Abstract
We assessed the rates of sperm retrieval and intracytoplasmic sperm injection outcomes, including the neonatal profile of infants conceived, in men with testicular failure. Three-hundred and sixty-five men with testicular failure who underwent micro-dissection testicular sperm extraction were included in this study. We compared their outcomes with 40 men with testicular failure who used donor sperm for injections due to failed retrieval, and 146 men with obstructive azoospermia who underwent percutaneous sperm retrieval. The retrieval rate in testicular failure was 41.4%, and the results were lower than the obstructed azoospermia (100%; adjusted odds ratio: 0.033; 95% CI: 0.007–0.164; P < 0.001). Live birth rates after sperm injections were lower in men with testicular failure (19.9%) compared with donor sperm (37.5%; adjusted OR: 0.377 (95% CI: 0.233–0.609, P < 0.001)) and obstructive azoospermia (34.2%; adjusted OR: 0.403 (95% CI: 0.241–0.676, P = 0.001). Newborn parameters of infants conceived were not significantly different among the groups. We concluded that the chances of obtaining sperm on retrieval and achieving a live birth after intracytoplasmic sperm injection (ICSI) are reduced in men with testicular failure. The profile of infants conceived after sperm injection does not seem to be negatively affected by testicular failure.
Keywords: infant, intracytoplasmic sperm injection, obstructive azoospermia, pregnancy outcome, sperm retrieval, testicular failure
INTRODUCTION
Azoospermia is a relatively common condition affecting infertile males.1 Many couples with azoospermia-related infertility rely on assisted reproductive techniques (ART) as the first line treatment for achieving biological parenthood.1,2 In such cases, sperm retrieval (SR) is performed in an attempt to obtain viable sperm for intracytoplasmic sperm injection (ICSI).3 Men diagnosed with azoospermia are broadly categorized as having a mechanical obstruction along the seminal tract or an intrinsic testicular impairment of sperm production (non-obstructive azoospermia).4 Despite being well preserved in obstructive azoospermia (OA), spermatogenesis is either minimal or absent in men with testicular failure, thus lowering the success rates of SR in the latter.3
Several studies have examined the outcomes of ICSI using non-ejaculated sperm.5,6,7,8,9 However, reports comparing both SR and ICSI results by making a clear distinction between the types of azoospermia are minimum.10,11 Still, ICSI data comparing the reproductive potential of the non-ejaculated gametes from men with testicular failure with the ejaculated sperm from fertile donors are lacking. Moreover, children conceived after ICSI by non-ejaculated sperms from fathers with normal and abnormal spermatogenesis are poorly studied,5,10,11,12,13 and concerns exist whether these spermatozoa impact the health of the offspring.14 Hence, a detailed assessment that combines: (i) data on sperm retrieval, (ii) clinical outcomes of sperm injection, and (iii) profile of infants conceived from such treatments can help doctors counsel the azoospermic patients before embarking on SR and assisted-reproductive techniques.
In this study, we assessed the sperm retrieval rates (SRR) and ICSI outcomes of azoospermic men with testicular failure who underwent micro-dissection testicular sperm extraction (micro-TESE). Neonatal outcomes of pregnancies originated from such interventions are provided. We compared these results with those of men with testicular failure who used donor sperm for injections, due to failed SR by micro-TESE, and men with normal spermatogenesis and azoospermia caused by obstruction.
MATERIALS AND METHODS
Patient selection
We retrospectively studied 365 consecutive men with testicular failure who underwent SR by micro-TESE in an attempt to find sperm for ICSI, over a 7-year period. Azoospermia was confirmed on at least two different centrifuged ejaculates. A thorough evaluation, including history, physical examination, hormone profile (serum FSH, LH and total testosterone) and genetic testing (Yq microdeletions and karyotyping) was available for all patients.4 Testicular failure was confirmed by histological evaluation of testicular biopsy specimens taken at the study center prior or during SR. Histological patterns of Sertoli cell-only (SCO), maturation arrest (MA) and hypo-spermatogenesis were found in 205 (57.6%), 67 (18.8%), and 84 (23.6%) men, respectively. Patients with hypogonadotropic hypogonadism and those with Yq microdeletions involving the AZFa and/or AZFb subregions were excluded. Eight patients (2.2%) having karyotype abnormalities (47, XXY) were included in this study. We used our previous report data of 146 consecutive men with OA for comparison, in which SR for ICSI were accomplished in the same period by the same surgeon.9 Only the first treatment cycle of each patient using fresh sperm for ICSI was included. Our institutional review board approved this study.
Sperm acquisition
Micro-dissection TESE, as described by Schlegel, was the method of sperm acquisition.15,16 Patients were asked to collect a semen specimen by masturbation immediately before SR. In all the study cases, azoospermia was confirmed after the analysis of centrifuged specimens. If we failed to retrieve sperm by micro-TESE in one testis, the method was used contra-laterally in the same operation. We performed retrievals on an outpatient basis under local anesthesia combined with intravenous propofol infusion.17,18 The extracted testicular fragments were immediately examined in the in vitro fertilization (IVF) laboratory.19 Successful retrievals were defined as the presence of sperm. The same senior urologist (SE) performed all the retrievals. Percutaneous aspirations were used in our previously reported group of men with OA.9
Sperm injections
Our methods for ovarian stimulation, oocyte collection, sperm injection, embryo culture and embryo transfer were previously described.18,19,20,21 In brief, we used recombinant gonadotropins for ovarian stimulation, in association with either gonadotropin-releasing hormone agonist or antagonist for LH surge suppression. Oocyte retrievals were performed prior to, but in the same day of SR. Spermatozoa from donors with proven fertility were offered to patients with testicular failure, as a back up for ICSI, before starting of treatment. On failure of sperm retrieval, ICSI would be carried out using donor sperm for couples on acceptance of the sperm donation. Sperm injections and embryo culture were carried out in a clean room IVF laboratory. Ultrasound-guided embryo transfer was performed on the third day of embryo development. There was no considerable difference in laboratory techniques during the study period. Embryologists and physicians performing procedures were also unchanged. Clinical pregnancy was confirmed by ultrasound at weeks 5 to 7. Miscarriage was determined by the presence of a nonviable clinical pregnancy on ultrasound in the first 20 weeks. The live birth rate was the ratio between the numbers of deliveries resulting in at least one live born infant and initiated ICSI cycles. Twin and triplets were deliveries resulting from two or three live births, respectively. Gestational age, weight and sex of newborns were registered at birth. Perinatal mortality included stillbirths and neonatal deaths.
Statistical analysis
Comparisons were performed with chi-square, Wilcoxon rank sum and Fisher's exact tests. Multivariate logistic and Poisson regression models were constructed for dichotomous outcomes and number of deliveries, respectively. For sperm retrieval, the covariates included male age and hormone levels. For embryonic and clinical outcomes, the covariates included male and female age, infertility duration, associated female infertility, and number of transferred embryos. Significance was considered at P < 0.05. Analyses were performed using R version 2.14.2 (Free Software Foundation, Boston, MA, USA).
RESULTS
The SRR by micro-TESE in men with testicular failure was 41.4%. Among men diagnosed with testicular failure, those with Sertoli cell-only and maturation arrest had lower SRR (SCO: 19.5%, n = 40/205; MA: 40.3%, n = 27/67) than the ones with hypo-spermatogenesis (100.0%; n = 84/84) based on testicular histological examination (P < 0.001). Compared to men with MA those with SCO had a lower SRR (P = 0.007). After adjusting for age and hormone levels, the odds ratio at obtaining sperm between the groups of testicular failure and OA was 0.033 (95% confidence interval (CI): 0.007 to 0.164, Wald P < 0.001). The overall complication rate following micro-TESE was 6.3%, and was not different from the 5.5% rate in percutaneous retrievals. Pain was the most common complaint (8 patients), followed by swelling (2 patients), and infection (2 patients). None of these patients required interventions but the use of analgesics and/or antibiotics. Cryopreservation of excess sperm retrieved by micro-TESE was carried out in 22.5% of cases, and the results were similar to those obtained in OA (Table 1).
Table 1.
Sperm retrieval rates in azoospermic men with testicular failure and obstructive azoospermia
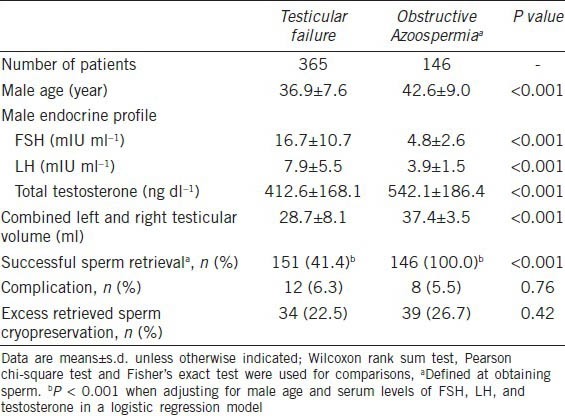
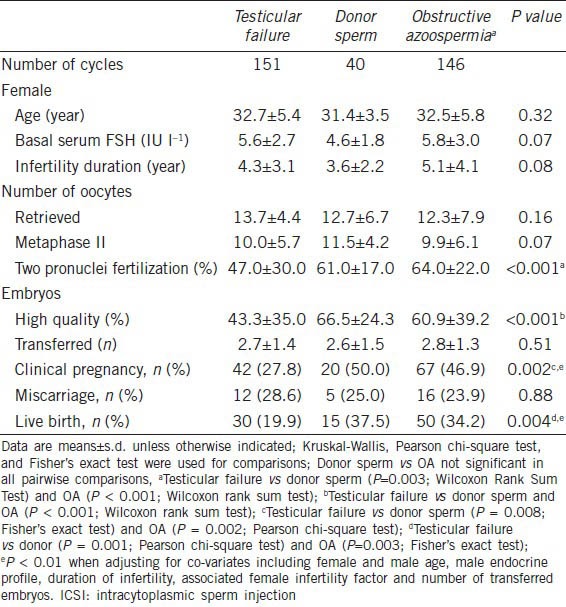
Sperm injections using testicular sperm retrieved from men with testicular failure were performed for 151 couples. We failed in obtaining sperm for 214 men with testicular failure (58.6%). Of these men, 40 used donor sperm for ICSI while the others had their treatments cancelled. In the latter, the retrieved oocytes were frozen (104 patients), donated (36 patients) or discarded (34 patients). The patient characteristics and ICSI outcomes are presented in Table 2. Female age, endocrine profile and duration of infertility were not different in the groups of testicular failure, donor sperm, and OA. There were no significant differences in the number and maturity of oocytes retrieved among the groups. The normal two-pronuclear zygote (2PN) and high quality embryos rates were lower in the testicular failure (47.0% and 43.3%, respectively) than donor sperm (61.0% and 66.5%, P < 0.001) and OA (64.0% and 60.9%, P < 0.001) groups, respectively. The mean number of transferred embryos was similar among the groups. Live birth rates were lower in the testicular failure (19.9%) than donor sperm (37.5%; P = 0.003) and OA (34.2%, P < 0.001) groups, whereas miscarriage rates did not differ among them. The adjusted odds ratio for clinical pregnancy and live birth by ICSI were 0.360 (95% CI: 0.176−0.736, Wald P = 0.005) and 0.377 (95% CI: 0.233−0.609, Wald P < 0.001) between testicular failure and donor sperm, and 0.388 (95% CI: 0.184−0.817, Wald P = 0.013) and 0.403 (95% CI: 0.241−0.676, Wald P = 0.001) between testicular failure and OA groups.
Table 2.
ICSI outcome in azoospermic men with testicular failure, stratified by successful and failed sperm retrieval (donor sperm), and obstructive azoospermia
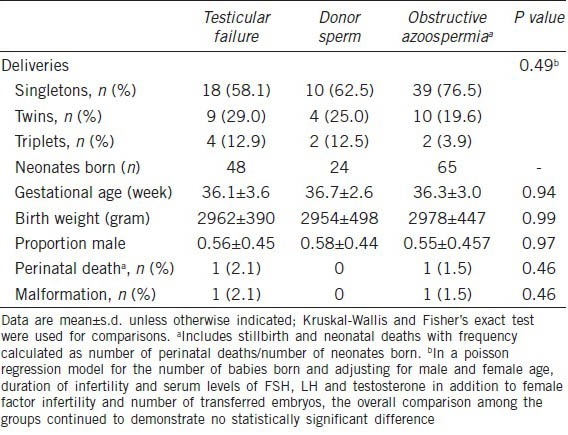
A total of 48 infants were delivered after ICSI with testicular sperm retrieved from men with testicular failure. Gestational age, birth weight and sex ratio of these children were comparable to those reported in the donor sperm and OA groups (Table 3). A total of 2 deliveries involved either a perinatal death or a malformation (cleft lip and palate) in the group of men with testicular failure, resulting in an overall adverse neonatal outcome of 4.1%.
Table 3.
Outcome of neonates born after ICSI in azoospermic men with testicular failure, stratified by successful and failed sperm retrieval (donor sperm), and obstructive azoospermia
DISCUSSION
We report SRR and sperm injection outcomes, including the profile of neonates conceived, of men with testicular failure. The chances of obtaining sperm were markedly reduced in men with testicular failure compared with OA, even when micro-TESE was used as the method of sperm acquisition. The odds ratio estimate represented the approximate halving in the testicular failure retrieval rate up to certain success in the OA patients. The magnitude of the OR estimate in the multivariable model demonstrated that covariate adjustment did not explain the large difference in retrieval rates between the testicular failure and OA. Sperm injections were carried out in all cases where SR had been successful. ICSI using donor sperm was also performed in a subgroup of men with testicular failure who had no sperm retrieved by micro-TESE. Zygote formation, embryo development and pregnancy rates were reduced when sperm from men with testicular failure were used in ICSI compared with donor sperm. Similarly, the outcomes were lower when ICSI was performed with non-ejaculated gametes obtained from men with testicular failure compared with OA. For live birth, the covariate adjusted-OR estimates of approximately 0.4 were consistent with the approximate halving in the success rates from roughly 35% to 20% in OA/donor sperm compared with testicular failure patients. Nevertheless, the neonatal profile of children conceived was not affected by testicular failure.
Azoospermia is a descriptive term for ejaculates that lack spermatozoa without implying a specific underlying cause.22 Under the clinical setting, patients presenting with azoospermia are classified with either non-obstructive azoospermia (NOA) or OA. Men with NOA have dysfunctional testes resulting from several conditions, including genetic and congenital abnormalities, post-infectious diseases, gonadotoxin exposure, trauma, endocrine disorders, and idiopathic causes.4,23 Although the fertility might be restored in the rare cases of spermatogenesis failure due to lack of appropriate stimulation by pituitary gonadotropins,24 the vast majority of these individuals have irreparable testicular failure. Since there are no treatment options in such cases, the alternative is to attempt SR and find viable testicular sperm for ICSI.3 The rationale of this approach relies on the fact that rare foci of sperm production exist in up to 60% of men with testicular failure.3,15,16,17
The recommended method for SR in men with testicular failure is testicular sperm extraction (TESE), which yields variable success rates of 25% to 60%.25 Because the presence and geographic location of islets of normal spermatogenesis are unpredictable, several authors have proposed micro-TESE as a better method for SR in such cases.3,15,16,17,18,26,27,28,29 Micro-TESE allows the identification of enlarged seminiferous tubules more likely to harbor sperm production. The minimal tissue extraction and preservation of intra-testicular blood supply are important features of micro-TESE, thus reducing the risk of testicular devascularization.30 In controlled series, micro-TESE performed better than TESE and percutaneous aspirations.16,27 For these reasons, micro-TESE was our preferred method for sperm acquisition in testicular failure. Given the fact that micro-TESE is an invasive procedure, we used strict criteria for classifying a patient as having testicular failure. Our method included semen analysis results, history and physical examinations, endocrine profiles, and genetic testing. In addition, we confirmed the diagnosis by histological evaluation. The combination of these parameters was shown to be highly accurate to diagnose testicular failure.31 In this series, the SRR of 41.4% and complication rate of 6.3% are comparable to those reported in the literature.3,15,16,17,18,26,27,28,29
Unlike testicular failure, OA is the endpoint of a mechanical blockage along the reproductive tract involving the vas deferens, epididymis, or ejaculatory duct.4,32 Treatment options in OA include microsurgical reconstruction and SR for ICSI.33 The SRR in OA is practically 100%, and are not influenced by the sperm collection method and cause of obstruction.9,34 In this study, we performed a logistic regression analysis to assess the odds of retrieving sperm by micro-TESE in testicular failure, and by percutaneous retrieval in OA. For this purpose, we used our published dataset of 146 men with normal spermatogenesis and azoospermia caused by obstruction, provided the procedures were performed by the same surgeon over the same period of time.9 Not surprisingly, we found that the odds of finding sperm with the aforesaid methods were significantly reduced given the presence of testicular failure (P < 0.001).
Testicular failure is considered to be an unfavourable prognostic condition for SR because spermatogenesis is disrupted.3,35 ICSI is widely used for patients with severe male infertility, but only few studies have addressed its outcomes by making a distinction between the type of azoospermia and considering the health of offspring.8,10,11,13 These aspects are important for evaluating the impact of spermatogenesis on ICSI outcomes. Our results of lower fertilization, embryo quality, and impaired pregnancy rates, using testicular sperm of men with defective spermatogenesis, were corroborated by others.5,8 They are also in agreement with a previous report from our group which assessed 835 infertile couples who underwent ICSI using either non-ejaculated or ejaculated sperm.10 In this aforementioned study, live birth rates were lower for azoospermic men with NOA (21.4%) compared with both azoospermic men with OA and men with severe male factor infertility whose ejaculated sperm was used for ICSI (37.5% and 32.3%, respectively; P = 0.003). The present series, however, substantially adds to our previous report. Of note, the studies differ in the population being assessed, albeit we acknowledge that some overlap exists. Here, we assessed SRR and defined micro-TESE as a selection criteria. Although the sperm acquisition method might not have influenced the ICSI outcomes for men with testicular failure, it would certainly have impacted the SRR.3,8,17,28,35 Hence, the effect of spermatogenesis on SR could be properly assessed by our logistic regression model. In addition, we controlled co-variates that might have influenced the SR and ICSI outcomes. Male and female age, hormone profile, duration of infertility, associated female infertility factor and number of transferred embryos were considered in the logistic regression analysis. Some of these factors reflect ovarian function and are robust predictors of pregnancy in ART.36 Importantly, we included a subgroup of men that used donor sperm for ICSI. To our knowledge, none of the published series compared ICSI outcomes between azoospermic patients and fertile donors. In this series, men with OA achieved similar results than fertile donors thus indicating that sperm integrity is not differentially affected by OA. In contrast, the sperm injection outcomes were negatively affected by testicular failure. These findings may be related to an increased risk of gametes extracted from men with severely impaired spermatogenesis to carry deficiencies involving centrioles and genetic material, which have been associated to decreased zygote formation and embryo development.14,37 Nevertheless, some investigators reported similar ICSI outcomes regardless of the azoospermia classification,6,7 which is in contrast to our observation and others as well.5,8 The reasons for these conflicting results remain unclear.
To date, only five studies compared the neonatal profile of babies born after ICSI by making a systematic distinction of OA and NOA.5,10,11,12,13 Similar to our findings, no major difference was noted in the neonatal outcomes and congenital malformation between the groups. Notwithstanding, a tendency towards lower gestational age and increased prematurity was observed for NOA in our previous study10 and in the series of Vernaeve et al.11 In this series, however, we could not confirm our previous results after controlling the co-variates, such as number of babies born and maternal age. Nevertheless, we add to the existing literature by comparing the neonatal outcomes of children conceived after ICSI from azoospermic fathers and fertile donors. From the limited group of 137 neonates born, we concluded that the gestational age, birth weight, perinatal death and malformation rates do not seem to be affected by testicular failure. Although the current data on pregnancy and postnatal ICSI outcomes using non-ejaculated sperm is reassuring, the limited population analyzed calls for continuous monitoring. Moreover, studies on the physical, neurological, and developmental outcomes of children conceived are still lacking. Future research should focus not only on the long-term outcomes of such children but also on collaborative multi-center studies that include large patient cohorts in an attempt to estimate the risk of less common outcomes, such as congenital malformations and imprinting disorders.
CONCLUSIONS
The results of this study serve as a counseling tool for doctors treating azoospermia-related infertility. Azoospermic patients with testicular failure should be advised that their chances of having sperm retrieved even when the best possible SR method is used, and of achieving a live birth with ICSI after a successful retrieval are negatively affected by their type of azoospermia. Once a live birth is achieved, no major differences are noted in the offspring's neonatal profile.
AUTHOR CONTRIBUTIONS
SCE designed the study, performed data analysis and prepared the manuscript. CP, BS, SV Jr, and CK participated in the acquisition of data, and helped to draft and revise the manuscript. AA participated in the design of the study, revised the manuscript and helped in coordination. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
Jeff Hammel performed the statistical analyses, and Fabiola Bento assisted with language revision.
REFERENCES
- 1.Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 2013;68:15–26. doi: 10.6061/clinics/2013(Sup01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan EA, Zegers-Hochschild F, Mansour R, Ishihara O, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Hum Reprod. 2013;28:1375–90. doi: 10.1093/humrep/det036. [DOI] [PubMed] [Google Scholar]
- 3.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 4.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected] Clinics (Sao Paulo) 2011;66:691–700. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palermo GD, Schlegel PN, Hariprashad JJ, Ergün B, Mielnik A, et al. Fertilization and pregnancy outcome with intracytoplasmic sperm injection for azoospermic men. Hum Reprod. 1999;14:741–8. doi: 10.1093/humrep/14.3.741. [DOI] [PubMed] [Google Scholar]
- 6.De Croo I, Van der Elst J, Everaert K, De Sutter P, Dhont M. Fertilization, pregnancy and embryo implantation rates after ICSI in cases of obstructive and non-obstructive azoospermia. Hum Reprod. 2000;15:1383–8. doi: 10.1093/humrep/15.6.1383. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem M, Bakr NI, Elgayaar MA, El Mongy S, Fathy H, et al. Comparison of the outcome of intracytoplasmic sperm injection in obstructive and non-obstructive azoospermia in the first cycle: a report of case series and meta-analysis. Int J Androl. 2005;28:16–21. doi: 10.1111/j.1365-2605.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 8.He X, Cao Y, Zhang Z, Zhao J, Wei Z, et al. Spermatogenesis affects the outcome of ICSI for azoospermic patients rather than sperm retrieval method. Syst Biol Reprod Med. 2010;56:457–64. doi: 10.3109/19396368.2010.513078. [DOI] [PubMed] [Google Scholar]
- 9.Esteves SC, Lee W, Benjamin DJ, Seol B, Verza S, Jr, et al. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol. 2013;189:232–7. doi: 10.1016/j.juro.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 10.Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: case series and systematic review. Clinics (Sao Paulo) 2013;68:141–50. doi: 10.6061/clinics/2013(Sup01)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernaeve V, Bonduelle M, Tournaye H, Camus M, Van Steirteghem A, et al. Pregnancy outcome and neonatal data of children born after ICSI using testicular sperm in obstructive and non-obstructive azoospermia. Hum Reprod. 2003;18:2093–7. doi: 10.1093/humrep/deg403. [DOI] [PubMed] [Google Scholar]
- 12.Fedder J, Gabrielsen A, Humaidan P, Erb K, Ernst E, et al. Malformation rate and sex ratio in 412 children conceived with epididymal or testicular sperm. Hum Reprod. 2007;22:1080–5. doi: 10.1093/humrep/del488. [DOI] [PubMed] [Google Scholar]
- 13.Belva F, De Schrijver F, Tournaye H, Liebaers I, Devroey P, et al. Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26:1752–8. doi: 10.1093/humrep/der121. [DOI] [PubMed] [Google Scholar]
- 14.Mateizel I, Verheyen G, Van Assche E, Tournaye H, Liebaers I, et al. FISH analysis of chromosome X, Y and 18 abnormalities in testicular sperm from azoospermic patients. Hum Reprod. 2002;17:2249–57. doi: 10.1093/humrep/17.9.2249. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 16.Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl. 2013;15:35–9. doi: 10.1038/aja.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteves SC, Miyaoka R, Orosz JE, Agarwal A. An update on sperm retrieval techniques for azoospermic males. Clinics (Sao Paulo) 2013;68:99–110. doi: 10.6061/clinics/2013(Sup01)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteves SC. Microdissection testicular sperm extraction (micro-TESE) as a sperm acquisition method for men with nonobstructive azoospermia seeking fertility: operative and laboratory aspects. Int Braz J Urol. 2013;39:440–1. doi: 10.1590/S1677-5538.IBJU.2013.03.21. [DOI] [PubMed] [Google Scholar]
- 19.Esteves SC, Varghese AC. Laboratory handling of epididymal and testicular spermatozoa: what can be done to improve sperm injections outcome. J Hum Reprod Sci. 2012;5:233–43. doi: 10.4103/0974-1208.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteves SC, Schertz JC, Verza S, Jr, Schneider DT, Zabaglia SF. A comparison of menotropin, highly-purified menotropin and follitropin alfa in cycles of intracytoplasmic sperm injection. Reprod Biol Endocrinol. 2009;7:111. doi: 10.1186/1477-7827-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteves SC, Bento FC. Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian Cells and Germinative Tissue Directive. Reprod Biomed Online. 2013;26:9–21. doi: 10.1016/j.rbmo.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Aziz N. The importance of semen analysis in the context of azoospermia. Clinics (Sao Paulo) 2013;68:35–8. doi: 10.6061/clinics/2013(Sup01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silber SJ. Microsurgical TESE and the distribution of spermatogenesis in non-obstructive azoospermia. Hum Reprod. 2000;15:2278–84. doi: 10.1093/humrep/15.11.2278. [DOI] [PubMed] [Google Scholar]
- 24.Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68:81–8. doi: 10.6061/clinics/2013(Sup01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donoso P, Tournaye H, Devroey P. Which is the best sperm retrieval technique for non-obstructive azoospermia. A systematic review? Hum Reprod Update. 2007;13:539–49. doi: 10.1093/humupd/dmm029. [DOI] [PubMed] [Google Scholar]
- 26.Reifsnyder JE, Ramasamy R, Husseini J, Schlegel PN. Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2012;188:532–6. doi: 10.1016/j.juro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Dobashi M, Yamazaki T, Hara I, Fujisawa M, et al. Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol. 2002;168:1063–7. doi: 10.1016/S0022-5347(05)64575-2. [DOI] [PubMed] [Google Scholar]
- 28.Nicopoullos JD, Gilling-Smith C, Almeida PA, Norman-Taylor J, Grace I, et al. Use of surgical sperm retrieval in azoospermic men: a meta-analysis. Fertil Steril. 2004;82:691–701. doi: 10.1016/j.fertnstert.2004.02.116. [DOI] [PubMed] [Google Scholar]
- 29.Talas H, Yaman O, Aydos K. Outcome of repeated micro-surgical testicular sperm extraction in patients with non-obstructive azoospermia. Asian J Androl. 2007;9:668–73. doi: 10.1111/j.1745-7262.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramasamy R, Yagan N, Schlegel PN. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology. 2005;65:1190–4. doi: 10.1016/j.urology.2004.12.059. [DOI] [PubMed] [Google Scholar]
- 31.Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167:197–200. [PubMed] [Google Scholar]
- 32.Practice Committee of the American Society for Reproductive Medicine. Sperm retrieval for obstructive azoospermia. Fertil Steril. 2008;90:S213–8. doi: 10.1016/j.fertnstert.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Baker K, Sabanegh E., Jr Obstructive azoospermia: reconstructive techniques and results. Clinics (Sao Paulo) 2013;68:61–73. doi: 10.6061/clinics/2013(Sup01)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyaoka R, Esteves SC. Predictive factors for sperm retrieval and sperm injection outcomes in obstructive azoospermia: do etiology, retrieval techniques and gamete source play a role? Clinics (Sao Paulo) 2013;68:111–9. doi: 10.6061/clinics/2013(Sup01)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14:109–15. doi: 10.1038/aja.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Loendersloot LL, van Wely M, Limpens J, Bossuyt PM, Repping S, et al. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–89. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 37.Vialard F, Bailly M, Bouazzi H, Albert M, Pont JC, et al. The high frequency of sperm aneuploidy in klinefelter patients and in nonobstructive azoospermia is due to meiotic errors in euploid spermatocytes. J Androl. 2012;33:1352–9. doi: 10.2164/jandrol.111.016329. [DOI] [PubMed] [Google Scholar]


