Abstract
Chronic pain is a major neurological disorder that can manifest differently between genders or sexes. The complex actions of sex hormones may underlie these differences; previous studies have suggested that elevated estrogen levels can enhance pain perception. The purpose of this study was to investigate the hypothesis that acute, activational effects of estradiol (E2) increase persistent inflammatory nociception, and anatomically where this modulation occurs. Spinal expression of Fos is widely used as a marker of nociceptive activation. This study used formalin-evoked nociception in ovariectomized (OVX) adult female rats and measured late-phase hindlimb flinching and Fos expression in the spinal cord, and their modification by acute estrogen supplementation similar to a proestrus surge. Six days after ovariectomy, female rats were injected subcutaneously (s.c.) with 10μg/kg E2 or vehicle. Twenty-four hours later, 50 μL of 1.25% or 100 μL of 5% formalin was injected into the right hindpaw; hindlimb flinches were counted, and spinal cords removed two hours after formalin injection. The numbers of Fos-expressing neurons in sections of the lumbar spinal cord were analyzed using immunohistochemistry. Formalin-induced inflammation produced a dose-dependent increase in late-phase hindlimb flinching, and E2 pretreatment increased flinching following 5%, but not 1.25% formalin injection. Despite the modification of behavior by E2, the number of spinal Fos-positive neurons was not altered by E2 pretreatment. These findings demonstrate that an acute proestrus-like surge in serum estrogen can produce a stimulus-intensity-dependent increase in inflammation-evoked nociceptive behavior. However, the lack of effect on spinal Fos expression suggests that this enhancement of nociceptive signaling by estrogen is independent of changes in peripheral activation of, expression of the immediate early gene Fos by, or signal throughput of spinal nociceptive neurons.
Keywords: Pain, Rat, Formalin Test, Spinal cord, Behavior, Estrogen
Introduction
Notable sex differences exist in the prevalence of pain disorders and the experience of pain [1–6]. Sex hormones (e.g., estrogens) are thought to contribute to these differences through organizational and/or activational effects. Estrogens appear to have complex activational –often pro-nociceptive – effects on innervation, synapse formation, and sensory function. Previous reports demonstrated that elevated serum estrogen levels enhance persistent inflammatory nociception [3, 7–14]. However, the literature is complicated by studies that do not directly address acute sensory modification by a single estrogen (i.e., E2) in female subjects. Studies of female hormone effects on nociception have investigated longer time scales than estrous cycle fluctuations [15, 16], directly manipulated multiple hormones [17], manipulated a hormone other than E2 [18], were conducted in males [19, 20], observed effects of endogenous hormones over the estrous cycle [7], or employed pain models other than formalin.
Therefore, the purpose of this study was to determine whether the acute, activational effects of E2 increase persistent inflammatory nociception. We postulated that sex differences in pain sensation and the disproportionate burden of inflammatory pain in women are due, at least in part, to direct, acute effects of E2. Thus, the working hypothesis was that acute administration of E2 would increase nociceptive behavior evoked by persistent inflammation in female rats. The model chosen was the intraplantar injection of dilute formalin, which is widely used to evoke spontaneous pain-related behaviors [21, 22]. For this study, acute fluctuations in serum estrogen levels on a time scale modeling the proestrus phase of the estrous cycle were produced by s.c. injection of E2 [23, 24] as a pretreatment 24 hours before the induction of inflammatory nociception with formalin.
This study also aimed to investigate the primary site(s) where E2 modifies nociception, hypothesizing that these acute, activational effects of E2 occur in peripheral, spinal and/or supraspinal sites involved in the transmission and perception of pain. There have been no reports of direct, systematic investigation into identification of the anatomical sites of action in the nervous system of the enhancement of pain by estrogens. As a first step in determining where this modulation occurs, this study addressed spinal nociceptive activation with the hypothesis that the acute modulatory effects of E2 target the peripheral and/or spinal nervous system and would manifest as increased numbers of spinal Fos-positive neurons. Previous studies demonstrated stimulus-intensity dependence of spinal Fos immunoreactivity following formalin injection [25–27]. The distribution of spinal Fos-positive neurons following noxious stimulation has been widely studied [26–30]. The number Fos-positive neurons in deeper spinal laminae correlated with the intensity of nociception-evoked behavior [31], suggesting the rationale for quantification of Fos within the dorsal laminae used in the current study.
Materials & Methods
A total of 106 adult female Sprague-Dawley rats (~11 weeks old, 200–230 g, Harlan, Indianapolis, IN) were housed on a 12-hour light/dark cycle, fed Harlan Teklad 8604 chow, and all procedures were performed during the light cycle. Rats were housed one per cage for days 1–4 post-surgery and two per cage otherwise. All procedures were approved by the KUMC Institutional Animal Care and Use Committee and followed the U.S. Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals [32].
The effects of estrogen on behavioral responses to formalin were investigated using two randomized groups of rats: 1) ovariectomized (OVX) receiving E2 (OVX + E2), and 2) OVX receiving an equivalent volume of vehicle (OVX + Veh). Six days after OVX, a surge in E2 produced by bolus s.c. injection was followed by nociceptive behavioral evaluation. Approximately 24 hours after E2 or vehicle injection (seven days after OVX), randomly-selected rats received a unilateral injection of 100 μL of 5% or 50 μL of 1.25% formalin into the right plantar hindpaw. Spontaneous hindpaw flinches were quantified 30–40 minutes post-injection (during the peak of the late-phase behaviors) in randomized order by an observer blind to E2 status/treatments.
In experiments assessing the spinal expression of Fos, a separate cohort of rats received identical E2 and formalin treatment as the behavioral cohort. A previous time-course study showed that maximum spinal Fos staining occurred 2 hours after formalin injection [30]. Accordingly, two hours after formalin injection, rats were anesthetized with ketamine/xylazine and perfused transcardially with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS, pH 7.4. Lumbar spinal cords were subsequently removed by laminectomy and post-fixed in paraformaldehyde at 4 °C, then in 30% sucrose at 4 °C before freezing.
In a separate control experiment to assess the efficacy of estrogen injection, 24 hours after E2 injection (seven days after OVX), rats were weighed then decapitated, adipose tissue was removed from uteri, and uteri were excised at the base and weighed wet.
Hormone manipulation
For ovariectomy (OVX), rats were anesthetized with ketamine (64 mg/kg, i.p.; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5.3 mg/kg, i.p.). Under aseptic conditions, both ovaries were externalized and excised. Muscle and skin layers were individually closed. Six days later, rats received a single s.c. injection of 10 μg/kg E2 benzoate (Sigma, E-9000) at 10 μg/mL, or an equivalent volume of vehicle (10% ethanol/90% corn oil). By this time point after OVX, serum E2 levels were shown to be below non-proestrus levels by a previous study [33]; this dose of E2 was chosen to mimic the surge in E2 observed during proestrus in rats [23, 24, 34–36] as previously described [23, 24].
Immunohistochemistry for Fos
For fluorescent immunohistochemistry, frozen lumbar spinal cords were cut into 20 μm-thick transverse sections and placed on charged glass microscope slides. Slides were washed in 0.4% Triton X-100 in PBS, pH 7.4, blocked with 5% normal donkey serum plus 1% bovine serum albumin in the same buffer, and incubated with primary rabbit anti-Fos antibody (1:1000, Calbiochem, Ab-5, Cat. No. PC38) overnight at 4 °C. They were then incubated with secondary fluorescein (FITC)-conjugated donkey anti-rabbit antibody (1:200, Jackson ImmunoResearch, Code No. 711-095-152) for 1 hour at room temperature, then mounted with Vectashield Hard Set mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Cat. No. H-1500). Sections were fluorescently co-labeled for NeuN to verify neuronal identification. NeuN co-labeling with Fos was not quantified, but Fos-positive cells appeared to be exclusively co-labeled with NeuN. Slides were then viewed on a Nikon 80i fluorescent microscope system by an observer blind to E2 status/treatments, and Fos-positive neurons counted in ipsilateral laminae I–VI of sections at least 60 μm apart in spinal lumbar 4th–5th vertebral segments, with at least five sections counted and averaged for each rat. Laminar divisions and spinal segments were based on gross landmarks of the lumbar enlargement [37]. Comparison of Fos immunoreactivity with DAPI staining during counting was used to confirm that only neurons displaying intact nuclei were quantified.
Data analyses
All data represent the mean ± SEM. Data were analyzed by Student’s t-test (SigmaPlot 10.0, Systat Software, Inc.). Significance was set at p ≤ 0.05 throughout all analyses.
Results
Measurement of uterine weights of E2-treated rats revealed that rats receiving E2 (OVX + E2; n = 18) had significantly higher uterine weight (197.7 ± 8.5 g) than controls receiving vehicle (OVX + Veh, n = 17; 130.5 ± 4.1 g; Student’s t-test, Welch-corrected).
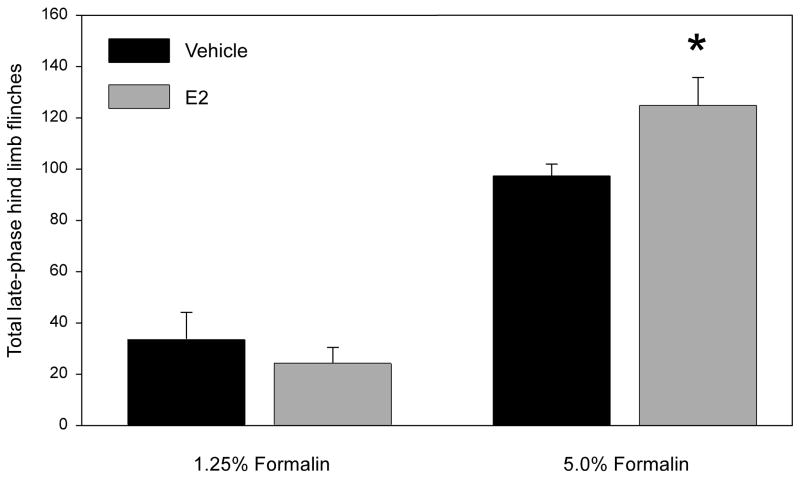
Behavioral analyses of formalin-injected OVX rats revealed that, for 1.25% formalin, the total number of late-phase flinches (30–40 min) for rats receiving E2 (67.2 ± 6.0) were not different than controls receiving vehicle (79.6 ± 7.2; Student’s t-test, n = 10 per group, Figure 1). In contrast, for 5% formalin, rats receiving E2 (140.3 ± 9.4) flinched significantly more than controls (112.0 ± 9.4; Student’s t-test, n = 12 per group, Figure 1). More detailed temporal analyses were performed to examine the effect of E2 on formalin-evoked hindlimb flinches 0–60 minutes post-injection for 1.25% formalin. Total flinches over the entire 60 minutes were: Vehicle, 286.4 ± 15.1; E2, 289.6 ± 16.1. When flinching behaviors were broken down into Early (0–5 min), Inter- (5–15 min) and Late (15–60 min) phases, total flinches for rats receiving E2 were not different than controls receiving vehicle for any time period or for the entire 60 minutes (Data not shown; Student’s t-test, n = 10 per group).
Figure 1.
Effect of E2 on (A) 1.25% or (B) 5% formalin-evoked flinching behavior 30–40 minutes post-formalin. Data represent the mean ± SEM (p ≤ 0.05, unpaired Student’s t-test; 1.25% n = 10; 5% n=12).
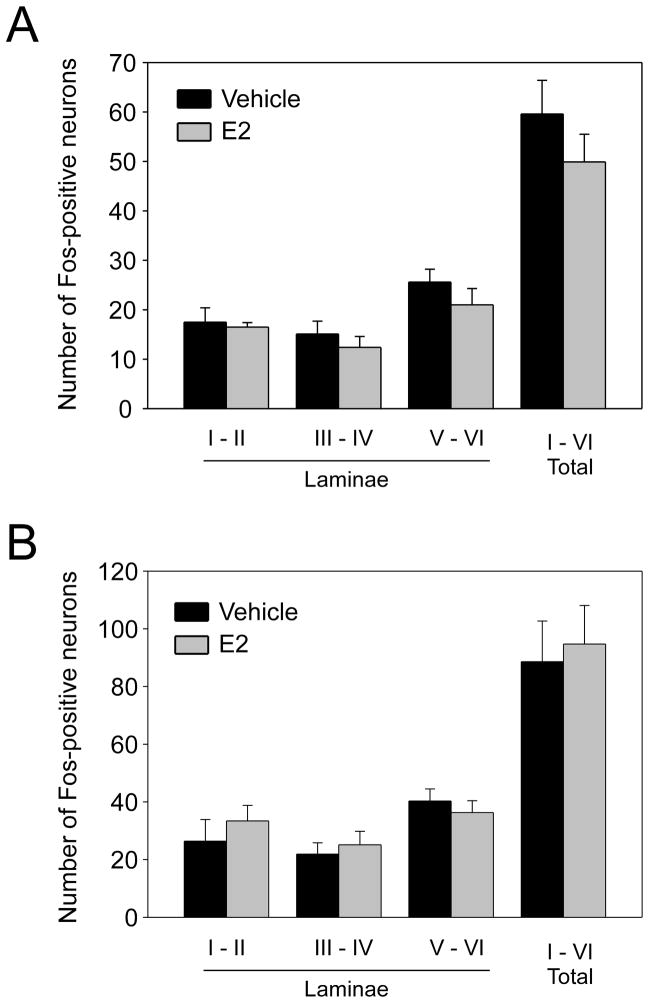
Hindpaw formalin injection elicited robust Fos expression in neurons throughout the ipsilateral lumbar dorsal horn after two hours (Figure 2). Formalin-evoked Fos expression was visible throughout the upper laminae of the ipsilateral dorsal horn; Fos staining in the contralateral dorsal horn was sparse. Fos-positive neurons were more prevalent in the medial part of the dorsal horn than laterally. Additionally, the number of Fos-positive neurons in the ipsilateral spinal cord dorsal horn was proportional to the stimulus intensity (Figure 3).
Figure 2.
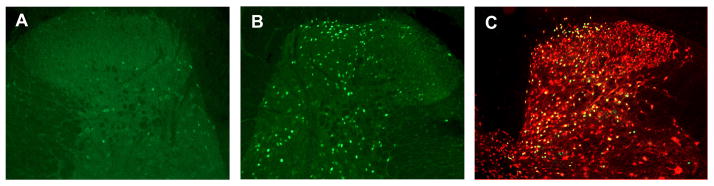
Representative photomicrographs of Fos fluorescent immunohistochemistry in lumbar spinal cord dorsal horns following formalin. Note that formalin-evoked Fos expression is visible throughout the upper laminae of the ipsilateral (right) dorsal horn (B) but is sparse in the contralateral dorsal horn (A). Fos-positive nuclei were verified as neuronal via co-labeling for NeuN (C). 10× magnification.
Figure 3.
Effect of E2 on (A)1.25% or (B) 5% formalin-evoked Fos immunohistochemistry in the ipsilateral spinal dorsal horn. Data represent the mean ± SEM. No significant differences were detected by unpaired Student’s t-test; n = 8 for 1.25% formalin; for 5% formalin Veh n = 5, E2 n = 6.
Comparison of rats receiving E2 (OVX + E2) with those receiving vehicle (OVX + Veh; n = 8 each) showed E2 administration did not alter the number of Fos-positive neurons in spinal cord laminae I–II, III–IV, V–VI, or the total across laminae I–VI evoked by 1.25% formalin (Figure 3A). At 5 % formalin, results also revealed no difference due to E2 for any of the laminar regions (Figure 3B; n = 5–6 per group). The hypothetical possibility of a ceiling effect on the number of Fos-positive neurons at 5% formalin initially investigated prompted further investigation of the same comparison at 1.25% formalin. This experiment revealed that 1.25% formalin also elicited robust Fos expression in the ipsilateral dorsal horn, with a pattern similar to that observed with 5% formalin, except lower in magnitude.
Discussion
For confirmation of systemic efficacy of E2, measurement of uterine weights revealed that ovariectomized rats receiving a single dose of E2 mimicing the proestrus surge of estrogen in intact female rats [23, 24, 34–36] had higher uterine weights than controls. Consistent with a previous report [24], these data demonstrated that the single dose of E2 produced systemic estrogenic (uterotrophic) effects evident 24 hours after E2 injection. These results confirmed the efficacy of this estrogen dose and timing regimen.
The current study tested the effects of E2 on nociception by using pain-related behavioral analysis. This study modeled effects of a proestrus E2 surge in modifying persistent inflammatory pain similar to that of chronic pain disorders. Results of E2 manipulation showed that a single injection of E2 enhanced late-phase formalin-induced hindpaw flinching at 5% formalin (Figure 1B); in contrast, no difference was observed at 1.25% (Figure 1A), even upon further, more detailed investigation of flinching for the entire hour post-injection. At the higher stimulus intensity, the proestrus-like E2 surge increased inflammatory pain-related behavior. This observation is consistent with several previous reports suggesting that elevated serum estrogen levels enhance persistent inflammatory nociception [3, 7–14]. However, the current results are difficult to directly compare or contrast with many previous studies that differed in the hormone manipulation, formalin dose, age and sex of rats, or pain model used. Previous reports have investigated the effect of gonadal hormones on persistent inflammatory nociception, but few have directly manipulated E2. In a gonadectomy study performed in rats, Aloisi et al. [38] reported that gonadal hormones in both sexes inhibited paw flexion duration following formalin, but did not affect flinching. In other studies [39, 40], females had higher flexion and licking duration than males following formalin. Another study showed gonadectomy for 3 months in female rats increased rubbing of a formalin-injected lip, but there was no change in flinches of an injected paw [41]. A different study demonstrated that E2 increased formalin-induced paw licking behavior in rats [19] –possibly corroborating the current findings – but was performed in male, not female subjects. A few studies showed an anti-nociceptive effect of E2 on formalin-related behaviors, but measured a different metric of paw movement and used longer E2 supplementation in younger rats [16, 42, 43]. This is the first report to demonstrate that an acute E2 manipulation previously shown to alter E2 levels and uterine innervation [24], is pronociceptive in the formalin model.
The observation that E2 increased flinching at 5%, but not 1.25% formalin indicates E2 is pronociceptive in a stimulus-intensity-dependent manner, and may require a threshold level of nociception. Stimulus-intensity dependence for sex differences in behavioral responses to formalin [44] and other noxious stimuli have been described [45]. This stimulus-intensity dependence likely extends to sex-hormone modulation of nociception – which current results suggest. The current results add to the existing knowledge of sex hormone modulation of persistent nociception by demonstrating that acute, direct manipulation of E2 in female rats increased nociceptive behavior evoked by 5% formalin.
This study, however, indicates a lack of effect of E2 on nociception-evoked spinal Fos expression. Results of immunohistochemical analysis revealed that formalin injection elicited robust Fos expression in neurons throughout the ipsilateral lumbar spinal cord dorsal horn after two hours (Figure 2). The number of Fos-positive neurons was directly related to the stimulus intensity (Figure 3), corroborating previous reports establishing Fos expression as a biomarker of nociceptive activation [25–27]. However, E2 did not alter the number of Fos-positive neurons in the ipsilateral dorsal spinal cord evoked by either formalin concentration (Figure 3), despite enhancing formalin-evoked flinching at the higher stimulus intensity. Together, these observations support the conclusion that, whereas an acute estrogen surge enhances pain-related behaviors evoked by persistent inflammatory nociception, estrogen may not significantly modulate peripheral or spinal nociceptive neuronal activation, and that these sites may not be primary targets for the pronociceptive effects of E2. Estrogen may enhance nociception at supraspinal sites where pain sensation is modified subsequent to spinal transmission. The increased flinching in E2-treated subjects may result from modification of nociceptive sensation or subsequent motor responses at sites subsequent to the first synaptic connection in the spinal dorsal horn.
Alternatively, the number of Fos-expressing spinal neurons may not fully reflect the degree of spinal activation by a persistent stimulus. There may be a ceiling on the number of Fos-expressing spinal nociceptive neurons available for activation above that evoked by 5% formalin or for “recruitment” under the effects of estrogen. Also, Fos expression may represent activation of both inhibitory and excitatory neurons, and both may respond to E2. Furthermore, immunohistochemical “positivity” quantified in this study may not reflect the intracellular degree of activation of individual neurons. E2 enhancement of neuronal function could be manifested as a cause or consequence of modification of expression of other genes without modulation of Fos. For example, a previous study showed that nociception-evoked gene expression in spinal cord dorsal horn was modulated by estrogen status [15], but this study used longer, constant E2 exposure and measured gene expression at later time points. Nonetheless, the current results suggest that behavioral enhancement by an E2 surge may be independent of spinal Fos modulation and/or the neuronal activation that it represents.
Conclusion
This study demonstrates that acute, direct manipulation of E2 in female rats increased formalin-evoked hindlimb flinching in a stimulus intensity-dependent manner. However, the numbers of nociception-evoked Fos-positive neurons in the spinal cord were not modified by an acute estrogen surge. Together these results suggest that non-spinal targets or mechanisms independent of spinal Fos modulation are responsible for the pronociceptive, acute activational effects of estrogen. These findings further the understanding of the modulation of pain by sex hormones, and how estrogen may contribute to the disproportionate burden of chronic inflammatory pain in women.
Effect of an estrogen surge on inflammatory nociception was studied in female rats.
Estrogen increased formalin-induced pain-related behavior.
Estrogen did not alter formalin-induced Fos-positive neuron counts in spinal cord.
Pronociceptive effects of acute estrogen may occur via non-spinal mechanisms
Acknowledgments
Supported by DA12505 (KMcC), Lied Foundation/KUMC Research Institute (KMcC), and KUMC Biomedical Research Training Program (AR). Core facility support provided by the KS IDDRC (HD02528). The authors thank Michelle Winter for her expert technical assistance and have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–80. doi: 10.1017/s0140525x97221485. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 2.Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;107:309–17. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lautenbacher S, Rollman GB. Sex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation method. Pain. 1993;53:255–64. doi: 10.1016/0304-3959(93)90221-A. [DOI] [PubMed] [Google Scholar]
- 4.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–7. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 5.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13:228–34. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–9. doi: 10.1016/s0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 8.Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59:512–20. doi: 10.1097/00006842-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Percept Mot Skills. 2003;96:201–11. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- 10.Isselee H, De Laat A, De Mot B, Lysens R. Pressure-pain threshold variation in temporomandibular disorder myalgia over the course of the menstrual cycle. J Orofac Pain. 2002;16:105–17. [PubMed] [Google Scholar]
- 11.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–61. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Meisler JG. Chronic pain conditions in women. J Womens Health. 1999;8:313–20. doi: 10.1089/jwh.1999.8.313. [DOI] [PubMed] [Google Scholar]
- 13.Somerville BW. The influence of progesterone and estradiol upon migraine. Headache. 1972;12:93–102. doi: 10.1111/j.1526-4610.1972.hed1203093.x. [DOI] [PubMed] [Google Scholar]
- 14.Tall JM, Crisp T. Effects of gender and gonadal hormones on nociceptive responses to intraplantar carrageenan in the rat. Neurosci Lett. 2004;354:239–41. doi: 10.1016/j.neulet.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 15.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–9. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 16.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–42. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Basoa MB, Gintzler AR. 17-Beta-estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Res. 1993;601:241–5. doi: 10.1016/0006-8993(93)91716-6. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Deleo JA. Neuregulin 1 is a pronociceptive cytokine that is regulated by progesterone in the spinal cord: implications for sex specific pain modulation. Eur J Pain. 2008;12:94–103. doi: 10.1016/j.ejpain.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Ceccarelli I, Fiorenzani P, Grasso G, Lariviere WR, Massafra C, Massai L, Muscettola M, Aloisi AM. Estrogen and mu-opioid receptor antagonists counteract the 17 beta-estradiol-induced licking increase and interferon-gamma reduction occurring during the formalin test in male rats. Pain. 2004;111:181–90. doi: 10.1016/j.pain.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Lu N, Zhao ZQ, Zhang YQ. Involvement of estrogen in rapid pain modulation in the rat spinal cord. Neurochem Res. 2012;37:2697–705. doi: 10.1007/s11064-012-0859-1. [DOI] [PubMed] [Google Scholar]
- 21.Porro CA, Cavazzuti M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog Neurobiol. 1993;41:565–607. doi: 10.1016/0301-0082(93)90044-s. [DOI] [PubMed] [Google Scholar]
- 22.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 23.Medlock KL, Forrester TM, Sheehan DM. Short-term effects of physiological and pharmacological doses of estradiol on estrogen receptor and uterine growth. J Recept Res. 1991;11:743–56. doi: 10.3109/10799899109064677. [DOI] [PubMed] [Google Scholar]
- 24.Zoubina EV, Mize AL, Alper RH, Smith PG. Acute and chronic estrogen supplementation decreases uterine sympathetic innervation in ovariectomized adult virgin rats. Histol Histopathol. 2001;16:989–96. doi: 10.14670/HH-16.989. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda T, Nishimoto C, Shiga Y, Toyooka H. The formalin test: effects of formalin concentration and short-term halothane inhalation. Reg Anesth Pain Med. 2001;26:407–13. doi: 10.1053/rapm.2001.25926. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko M, Mestre C, Sanchez EH, Hammond DL. Intrathecally administered gabapentin inhibits formalin-evoked nociception and the expression of Fos-like immunoreactivity in the spinal cord of the rat. J Pharmacol Exp Ther. 2000;292:743–51. [PubMed] [Google Scholar]
- 27.Khanna S, Chang LS, Jiang F, Koh HC. Nociception-driven decreased induction of Fos protein in ventral hippocampus field CA1 of the rat. Brain Res. 2004;1004:167–76. doi: 10.1016/j.brainres.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 28.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 29.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–4. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 30.Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–35. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bon K, Wilson SG, Mogil JS, Roberts WJ. Genetic evidence for the correlation of deep dorsal horn Fos protein immunoreactivity with tonic formalin pain behavior. J Pain. 2002;3:181–9. doi: 10.1054/jpai.2002.123710. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council (U.S.) Guide for the Care and Use of Laboratory Animals. 7. The National Academies Press; 1996. [PubMed] [Google Scholar]
- 33.Lesclous P, Guez D, Llorens A, Saffar JL. Time-course of mast cell accumulation in rat bone marrow after ovariectomy. Calcif Tissue Int. 2001;68:297–303. doi: 10.1007/BF02390837. [DOI] [PubMed] [Google Scholar]
- 34.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 35.Nag S, Mokha SS. Activation of alpha2-adrenoceptors in the trigeminal region produces sex-specific modulation of nociception in the rat. Neuroscience. 2006;142:1255–62. doi: 10.1016/j.neuroscience.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; San Diego: 2005. [Google Scholar]
- 38.Aloisi AM, Ceccarelli I, Herdegen T. Gonadectomy and persistent pain differently affect hippocampal c-Fos expression in male and female rats. Neurosci Lett. 2000;281:29–32. doi: 10.1016/s0304-3940(00)00819-3. [DOI] [PubMed] [Google Scholar]
- 39.Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett. 2004;361:262–4. doi: 10.1016/j.neulet.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett. 1994;179:79–82. doi: 10.1016/0304-3940(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 41.Pajot J, Ressot C, Ngom I, Woda A. Gonadectomy induces site-specific differences in nociception in rats. Pain. 2003;104:367–73. doi: 10.1016/s0304-3959(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 42.Kuba T, Kemen LM, Quinones-Jenab V. Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res. 2005;1047:119–22. doi: 10.1016/j.brainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49:441–9. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Aloisi AM, Sacerdote P, Albonetti ME, Carli G. Sex-related effects on behaviour and beta-endorphin of different intensities of formalin pain in rats. Brain Res. 1995;699:242–9. doi: 10.1016/0006-8993(95)00912-a. [DOI] [PubMed] [Google Scholar]
- 45.Aloisi AM. Sex differences in pain-induced effects on the septo-hippocampal system. Brain Res Brain Res Rev. 1997;25:397–406. doi: 10.1016/s0165-0173(97)00030-1. [DOI] [PubMed] [Google Scholar]