Abstract
In spite of the wide application potential of 1,2,4,5-tetrazines, particularly in live-cell and in-vivo imaging, a major limitation has been the lack of practical synthetic methods. Here we report the in situ synthesis of (E)-3-substituted-6-alkenyl-1,2,4,5-tetrazine derivatives via an elimination-Heck cascade reaction. Using this strategy, we provide 24 examples of π-conjugated tetrazine derivatives that can be conveniently prepared from tetrazine building blocks and related halides. These include tetrazine analogs of biological small molecules, highly conjugated buta-1,3-diene substituted tetrazines, and a diverse array of fluorescent probes suitable for live-cell imaging. These highly conjugated probes show dramatic fluorescent turn-on (up to 400-fold) when reacted with dienophiles such as cyclopropenes and trans-cyclooctenes, and we demonstrate their application for live-cell imaging. This work provides an efficient and practical synthetic methodology for tetrazine derivatives and will facilitate the application of conjugated tetrazines, particularly as fluorogenic probes for live-cell imaging.
Keywords: bioorthogonal, fluorophore, cycloaddition, cellular imaging, heterocycle
The chemistry of 1,2,4,5–tetrazines has gained growing interest in the last decade, owing to their unique physicochemical characteristics.[1] Tetrazines have seen expanding use in chemical biology, material science, natural product synthesis, coordination chemistry, electrochemistry, photovoltaics, and explosives research.[1a, 1b, 2] Of particular interest has been the use of tetrazines for bioorthogonal live-cell imaging applications.[1b, 1c, 3] In spite of the application potential of tetrazines, a major limitation has been the lack of practical synthetic methods. This has hampered the development of new fluorescent tetrazine probes, particularly those with fluorogenic properties.[4][5] To address this problem, herein we report the in situ synthesis of (E)-3-substituted-6-alkenyl-1,2,4,5-tetrazine derivatives via an elimination-Heck cascade reaction. This method enables convenient introduction of 3-substituted-6-alkenyl-1,2,4,5- tetrazine moieties onto a diverse array of functional molecules. These include unnatural nucleotides and amino acids that are relevant to bioorthogonal chemistry applications. The technique can also be used to readily prepare unique π-conjugated 1,2,4,5-tetrazine derivatives that are either difficult or not possible to prepare using alternative synthetic strategies, facilitating the future use of π-conjugated tetrazines as electron-deficient components in molecular electronics, photovoltaics, and non-linear optics.[1a, 6] Finally, we demonstrate the ability to synthesize a diverse set of tetrazine fluorogenic probes, both from xanthene and BODIPY precursors. Due to conjugation between the alkenyl tetrazine and the fluorescent core, these dyes show excellent fluorogenic properties after reaction with dienophiles, with turn-on ratios up to 400-fold. We demonstrate their suitability for live-cell imaging applications by detecting dienophile modified cell surface markers.
Recently we developed a metal–catalyzed one–pot procedure to prepare symmetric and unsymmetric tetrazines from aliphatic nitriles and anhydrous hydrazine.[7] Nevertheless, this technique has limitations. Synthesis requires excess anhydrous hydrazine and heating, conditions that are not compatible with several functional groups such as carbonyls and alkyl halides, that are susceptible to either nucleophilic addition or reduction.[8] It is therefore difficult to directly introduce 1,2,4,5-tetrazine onto relatively complex molecules such as fluorophores using this method. Conjugated alkenyl substituted 1,2,4,5-tetrazines were not obtainable from the corresponding alkenyl-nitriles. Additionally, there is limited commercial availability of anhydrous hydrazine in Europe and China due to safety concerns, further encumbering methods that require anhydrous hydrazine every time a new tetrazine derivative is synthesized.
We envisioned developing a simple tetrazine building block that was stable, could be easily synthesized, and readily installed onto complex substrates, including commonly used fluorescent probes, under mild conditions. In previous studies, s-dichlorotetrazine and s-dithiomethyltetrazine were regarded as typical tetrazine building blocks and have been used to prepare numerous functional s-tetrazines via nucleophilic displacement.[1a, 2f] Related tetrazines undergo SNAr reactions with carbanions and limited cross–coupling reactions; however, the reactions take place only if the tetrazine is deactivated by one donating substituent (alkylamino, alkoxy, or alkylthio), and the desired products are obtained in moderate yield, greatly restricting the possible tetrazine derivatives and potential applications.[9] For instance, although 1,2,4,5-tetrazines have been widely used in bioorthogonal reactions, owing to their high reactivity in inverse-electron demand Diels-Alder cycloadditions, mono(bis)-alkylamino (alkoxy, alkylthio) substituted tetrazine derivatives are not expected to be rapidly reacting due to the electronic effects of the electron-donating groups.[10]
We hypothesized that 3-substituted-6-vinyl tetrazines could be versatile tetrazine building blocks readily appended onto a diverse array of molecules using the Heck coupling reaction. However, there have been very few reports of alkenyl-modified tetrazines, and unsymmetric vinyl tetrazines are unknown, likely due to the previous difficulty in synthesizing unsymmetric tetrazines as well as the potential volatility of simple vinyl-tetrazines.[11] As mentioned, we recently disclosed a straightforward route to unsymmetric tetrazines, and using this technique we synthesized 3-hydroxyethyl-6-methyl-tetrazine in one-pot fashion from commercially available starting materials.[7] Mesylation of 3-hydroxyethyl-6-methyl-tetrazine, to form 1a, followed by elimination led to 3-methyl-6-vinyl tetrazine. As expected, the resulting vinyl tetrazine was very volatile and not convenient to isolate. In contrast, tetrazine 1a is a stable and easily handled pink powder (see TOC graphic), which could be stored at −20°C for several months without noticeable decomposition (see Supporting Information). We therefore turned to precursor 1a as a substitute of 3-methyl-6-vinyl tetrazine and explored an in situ elimination-Heck cascade reaction.[12]
We initially screened the reaction conditions for the Heck cascade reaction of 1a with iodobenzene using common catalysts, ligands, bases, and solvents. However, using 1.5 eq iodobenzene, 10% Pd(PPh3)4/Et3N/DMF and heating to 80°C for 90 min, led to no observable product (Table 1, Entry 1). This result is in agreement with past difficulties in using standard Heck coupling conditions with tetrazines.[9a] In recent years, microwave irradiation has been widely used to improve yields in cross-coupling reactions and we therefore decided to explore the use of microwave activation for the Heck cascade reaction.[13] After a screening of catalysts, ligands[14] and bases (Table 1, Entry 2–5), we found use of Ligand 3 at 3% loading enabled the isolation of 2a in nearly quantitative yield from both iodobenzene and bromobenzene (Table 1, Entry 5).
Table 1.
Optimization of the reaction conditions.[a]
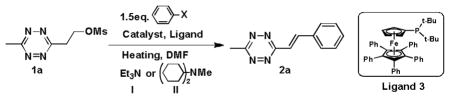
| ||||
|---|---|---|---|---|
| Entry | Cat., Ligand | Heat, Time | Base | Yield (%)[d] |
| 1[b] | 10% Pd(PPh3)4 | 80°C, 90 min | I | 0 |
| 2[b] | 10% Pd(PPh3)4 | 50°C, MW 30 min | II | 55 |
| 3[b] | 10%Pd2(dba)340% P(o-Tol)3 | 50°C, MW 30 min | II | 80 |
| 4[c] | 3%Pd2(dba)3,12%(t-Bu)3P+BF-4 | 60°C, MW 40 min | II | 58 |
| 5[b], [c] | 3%Pd2(dba)312% ligand 3 | 50°C, MW 30 min | II | 99 |
All reactions were carried out on a 0.02 mmol scale in 1.5 mL DMF. Ms – Mesyl group. MW – microwave.
Iodobenzene as starting material.
Bromobenzene as starting material.
Isolated yield based on 1a, no (Z)-3-methyl-6-styryl-s-tetrazine was observed.
With these optimized conditions in hand, we surveyed the substrate scope of the in-situ elimination-Heck reaction (Table 2). Substitutions at the 3-position of the alkenyl-tetrazines could be introduced by the synthesis and use of alternative vinyl tetrazine precursors. In this fashion, t-butyl, unsubstituted, phenyl, heterocyclic, and protected-amine alkenyl-tetrazine coupling products could be obtained in high yields (2b – 2f). Diversified phenyl bromides possessing sterically bulky, electron-donating, electron-withdrawing and heterocyclic substituents were also tolerated by the reaction conditions and gave the corresponding alkenyl tetrazines in excellent to good yields (2g – 2n). Interestingly, reduction of the double bond of alkenyl tetrazines such as 2a through hydrogenation was possible, providing a novel route to unsymmetric alkyl substituted 1,2,4,5-tetrazines such as 11 (Supporting Information).
Table 2.
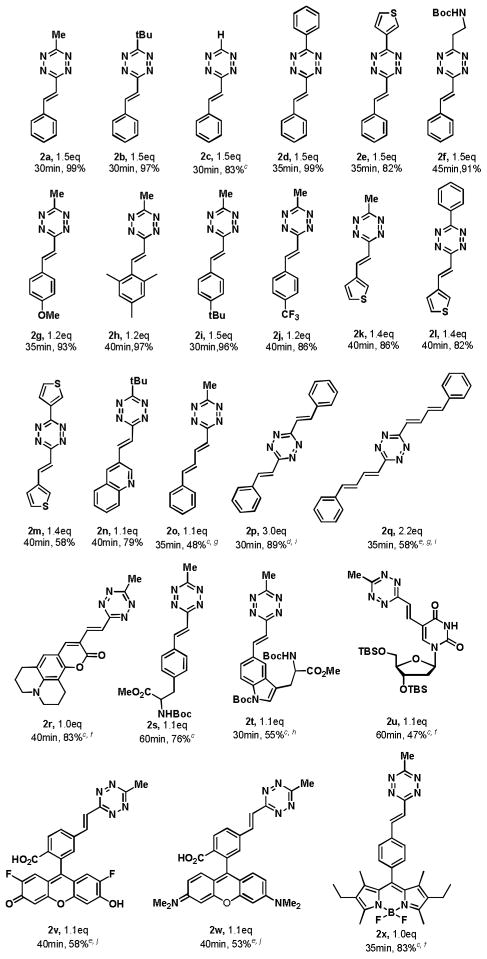
|
Conducted on 0.02 mmol scale. Reaction time and equivalents of bromide compounds shown under each product, 3 mol% Pd2(dba)3, 12 mol% ligand 3, 3 eq. Cy2NMe, microwave 50°C.
Isolated yield.
5 mol% Pd2(dba)3, 20 mol% ligand 3.
6 mol% Pd2(dba)3, 24 mol% ligand 3.
10 mol% Pd2(dba)3, 40 mol% ligand 3.
Iodide as starting material.
microwave 55°C.
microwave 60°C.
6 eq. Cy2NMe.
4 eq. Cy2NMe.
Despite significant interest in incorporating electron deficient tetrazine heterocycles in conjugated bridges, a roadblock has been the lack of accessible methods to synthesize π-conjugated tetrazines, particularly alkenyl substituted tetrazines, of which there are very few reported[11, 15] and longer conjugated buta-1,3-diene substituted tetrazines which, to our knowledge, are unknown in the literature. Remarkably, using the Heck cascade reaction, we were also able to readily synthesize conjugated mono-phenylbutadiene, bistyryl and biphenylbutadiene substituted s-tetrazines (2o -2q) in moderate to good yield.
We next examined the installation of bioorthogonal tetrazine handles on several biologically relevant and functionally complex molecules such as coumarin, deoxyribose, and amino acid derivatives.[1c, 3–4, 16] Under the modified Heck reaction conditions, these substrates smoothly reacted with 1a and delivered 2r - 2u in 47% – 83% yield. Deprotection of 2s - 2u, gave unnatural tetrazine modified deoxyuridine 12, DL-phenylalanine 13 and DL-tryptophan 14 (see Supporting Information). We evaluated the stability of (E)-3-substituted-6-styryl-s-tetrazines 2a–c in aqueous solutions and in the presence of biologically relevant nucleophiles such as thiols. Additionally, we measured the reaction kinetics between alkenyl tetrazines 2a–c and a highly strained trans-cyclooctene (TCO) dienophile. Stability and reactivity trends were consistent with past observations (Supporting Information).[17]
A major application of tetrazine ligations has been the live-cell imaging of dienophile tagged small molecules, including proteins, lipids, sugars, and drug analogs. [2b, 3a, 3b, 16a, 16b, 18] These applications are aided by the existence of fluorogenic tetrazines, which consist of popular fluorophores for cellular imaging such as xanthene and BODIPY dyes quenched through energy transfer by a tetrazine handle.[19] Unfortunately, when tetrazines are appended through aliphatic linkers, fluorescence increases after ligation are typically 10-fold and background signal from unreactive fluorophore limits the sensitivity of detection.[4] Fluorogenic tetrazine probes possesing fluorescence intensity increases greater than 100 fold after ligation would enable far more sensitive detection of dienophile targets. Recently, it was demonstrated that tetrazines could be appended directly onto BODIPY fluorophores resulting in highly fluorogenic probes that were quenched by through bond energy transfer (TBET).[5] Unfortunately, the harsh conditions required for heterocycle synthesis resulted in low yields and the technique was only demonstrated for a green emitting BODIPY dye. Conventional BODIPY probes have several drawbacks, including a small Stokes shift[20] and poor aqueous solubility.[21] In contrast, xanthene dyes, such as fluorescein and rhodamine derivatives, are arguably the most popular class of fluorescent probes for cellular imaging and are typically highly soluble in aqueous solutions. However, to date, highly quenched tetrazine xanthene dyes have not been demonstrated.
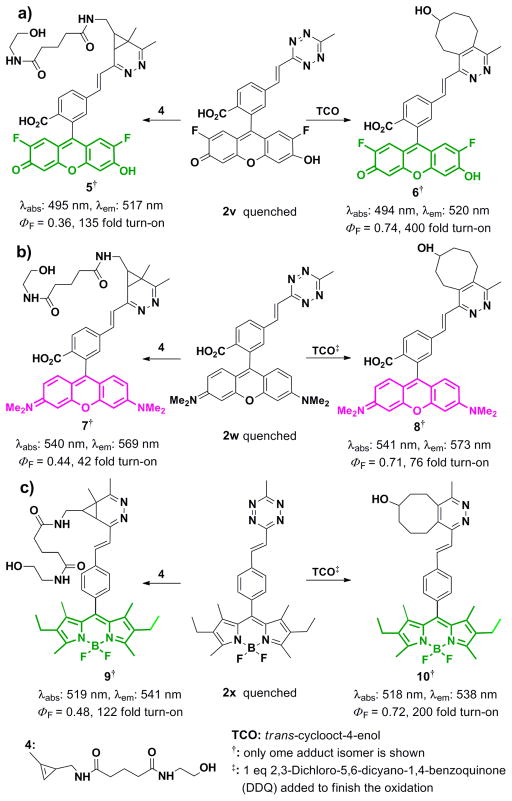
We hypothesized that the mild in-situ Heck reaction could be used to directly conjugate tetrazines through π-conjugation to a diverse series of fluorescent dyes, potentially enabling highly efficient quenching by TBET. [22] To our delight, we were able to apply our method to synthesize tetrazine-conjugated xanthene dyes such as 2′,7′-Difluorofluorescein (Oregon-Green)-tetrazine derivative 2v and Tetramethylrhodamine-tetrazine 2w in 58% and 53% yield respectively. Additionally, BODIPY-tetrazine dye 2x could be synthesized in 83% yield. All fluorophore-alkenyl-tetrazines were highly quenched, but became strongly emissive upon reaction with dienophiles (Scheme 1). Oregon-Green-tetrazine derivative 2v showed the highest turn-on with 135-fold and 400-fold increases in fluorescence intensity after reaction with a cyclopropene[23] and a TCO respectively (Scheme 1a, Figure 1a). The alkenyl-tetrazine fluorophores were stable when stored at −80°C for 1 month and remained stable in solution over 24 hours (Supporting Information). Rhodamine 2w also showed significant turn-on (up to 76-fold), with maximimum emission in the red (569–573 nm) after reaction with dienophiles. This indicates that appropriately conjugated tetrazines can significantly quench both green and red emitting dyes, opening up the possibility of two-color imaging using highly fluorogenic tetrazine probes.
Scheme 1.
a) Fluorogenic reaction of 2v with cyclopropene 4 and TCO along with observed optical properties in phosphate-buffered saline (PBS) at pH 7.4 (2μM). Fluorescein (in 0.1M NaOH) was used as the standard for quantum yield measurement. b) Fluorogenic reaction of 2w with cyclopropene 4 and TCO along with observed optical properties in EtOH (2μM). Rhodamine 6G (in EtOH) was used as the standard for quantum yield measurement c) Fluorogenic reaction of 2x with cyclopropene 4 and TCO along with observed optical properties in EtOH (2μM). Fluorescein (in 0.1M NaOH) was used as the standard for quantum yield measurement. Note that the reaction product of tetrazine with TCO can readily aromatize with oxidation.
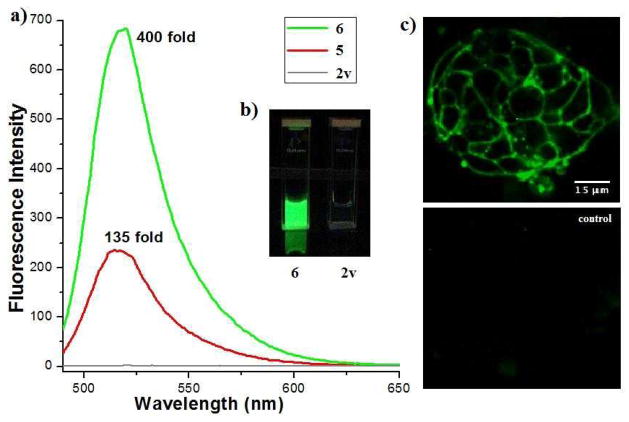
Figure 1.
a) Fluorescence emission spectra for compound 2v in PBS (grey line) and compound 5 (red line) and 6 (green line) in PBS; excitation at 480 nm. b) Equimolar solutions of compound 6 and 2v under excitation by a handheld UV lamp. c) Live-cell imaging of LS174T cells. Top: Cells were incubated (t = 1h) with 200 nM TCO-conjugated A33 antibodies, washed, and then imaged 30 minutes after the addition of 5 μM 2v. Bottom: cells lacking TCO were treated with 5 μM 2v for 30 minutes. Scale bar = 15 μm.
To demonstrate the suitability of quenched alkenyl-tetrazine probes for live-cell imaging, A33 antigens on live LS174T human colon carcinoma cells were pretargeted with TCO monoclonal antibodies.[24] Exposure to 2v followed by confocal imaging readily revealed the location of targeted dienophiles (Figure 1c), while cells lacking targeted dienophile showed negligible background staining, a benefit of the highly fluorogenic nature of 2v.
In summary, we have developed a series of novel tetrazine building blocks, which can smoothly react with aryl halides via a mild and high-yielding in situ elimination-Heck cascade reaction leading to the formation of (E)-3-substituted-6-alkenyl-1,2,4,5-tetrazines. This method enables convenient preparation of highly conjugated 1,2,4,5-tetrazines, including previously unreported buta-1,3-diene substituted 1,2,4,5-tetrazines. Moreover, this methodology provides a new strategy to prepare highly quenched fluorogenic tetrazines, including derivatives of popular xanthene and BODIPY dyes suitable for live-cell imaging applications. We believe this methodology will greatly facilitate the study of 1,2,4,5-tetrazines, advancing their further application in chemical biology, material science, electrochemistry, photovoltaics, nonlinear optics, and particularly live-cell imaging.
Supplementary Material
Footnotes
We acknowledge helpful discussions with Prof. Carlos Guerrero. This work was partially funded by the University of California, San Diego, and the NIH under grant number K01EB010078
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx. ((Please delete if not appropriate))
References
- 1.a) Clavier G, Audebert P. Chem Rev. 2010;110:3299–3314. doi: 10.1021/cr900357e. [DOI] [PubMed] [Google Scholar]; b) Devaraj NK, Weissleder R. Acc Chem Res. 2011;44:816–827. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Budin G, Yang KS, Reiner T, Weissleder R. Angew Chem Int Ed. 2011;50:9378–9381. doi: 10.1002/anie.201103273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Chavez DE, Hanson SK, Veauthier JM, Parrish DA. Angew Chem Int Ed. 2013;52:6876–6879. doi: 10.1002/anie.201302128. [DOI] [PubMed] [Google Scholar]; b) Yang J, Seckute J, Cole CM, Devaraj NK. Angew Chem Int Ed. 2012;51:7476–7479. doi: 10.1002/anie.201202122. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hamasaki A, Zimpleman JM, Hwang I, Boger DL. J Am Chem Soc. 2005;127:10767–10770. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kaim W. Coordin Chem Rev. 2002;230:127–139. [Google Scholar]; e) Li Z, Ding JF, Song NH, Lu JP, Tao Y. J Am Chem Soc. 2010;132:13160–13161. doi: 10.1021/ja106052e. [DOI] [PubMed] [Google Scholar]; f) Saracoglu N. Tetrahedron. 2007;63:4199–4236. [Google Scholar]
- 3.a) Yang KS, Budin G, Reiner T, Vinegoni C, Weissleder R. Angew Chem Int Ed. 2012;51:6598–6603. doi: 10.1002/anie.201200994. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Plass T, Milles S, Koehler C, Szymanski J, Mueller R, Wiessler M, Schultz C, Lemke EA. Angew Chem Int Ed. 2012;51:4166–4170. doi: 10.1002/anie.201108231. [DOI] [PubMed] [Google Scholar]; c) Lang K, Davis L, Torres-Kolbus J, Chou CJ, Deiters A, Chin JW. Nat Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew Chem Int Ed. 2010;49:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson JCT, Meimetis LG, Hilderbrand SA, Weissleder R. Angew Chem Int Ed. 2013;52:6917–6920. doi: 10.1002/anie.201301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Albert IDL, Marks TJ, Ratner MA. J Am Chem Soc. 1997;119:6575–6582. [Google Scholar]; b) Audebert P, Sadki S, Miomandre F, Clavier G. Electrochem Comm. 2004;6:144–147. [Google Scholar]; c) Audebert P, Sadki S, Miomandre F, Clavier G, Vernieres MC, Saoud M, Hapiot P. New J Chem. 2004;28:387–392. [Google Scholar]; d) Ma WB, Wu YQ, Gu DH, Gan FX. J Mol Struc-Theochem. 2006;774:13–18. [Google Scholar]
- 7.Yang J, Karver MR, Li W, Sahu S, Devaraj NK. Angew Chem Int Ed. 2012;51:5222–5225. doi: 10.1002/anie.201201117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrkit GD, Michalek GA. Ind Eng Chem. 1950;42:1862–1875. [Google Scholar]
- 9.a) Leconte N, Keromnes-Wuillaume A, Suzenet F, Guillaumet G. Synlett. 2007:204–210. [Google Scholar]; b) Novak Z, Kotschy A. Org Lett. 2003;5:3495–3497. doi: 10.1021/ol035312w. [DOI] [PubMed] [Google Scholar]
- 10.Knall AC, Slugovc C. Chem Soc Rev. 2013;42:5131–5142. doi: 10.1039/c3cs60049a. [DOI] [PubMed] [Google Scholar]
- 11.Pican S, Lapinte V, Pilard JF, Pasquinet E, Beller L, Fontaine L, Poullain D. Synlett. 2009:731–734. [Google Scholar]
- 12.a) Praquin CFB, Koning PD, Peach PJ, Howard RM, Spencer SL. Org Proc Res Dev. 2011;15:1124–1129. [Google Scholar]; b) Prakash GKS, Jog PV, Krishnan HS, Olah GA. J Am Chem Soc. 2011;133:2140. doi: 10.1021/ja111462h. [DOI] [PubMed] [Google Scholar]; c) Saiyed AS, Bedekar AV. Tetrahedron Letters. 2010;51:6227–6231. [Google Scholar]
- 13.Mehtaa VP, Van der Eycken EV. Chem Soc Rev. 2011;40:4925–4936. doi: 10.1039/c1cs15094d. [DOI] [PubMed] [Google Scholar]
- 14.a) Stambuli JP, Stauffer SR, Shaughnessy KH, Hartwig JF. J Am Chem Soc. 2001;123:2677–2678. doi: 10.1021/ja0058435. [DOI] [PubMed] [Google Scholar]; b) Netherton MR, Fu GC. Org Lett. 2001;3:4295–4298. doi: 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]; c) Shelby Q, Kataoka N, Mann G, Hartwig J. J Am Chem Soc. 2000;122:10718–10719. [Google Scholar]; d) Kataoka N, Shelby Q, Stambuli JP, Hartwig J. J Org Chem. 2002;67:5553–5566. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 15.Lifka T, Meier H. J Prak Chem-Chem Ztg. 1995;337:641–646. [Google Scholar]
- 16.a) Seckute J, Yang J, Devaraj NK. Nucleic acids research. 2013;41:e148. doi: 10.1093/nar/gkt540. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. J Am Chem Soc. 2012;134:10317–10320. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schoch J, Wiessler M, Jaschke A. J Am Chem Soc. 2010;132:8846–8847. doi: 10.1021/ja102871p. [DOI] [PubMed] [Google Scholar]
- 17.Karver MR, Weissleder R, Hilderbrand SA. Bioconjugate Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole CM, Yang J, Seckute J, Devaraj NK. Chembiochem. 2013;14:205–208. doi: 10.1002/cbic.201200719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumas-Verdes C, Miomandre F, Lepicier E, Galangau O, Vu TT, Clavier G, Meallet-Renault R, Audebert P. Eur J Org Chem. 2010:2525–2535. [Google Scholar]
- 20.Ulrich G, Goze C, Guardigli M, Roda A, Ziessel R. Angew Chem Int Ed. 2005;44:3694–3698. doi: 10.1002/anie.200500808. [DOI] [PubMed] [Google Scholar]
- 21.Loudet A, Burgess K. Chem Rev. 2007;107:4891–4932. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 22.a) Burghart A, Thoresen LH, Chen J, Burgess K, Bergstrom F, Johansson LBA. Chem Comm. 2000:2203–2204. [Google Scholar]; b) Jiao GS, Thoresen LH, Burgess K. J Am Chem Soc. 2003;125:14668–14669. doi: 10.1021/ja037193l. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Liang Y, Seckute J, Houk KN, Devaraj NK. Chem a Eur J. 2013 doi: 10.1002/chem.201304225. [DOI] [Google Scholar]
- 24.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, Weissleder R. P Natl Acad Sci USA. 2012;109:4762–4767. doi: 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.