Abstract
We investigated whether inhibiting phosphorylated p70S6K (p-p70S6K) suppresses the proliferation and growth of noninvasive low-grade urothelial carcinoma (LG-URCa) in vitro and whether p-p70S6K can serve as a predictive biomarker for the recurrence of noninvasive LG-URCa of the bladder in patients. We constructed a tissue microarray (TMA) for 95 LG-URCa and 35 benign urothelium samples and performed immunohistochemical staining for p-p70S6K and p-4E-BP1. A Cox regression model was used to investigate the predictive factors for recurrence of LG-URCa. We investigated the dose-dependent antiproliferative effect of rapamycin, its antiproliferative effect and the growth-inhibition effect of p70S6K siRNA transfection in RT4 and 253J cell lines. The pT1 staged group (P < 0.05; hazard ratio (HR), 2.415) and the high p-p70S6K staining group (P < 0.05; HR, 2.249) were independent factors for predicting recurrence. Rapamycin inhibited RT4 and 253J cell proliferation in a dose-dependent manner (r = −0.850, P < 0.001 in RT4 cells; r = −0.835, P < 0.001 in 253J cells). RT4 and 253J cell proliferation and growth were inhibited by the transfection of p70S6K siRNA and rapamycin, respectively (P < 0.05). Transfection of p70S6K siRNA resulted in inhibitory effects on cell proliferation and growth that were similar to those of rapamycin. Our results suggest that inhibiting p70S6K phosphorylation is important to prevent recurrence and that p70S6K phosphorylation can be used as a molecular biomarker to predict recurrence of certain LG-URCa of the bladder.
Keywords: bladder, cancer, p70S6K
INTRODUCTION
Approximately 70%–80% cases of urothelial carcinoma (URCa) of the bladder present as non-muscle invasive tumors (pTa and pT1). Such non-muscle invasive URCa is treated with transurethral resection of bladder and intravesical instillation of bacillus Calmette-Guerin, with a favorable prognosis and a 5-year survival rate of approximately 90%.1 However, recurrence is observed in 50%–90% of cases and 10%–20% of these progresses to more invasive URCa.1 Therefore, the outcomes of patients with non-muscle invasive URCa is related to recurrence and progression and precise prediction of recurrence and progression is largely dependent on URCa pathologic grade. URCa is classified into four categories: papilloma, papillary urothelial neoplasm of low malignant potential, low-grade URCa (LG-URCa) and high-grade URCa (HG-URCa).
Despite treatment with transurethral resection of bladder and appropriate therapy, the recurrence rate for non-muscle invasive HG-URC is >80% and >50% of recurrent cases progress to a more invasive form. The rate of invasion, progression or metastasis is low in non-muscle invasive LG-URCa treated with transurethral resection of bladder and appropriate therapy, although recurrence is observed in approximately 50% of cases.2 However, the mechanism of non-muscle invasive LG-URCa recurrence is not yet known. Laboratory methods that predict the risk of recurrence and progression include examination of proliferating cell nuclear antigens, the ras gene, and p21, p53 and Rb expression. Such factors are significantly expressed mostly in HG-URCa or invasive URCa, but not in LG-URCa; therefore, identifying high-risk factors that predict disease recurrence is very important.3
The phosphatidylinositol 3’-kinase (PI3K) pathway activates a number of signaling molecules and the Akt/mammalian target of the rapamycin (mTOR) pathway is of particular interest because of its role in inhibiting apoptosis and promoting cell proliferation.4 mTOR controls protein synthesis by phosphorylating downstream substrates, including p70S6 kinase (p70S6K) and eukaryotic initiation factor (elF) 4E binding protein 1 (4E-BP1).5 The p70S6K protein phosphorylates the 40S ribosomal protein S6 and is proposed to play a crucial role in the translation of 5’-terminal oligopyrimidine tract mRNAs.6 Phosphorylation by mTOR 4E-BP1 disrupts its binding to elF4E, a protein that binds the 5’-cap structure of mRNA. The released elF4E allows the formation of a functional translation initiation complex, thereby allowing translation.7
We previously reported that activating the mTOR pathway, such as phosphorylating p70S6K, is related to high recurrence and disease progression in non-muscle invasive URCa of the bladder.8 Several studies have revealed that the PI3K/AKT/mTOR pathway is largely responsible for promoting cell growth in non-muscle invasive LG-URCa of the bladder.9,10 Therefore, we investigated whether inhibiting phosphorylated p70S6K suppresses the proliferation and growth of non-muscle invasive LG-URCa in vitro models and whether phosphorylated p70S6K is a predictive biomarker for recurrence and progression of noninvasive LG-URCa of the bladder in a patient model.
MATERIALS AND METHODS
Human noninvasive LG-URCa tissue microarray (TMA) construction and immunohistochemistry
After obtaining institutional review board approval, we retrieved 95 LG-URCa bladder specimens collected at Chung-Ang University Hospital between 1989 and 2007. Paraffin blocks were available in 95 cases and TMAs were constructed using a manual array device (TMA set, Labro, Seoul, Korea) for the 95 LG-URCa samples and 35 benign urothelium samples, as described previously.8 All clinicopathological patient data were retrieved from electronic medical records and included demographics, pathological stage, tumor size, tumor multiplicity and concurrent carcinoma in situ. Follow-up data on postoperative intravesical bacillus Calmette-Guerin treatment, disease recurrence and progression were obtained via a review of patient medical records and letters of inquiry to patients. Immunohistochemistry sections were incubated with the primary antibody (anti-p-p70S6K (Ser371): diluted 1:100 and anti-p-4E-BP1: diluted 1:400), followed by a peroxidase-conjugated secondary antibody. Samples were assessed in a blind manner by one pathologist with no knowledge of the patients’ clinicopathological data. We modified standard H-score to evaluate immunohistochemical positivity. The standard H-score (scale of 0–300) was calculated based on the product of the percentage of cells showing cytoplasmic labeling (0–100) multiplied by the intensity of labeling (1 = weak; 2 = moderate and 3 = strong).11 And then a final H-score was generated by calculating the average of three tissue samples. A cutoff was used for the expression of each marker based on the median tumor H-score (H-score ≥10 and 93 for p-p70S6K and p-4E-BP1, respectively). We defined high expression of a marker as the cutoff and higher and low expression as none or less than the cutoff.
Cell cultures and reagents
The human non-muscle invasive LG-URCa cell lines (RT4 and 253J) were purchased from the American Type Culture Collection (Manassas, VA, USA). Rapamycin was purchased from Sigma (St Louis, MO, USA) and the following antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA): mTOR, phosphorylated-mTOR (p-mTOR, Ser 2448), p70 S6 kinase, phosphorylated-p70 S6 kinase (p-p70 S6 kinase, Ser371) and β-actin.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered saline and trypsinized and lysis buffer (Intron, Seoul, Korea) was added. The lysates were stored at −20 °C until analysis. The amount of protein was quantified by the Bradford assay (Bio-Rad, Hercules, CA, USA). Equal amounts of protein were loaded onto Readygels (4%–20% Tris-HCL; Bio-Rad) and electrophoresis was performed according to the manufacturer's instructions. Proteins were blotted onto polyvinylidene fluoride (PVDF) membranes (Invitrogen, Carlsbad, CA, USA) and incubated for 1 h at room temperature in 5% skim milk for blocking. Blots were incubated with primary antibodies overnight at 4 °C and with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The membranes were developed using enhanced chemiluminescence.
siRNA, plasmid DNA construct and transfection
siRNA oligonucleotides against p70S6K (siRNA I/II) were designed by and purchased from Cell Signaling Technologies (Beverly, MA, USA). Transient transfections into RT4 and 253J cells were performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Cells were harvested at 48 h for subsequent experiments. A plasmid containing the coding sequence of Homo sapiens p70S6K (GenBank: NM 003161) was prepared by cloning into the pcDNA3.1 vector (Invitrogen). The coding region of the p70S6K gene was PCR amplified from pCMV-SPORT6. Next, the p70S6K fragment and the pcDNA3.1 vector (Invitrogen) were digested with the EcoRI and Xhol restriction enzymes and ligated by infusion technology to build the pcDNA3.1-p70S6K plasmid. Competent Escherichia coli DH5α cells were transformed and positive clones were identified by PCR. The PCR products were digested and sequenced. After sequencing, positive clones were selected to grow and propagate. The pcDNA3.1-p70S6K plasmid was purified and transfected into RT4 and 253J cells using Lipofectamine 2000 in Opti-minimal essential medium (Invitrogen) according to the manufacturer's protocol. RT4 and 253J cells were grown in six-well plates until they reached 85%-90% confluence. One microliter of Lipofectamine 2000 reagent and 0.4 mg pcDNA3.1-p70S6K was used to transfect each well of cells in the absence of serum. The cells were harvested for Western blot analysis 24 h after transfection.
Cell proliferation assay
Cell proliferation was determined using a EZ-Cytoz viability assay kit (Dail lab, Seoul, Korea) according to the manufacturer's instructions. This assay is based on the cleavage of tetrazolium salt to water-soluble formazan by the succinate-tetrazolium reductase system. Briefly, RT4 and 253J cells were seeded in 96-well plates at a density of 2 × 104 cells per well. After 24 h in culture, the cells were treated with 100 nmol l−1 p70S6K siRNA, 0.5 mg pcDNA3.1-p70S6K plasmid and 10 μmol l−1 rapamycin for 48 h. The cell proliferation assay was performed 1, 2 or 3 days after treatment. The medium was replaced with fresh medium (200 μl) and 10 μl EZ-cytox reagent was added to each well. The cell culture was continued for 1 h and the culture medium was then removed to wells of a new plate. Optical density was quantified at a wavelength of 450 nm.
Crystal violet growth assay
RT4 and 253J cells were seeded in 24-well plates at a density of 2 × 105 cells per well. After 24 h in culture, the cells were treated with 100 nmol l−1 p70S6K siRNA, 0.5 μg pcDNA3.1-p70S6K plasmid and 10 μmol l−1 rapamycin for 24 h. Growth inhibition was determined using the crystal violet method. Briefly, after 2 days of treatment, the cells were fixed in 5% glutaraldehyde in phosphate-buffered saline, rinsed with distilled water and dried completely. The cells were incubated in a 1:1 volume mixture of 200 μmol l−1 3-(cyclohexylamino)-1-propanesulfanoic acid, pH = 9.5 and 0.2% crystal violet at 25 °C for 30 min and then washed and dried. Crystal violet stain was subsequently dissolved in 10% acetic acid (Sigma, MO, USA) and the absorbance was read at 560 nm following background subtraction. Cell growth was determined and photographed using a Zeiss Axiovert 200M cell live microscope.
Statistical analysis
Statistical Package for Social Sciences software package version 14.0 (SPSS Inc, Chicago, IL, USA) was used for all statistical analyses. The relationships between ordinary variables were investigated using a two-tailed Student's t-test and analysis of variance. Spearman's correlation coefficients were calculated to test the relationships among parameters. Recurrence-free survival curves were estimated using the Kaplan–Meier method and any differences in the survival curves were compared using log-rank tests. A Cox regression model was used to investigate predictive factors for the recurrence of LG-URCa in a multivariate analysis. A P < 0.05 was considered significant.
RESULTS
p-p70S6K and p-4E-BP1 expression in human LG-URCa TMA
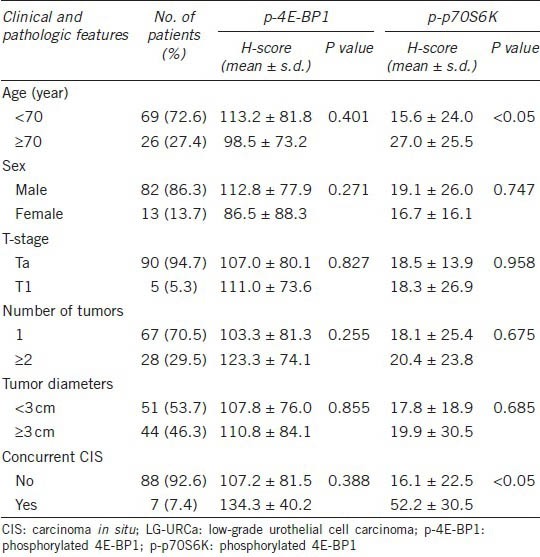
The median age of diagnosis for the patient cohort was 64 (29–85) years, with a male: female ratio of 6.3:1 and a median follow-up period of 84 months (range, 12–120 months). Recurrence was observed in 37 patients (39.3%). No relationship was observed between p-4E-BP1 H-scores and clinicopathological variables (age, sex, pathological stage, tumor size, tumor multiplicity and concurrent carcinoma in situ), but the p-p70S6K H-scores were higher for older age (15.6 ± 24.0 vs 27.0 ± 25.5, P < 0.05) and concurrent carcinoma in situ (16.1 ± 22.5 vs 52.2 ± 30.5) (Table 1).
Table 1.
Clinicopathological characteristics of the 95 patients with LG-URCa and a summary of p-p70S6K and p-4E-BP1 expression (mean H-score), categorized by clinicopathological parameters
As shown in Figure 1a, p-p70S6K and p-4E-BP1 were expressed in the cytoplasm of LG-URCa of the bladder and in benign epithelium and p-p70S6K staining was less intense in low-grade superficial umbilical cord cell tissue compared to that in the benign urothelium. However, p-4E-BP1 staining was greater in LG-URCa tissue. The mean p-p70S6K H-score was lower in the LG-URCa cohort than in the benign cohort (27.4 ± 4.6 vs 183.8 ± 16.0, P < 0.05), whereas the mean p-4E-BP1 score was higher in the LG-URCa cohort than in the benign cohort (117.8 ± 7.9 vs 61.2 ± 14.1, P < 0.05) (Figure 1b). The median tumor H-scores were 10 and 93 for p-p70S6K and p-4E-BP1, respectively. We investigated the correlation between p-p70S6K and p-4E-BP1 expression and recurrence rates. In the Kaplan-Meier survival analysis, LG-URCa, with high p-p70S6K immunohistochemical staining, exhibited higher recurrence (P < 0.05, log-rank test), although p-4E-BP1 status was not related to recurrence (Figure 1c and 1d).
Figure 1.
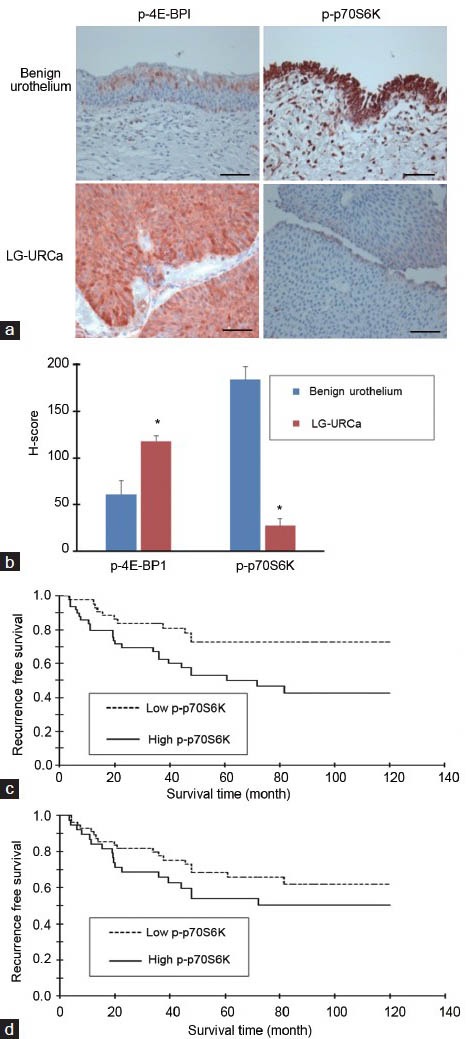
Expression of phosphorylated-p70S6K (p-p70S6K) and p-4E-BP1 in bladder tissue. (a) Immunohistochemical staining of p-p70S6K and p-4E-BP1 in paraffin-embedded sections of benign urothelium and low-grade urothelial carcinoma (LG-URCa) tissues. P-p70S6K staining (red) was lower in LG-URCa tissues than in benign urothelial tissues, but p-4E-BP1 staining (red) was higher in LG-URCa tissues. (b) Graph displaying the comparisons between the H-scores of p-p70S6K and p-4E-BP1 with respect to benign and LG-URCa tissues. The mean H-scores for p-4E-BP1 were significantly higher in LG-URCa tissues and the mean H-scores for p-p70S6K were significantly lower (*P < 0.05). (c) LG-URCa with high p-p70S6K immunohistochemical staining exhibited a higher risk of recurrence (P < 0.05). (d) LG-URCa with p-4E-BP1 status was not related to recurrence in LG-URCa. (P = 0.314). Scale bars = 100 μm.
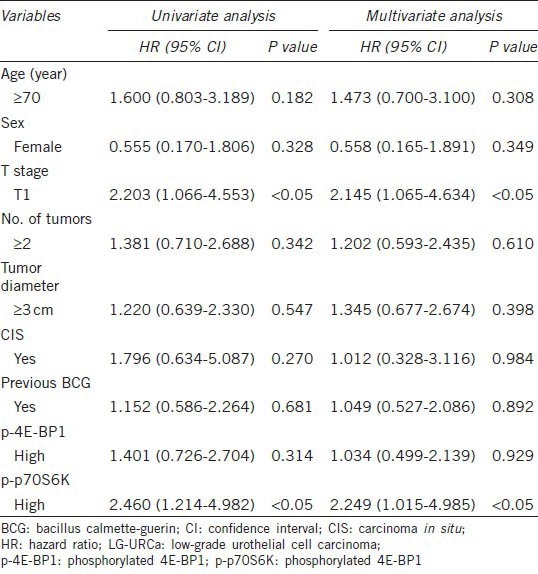
Table 2 shows the results of univariate and multivariate analyses using the Cox regression model for recurrence-free survival. T1 staging group (P < 0.05; hazard ratio (HR), 2.145) and high p-p70S6K staining group (P < 0.05; HR, 2.249) were independent factors predicting recurrence. Therefore, we decided not to investigate the 4E-BP-1 pathway in vitro because of the lack of a correlation between p-4E-BP-1 expression and recurrence in vivo.
Table 2.
Results of univariate and multivariate analyses of clinicopathological variables and the expression levels of p-p70S6K and p-4E-BP1 in relation to recurrence-free survival in 95 patients with LG-URCa
Western blot analysis for mTOR pathway protein expression in the URCa cell lines
We analyzed the expression and activation status of the mTOR and p70S6K proteins in the RT4 and 253J cell lines. As shown in (Figure 2a), mTOR and p70S6K were expressed in both URCa cells and mTOR and p70S6K were phosphorylated in RT4 and 253J cells. Thus, both low-grade non-muscle invasive URCa cell lines exhibited constitutively activated mTOR/p70S6K signaling.
Figure 2.
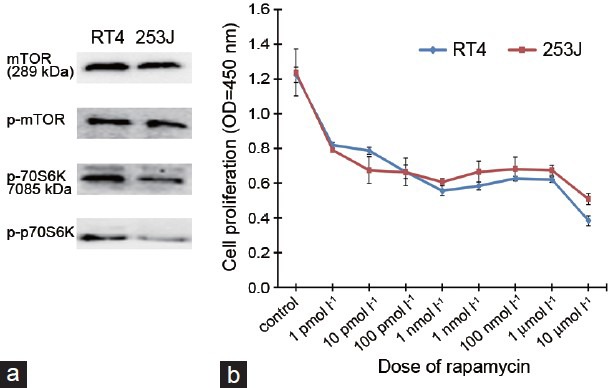
Western blot analysis for mTOR pathway protein expression in LG-URCa cell lines and the antiproliferative effect of rapamycin in RT4 and 253J cells. (a) Cell extracts from four URCa cell lines (RT4 and 263J) were subjected to immunoblotting using antibodies directed against mTOR, p-mTOR, p70S6K and p-p70S6K. Protein loading was normalized using an antibody recognizing β-actin. RT4 and 253J cells expressed phosphorylated mTOR and p70S6K proteins. Blots representative of three separate experiments are shown. (b) Incubating RT4 and 263J cells with increasing doses of rapamycin demonstrated a dose-dependent reduction in cell proliferation at 3 days after treatment (r = −0.850, P < 0.001 in RT4 and r = -0.835, P < 0.001 in 253J cells).
Dose-dependent antiproliferative effect of rapamycin in RT4 and 253J cells
RT4 and 253J cells were treated with rapamycin (1 pmol l−1, 10 pmol l−1, 100 pmol l−1, 1 nmol l−1, 10 nmol l−1, 100 nmol l−1, 1 μmol l−1 and 10 μmol l−1) for 48 h. The cell proliferation assay was performed 1, 2 or 3 days after treatment. Each of the concentrations above was considered as one treated group and there was no rapamycin in the control group. Rapamycin had demonstrated inhibitory effects on RT4 and 253J cell proliferation in a dose-dependent manner at 3 days after treatment. Cell proliferation was correlated with rapamycin concentration and the Spearman's correlation values for these two parameters were r = −0.850, P < 0.001 for RT4 and r = −0.835, P < 0.001 for 253J cells (Figure 2b).
The cell proliferation-inhibition effect of p70S6K siRNA transfection and rapamycin in RT4 and 253J cells
Figure 3a shows that p70S6K siRNA mediated the downregulation of p70S6K and its phosphorylated counterpart and that pcDNA3.1-p70S6K-mediated the upregulation of p70S6K and its phosphorylated counterpart in RT4 and 253J cells, although the downregulatory effect of p70S6K siRNA was not absolute. Rapamycin inhibited p70S6K phosphorylation in RT4 and 253J cells.
Figure 3.
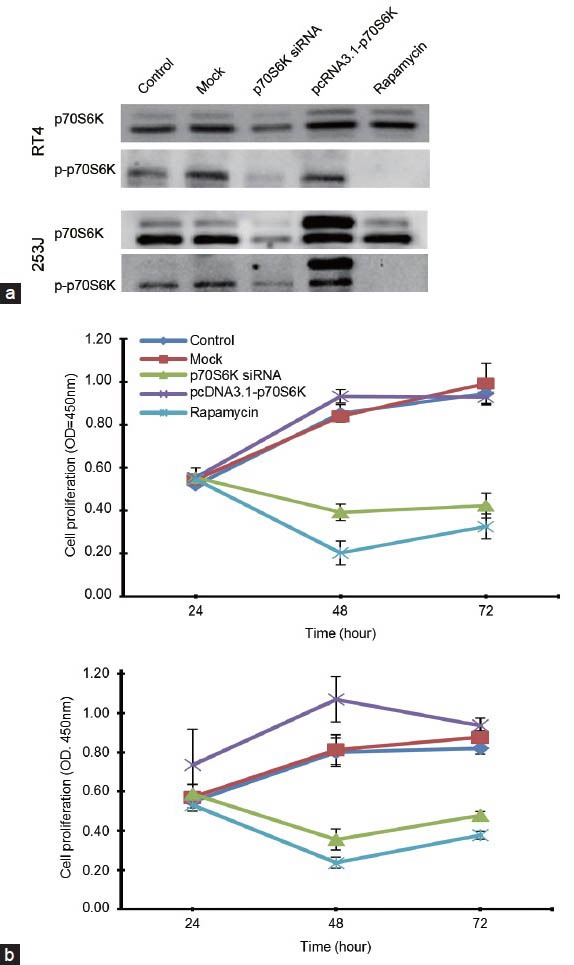
The p70S6K siRNA and pcDNA3.1-p70S6K transfection and the cell proliferation inhibitory effect of p70S6K siRNA transfection and rapamycin in RT4 and 253J cells. (a) Western blot analysis of p70S6K and p-p70S6K protein expression after p70S6K siRNA and pcDNA3.1-p70S6K transfection into RT4 and 253J cells. Cells transfected with p70S6K siRNAs and/or pcDNA3.1-p70S6K were analyzed by immunoblotting for the indicated proteins and rapamycin after 48 h. (b) Cell proliferation assays for RT4 and 253J treated with p70S6K siRNAs and/or pcDNA3.1-p70S6K and rapamycin. p70S6K siRNA and rapamycin significantly (P < 0.05) inhibited cell proliferation as compared with the negative control series at 48 and 72 h after exposure.
RT4 and 253J cells were treated with 100 nmol l−1 p70S6K siRNA, 100 nmol l−1 pcDNA3.1-p70S6K and 10 nmol l−1 rapamycin for 48 h (Figure 3b). RT4 and 253J cell proliferation was inhibited by the transfection of p70S6K siRNA, respectively (P < 0.05), as compared with that in the negative control at 48 and 72 h after transfection. Rapamycin treatment resulted in reduced cell proliferation (P < 0.05). The p70S6K siRNA transfection showed inhibitory effects similar to those of rapamycin. pcDNA3.1-p70S6K treatment tended to result in higher cell proliferation at 48 h than that in the control and mock groups, although it was not significant in RT4 or 253J cells.
The cell growth-inhibition effect of p70S6K siRNA transfection and rapamycin in RT4 and 253J cells
In the crystal violet growth assay, RT4 and 253J cell growth was inhibited by the transfection of p70S6K siRNA and rapamycin, respectively (P < 0.05), as compared with that in the negative control. The p70S6K siRNA transfection showed inhibitory effects similar to those of rapamycin (Figure 4a). pcDNA3.1-p70S6K tended to induce cell growth as compared with that in the control and mock groups, although it was not significant in RT4 and 253J cells. The morphological changes were in concordance with the treatment effects on cell growth (Figure 4b).
Figure 4.
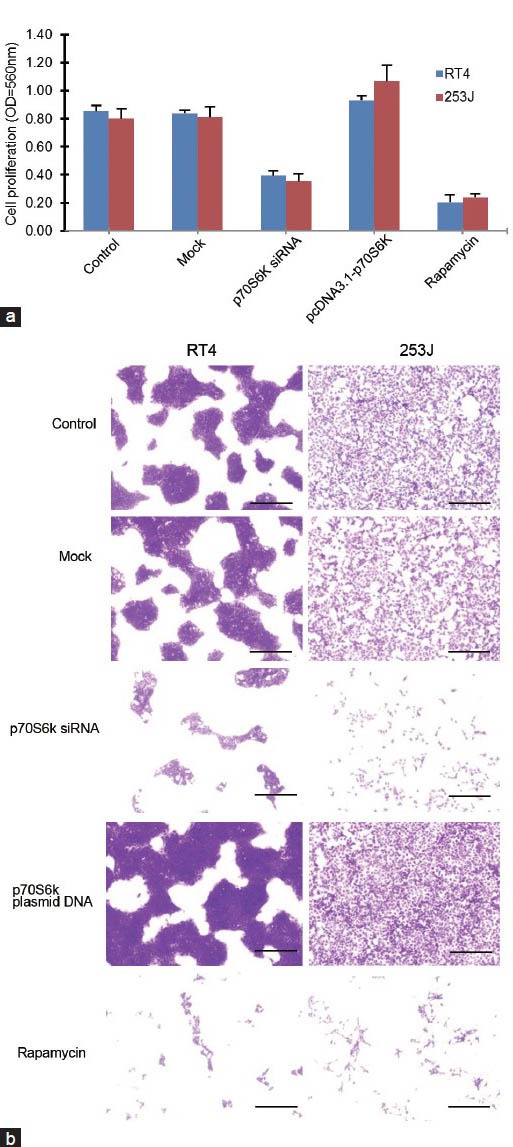
The growth-inhibitory effect of p70S6K siRNA transfection and rapamycin in RT4 and 253J cells. (a) RT4 and 253J cell growth was inhibited by the transfection of p70S6K siRNA and rapamycin, respectively (P < 0.05), as compared with that in the negative control. The transfection of p70S6K siRNA tended to show inhibitory effects similar to those of rapamycin. (b) Morphological changes were in accordance with the treatment effects on cell growth in RT4 and 253J cells. Scale bars = 100 mm.
DISCUSSION
URCa of the bladder is a unique epithelial tumor in terms of the two divergent pathways of tumorigenesis, which correspond to phenotypically distinct; non-muscle invasive, LG-URCa and muscle invasive, HG-URCa.12 LG-URCa frequently exhibits activating mutations in the HRAS gene and the fibroblast growth factor receptor 3 gene, whereas most HG-URCa contains inactivated p53 and/or deletion of the retinoblastoma gene.3 Despite the two distinct molecular pathways of progression, a subset of LG-URCa progress to a more aggressive, higher-grade and muscle-invasive disease, usually after a course of multiple recurrences. Therefore, identifying biomarkers that would help in the recurrence of LG-URCa is an area of active research.
In our study, high p-p70S6K expression, as shown by the immunohistochemical staining, predicted recurrence for LG-URCa treated by transurethral resection, demonstrating that the mTOR signaling pathway thorough p-p70S6K is involved in LG-URCa recurrence. Using p-p70S6K enabled us to identify a subset of patients with LG-URCa who had an increased risk for recurrence. These results were similar to those of our previous study in a non-muscle invasive bladder cancer cohort, in which the high expression of p-p70S6K was a predictive factor for recurrence of early breast cancer and lung cancer.8,13,14 Sun et al.,15 reported that high expression of p-S6, a substrate of p-p70S6K, predicts the progression of non-muscle-invasive URCa of the bladder treated by transurethral resection in immunohistochemical staining of 266 human UC samples, and that the unfavorable prognostic findings of p-p70S6K overexpression have been reported in other solid tumors.13,16
We demonstrated that p-4E-BP1 and p70S6K revealed a diverse pattern regarding their association with tumor recurrence. This finding suggests that p-4E-BP1 and p70S6K are not activated equally in the pathway. Increased p-4E-BP1 expression has been associated with a worse prognosis in a few types of neoplasms.17,18 By contrast, p-4E-BP1 seems to be less reliable than other markers as an independent, recurrence-predictive factor for LG-URCa. We failed to demonstrate an association between the status of the marker and recurrence in both univariate and multivariate analyses.
We demonstrated that the URCa cell line, a line derived from the LG-URCa of the bladder, expressed the p-mTOR and p-p70S6K proteins and that rapamycin inhibited RT4 and 253J cell proliferation dose dependently. These results are similar to those of previous preclinical studies that have investigated the role of the mTOR signaling pathway in URCa.19,20,21 Indeed, rapamycin and its derivative RAD001 (everlimus) inhibit URCa cell proliferation and induce G0-G1 cell-cycle arrest without apoptosis in URCa cell lines.19,20,21 Previous studies have focused on invasive URCa cell lines, whereas we focused on LG-URCa cell lines (RT4 and 253J). Although a considerable number of cell models exist for advanced URCa, the majority of URCa are diagnosed as low-grade, non-muscle invasive tumors.1 Recent genetic analyses have identified RT4 and 253J cells as clinically relevant URCa cell lines, representative of low-grade, non-muscle invasive characteristics.22 Moreover, the RT4 and 253J gene expression profile correlates with long-term patient survivorship, a hallmark of LG-URCa.22,23
We revealed that p70S6K siRNA suppressed RT4 and 253J cell proliferation and growth and that the efficacy of p70S6K siRNA was similar to that of rapamycin. These findings suggest that effectively inhibiting p70S6K by mTOR siRNA, p70S6K siRNA and rapamycin-inhibited URCa cell proliferation and growth. Moreover, we showed increased cell proliferation and growth by transient transfection using pcDNA3.1-p70S6K, indicating that the phosphorylation of p70S6K, which is downstream of the mTOR pathway, is essential for the proliferation and growth of LG-URCa in vitro. These results correlated with our results from the LG-URCa TMA immunohistochemistry in vivo. Therefore, recurrence of LG-URCa can be prevented by inhibiting p70S6K phosphorylation and p-p70S6K may be a biomarker to predict the recurrence of LG-URCa.
There are several limitations of our study. First, phos4E-BP1 expression was higher and p-p70S6K expression was lower in the bladder cancer cohort than in the benign cohort. However, we did not show these results in vitro because a normal urothelial cell line could not be obtained. Schultz et al.24 reported a lower p-p70S6K expression in the bladder cancer cohort compared with benign urothelium in an immunohistochemical study of 144 patients with bladder cancer who had undergone radical cystectomy. They suggested that these findings were consistent with downregulation of the mTOR downstream effect. However, in our study, the bladder cancer cohort exhibited higher expression of p-4E-BP1 that that in benign urothelium, indicating that the mTOR pathway was consistently processed; but that the pathway was shifted from p70S6K phosphorylation to 4E-BP1 phosphorylation. Therefore, we hypothesize that high phos4E-BP1 expression may be related to the tumorigenesis of bladder cancer. 4E-BP1 is a protein that binds to elF-4E and plays a critical role in the regulation of gene expression. When 4E-BP1 is phosphorylated, elF-4E is released and the initiation complex may be formed to promote translation.25 Several studies have implicated components of the protein initiation synthesis apparatus in carcinogenesis. For example, elF-4E has been revealed to induce malignant transformation when overexpressed in mammalian cells.26 Further study is required to evaluate these mechanisms. Finally, a rigorous validation process must precede the incorporation of novel molecular biomarkers into clinical management. Therefore, we need further prospective investigations using a larger number of patients.
Despite several limitations, this is one of the few preclinical and clinical studies indicating that inhibiting p70S6K phosphorylation in the LG-URCa is important in preventing recurrence. Our results suggest that phosphorylated p70S6K may be useful as a molecular biomarker to predict recurrence in certain LG-URCas of the bladder.
AUTHOR CONTRIBUTIONS
IHC and JHK concevied and designed the experiments. SJK and KML performed the experiments. SJK, HSJ and IHC analyzed the data. TJL contributed reagents/materials/analysis tools. SJK and IHC wrote the paper.
COMPETING INTERESTS
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This study was supported by the Department of Convergence Medicine and Pharmaceutical Biosciences Research Scholarship Grants, Chung-Ang University in 2013.
REFERENCES
- 1.Pashos CL, Botteman MF, Laskin BL, Redaelli A. Bladder cancer: epidemiology, diagnosis, and management. Cancer Pract. 2002;10:311–22. doi: 10.1046/j.1523-5394.2002.106011.x. [DOI] [PubMed] [Google Scholar]
- 2.Holmang S, Hedelin H, Anderstrom C, Holmberg E, Busch C, et al. Recurrence and progression in low grade papillary urothelial tumors. J Urol. 1999;162:702–7. doi: 10.1097/00005392-199909010-00019. [DOI] [PubMed] [Google Scholar]
- 3.Spruck CH, 3rd, Ohneseit PF, Gonzalez-Zulueta M, Esrig D, Miyao N, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994;54:784–8. [PubMed] [Google Scholar]
- 4.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 5.Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, et al. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999;274:34493–8. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- 6.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, et al. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–20. [PubMed] [Google Scholar]
- 8.Park SJ, Lee TJ, Chang IH. Role of the mTOR pathway in the progression and recurrence of bladder cancer: an immunohistochemical tissue microarray study. Korean J Urol. 2011;52:466–73. doi: 10.4111/kju.2011.52.7.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo L, Zheng X, Huang HY, Shapiro E, Lepor H, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117:314–25. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Knowles E, Hernandez S, Malats N, Kogevinas M, Lloreta J, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401–4. doi: 10.1158/0008-5472.CAN-06-1182. [DOI] [PubMed] [Google Scholar]
- 11.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 13.van der Hage JA, van den Broek LJ, Legrand C, Clahsen PC, Bosch CJ, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004;90:1543–50. doi: 10.1038/sj.bjc.6601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starnes SL, Pathrose P, Wang J, Succop P, Morris JC, et al. Clinical and molecular predictors of recurrence in stage I non-small cell lung cancer. Ann Thorac Surg. 2012;93:1606–12. doi: 10.1016/j.athoracsur.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumor progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology. 2011;58:1054–63. doi: 10.1111/j.1365-2559.2011.03856.x. [DOI] [PubMed] [Google Scholar]
- 16.Baba HA, Wohlschlaeger J, Cicinnati VR, Hilgard P, Lang H, et al. Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int. 2009;29:399–405. doi: 10.1111/j.1478-3231.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, et al. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–88. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 18.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–7. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fechner G, Classen K, Schmidt D, Hauser S, Muller SC. Rapamycin inhibits in vitro growth and release of angiogenetic factors in human bladder cancer. Urology. 2009;73:665–8. doi: 10.1016/j.urology.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 20.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, et al. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–47. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 21.Pinto-Leite R, Botelho P, Ribeiro E, Oliveira PA, Santos L. Effect of sirolimus on urinary bladder cancer T24 cell line. J Exp Clin Cancer Res. 2009;28:3. doi: 10.1186/1756-9966-28-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dancik GM, Ru Y, Owens CR, Theodorescu D. A framework to select clinically relevant cancer cell lines for investigation by establishing their molecular similarity with primary human cancers. Cancer Res. 2011;71:7398–409. doi: 10.1158/0008-5472.CAN-11-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawinski A, Sylvester R, Kurth KH, Bouffioux C, van der Meijden A, et al. A combined analysis of european organization for research and treatment of cancer, and medical research council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European organization for research and treatment of cancer genitourinary tract cancer cooperative group and the medical research council working party on superficial bladder cancer. J Urol. 1996;156:1934–40. [PubMed] [Google Scholar]
- 24.Schultz L, Albadine R, Hicks J, Jadallah S, DeMarzo AM, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010;116:5517–26. doi: 10.1002/cncr.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–35. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 26.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5’ cap. Nature. 1990;345:544–7. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]


