scholar]
- 80.Rastrelli G, Corona G, Fisher AD, Silverii A, Mannucci E, et al. Two unconventional risk factors for major adverse cardiovascular events in subjects with sexual dysfunction: low education and reported partner's hypoactive sexual desire in comparison with conventional risk factors. J Sex Med. 2012;9:3227–38. doi: 10.1111/j.1743-6109.2012.02947.x. [DOI] [PubMed] [Google Scholar]
- 81.Hermans MP, Fruchart JC. Reducing residual vascular risk in patients with atherogenic dyslipidemia: where do we go from here? Clin Lipidology. 2010;5:811–26. [Google Scholar]
- 82.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–13. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo W, Liao C, Zou Y, Li F, Li T, et al. Erectile dysfunction and risk of clinical cardiovascular events: a meta-analysis of seven cohort studies. J Sex Med. 2010;7:2805–16. doi: 10.1111/j.1743-6109.2010.01792.x. [DOI] [PubMed] [Google Scholar]
- 84.Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–85. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 85.Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6:99–109. doi: 10.1161/CIRCOUTCOMES.112.966903. [DOI] [PubMed] [Google Scholar]
- 86.Corona G, Rastrelli G, Monami M, Melani C, Balzi D, et al. Body mass index regulates hypogonadism-associated CV risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011;8:2098–105. doi: 10.1111/j.1743-6109.2011.02292.x. [DOI] [PubMed] [Google Scholar]
- 87.McGregor JC, Kim PW, Perencevich EN, Bradham DD, Furuno JP, et al. Utility of the chronic disease score and charlson comorbidity index as comorbidity measures for use in epidemiologic studies of antibioticresistant organisms. Am J Epidemiol. 2005;161:483–93. doi: 10.1093/aje/kwi068. [DOI] [PubMed] [Google Scholar]
- 88.Corona G, Monami M, Boddi V, Cameron-Smith M, Fisher AD, et al. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010;7(4 Pt 1):1557–64. doi: 10.1111/j.1743-6109.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 89.Corona G, Rastrelli G, Boddi V, Monami M, Melani C, et al. Prolactin levels independently predict major cardiovascular events in patients with erectile dysfunction. Int J Androl. 2011;34:217–24. doi: 10.1111/j.1365-2605.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 90.Rastrelli G, Corona G, Monami M, Melani C, Balzi D, et al. Poor response to alprostadil ICI test is associated with arteriogenic erectile dysfunction and higher risk of major adverse cardiovascular events. J Sex Med. 2011;8:3433–45. doi: 10.1111/j.1743-6109.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- 91.Corona G, Monami M, Boddi V, Rastrelli G, Melani C, et al. Pulse pressure independently predicts major cardiovascular events in younger but not in older subjects with erectile dysfunction. J Sex Med. 2011;8:247–54. doi: 10.1111/j.1743-6109.2010.01966.x. [DOI] [PubMed] [Google Scholar]
- 92.Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, et al. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011;8:3418–32. doi: 10.1111/j.1743-6109.2011.02473.x. [DOI] [PubMed] [Google Scholar]


