Introduction
Cytarabine (ara-C, [1-β-D-arabinofuranosylcytosine]), enters cells primarily as a false substrate through specialized nucleoside transporter proteins, predominantly hENT1 (the human equilibrative nucleoside transporter. Reduced hENT1 expression and activity is associated with adverse therapeutic outcomes and reduced cytotoxicity for patients treated with cytarabine (1, 2). Patients with AML and low hENT1 expression have reduced disease- free survival (1, 3). Elacytarabine (CP-4055) (Figure 1), the lipophilic 5′-elaidic acid ester of cytarabine, enters cells independently of hENT1. Preclinical studies of elacytarabine demonstrate activity in cytarabine-resistant cell lines and in animal models with tumor xenografts (4, 5). Elacytarabine may thus be active in patients with leukemia for whom cytarabine is ineffective (6-8). We conducted a study to determine DLT, establish the pharmacokinetics (PK) and toxicity profiles and to obtain a preliminary assessment of activity of elacytarabine CIV combined with idarubicin in adult patients with refractory AML.
Figure 1.
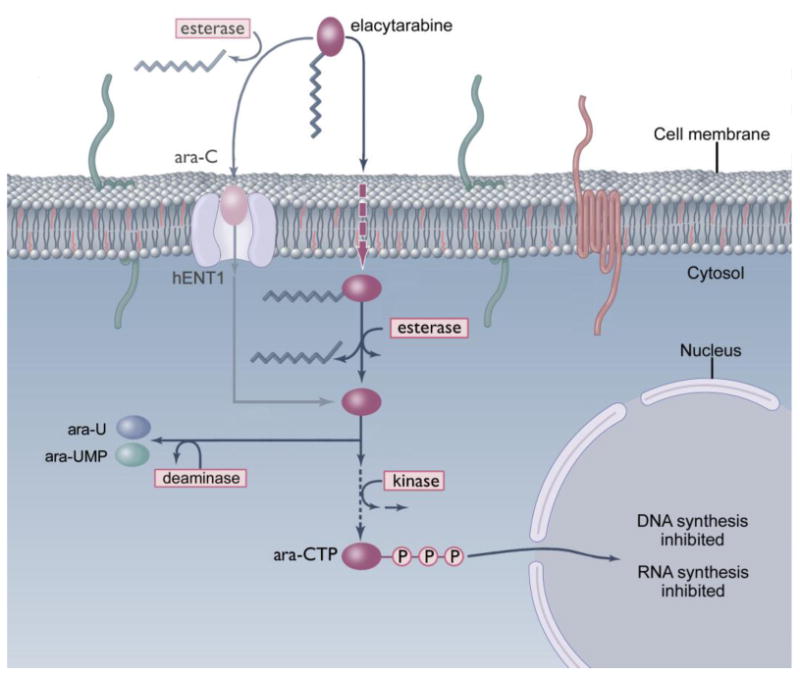
Elacytarabine mechanism of action relative to that of cytarabine (ara-C) The cytotoxic activity of elacytarabine relative to that of cytarabine is mediated through increased cell uptake, decreased deactivation to ara-U, prolonged exposure to ara-CTP and transient inhibition of RNA synthesis.
Materials and Methods
Adult patients (≥18 years of age) with relapsed or refractory AML were enrolled in this open-label, non-randomized, multicenter, phase I study (CP4055-106/NCT00405743) of elacytarabine combined with idarubicin. Patients had an ECOG-WHO performance status ≤2; persistent chronic clinically significant symptoms from prior chemotherapy no more severe than grade 1, according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0), and were not receiving any other cytotoxic treatment. The protocol and study documents were approved by the appropriate Institutional Review Board or Ethics Committees. Written informed consent was obtained from all patients before doing any study specific procedure. Elacytarabine was administered CIV days 1-5 in 3-week courses. Idarubicin was administered intravenously at a dose of 12 mg/m2/day on days 2-4. The starting dose of CIV elacytarabine was 1000 mg/m2/day based on data from a single agent elacytarabine phase I study in patients with refractory hematologic malignancies (9). Planned elacytarabine dose escalation was in 15% increments until DLT was observed. Standard definitions were used to evaluate response. The safety endpoint was the incidence of DLTs during course 1 of elacytarabine and idarubicin treatment. The concentrations of elacytarabine, ara-C and ara- U in plasma were determined.
Results
15 patients (median 57 years – range 21-76 years, 9 male, 9 ECOG 2) with refractory AML), were enrolled in the study. DLT (grade 3 typhlitis and/or hand-foot syndrome) were observed in 3 of 4 patients at an elacytarabine dose level of 1150 mg/m2/day. The initial dose level of elacytarabine 1000 mg/m2/day was thus expanded to include a minimum of 10 evaluable patients - a total of 11 patients were enrolled at this dose level as one patient was inevaluable due to inadequate bone marrow aspirates. Therapy-related AEs occurring in ≥10% of patients included diarrhoea, nausea, abdominal pain, thrombocytopenia, constipation, hyperbilirubinemia and decreased appetite.
Elacytarabine and cytarabine were detected in plasma up to 24 hours after end of infusion (last sampling time-point). The concentration of ara-U declined slowly after the infusion, and this metabolite was also detected in plasma up to 24 hours after end of infusion for most patients. The AUC of cytarabine increased proportional with the dose of elacytarabine. The concentration of ara-C is approximately 2% of the concentration of elacytarabine at steady state. For most patients, the Cmax (maximum plasma concentration) of elacytarabine was observed at or shortly before the end of infusion while Cmax for ara-C and ara-U was observed at 48 hours after start of infusion. The elacytarabine t 1/2 initial was 0.6 - 2.0 hours. For elacytarabine there seems to be a trend towards lower clearance with higher doses and higher AUC.
Anti-leukemia activity (2 CR, 2 CRp) was observed in 4/10 evaluable patients receiving the 1000mg/m2/day elacytarabine schedule – 3 of these 4 patients had relapsed AML after prior cytarabine and idarubicin therapy. A 22 year male whose AML has failed to respond to two prior cycles of cytarabine and idarubicin obtained a CR of 6 months duration after his first course of therapy on study and received 2 consolidation courses of elacytarabine at doses of 750 and 500 mg/m2/day respectively. A 21 year old female, with AML failure to respond to an immediate prior course of cytarabine and idarubicin, obtained a CR of 5 months duration following first course of study therapy, prior to her receiving stem cell transplantation. A CRp of 5 months following first course of study therapy was observed in a 74 year old female who had received decitabine and sapacitabine prior therapies – she received a single consolidation course of elacytarabine at a dose of 500 mg/m2/day. A 60 year old female, with AML failure to respond to multiple prior therapies, including cytarabine and idarubicin, obtained a CRp of 5 months duration following first course of study therapy, prior to her receiving stem cell transplantation.
Discussion
This study of elacytarabine and idarubicin in patients with refractory AML demonstrates encouraging clinical activity in this population. For the chosen phase II dose, both elacytarabine and cytarabine were detected in plasma up to 24 hours after the end of infusion. AUC values for both substances and the increased initial half-life of cytarabine after elacytarabine administration (2.0 hours versus 0.1-0.2 hour), indicate that treatment with elacytarabine provides plasma levels of both elacytarabine and cytarabine in the concentration range associated with cytotoxicity, i.e. concentrations of cytarabine that have been shown to be effective in patients with AML. This enables cancer cells to maintain a high level of intracellular cytarabine – and consequently also a high level of the active metabolite ara-CTP. Our findings are supported by in vitro data in tumor cells after treatment with elacytarabine (4). Elacytarabine administered at the recommended dose and schedule may provide a more optimal exposure of leukemic cells to the active nucleoside analogue (ara-CTP) than that provided by cytarabine. The fact that complete remission (CR or CRp) was achieved in patients with recent failure to cytarabine and idarubicin therapy, provides support for further focused investigation of elacytarabine in AML populations with hENT1 deficiency; i.e. reduced cell uptake of nucleoside analogues. Measuring hENT1 expression levels is currently not standard of care and would require a rapid, accessible and validated method in order to guide on type of therapy. In patients who are newly diagnosed with AML, flow cytometry is essential to further characterize the disease, and measuring hENT1 expression by this method may allow for more personalized therapy in AML. In conclusion, the recommended phase II dose of elacytarabine is 1000 mg/m2/day administered as CIV during 5 days in 3-week courses given with idarubicin 12 mg/m2/day on days 2 to 4. The acceptable safety profile and promising clinical anti- leukemic activity documented on this study supports the continued clinical evaluation of this combination. Fatty acid esterification of nucleoside analogues is a promising approach to circumvent clinically important mechanisms of resistance in patients with leukemia.
Acknowledgments
Clavis Pharma provided elacytarabine for this study. FG, DR, TFJ, were involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the article critically for important intellectual content, and final approval of the version to be submitted. TFJ was involved in involved in acquisition of data, drafting and revising the article critically for important intellectual content, and final approval of the version to be submitted.
Footnotes
Conflict of Interest – FG, DR, and SoB have received research support from Clavis Pharma. TFJ is an employee of Clavis Pharma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galmarini CM, Thomas X, Calvo F, Rousselot P, El Jafaari A, Cros E, et al. Potential mechanisms of resistance to cytarabine in AML patients. Leuk Res. 2002 Jul;26(7):621–629. doi: 10.1016/s0145-2126(01)00184-9. [DOI] [PubMed] [Google Scholar]
- 2.Hubeek I, Stam RW, Peters GJ, Broekhuizen R, Meijerink JP, van Wering ER, et al. The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br J Cancer. 2005 Dec 12;93(12):1388–1394. doi: 10.1038/sj.bjc.6602881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galmarini CM, Thomas X, Calvo F, Rousselot P, Rabilloud M, El Jaffari A, et al. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br J Haematol. 2002 Jun;117(4):860–868. doi: 10.1046/j.1365-2141.2002.03538.x. [DOI] [PubMed] [Google Scholar]
- 4.Adema AD, Laan AC, Myhren F, Fichtner I, Verheul HM, Sandvold ML, et al. Cell cycle effects of fatty acid derivatives of cytarabine, CP-4055, and of gemcitabine, CP-4126, as basis for the interaction with oxaliplatin and docetaxel. Int J Oncol. 2010 Jan;36(1):285–294. [PubMed] [Google Scholar]
- 5.Galmarini CM, Myhren F, Sandvold ML. CP-4055 and CP-4126 are active in ara-C and gemcitabine-resistant lymphoma cell lines. Br J Haematol. 2009 Nov 19;144(2):273–275. doi: 10.1111/j.1365-2141.2008.07467.x. [DOI] [PubMed] [Google Scholar]
- 6.Adema AD, Losekoot N, Smid K, Kathmann I, Myhren F, Sandvold ML, et al. Induction of resistance to the lipophilic cytarabine prodrug elacytarabine (CP-4055) in CEM leukemic cells. Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):394–399. doi: 10.1080/15257771003741166. [DOI] [PubMed] [Google Scholar]
- 7.Breistol K, Balzarini J, Sandvold ML, Myhren F, Martinsen M, De Clercq E, et al. Antitumor activity of P-4055 (elaidic acid-cytarabine) compared to cytarabine in metastatic and s.c. human tumor xenograft models. Cancer Res. 1999 Jun 15;59(12):2944–2949. [PubMed] [Google Scholar]
- 8.Sandvold ML, Galmarini C, Myhren F, Peters G. The activity of the lipophilic nucleoside derivatives elacytarabine and CP-4126 in a panel of tumor cell lines resistant to nucleoside analogues. Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):386–393. doi: 10.1080/15257771003729625. [DOI] [PubMed] [Google Scholar]
- 9.Giles F, Vey N, Rizzieri D, Ravandi F, Prebet T, Borthakur G, et al. Phase I and pharmacokinetic study of elacytarabine, a novel 5′-elaidic acid derivative of cytarabine, in adults with refractory hematologic malignancies. Leukemia. 2012 doi: 10.1038/leu.2012.1. In Press. [DOI] [PubMed] [Google Scholar]


