Abstract
Background and Aims
Visceral hypersensitivity and symptom severity in Irritable Bowel Syndrome (IBS) are both exacerbated by stress. The eye-blink startle response represents a noninvasive measure of central defensive responding. Evidence for central hyperexcitability was studied in IBS patients by examining potentiation of the startle reflex to a nociceptive threat.
Methods
Acoustic startle responses were examined in female IBS patients (n = 42) and healthy controls (n = 22) during cued periods in which an aversive abdominal or biceps stimulation was impossible (safe), possible (imminent threat) or anticipated (period just before the imminent threat), and during a threatening context (muscle stimulation pads attached but no cues for stimulation).
Results
Both groups showed potentiation of startle responses during the imminent threat condition compared with both the anticipation and safe conditions. Compared with controls, IBS subjects showed significantly larger startle responses during anticipation and imminent threat conditions after receiving an initial aversive stimulation. There were no group differences during the context threat manipulation. Moreover, in IBS patients but not controls, higher neuroticism was associated with larger startle responses during safe and anticipation conditions but not imminent threat, whereas anxiety symptoms were negatively associated with startle magnitude during imminent threat.
Conclusions
Female IBS patients show increased startle responses to threat of aversive stimulation at both abdominal and nonabdominal sites compared with controls. The data represent the first demonstration of altered threat potentiated startle in a functional pain condition and provide support for the use of these paradigms in further evaluation of affective mechanisms in these disorders.
Keywords: irritable bowel syndrome, functional bowel disorders, acoustic startle response, fear-potentiated startle
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder characterized by recurrent abdominal pain or discomfort combined with alterations in bowel function (1). Perceptual hypersensitivity to visceral stimuli may underlie symptoms of chronic pain and discomfort in IBS and is thought to be related in part to central pain amplification (2–5). Both hypervigilance to GI sensations as well as symptom specific fears or anxiety are thought to play a role in this mechanism. Visceral hypersensitivity in IBS has been shown to increase during laboratory stress (6) and to decrease with repeated exposure to rectal distension (7) further supporting the important role of cognitive/affective processes in IBS hypersensitivity. Recent brain imaging studies have also provided support for the role of these processes by showing increased activity in brain regions mediating arousal and affect, including the amygdala complex and the locus coeruleus, in IBS patients compared with controls, during colorectal balloon distension and during expectation of visceral discomfort (5,8,9). These latter findings have led us to pursue the hypothesis that IBS patients may show abnormal arousal and defensive responses during expected aversive stimuli, using the startle reflex.
The startle reflex is a cross species defensive motor response to sudden intense stimuli. Its magnitude is highly influenced by the presence or absence of aversive conditions (10). Enhancement of startle magnitude under aversive or threatening conditions is mediated by the amygdala complex. Animal research has shown that startle reflex potentiation to a conditioned cue (such as that which reliably signals a shock) rapidly habituates after the shock contingency is withdrawn and is mediated primarily by the central nucleus of the amygdala, while a longer lasting potentiation of startle that occurs during unpredictable or contextual stressors involves more the bed nucleus of the stria terminalis (11). These two paradigms are thought to resemble the differentiation between human experiences of fear and anxiety, respectively, (11) and have led to the development of human experimental protocols for enhanced startle reflexes to cued fear responses as well as contextual threat or anxiety-like responses (12).
In the present study, we utilize both context and cued threat procedures to examine differences in acoustic startle responses (ASRs) between IBS patients and healthy controls. Context threat was studied using procedures reported in previous studies of anxiety by the placement of the electrical stimulation pads on the subject before any specific discussion of when actual stimulation will occur (13). Three levels of cued threat were also studied; periods during which subjects were informed they might receive electrical stimulation to either the biceps or abdomen (imminent threat), periods during which subjects were informed they would not receive stimulation but which were part of the countdown to the imminent threat period (anticipation) and trials in which they would definitely not receive a stimulation (safe).
Based on the overall hypothesis of a hyperresponsivity of arousal and defensive circuits in IBS patients compared with healthy control subjects, the study was designed to test the following specific hypotheses: 1) IBS patients would show greater responsiveness to contextual threat, evidenced by increased ASRs immediately following stimulation pad placement, relative to preceding baseline periods. 2) IBS patients would show greater responsiveness to cued threat compared with controls, evidenced by increased ASRs during anticipation and imminent threat periods relative to safe periods. 3) IBS patients would show larger ASRs to abdominal compared with nonabdominal threat. In addition to these primary hypotheses, we also examined neuroticism and current anxiety symptoms as moderators of the increases in ASRs since anxiety has been associated both with IBS presentation and startle potentiation (14).
METHODS
Subjects
A sample of 42 female IBS patients and 22 female healthy controls were recruited by advertisement (see Table 1). IBS diagnosis was confirmed during a clinical exam by a gastroenterologist or nurse practitioner experienced in functional GI disorders using Rome II criteria (15). Bowel habit predominance was defined according to the Rome II supportive symptoms question list with 15 of the subjects determined to be constipation predominant, 18 diarrhea predominant, and 9 with mixed bowel habits. A total of 2.4% IBS patients rated their usual symptoms as mild (can be ignored if you don’t think about it), 45.2% as moderate (cannot be ignored but does not affect your lifestyle), 47.6% as severe (affects your lifestyle), and 4.8% as very severe (markedly affects your lifestyle).
TABLE 1.
Age, Anxiety, and Stimulus Ratings
| Mean | SD | t | p | |
|---|---|---|---|---|
| Age | ||||
| Controls (n = 22) | 28.71 | 8.55 | ns | |
| IBS (n = 42) | 32.62 | 11.72 | ||
| Neuroticism (EPQ-N) | ||||
| Controls (n = 18) | 4.28 | 4.00 | 5.101 | <.001 |
| IBS (n = 33) | 11.59 | 5.30 | ||
| Anxiety symptoms (HAD-A) | ||||
| Controls | 4.39 | 2.20 | 5.721 | <.001 |
| IBS | 9.17 | 3.18 | ||
| Abd. Stim. intensity | ||||
| Controls | 11.58 | 3.78 | 3.169 | .003 |
| IBS | 14.56 | 3.05 | ||
| Abd. Stim. unpleasantness | ||||
| Controls | 8.32 | 3.58 | 3.202 | .002 |
| IBS | 11.40 | 3.31 | ||
| Bicep Stim. intensity | ||||
| Controls | 13.16 | 2.93 | 3.127 | .003 |
| IBS | 15.43 | 2.35 | ||
| Bicep Stim. unpleasantness | ||||
| Controls | 9.95 | 3.01 | 2.055 | .045 |
| IBS | 11.93 | 3.59 |
Abd. = abdominal; Stim. = muscle contraction stimulus.
Unless otherwise stated, group means are from 19 controls and 36 IBS patients. All t values are from 2-tailed independent samples t-tests. Muscle contraction stimulus ratings are based on 20-point maximum scales.
Inclusion criteria for all subjects were: 1) no current use of psychoactive medication; 2) no current or past psychotic, major depressive, or substance abuse disorder; and 3) no major medical or neurological conditions (e.g., epilepsy, diabetes, heart disease) or major pain conditions that might be associated with abnormal eyeblink responses (with the exception of IBS for the IBS group). All study protocols were performed after approval by the UCLA and VA Greater Los Angeles Health Care System Institutional Review Boards. Informed consent was obtained from all subjects and the study was completed over a 3-year period.
Measures
Psychometric Instruments
Before beginning the study, subjects filled out the Hospital Anxiety and Depression Scales (HADS) (16) and the Neuroticism scale from the Eysenck Personality Questionnaire (EPQ-N) (17). The HADS is a screening measure of current anxiety and depression symptoms validated for nonpsychiatric samples. The EPQ-N has been validated as a measure of trait anxiety or anxiety vulnerability (17).
Electrophysiological Materials, Equipment and Data Acquisition
Auditory startle stimuli (105 dB, zero rise time, 50-ms white noise bursts) were presented binaurally through stereophonic headphones. The stimulations to the biceps muscle and area over the sigmoid colon were delivered by a Digital 807 Electrical Muscle Stimulation Device (Everyway Medical Instruments Co.). Stimulations consisted of a 1-second duration, 20.4 mA peak current passing between two pads (equating to a 50 V peak). This intensity level was based on pilot testing to represent an uncomfortable but not painful intensity of muscle contraction for most subjects. Use of a standardized intensity was chosen over that of individually determined values primarily because of concerns that preexposure (during the work-up procedure) to the stimulation would decrease the “threat” value of the stimulation during the cued threat procedure.
Electromyogram (EMG) activity of the orbicularis oculi associated with the startle response was recorded from electrodes placed beneath the right eye approximately 10 mm apart edge to edge, and 8 mm below the lower lid margin. The medial electrode was placed directly under the pupil. A ground electrode was placed in the center of the forehead. The impedance level of electrodes was 20 kOhm or less. EMG was full-wave rectified with low and high frequency cut-off values of 30 Hz and 1000 Hz, respectively. A vertical electrooculogram (EOG) was recorded from electrodes above and below the left eye to facilitate recognition of spontaneous blinks and eye movements.
Subjective Ratings
At completion of the experiment, subjects rated the intensity and unpleasantness of the biceps and the abdominal stimulation separately, on validated 20-point verbal descriptor anchored visual analog scales, with higher scores reflecting greater intensity (faint to extremely intense) and unpleasantness (neutral to very intolerable) (18).
Procedure
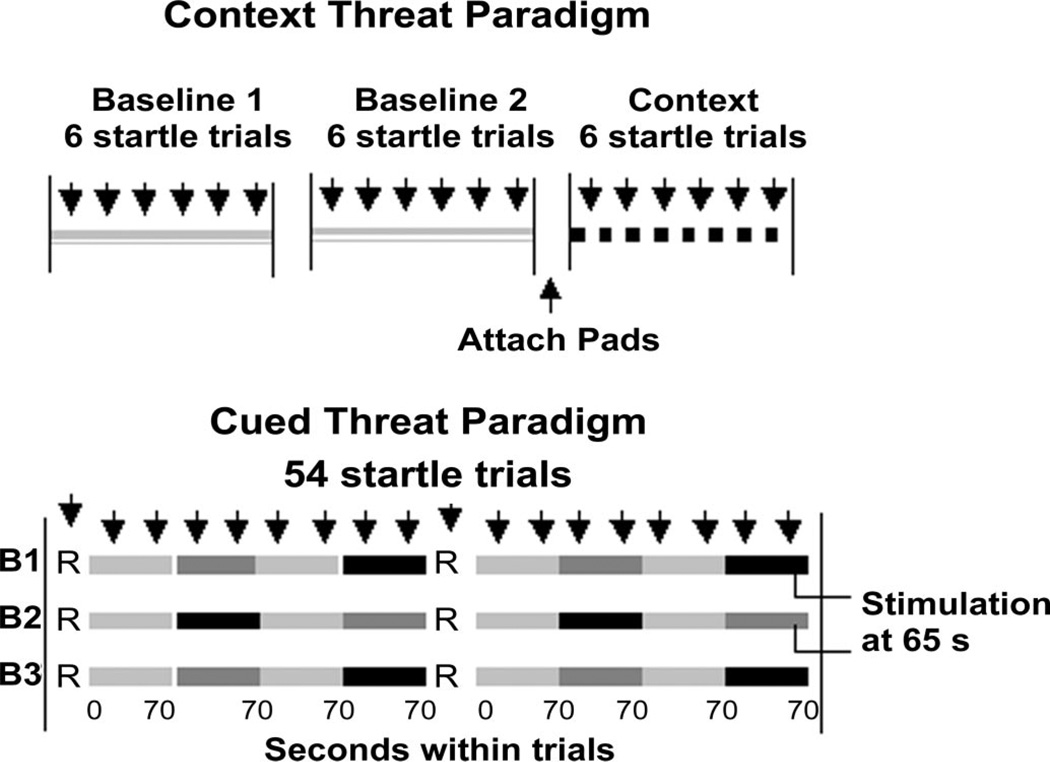
During the experiment, subjects were seated upright in a sound attenuated room adjacent to the experimental room, interconnected via intercom and closed-circuit video cameras that provided a view of the subjects from two angles. Behavioral observations permitted identification of trials involving excessive body movements or drowsiness for data inspection and rejection if necessary. Figure 1 illustrates the experimental protocol including two initial baseline conditions, the context threat, and the cued threat conditions. During each of the baseline and context threat periods, six startle stimuli were delivered with a mean inter-stimulus interval of 22 seconds, while subjects focused on a fixation cross in the center of the computer monitor. The breaks between the two baseline periods and before the context threat period were comparable except that before the context threat period, subjects were fitted with two pairs of stimulation pads, one pair attached to the biceps muscle of the arm and the other to their abdomen over the sigmoid colon. Context threat was generated by informing subjects that the connections would be used for stimulation and by showing them a diagram of the placements that emphasized that the abdominal location was over the sigmoid colon. They were also told that no stimulations would be given during the next period (context threat).
Figure 1.
Experimental design depicting the first and second baseline periods and the context period, followed by the cued threat procedure. R = rest periods; B = Block; light gray bars = safe periods; dark gray bars = abdominal threat periods; black bars = biceps threat periods; downward arrows = startle stimuli.
During the cued threat component of the experiment, subjects were shown on a computer monitor both a key word and a colored bar that gradually moved across the screen over the 70 seconds period. They were informed that no stimulations would be given when a ‘Safe’ message was displayed but when the ‘Danger’ message was displayed they might receive a stimulation to either the abdomen or biceps. They were also informed that during the safe period the progressing bar would just indicate the passage of time but during the ‘Danger’ periods, stimulation, if it occurred, would only happen when the bar turned red (between 45 and 70 seconds). The first part of the ‘Danger’ period when the bar was pink is considered an anticipation period whereas the latter part with the red bar is the imminent threat period. Lastly, subjects were informed that during the entire procedure they may get the stimulation up to three times at each site, and that after they received a stimulation, the next stimulation would be a little stronger. Actually, each subject received only one abdominal and one biceps stimulation.
As shown in Figure 1, during the cued threat procedure, 12 safe, 6 abdominal threat and 6 biceps threat periods each of 70 seconds duration were presented. These periods were grouped into three blocks with each consisting of a rest period presented first, followed by two alternating safe and threat periods, a rest period and a further two alternating safe and threat periods. The order of abdominal versus bicep threat periods was counterbalanced across subjects. Subjects were given a total of 54 startle stimuli during the cued threat procedure. One startle stimulus (SS) was delivered during each of the six rest periods (data not presented). Two startle stimuli were presented within each of the 12 safe and 12 threat periods (six abdominal and six biceps), one between 19 and 24 seconds and one between 54 and 59 seconds with the precise timing varying at random within these intervals so that subjects did not learn to anticipate when startle stimuli would be delivered. The mean time between startle trials was 30 seconds. Abdominal and biceps stimulations were given at 65 seconds during the last threat periods of the first two blocks and the delivery of the actual stimulation was counterbalanced across subjects.
After electrode removal, subjects rated the abdominal and arm stimulation, their hearing was tested and they were debriefed. No subjects were excluded from the study due to hearing deficits.
Data Analysis
Startle Blink Responses
The EMG activity of the orbicularis oculi was used to measure the ASR. After applying a 2 ms moving average filter, amplitudes of EMG were defined as the difference between the mean amplitude of the 200 ms of EMG preceding the SS and the peak of the response, expressed in microvolts (µV) (19). A total of 6.4% trials were rejected for artifact associated with movement, a spontaneous blink just before or after the SS or excessive EMG or EOG activity during the 200 ms baseline. Trials were scored as zero (5.1% of all trials) if no observable blink activity was evident in the EMG channel during the 20 to 80 ms response onset window following the SS and there was no reason to reject the trial. Subjects with more than 20 rejected trials (n = 3) or with 18 or more zero trials (n = 2) were excluded from analyses. Data from a further four subjects (two from each group) failed to complete the cued threat component. Thus, the context analysis was based on data from 38 IBS patients and 21 healthy controls and the cued threat analysis was based on data from 36 IBS patients and 19 healthy controls.
Statistical Analyses
The main analyses were performed using the Proc Mixed procedure in SAS (SAS Institute Incorporated, Carey, NC) on square root transformed EMG data due to positive skew. The primary approach was individual growth curve modeling (IGCM) for repeated measures data. IGCM has a number of advantages including missing data handling, precision of parameter estimates, and the ability to make better statistical inference compared with other repeated measures approaches (20,21).
For the contextual threat analyses, a two Group (IBS; controls) × three Condition (baseline 1; baseline 2; context) × Trial Number (6 per condition) mixed models analysis of variance (ANOVA) was used. For the cued threat analyses, ASRs elicited during the 19 to 24 seconds interval of ‘Danger’ periods (i.e., when the bar is still pink and subjects know that an electrical stimulation will not be given) were considered to be elicited during the anticipation condition. ASRs elicited during the 54 to 59 seconds interval of the ‘Danger’ periods (i.e., when the bar is red and subjects know that an electrical stimulation, if one will be given, will occur during this interval) were considered as imminent threat condition responses. ASRs recorded during the 19 to 24 seconds and the 54 to 59 seconds intervals of safe periods were used to represent the safe condition as they did not differ from each other. Thus, the cued threat procedure yielded three conditions: safe, anticipation, and imminent threat. ASRs elicited during these three conditions were analyzed as a function of the three experimental blocks in order to examine time course effects and the effect of having received an aversive stimulation. The analysis used a two Group (IBS; Control) × three Condition (safe, anticipation, imminent threat) × two Threat Site (abdominal; arm) × three Block (first; second; third) design. Post hoc comparisons were Sidak adjusted.
In addition, exploratory analyses examined the influence of anxiety symptoms (measured by the HADS) and neuroticism (measured by the EPQ-N) on ASRs during cued threat in the IBS and control subjects. The impact of the anxiety measures was tested first by adding them separately as covariates to the cued threat analysis described above. This was followed by separate analyses of IBS and control subjects to examine more specifically how these measures might alter startle within the two groups since the groups differed in size and distribution of these measures (controls had lower values and much less variability). In all analyses, an autoregressive, AR(1) covariance structure yielded the best fit among the commonly used variance-covariance structures as indicated by Akaike’s Information Criteria. We modeled the within person change over time by specifying intercepts as a random effect and time as a repeated effect. The most parsimonious specification for the effects of time on ASRs was determined using the recommended model-fitting approach which comprises a step-wise deletion process to simplify the form of the slope parameters (22).
RESULTS
Stimulation Ratings
IBS patients rated the intensity and unpleasantness of the abdominal stimulation and the unpleasantness and intensity of the biceps stimulation significantly greater than controls (Table 1).
Context Threat—The Effects of Stimulation Pad Attachment
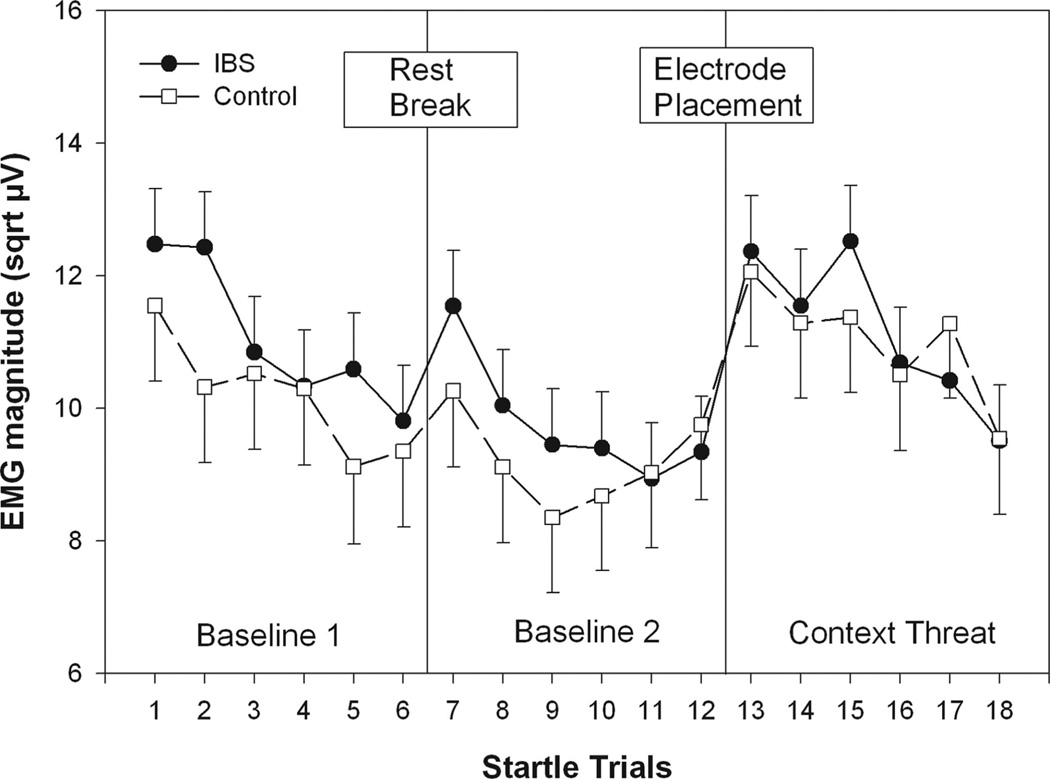
Figure 2 shows ASR magnitude during the first and second set of six baseline startle trials and the six context threat trials. ASR declined due to habituation from baseline 1 to baseline 2 and increased again during context threat, resulting in a significant Condition main effect (F(2, 295) = 24.84, p < .001) in the two Group (IBS; controls) × three Condition (baseline 1; baseline 2; context) × six Trials (per condition) ANOVA. This was confirmed by post hoc contrasts showing ASR in baseline 1 and context was significantly greater than that during baseline 2 (p values <.001). No significant difference was found between baseline 1 and context (p > .05). Both groups showed significant habituation of ASRs across startle trials within each condition and an initial increase in ASR magnitude on the first context trial following stimulation pad placement. This resulted in a main effect for Trial, Number (F(5,762) = 13.77, p <.001) and a Condition × Trial Number interaction (F(10,811) = 2.35, p = .0099). There were no significant effects involving Group.
Figure 2.
Context manipulation results. ASR magnitude during the first and second set of six baseline startle trials and the six context threat trials. Values are estimated means (with standard error bars) for each startle trial. sqrt µV = square root transformed microvolts.
Cued Threat—The Effects of Safety, Anticipation, and Imminent Threat
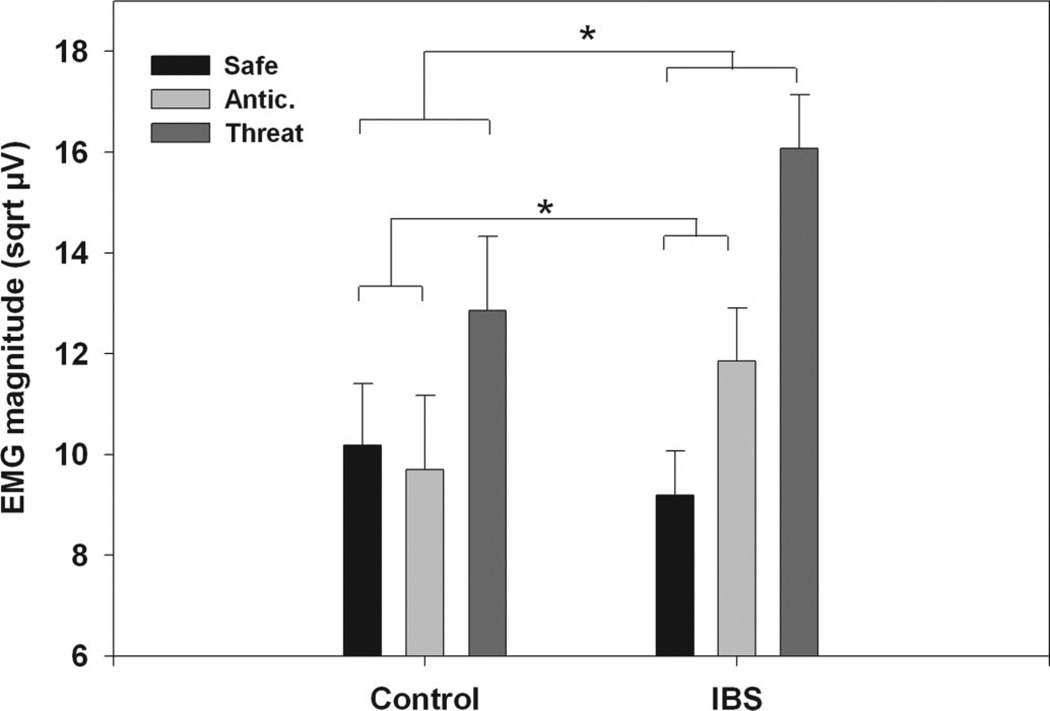
The two Group (IBS; Control) × three Condition (safe; anticipation; imminent threat) × two Threat Site (abdominal; arm) × three Block (first; second; third) mixed model ANOVA initially revealed no differences as a function of threat location (abdominal or arm). Therefore, Threat Site was collapsed in subsequent analyses. For both groups there were significantly larger ASRs during the imminent threat condition compared with both the anticipation and safe conditions (p values <.001), but no significant difference between the safe and anticipation condition (p > .05). This was reflected in the two Group (IBS; Control) × three Condition (safe, anticipation, imminent threat) × three Block (first; second; third) ANOVA showing a significant main effect for Condition (F(2,2031) = 17.37, p < .001). There was also a significant Condition × Group interaction (F(2,2031) = 4.01, p = .018). A post hoc contrast of condition effects across the groups indicated that the IBS group showed a significantly greater difference in ASR magnitude between the safe and imminent threat conditions relative to controls (p = .01). The IBS group also showed a significantly greater difference in ASR magnitude from the safe to the anticipation condition relative to controls (p = .05). These results are shown in Figure 3.
Figure 3.
ASRs for the cued threat procedure averaged over Blocks. Values are estimated means (with standard error bars) for each condition, Anticipation and threat conditions are averaged over threat type (abdominal and arm). Safe includes both the early and late trials of the safe period. Significance is indicated for group differences in magnitude of ASRs between safe and anticipation and safe and imminent threat conditions. sqrt µV = square root transformed microvolts.
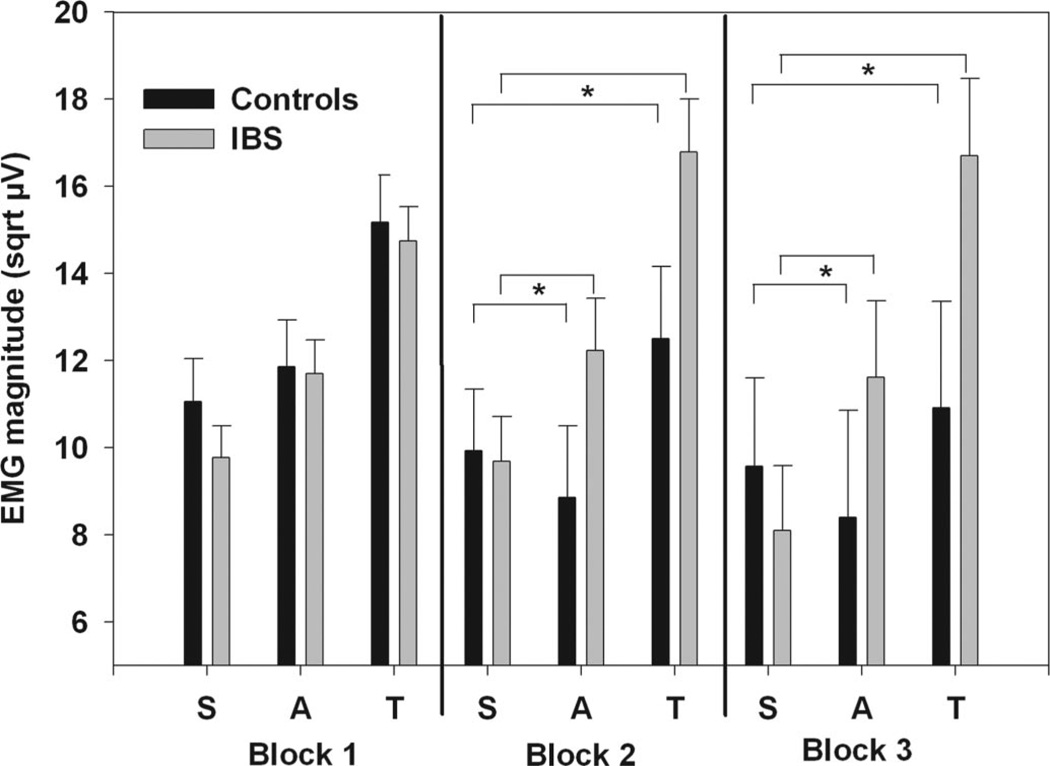
These effects, however, did differ over the three blocks in the cued threat component. Figure 4 shows the estimated ASRs during the safe, anticipation and imminent threat conditions for the IBS and control subjects during the three blocks. There were no significant Block main effects, or interactions between Block and Condition; however, there was a significant interaction between Group and Block (F(2,964) = 3.73, p = .024) as well as a three-way interaction between Group, Condition, and Block (F(4,2104) = 2.39, p = .049). Post hoc contrasts of condition effects across groups demonstrated that IBS patients compared with controls showed a significantly greater increase in ASR magnitude from safe to imminent threat for Blocks 2 (p = .0074) and 3 (p = .0081) but not Block 1 (p > .1). Similarly, IBS patients tended to show a significantly greater increase in ASRs from safe to anticipation during Blocks 2 (p = .027) and 3 (p = .082) but not in Block 1 (p > .1). Thus it seems the major group differences emerged after subjects experienced an actual stimulation, regardless of site.
Figure 4.
ASRs for the cued threat procedure including Block. Values are estimated means (with standard error bars) for each condition over the three blocks. Anticipation and threat conditions are averaged over threat type (abdominal and arm). Safe includes both the early and late trials of the safe period. Significance is indicated for group differences in magnitude of ASRs between safe and anticipation and safe and imminent threat conditions. sqrt µV = square root transformed microvolts.
Moderation of ASR Potentiation as a Function of Neuroticism and Anxiety Symptoms
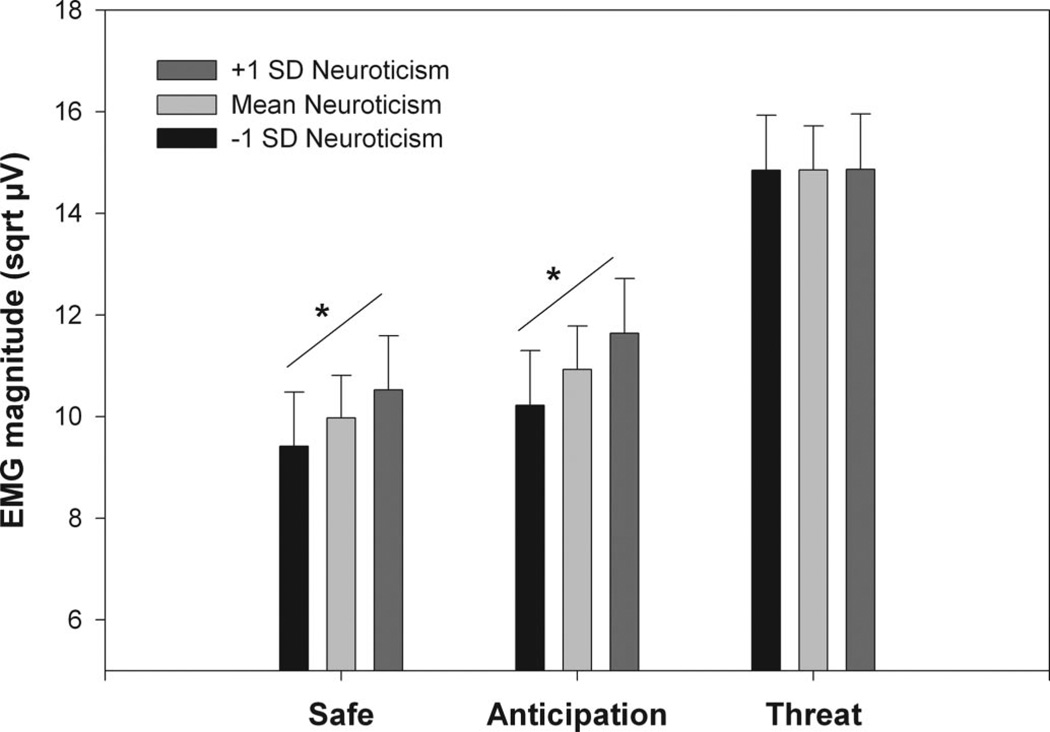
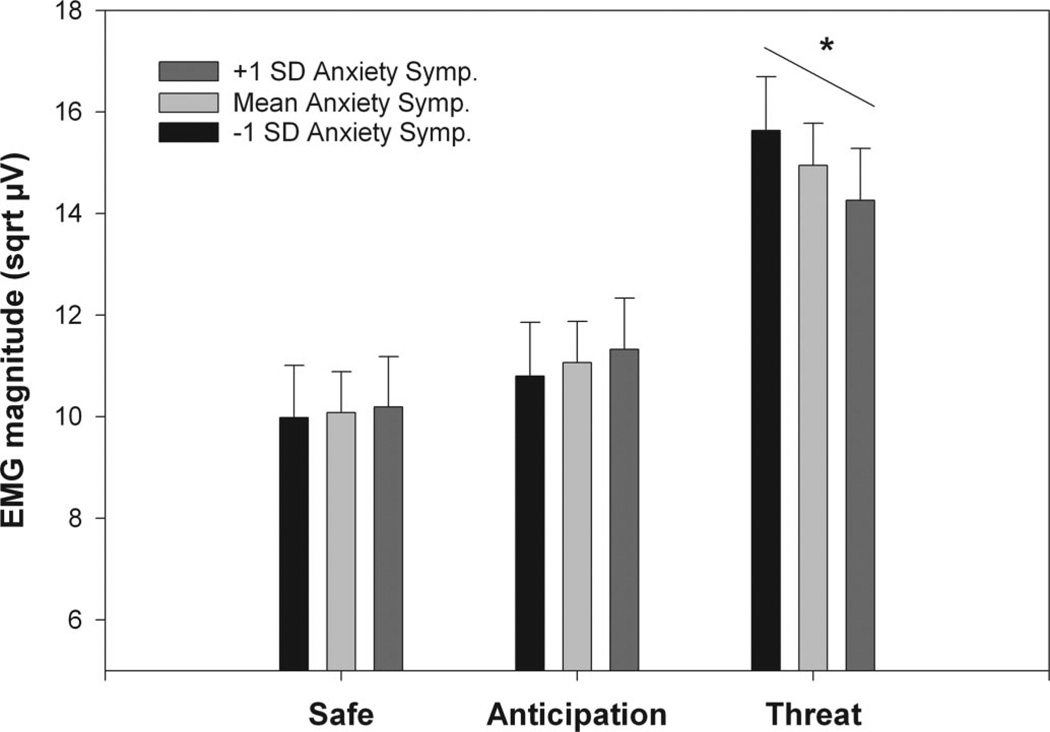
As expected, the IBS patients reported significantly greater anxiety symptoms and neuroticism compared with controls despite selection for absence of psychiatric disorders (Table 1). Mixed model analyses of covariance (ANCOVAs) were first used to examine the effects of neuroticism and anxiety scores on ASR modulation across the groups. After inclusion of neuroticism or anxiety symptoms, the significant Group × Condition interactions found in the analyses described above remained, indicating the primary group differences in ASR were not due to elevated anxiety symptoms or neuroticism in the IBS subjects. To further explore the impact of these variables on ASR, Condition × Block mixed model ANCOVAs were performed for each group separately. For IBS there was a greater positive slope between neuroticism and ASRs during both the safe and anticipation conditions than during the imminent threat condition (p values <.005). This resulted in a significant Neuroticism × Condition interaction (F(2,1192) = 7.03, p < .001). Figure 5 illustrates this interaction by showing the estimated ASRs at three levels of neuroticism: mean neuroticism, one SD above the mean, and one SD below the mean. A similar analysis for the HAD anxiety scale in IBS (Figure 6) also yielded a significant Anxiety × Condition interaction (F(2,1290) = 15.15, p < .001). However, this was due to there being a greater negative slope between anxiety and ASRs during the imminent threat condition compared with both the safe and anticipation conditions (p values <.005), indicating increased ASR with lower anxiety symptoms. For controls, the same mixed model ANCOVAs did not yield any significant neuroticism or anxiety symptom effects or interactions (p values >.05).
Figure 5.
Estimated mean ASRs (and standard errors) for IBS patients within 1 SD of the mean and those >1 SD above and below the mean on neuroticism (EPQ-R) for the three cued threat conditions. sqrt µV = square root transformed microvolts.
Figure 6.
Estimated mean ASRs (and standard errors) for IBS patients within 1 SD of the mean and those >1 SD above and below the mean on anxiety symptoms HAD-A) for the three cued threat conditions. sqrt µV = square root transformed microvolts.
DISCUSSION
Enhancement of the defensive startle reflex is a sensitive marker of limbic circuit (e.g., extended amygdala) activation associated with aversive events and contexts. In this experiment, all subjects showed startle magnitude increases from the context threat of placement of the stimulation pads as well as greater startle magnitude with increasing imminence of an actual stimulation, regardless of site. Startle was greatest during the imminent threat condition (in which a stimulation was actually possible), substantially less during the period in which the stimulation could be anticipated and smallest during the safe condition. Compared with the controls, the IBS subjects showed significantly greater startle magnitude during the anticipation and imminent threat periods, relative to the safe periods, once they had received an actual stimulation to either the abdomen or biceps. As expected the IBS patients also rated the severity of the abdominal and the biceps stimulations higher than did the controls. These findings are discussed in terms of the three major hypotheses stated in the Introduction.
IBS Patients Show Greater Responsiveness to Contextual Threat
The IBS patients did not show differences in startle magnitude compared with controls during either baseline period or during the startle trials following the application of the stimulation pads (context threat). Both groups showed a small increase in startle magnitude associated with the pad application. This finding is in contrast with previous studies showing greater enhancement of ASR during contexts associated with threat but not during cued threat conditions in patients with anxiety and posttraumatic stress disorder (23). Since baselines were obtained during a session in which the subject knew a noxious stimulation might occur later in the experimental procedure, it is possible that this knowledge may have elevated baseline startle responses, leading to lowered context potentiation and therefore a decreased likelihood of observing group differences.
IBS Patients Show Greater Responsiveness to Cued Threat
This hypothesis was confirmed. IBS patients compared with controls showed increased startle potentiation during both the anticipation and the imminent threat conditions. However, this group difference was only evident following actual experience of the stimulation, regardless of site. Although there is limited data showing increased responses during cued threat conditions in patient groups, a study of individuals with specific phobias has shown a significantly enhanced startle response during contact with a representation of the phobic object (24). In addition, Grillon and Davis (25) have shown a significant relationship between self-reported fear of aversive stimulation and startle responses during threat periods, but not baseline periods, of a fear potentiation protocol.
The group differences found during the imminent threat condition suggests that IBS patients show increased fear-potentiated startle. However, despite the lack of group differences during the explicit context manipulation and during the safe trials of the cued threat procedure, the increase in startle during the anticipation condition indicates IBS patients may also be responding to the context of the danger signal, even during a period in which they know they will not receive the noxious stimulation. This effect was evident after experiencing the stimulation and suggests in IBS patients some generalized heightened responsiveness evoked by the experimental context, but only after they have been primed through receiving an actual aversive stimulation.
IBS Patients Show Increased Responsiveness to the Abdominal Compared With the Nonabdominal Pain Threat
Although by questionnaire, IBS patients show evidence for visceral specific anxiety, (4) in this study the IBS group showed similar startle potentiation to both the abdominal and nonabdominal threat conditions. This would imply that the increased responses in IBS are generalized across physical threats to all parts of the body. The finding is consistent with the literature showing hypervigilance in IBS to both visceral and somatic experimental stimuli (26,27) as well as with evoked potential data showing overall increased responses to nonvisceral stimuli in terms of enhanced P1, preattentive processing (28). IBS patients also showed perceptual hypersensitivity based on their ratings to both the abdominal and biceps stimulation.
Effects of Neuroticism and Anxiety Symptoms
An exploratory analysis was run to examine if group differences in startle potentiation during the cued threat portion of the study might be related to either a trait measure of neuroticism or current anxiety symptoms. Although neither measure accounted for the group differences in startle magnitude, neuroticism was positively associated with startle in the IBS subjects, but only during the nonimminent threat conditions (safe and anticipation). This is a pattern consistent with studies of anxiety disorders and PTSD, namely an increase in startle magnitude during the nonimminent threat conditions and baseline periods (23). Hence, the impact of anxiety-proneness on startle magnitude may be similar in the IBS group as in other populations. The negative relationship between startle magnitude and the extent of anxiety symptoms was different and surprising. For IBS patients, increased anxiety symptoms were associated with decreased startle potentiation and this occurred only during the imminent threat condition. One explanation for this finding is based on data showing that conscious modulation of emotion also modulates startle (29,30). We might speculate that the IBS subjects with greater anxiety symptoms may have used increased emotional suppression strategies to cope with the threatened noxious stimulation, leading to decreased startle responses. In any case, the exploratory analyses of neuroticism and anxiety clearly indicate that increased startle magnitude in IBS patients compared with controls during imminent threat is not a result of greater anxiety and that these findings warrant further exploration.
Relevance of Altered Startle Responses to IBS Pathophysiology
IBS is strongly associated with hypersensitivity to visceral, and in some cases, somatic pain-related stimuli. It is presumed that this hypersensitivity plays a significant role in symptom generation, and that regardless of possible peripheral mechanisms of hypersensitivity, central processes of pain amplification are highly relevant in IBS as well as other functional pain conditions (5). Recent studies have identified the amygdala and related regions of the emotional arousal circuit as important components in the brain’s preparatory response to expected aversive stimuli, as well as the response to the delivered stimulus. In healthy subjects, it has been shown that increased negative affect induced by viewing unpleasant pictures leads to both increased startle responses as well as an enhanced RIII peripheral nociceptive reflex, which is a surrogate marker of diminished descending pain inhibition (31,32). Results from several brain imaging studies also support the role of the amygdala and other limbic structures in pain modulation (33). Using Positron Emission Tomography imaging we reported increased responses in IBS patients compared with controls in the amygdala as well as in the dorsal anterior cingulate cortex, during expected and actual visceral stimulation, (8) and decreases in amygdala and other limbic responses as well as perceived intensity, following multiple exposures to visceral distension testing (7). More recently, Berman et al.(9), using functional MRI demonstrated a failure of IBS patients to deactivate emotional arousal circuits during cued expectation of an aversive visceral stimulus. Hence, the increased fear-potentiated startle found for the IBS patients in this study is consistent with limbic hyperreactivity and related hypervigilance to potential negative sensations.
Limitations
Several limitations of the current study should be mentioned. First, only female IBS patients were included and there may be significant sex differences within the IBS population on these measures as has previously been shown for autonomic responses and perceptual and brain imaging responses to visceral stimulation (34–36). Second, there were no controls for menstrual cycle or sex hormone levels in the study. Menstrual cycle has been shown to influence modulation of startle by prepulse inhibition; however, there are no data on its possible influence on fear-potentiated startle as used here (37).
CONCLUSIONS
In conclusion, this study demonstrated increased defensive responding to a threat of aversive stimulation in female IBS patients compared with controls. This hyperresponsiveness was apparent after priming by actual experience of the aversive stimulation and occurred during both imminent threat and anticipation of threat. The data indicate that startle modulation studies offer a unique, noninvasive, and translational approach to assessing the sensitivity of limbic circuits to threat of, and potentially the presence of, uncomfortable or painful sensations. Hypersensitivity and pain fear are characteristic of many functional pain disorders and, therefore, the inclusion of startle reflex measures may be useful for testing affective mediation of perceptual and physiological responses as well as individual difference variables in these groups.
Acknowledgment
Supported in part by NIH Grants NR007768 (to B.N.), P50 DK64539 (to E.A.M.), R24 AT002681 (to E.A.M.), VA Medical Research (to B.N.), and a gift from the Virginia Friedhofer Charitable Trust (to E.O.).
Glossary
- ASR
acoustic startle response
- IBS
irritable bowel syndrome
- GI
gastrointestinal
- HADS
Hospital Anxiety and Depression Scales
- EPQ-N
Neuroticism scale from the Eysenck Personality Questionnaire
- EMG
electromyogram
- EOG
electrooculogram
- SS
startle stimulus
- IGCM
individual growth curve modeling
- ANOVA
analysis of variance
- ANCOVA
analysis of covariance
Footnotes
None of the authors report any biomedical financial interests or conflicts of interest relevant for this study.
REFERENCES
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC, Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III: the functional gastrointestinal disorders. Vol. 3. McLean, VA: Degnon Associates; 2006. Functional bowel disorders; pp. 487–556. [Google Scholar]
- 2.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distension testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 3.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, Fass R, Chang L, Mayer M, Naliboff BD. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 7.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Naliboff BD, Derbyshire SWG, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in irritable bowel syndrome patients and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Berman SM, Naliboff BD, Suyenobu B, Stains J, Gordon W, Bueller JA, Ohning G, Mayer EA. Activations and deactivations of the human amygdala associated with visceral pain: an FMRI study. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 11.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 12.Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Grillon C, Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. Int J Psychophysiol. 1998;28:223–231. doi: 10.1016/s0167-8760(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 14.Mayer EA, Craske MG, Naliboff BD. Depression, anxiety and the gastrointestinal system. J Clin Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- 15.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA, Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II: the functional gastrointestinal disorders diagnosis, pathophysiology and treatment: a multinational consensus. Vol. 2. McLean: Degnon Associates; 2000. C. Functional bowel disorders and D. Functional abdominal pain; pp. 351–432. [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire: (EPQ-R Adult) comprising the EPQ-Revised (EPQ-R) and EPQ-R short scale. San Diego: Educational and Industrial Testing Service; 1994. [Google Scholar]
- 18.Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 19.Ornitz EM, Guthrie D, Lane SJ, Sugiyama T. Maturation of startle facilitation by sustained prestimulation. Psychophysiology. 1990;27:298–308. doi: 10.1111/j.1469-8986.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 20.Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13–20. [PubMed] [Google Scholar]
- 21.Singer JD, Willet JB. Applied longitudinal data analysis. Oxford: Oxford University Press; 2003. [Google Scholar]
- 22.SAS for proc mixed. 2nd ed. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- 23.Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 24.Jong PD, Visser S, Merckelback H. Startle and spider phobia: unilateral probes and the prediction of treatment effects. J Psychophysiol. 1996;10:150–160. [Google Scholar]
- 25.Grillon C, Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 26.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Mayer EA, Johnson T, FitzGerald L, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 28.Berman SM, Naliboff BD, Chang L, FitzGerald L, Antolin T, Camplone A, Mayer EA. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am J Gastroenterol. 2002;97:2791–2797. doi: 10.1111/j.1572-0241.2002.07024.x. [DOI] [PubMed] [Google Scholar]
- 29.Muhlberger A, Wieser MJ, Pauli P. Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biol Psychol. 2008;77:47–52. doi: 10.1016/j.biopsycho.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- 31.Rhudy JL, Williams AE, McCabe KM, Nguyen MA, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 32.Bradley MM, Cuthbert BN, Lang PJ, Dawson ME, Schell AM, Bohmelt AH. Startle modification: implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. Affect and the startle reflex; pp. 157–183. [Google Scholar]
- 33.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 34.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex-specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang L, Naliboff BD, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Mayer EA. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 36.Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]