Abstract
Objectives
We aimed to characterize use of cardiovascular testing for patients with incident heart failure (HF) hospitalization participating in the NHLBI-sponsored Cardiovascular Research Network PRESERVE study.
Background
HF is a common cause of hospitalization, and testing and treatment patterns may differ substantially between providers. Testing choices have important implications for the cost and quality of care.
Methods
Crude and adjusted cardiovascular testing rates were calculated for each participating hospital. Cox proportional hazards regression models were used to examine hospital testing rates after adjustment for hospital-level patient case-mix.
Results
Of the 37,099 patients in the PRESERVE study, 5,878 patients were hospitalized with incident HF between 2005 and 2008. Of these, evidence of cardiovascular testing was available for 4,650 (79.1 %) over the time period between 14 days before the incident HF admission and ending six months following the incident discharge. We compared crude and adjusted cardiovascular testing rates at the hospital level because the vast majority of testing occurred during the incident HF hospitalization. Of patients for whom testing was performed, 4,085 (87.9%) had an echocardiogram, 4,345 (93.4%) had a systolic function assessment, and 1,714 (36.9%) had a coronary artery disease assessment. Crude and adjusted testing rates varied markedly across the profiled hospitals, for individual testing modalities (e.g., echocardiography, stress echocardiography, nuclear stress testing, and left heart catheterization) and for specific clinical indications (e.g., systolic function assessment and coronary artery disease assessment).
Conclusions
For patients with newly diagnosed heart failure, we did not observe widespread overuse of cardiovascular testing in the six months following incident HF hospitalization relative to existing HF guidelines. Variations in testing were greatest for assessment of ischemia, where testing guidelines are less certain.
Keywords: Heart Failure, Cardiovascular Testing, Geographic Variations
INTRODUCTION
Over the past several decades, advances in the prevention and treatment of cardiovascular disease have led to important declines in age-adjusted cardiovascular-related mortality (1). At the same time, cardiovascular imaging has proliferated (2,3). A recent review of Medicare billing data revealed a doubling of expenditures on medical imaging from $6.89 billion in 2000 to $14.1 billion in 2005, approximately a third of which is cardiovascular (4). Medicare expenditures for diagnostic imaging have grown more rapidly than any other component of medical care (5). However, relatively few data link cardiovascular imaging to improved patient outcomes, and concern is growing that these tests have been adopted at extraordinary cost with insufficient evidence of benefit (6,7).
In response to this dramatic growth in imaging, professional groups have promulgated clinical practice guidelines and appropriate use criteria (AUC) (8–12). However, the AUC are not supported by randomized trial evidence, and guidelines rarely consider cost-effectiveness (13). AUC are limited in their discussion of how multiple testing modalities are most efficiently combined where multiple overlapping testing indications exist. Non-invasive imaging techniques may be interchangeable in some instances, and diminishing returns to overlapping imaging studies are likely. Therefore, there is a critical need to better understand how imaging combinations are used in clinical practice.
There are more than one million hospitalizations for acute heart failure (HF) annually, and the inpatient cost for these patients was estimated at $20.1 billion in 2009 (1,14). Testing and treatment patterns for newly diagnosed HF may differ substantially between providers and may have important implications for the cost and quality of care (15–17). In this study, we describe the type and frequency of cardiovascular testing in the first six months following hospitalization for incident HF in a large, diverse cohort of patients derived from the Cardiovascular Research Network PRESERVE Study.
METHODS
Source population
The source population included members from three participating health plans within the NHBLI-sponsored Cardiovascular Research Network (CVRN) (1,18,19). Sites included hospitals participating in the Kaiser Permanente Northern California, Kaiser Permanente Colorado, and Kaiser Permanente Northwest regions. These sites are integrated health care delivery systems that provide comprehensive care to ethnically, socioeconomically, and geographically diverse populations across various practice settings. They systematically track care provided and outcomes experienced within and outside of owned facilities. Each site has a Virtual Data Warehouse (VDW) that serves as the primary data source for subject identification and characterization (19). The VDWs are comprised of electronic datasets populated with linked demographic, administrative, and health care utilization data. Utilization data include ambulatory visits, as well as network and non-network hospitalizations with diagnoses and procedures. Institutional review boards at participating sites approved the study.
Study sample
We identified all persons aged ≥21 years who were hospitalized with newly diagnosed HF from 2005 through 2008. We used the following International Classification of Diseases, 9th Edition (ICD-9) codes: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9. Prior studies have shown a positive predictive value of >95% for admissions with a primary discharge diagnosis of HF based on these codes when compared against chart review and Framingham clinical criteria (20–22). Hospitalizations for HF were identified from each site’s VDW based on a primary ICD-9 discharge diagnosis for HF. We defined incident HF as an eligible HF hospitalization within the sampling frame that was not preceded by any other inpatient or outpatient HF diagnosis within the previous five years.
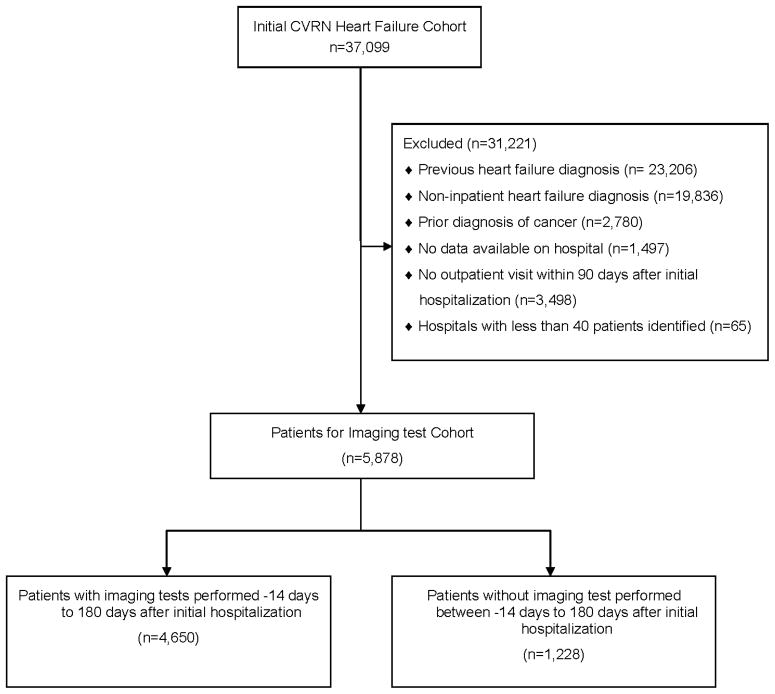
We excluded patients who did not have continuous health plan membership and pharmacy drug benefits during the 12 months before their index HF admission. We excluded patients who did not have at least one outpatient visit within three months of their index HF admission to ensure more complete data on post-discharge medical care. Finally, we excluded patients with a diagnosis of systemic cancer as serial imaging may be indicated to assess the safety of chemotherapy administration, even in the absence of symptomatic HF (Figure 1) (8,23).
Figure 1.
Cohort assembly for patients with incident heart failure from the Cardiovascular Research Network PRESERVE Study.
CVRN = Cardiovascular Research Network.
We identified all cardiovascular testing that occurred between 14 days prior to and 180 days following the incident HF hospitalization. Administrative records were searched for any evidence of testing. Imaging reports were also searched for evidence of an associated report from an imaging study that was performed despite no available administrative bill. For cases where no evidence of testing was identified through either administrative records or study report, the medical record was manually reviewed to identify if any testing occurred. This procedure was intended to capture studies that may have been performed at another hospital. Cardiovascular testing included transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), stress echocardiography, single positron emission tomography (SPECT), positron emission tomography (PET), cardiac magnetic resonance imaging (MRI), nuclear scintigraphy, left ventriculography, left heart catheterization, right and left heart catheterization and cardiac computed tomography angiography (CCTA). We considered all tests performed between 14 days prior to and 30 days after the incident heart failure admission to represent the initial testing strategy. We included testing prior to the index admission because outpatient testing may have prompted the index hospitalization. The Centers for Medicare & Medicaid Services (CMS) use a similar rationale to bundle payment for heart failure episodes of care in their Bundled Payments for Care Initiative (BPCI) (24). A systolic function assessment included any of the following tests individually or in combination: TTE, TEE, stress echocardiography, SPECT, PET, MRI, nuclear scintigraphy, or left ventriculography. A coronary artery disease (CAD) assessment included: stress echocardiography, SPECT, PET, left heart catheterization, right and left heart catheterization, or CCTA. Administrative data were searched for the following procedural codes: 76620, 76625, 76627, 76628, 76632, 93303, 93304, 93306, 93307, 93308, 93320, 93321, 93325, X3307, 7662A, 7662B, 7662C, 7662D, 7662E, 7662F, 7662G, 7663A, 7663B, 9331B, 9332A, X3308, 93350, 93312, 93313, 93314, 93318, 9331A, X3312, 93510, 93511, 93539, 93540, 93545, 93543, 75552, 75553, 75554, 75555, 75556, 75557, 75558, 75559, 75560, 75561, 75562, 75563, 75564, 78496, 78459, 78491, 78492, 93526, 78464, 78465, 78468, 78469, 78472, 78473, 78478, 78480, 78481, 78483, 78494, 93015, 93016, 93017, and 93018.
We identified hospital characteristics from the American Heart Association (AHA) Hospital Statistics for 2009 (25). We ascertained characteristics of hospitals not included in the AHA database by manually calling hospital administrators at the included sites.
Covariates
Information on coexisting illnesses was based on relevant ICD-9 and CPT codes, laboratory results, or filled outpatient prescriptions from health plan pharmacy databases. We chose laboratory values closest to the index date. Information was also obtained from site-specific cancer registries (26). We collected baseline data on diagnoses of acute myocardial infarction, unstable angina, coronary artery revascularization, hypertension, diabetes mellitus, chronic lung disease, and systemic cancer based on ICD-9 and CPT codes (26).
Statistical analysis
All analyses were conducted using SAS statistical software, version 9.3 (Cary, N.C.). Because most cardiovascular testing occurred during the incident HF hospitalization, the hospital was designated as the unit of analysis. To create statistically valid hospital testing profiles, we restricted the analysis to the 31 hospitals with a minimum of 40 incident HF admissions. This threshold was chosen because 96.7% of cardiovascular tests were performed in these 31 hospitals, and the number of incident HF admissions per hospital dropped sharply below this cut point (data not shown). Crude and adjusted cardiovascular testing rates were calculated for each hospital in the final dataset. Cox proportional hazards regression models were used to examine hospital testing rates after adjustment for hospital-level patient case-mix and to account for differential time of follow-up and censoring. Patients who died, disenrolled, ended participation in the PRESERVE study, or had a transplant were censored. Adjusted hospital testing rates were compared with the facility that had the highest rate of echocardiography testing. Case mix was defined using administrative data. Covariates included age, gender, diabetes, hypertension, dyslipidemia, coronary disease, and end-stage renal disease.
RESULTS
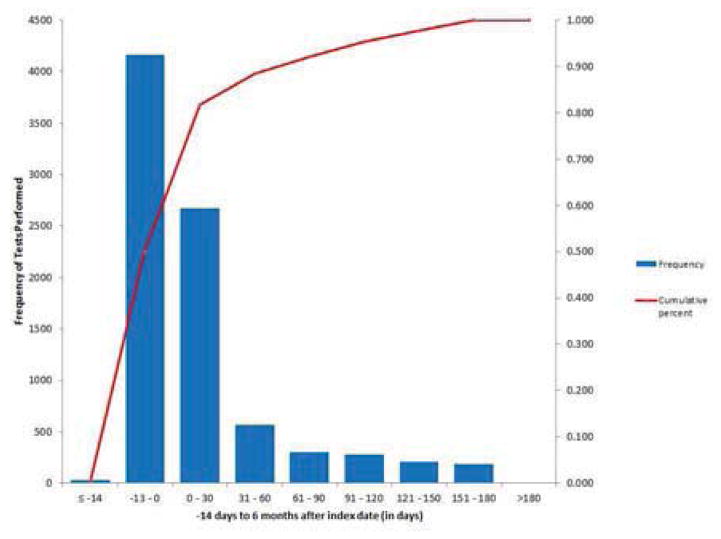
Of the 37,099 patients included in the PRESERVE study, we identified 5,878 patients hospitalized for incident HF between 2005 and 2008. Of these, cardiovascular testing was performed for 4,650 (79.1%) patients over the time period beginning 14 days before the incident HF admission and ending six months following discharge. Patients with and without testing differed from each other in a number of important respects. Patients with identifiable testing were younger, more likely to be men, and had fewer comorbidities (Table 1). For those patients with available testing, the majority of tests were completed during or immediately following the index HF admission (Figure. 2).
Table 1.
Baseline Characteristics among Patients Hospitalized for Incident HF (2005–2008)
| Overall N = 5,878 | Imaging test available N = 4,650 | Imaging test not available N = 1,228 | P-Value | |
|---|---|---|---|---|
|
|
||||
| Mean (SD) age in years | 73.4 (13.8) | 72.2 (13.9) | 78.1 (12.6) | <0.001 |
| Female gender, n (%) | 3039 (51.7) | 2319 (49.9) | 720 (58.6) | <0.001 |
| Medical History, n (%) | ||||
| Acute myocardial Infraction | 350 (6.0) | 239 (5.1) | 111 (9.0) | <0.001 |
| Unstable angina | 193 (3.3) | 140 (3.0) | 53 (4.3) | 0.02 |
| Coronary artery bypass surgery | 169 (2.9) | 131 (2.8) | 38 (3.1) | 0.61 |
| Percutaneous coronary intervention | 318 (5.4) | 239 (5.1) | 79 (6.4) | 0.07 |
| Ischemic stroke or transient ischemic attack | 421 (7.2) | 296 (6.4) | 125 (10.2) | <0.001 |
| Cerebrovascular disease | 977 (16.6) | 710 (15.3) | 267 (21.7) | <0.001 |
| Other thromboembolic event | 37 (0.6) | 25 (0.5) | 12 (1.0) | 0.08 |
| Atrial fibrillation or atrial flutter | 1744 (29.7) | 1294 (27.8) | 450 (36.6) | <0.001 |
| Ventricular fibrillation or ventricular tachycardia | 85 (1.4) | 68 (1.5) | 17 (1.4) | 0.84 |
| Mitral and/or aortic valvular disease | 728 (12.4) | 510 (11.0) | 218 (17.8) | <0.001 |
| Peripheral arterial disease | 418 (7.1) | 302 (6.5) | 116 (9.4) | <0.001 |
| Rheumatic heart disease | 179 (3.0) | 142 (3.1) | 37 (3.0) | 0.94 |
| Cardiac resynchronization therapy | 3 (0.1) | 2 (0.0) | 1 (0.1) | 0.60 |
| Implantable cardioverter defibrillator | 28 (0.5) | 24 (0.5) | 4 (0.3) | 0.39 |
| Pacemaker | 202 (3.4) | 141 (3.0) | 61 (5.0) | 0.001 |
| Dyslipidemia | 3481 (59.2) | 2741 (58.9) | 740 (60.3) | 0.40 |
| Hypertension | 4536 (77.2) | 3484 (74.9) | 1052 (85.7) | <0.001 |
| Diabetes mellitus | 1079 (18.4) | 845 (18.2) | 234 (19.1) | 0.48 |
| Hospitalized bleeds | 291 (5.0) | 200 (4.3) | 91 (7.4) | <0.001 |
| Chronic lung disease | 2037 (34.7) | 1583 (34.0) | 454 (37.0) | 0.06 |
| Chronic liver disease | 212 (3.6) | 173 (3.7) | 39 (3.2) | 0.36 |
| Baseline estimated GFR category, n (%) | <0.001 | |||
| > 130 | 13 (0.2) | 10 (0.2) | 3 (0.2) | |
| 90–130 | 703 (12.0) | 610 (13.1) | 93 (7.6) | |
| 60–89 | 2107 (35.8) | 1704 (36.6) | 403 (32.8) | |
| 45–59 | 1255 (21.4) | 967 (20.8) | 288 (23.5) | |
| 30–44 | 831 (14.1) | 601 (12.9) | 230 (18.7) | |
| 15–29 | 343 (5.8) | 250 (5.4) | 93 (7.6) | |
| <15 | 64 (1.1) | 52 (1.1) | 12 (1.0) | |
| Dialysis | 150 (2.6) | 87 (1.9) | 63 (5.1) | |
| Missing | 412 (7.0) | 369 (7.9) | 43 (3.5) | |
| Baseline estimated hemoglobin category, n (%) | <0.001 | |||
| ≥16.0 | 343 (5.8) | 299 (6.4) | 44 (3.6) | |
| 15.0 – 15.9 | 503 (8.6) | 419 (9.0) | 84 (6.8) | |
| 14.0 – 14.9 | 924 (15.7) | 747 (16.1) | 177 (14.4) | |
| 13.0 – 13.9 | 1083 (18.4) | 846 (18.2) | 237 (19.3) | |
| 12.0 – 12.9 | 1006 (17.1) | 777 (16.7) | 229 (18.6) | |
| 11.0 – 11.9 | 689 (11.7) | 502 (10.8) | 187 (15.2) | |
| 10.0 – 10.9 | 455 (7.7) | 339 (7.3) | 116 (9.4) | |
| 9.0 – 9.9 | 182 (3.1) | 131 (2.8) | 51 (4.2) | |
| <9.0 | 107 (1.8) | 76 (1.6) | 31 (2.5) | |
| Missing | 586 (10.0) | 514 (11.1) | 72 (5.9) | |
| Systolic blood pressure category, mmHg, n (%) | 0.02 | |||
| ≥180 | 250 (4.3) | 201 (4.3) | 49 (4.0) | |
| 160 – 179 | 571 (9.7) | 430 (9.2) | 141 (11.5) | |
| 140 – 159 | 1332 (22.7) | 1038 (22.3) | 294 (23.9) | |
| 130 – 139 | 1294 (22.0) | 1038 (22.3) | 256 (20.8) | |
| 121 – 129 | 857 (14.6) | 686 (14.8) | 171 (13.9) | |
| 110 – 120 | 1100 (18.7) | 866 (18.6) | 234 (19.1) | |
| 100 – 109 | 211 (3.6) | 168 (3.6) | 43 (3.5) | |
| <100 | 90 (1.5) | 69 (1.5) | 21 (1.7) | |
| Missing | 173 (2.9) | 154 (3.3) | 19 (1.5) | |
| Diastolic blood pressure category, mmHg, n (%) | <0.001 | |||
| ≥110 | 110 (1.9) | 98 (2.1) | 12 (1.0) | |
| 100–109 | 197 (3.4) | 161 (3.5) | 36 (2.9) | |
| 90–99 | 475 (8.1) | 387 (8.3) | 88 (7.2) | |
| 85–89 | 370 (6.3) | 304 (6.5) | 66 (5.4) | |
| 81–84 | 457 (7.8) | 371 (8.0) | 86 (7.0) | |
| ≤80 | 4096 (69.7) | 3175 (68.3) | 921 (75.0) | |
| Missing | 173 (2.9) | 154 (3.3) | 19 (1.5) | |
| HDL‡ cholesterol category, g/dL, n (%) | 0.25 | |||
| ≥60 | 1057 (18.0) | 809 (17.4) | 248 (20.2) | |
| 50–50.9 | 1046 (17.8) | 840 (18.1) | 206 (16.8) | |
| 40–49.9 | 1471 (25.0) | 1167 (25.1) | 304 (24.8) | |
| 35–39.9 | 698 (11.9) | 546 (11.7) | 152 (12.4) | |
| <35 | 720 (12.2) | 577 (12.4) | 143 (11.6) | |
| Missing | 886 (15.1) | 711 (15.3) | 175 (14.3) | |
| LDL§ cholesterol category, g/dL, n (%) | 0.12 | |||
| ≥200 | 62 (1.1) | 55 (1.2) | 7 (0.6) | |
| 160–199.9 | 263 (4.5) | 206 (4.4) | 57 (4.6) | |
| 130–159.9 | 668 (11.4) | 522 (11.2) | 146 (11.9) | |
| 100–129.9 | 1362 (23.2) | 1087 (23.4) | 275 (22.4) | |
| 70–99.9 | 1789 (30.4) | 1411 (30.3) | 378 (30.8) | |
| <70 | 798 (13.6) | 610 (13.1) | 188 (15.3) | |
| Missing | 936 (15.9) | 759 (16.3) | 177 (14.4) | |
SD = standard deviation; GFR = glomerular filtration rate; HDL = high-density lipoprotein; LDL = low-density lipoprotein
Figure 2. Frequency of cardiovascular testing between 14 days before and 180 days following the incident heart failure admission.
Pareto chart of cardiovascular testing (in days) from 14 days prior to the incident heart failure admission through 180 days following the incident heart failure admission. The frequency of testing is represented in the bar charts. The cumulative percentage of testing is represented in the plot.
All but two of the hospitals were not-for-profit, and 13 (42%) were teaching facilities. Eighteen (58%) offered on-site cardiac catheterization and 17 (55%) offered on-site cardiac surgery. All hospitals without onsite cardiac catheterization had referral agreements with centers that offered this service. Mean hospital bed size was 165.8 (standard deviation [SD] = 99.1), with median 150 (interquartile range [IQR] 96.2). Median household income for the country in which the hospital was located ranged from $41,390 to $78,009. All but three hospitals were located in counties above the median household income nationwide ($46,326) and all in counties below the 80th percentile. Twenty-four of the hospitals were located in California, three were located in Oregon, two were located in Colorado, and two were located in Washington state.
For patients with available results, 4,085 (87.9 %) had an echocardiogram, 4,345 (93.4 %) had a systolic function assessment, and 1,714 (36.9 %) had a CAD assessment. A total of 1,213 (26.1%) had multiple tests during the study period (Table 2). Repeat testing was infrequent in the six months following incident HF admission across all sites. Between 30 and 180 days following their incident heart failure admission, only 51 (1.1%) patients had a repeat echocardiogram and 677 (14.6 %) patients had any additional cardiovascular test. The rate of repeat testing was low, even though 712 (12.1%) of patients were readmitted within 30 days following discharge and 2,236 (38.0%) of patients were readmitted within six months following discharge (Table 3). Only 710 (36.7%) of readmitted patients had more than one test performed during the study period.
Table 2.
Frequency of testing combinations employed for patients with incident heart failure.
| Testing Combination | Number | Percentage |
|---|---|---|
| Echo | 2,453 | 52.8% |
| Stress Echo + SPECT | 528 | 11.4% |
| Echo + SPECT | 244 | 5.2% |
| SPECT | 212 | 4.6% |
| Stress Echo | 212 | 4.6% |
| RHC + LHC | 148 | 3.2% |
| LHC | 146 | 3.1% |
| Echo + LHC | 141 | 3.0% |
| Echo + RHC + LHC | 122 | 2.6% |
| Other | 441 | 9.5% |
Echo = transthoracic echocardiogram; Stress Echo = stress echocardiogram or dobutamine stress echocardiogram; SPECT = single photon emission tomography; LHC = left heart catheterization; RHC = right heart catheterization.
Table 3.
Frequency and timing of hospital readmissions.
| Category | Number | Percentage |
|---|---|---|
| 30 day hospital readmission | 712 | 12.1 |
| 180 day hospital readmission | 2,236 | 38.0 |
| Count of number of readmissions per patient. | |
|---|---|
| Frequency of hospital readmission | Count of patients, n (%) |
| 0 | 3,642 (62.0) |
| 1 | 1,378 (23.4) |
| 2 | 507 (8.6) |
| 3 | 228 (3.9) |
| ≥ 4 | 123 (2.1) |
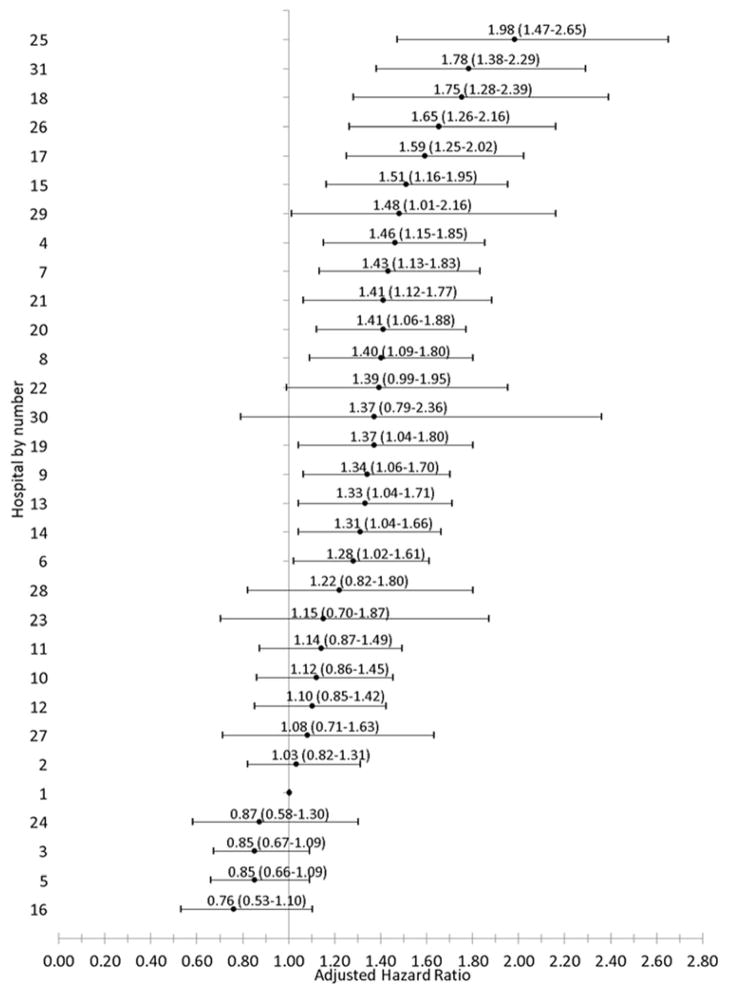
Crude testing proportions varied substantially across the hospitals for individual testing modalities and for testing by indication; and these differences persisted following multivariable adjustment for potential confounders (Table 4). When all testing methods were considered together, rates of systolic function assessment ranged from 53.9 to 242.7 per 100 patient years (adjusted HR 0.69 to 1.29) and rates of ischemia assessment ranged from 31.1 to 140.5 per 100 patient years (adjusted HR 0.76 to 1.98) (Figures 3 – 5).
Table 4.
Testing rates for individual testing modalities.
| Modality | Crude rate per 100 patient years | Adjusted rate per 100 patient years |
|---|---|---|
| Echocardiography | 24.8 – 62.0 | 22.3 – 161.8 |
| Stress echocardiography | 3.1 – 27.9 | 3.5 – 37.2 |
| SPECT | 3.1 – 27.9 | 1.3 – 63.9 |
| LHC | 5.8 – 27.4 | 9.3 – 63.4 |
SPECT = single photon emission tomography; LHC = left heart catheterization; RHC = right heart catheterization.
Figure 3. Adjusted hazard ratio and 95% confidence interval of any cardiovascular test use among 5,878 adults with incident heart failure by hospital (2005–2008).
Adjusted hazard ratio of cardiovascular testing relative to the hospital with the highest rate of echocardiography.
Figure 5. Adjusted hazard ratio and 95% confidence interval of CAD test use among 4,650 adults with incident heart failure and at least one available imaging test by hospitals (2005–2008).
Adjusted hazard ratio of cardiovascular testing relative to the hospital with the highest rate of echocardiography. A coronary artery disease (CAD) assessment included: stress echocardiography, SPECT, PET, left heart catheterization, right and left heart catheterization, or CCTA.
DISCUSSION
In this study, we examined hospital-level variations in use of cardiovascular testing for patients hospitalized for incident HF. To our knowledge, no previous published study has comprehensively examined patterns of use of all major testing modalities simultaneously among newly diagnosed HF patients. Similar to previous work on geographic variations in healthcare utilization, our findings demonstrate wide hospital variations in testing (27,28). Our study extends previous work by assessing both individual testing modalities and clinical indications (e.g., assessment of systolic function, assessment of CAD) in adults with incident HF.
One of four core HF performance measures promoted by the Joint Commission is the “documentation in the hospital record that left ventricular systolic function was evaluated before arrival, during hospitalization, or is planned for after discharge” (29). Recent Medicare reimbursement reductions for outpatient echocardiography and nuclear stress testing reflect a widespread belief that these tests are overused generally (30,31). However, we found less than one evaluation of systolic function per patient, very low rates of multiple testing, and very infrequent repeat testing in the initial six months following incident HF hospitalization within participating health care delivery systems. Our findings do not represent overuse relative to existing HF guidelines for systolic function assessment (32).
Echocardiography is the mainstay of systolic function assessment in incident HF and carries both an American College of Cardiology (ACC)/American Heart Association (AHA) Class IC recommendation (11) and an “appropriate” rating in the American College of Radiology/American College of Cardiology (ACR/ACC) report on appropriate use of cardiovascular imaging in HF (33). Although a recent study suggests that even clinically “appropriate” studies may not be clinically useful, a complete evaluation of HF requires an assessment of cardiac structure and function, which involves imaging (34,35). The results of imaging are essential for selection of evidence-based therapies for heart failure and are useful for prognostication (36,37). Though the sensitivity and specificity of ICD-9-CM codes is imperfect, not all patients in this cohort appear to have received an echocardiogram during the ascertainment window of interest. Furthermore, both crude and adjusted rates of echocardiography differed substantially between the 31 hospital sites profiled.
Repeat echocardiographic assessment may be appropriate when there is a change in clinical status, for assessment of response to medical therapy, and to determine eligibility for advanced HF interventions such as implantable cardioverter defibrillators or biventricular pacing (38). In this study, 38% of patients were readmitted during follow-up, and some of these hospitalizations may have represented a change in clinical status that justified additional cardiovascular testing (39). Even so, there was a very low rate of repeat imaging for readmitted patients in this cohort, and only 1.1% of patients overall had a repeat echocardiogram during short-term follow-up despite a high rate of hospital readmissions. This low rate of repeat testing may reflect the advanced electronic medical record available in all participating health systems which readily provided prior imaging results, strong incentives to be treated within a network facility, and close follow-up which characterizes the integrated health care delivery model.
In this patient population, there was significantly more variation in rates of assessment of CAD than for assessment of systolic function. Although not codified as a quality measure by the Joint Commission, performance of coronary arteriography to exclude CAD as the basis of left ventricular systolic function is an ACC/AHA Class IB recommendation for patients with known or suspected CAD (11,12). The indications for CAD assessment in patients without clinical, electrocardiographic, or imaging findings of CAD are uncertain (9,11,12,33,40). The lack of high-quality evidence has led to imprecise use of cardiovascular imaging in this clinical situation. Not surprisingly, there was marked between-hospital variation in the rate and method of ischemia assessment. Differences in test availability and physician expertise between hospital sites may have played a role in variability of ischemia testing, particularly for cardiac catheterization. Several testing types were rarely used, including PET, cardiac MRI, and CCTA. However, stress echocardiography and stress SPECT testing are commonly available, and while most testing occurred during the incident hospitalization, our testing profiles extended to six months following incident diagnosis so all patients had access to cardiac catheterization. Although all patients in this cohort had access to cardiac catheterization within the network, the availability of left heart catheterization at the presenting hospital may have influenced test selection. We are unable to identify which patients had signs, symptoms, or findings of ischemia on initial testing and who are most likely to benefit from an ischemia evaluation.
The ACR/ACC appropriate utilization of cardiovascular imaging in HF guideline supports a sequential testing approach in newly diagnosed HF. Even so, few patients underwent multiple testing in this patient population. Different cardiovascular testing approaches offer overlapping information, are not clinically interchangeable, and differ considerably with regard to cost and invasiveness. Each modality offers unique information, and a variety of testing combinations are possible, with considerable implications for cost and cost-effectiveness. We are unable to assess why 11.4% of patients underwent both a stress echocardiogram and a SPECT study. This combination may reflect poor endocardial definition or failure to reach the target heart rate on the stress echocardiogram but could also reflect perceived complementarity between these tests. Also, we did not search records for exercise treadmill testing without imaging, and the rates of ischemic evaluation may therefore be higher than reported here. Further study is needed to determine the most cost-effective approach to initial assessment of incident HF, particularly where CAD is suspected.
The CVRN PRESERVE cohort offers a source of “real world” data for a large and diverse patient cohort that was hospitalized for incident HF. However, because the majority of patients were treated within integrated health care delivery systems with comprehensive coverage plans for many patients, some imaging studies that were actually performed may not have been coded in the electronic databases. To minimize this potential bias, we augmented our analysis of administrative procedure coding data with a review of imaging specific reports and a manual review of patient records. Even so, some cardiovascular testing may have been performed without generating a formal report (e.g., bedside limited echocardiogram) or documentation and some tests may not have been available in any of the data sources used. We were unable to identify the reasons behind the testing variations seen in this study. Further, all patients in our sample had health insurance, including a pharmacy drug benefit, and the availability of advanced electronic medical record systems, which may have substantially reduced duplicate testing. Therefore, these findings may not be generalizable to other patient populations and settings. However, extensive national investments in electronic medical records with “meaningful use” (41) and pilot programs in “accountable care” may mitigate these historical differences (24,42,43).
In a contemporary population of adults hospitalized for incident HF, we found significant hospital-level variations in cardiovascular testing that did not appear to be explained by patient case-mix. The greatest variations occurred in testing modalities for CAD where less rigorous evidence exists for their clinical utility. More research is needed to clarify the most cost-effective test or testing combination for patients with incident HF.
Figure 4. Adjusted hazard ratio and 95% confidence interval of systolic assessment test use among 4,650 adults with incident heart failure and at least one available imaging test by hospitals (2005–2008).
Adjusted hazard ratio of cardiovascular testing relative to the hospital with the highest rate of echocardiography. A systolic function assessment included any of the following tests individually or in combination: echocardiography, transesophageal echocardiogram, stress echocardiography, SPECT, PET, MRI, nuclear scintigraphy, or left ventriculography.
Acknowledgments
Financial Support: This study was conducted within the Cardiovascular Research Network sponsored by the National Heart Lung and Blood Institute (NHLBI) (U19 HL91179-01) and the American Recovery and Reinvestment Act OF 2009 (NHLBI Grant # 1RC1HL099395-01).
ABBREVIATIONS LIST
- AUC
Appropriate Use Criteria
- CCTA
cardiac computed tomography angiography
- CMS
Centers for Medicare and Medicaid Services
- HF
heart failure
- MPI
myocardial perfusion imaging
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single positron emission tomography
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
Footnotes
Disclosures: The authors declare no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374–9. doi: 10.1161/CIRCULATIONAHA.105.560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearlman AS, Ryan T, Picard MH, Douglas PS. Evolving trends in the use of echocardiography: a study of Medicare beneficiaries. J Am Coll Cardiol. 2007;49:2283–91. doi: 10.1016/j.jacc.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 4.Medicare Part B Imaging Services. Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. Washington, DC: Government Accounting Office; 2008. pp. 1–50. [Google Scholar]
- 5.Iglehart JK. The new era of medical imaging--progress and pitfalls. N Engl J Med. 2006;354:2822–8. doi: 10.1056/NEJMhpr061219. [DOI] [PubMed] [Google Scholar]
- 6.Douglas PS, Taylor A, Bild D, et al. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Am Soc Echocardiogr. 2009;22:766–73. doi: 10.1016/j.echo.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonow RO. 2009 ASNC keynote lecture: measuring cost, cost-effectiveness, and quality in cardiovascular imaging. J Nucl Cardiol. 2010;17:362–9. doi: 10.1007/s12350-010-9224-4. [DOI] [PubMed] [Google Scholar]
- 8.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance. Endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Soc Echocardiogr. 2007;20:787–805. doi: 10.1016/j.echo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol. 2008;51:1127–47. doi: 10.1016/j.jacc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine: endorsed by the American College of Emergency Physicians. Circulation. 2009;119:e561–87. doi: 10.1161/CIRCULATIONAHA.109.192519. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA American College of C, American Heart Association Task Force on Practice G. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Redberg RF. Evidence, appropriateness, and technology assessment in cardiology: a case study of computed tomography. Health Aff (Millwood) 2007;26:86–95. doi: 10.1377/hlthaff.26.1.86. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Zhang Z, Ayala C, Wall HK, Fang J. Costs of heart failure-related hospitalizations in patients aged 18 to 64 years. The American journal of managed care. 2010;16:769–76. [PubMed] [Google Scholar]
- 15.Patterson ME, Hernandez AF, Hammill BG, et al. Process of care performance measures and long-term outcomes in patients hospitalized with heart failure. Med Care. 2010;48:210–6. doi: 10.1097/MLR.0b013e3181ca3eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Yancy CW, Heywood JT. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–77. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168:2415–21. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 19.Magid DJ, Gurwitz JH, Rumsfeld JS, Go AS. Creating a research data network for cardiovascular disease: the CVRN. Expert Rev Cardiovasc Ther. 2008;6:1043–5. doi: 10.1586/14779072.6.8.1043. [DOI] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, Mcnamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–11. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 23.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. [Accessed November 4, 2013];Bundled Payments for Care improvement (BPCI) Initiative: General Information. http://innovation.cms.gov/initiatives/bundled-payments.
- 25.AHA Hospital Statistics™ 2009 Edition. American Heart Association; Chicago, IL: [Google Scholar]
- 26.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed May 8, 2011];Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org/
- 28.Geographic Variation in Health Care Spending. Washington, D.C: Congressional Budget Office; 2008. [Google Scholar]
- 29. [Accessed April 21, 2012];Specifications Manual for Joint Commission National Quality Core Measures. 2010B http://manual.jointcommission.org/releases/Archive/TJC2010B1/MIF0029.html.
- 30.Shaw LJ, Min JK, Hachamovitch R, et al. Cardiovascular imaging research at the crossroads. J Am Coll Cardiol Img. 2010;3:316–24. doi: 10.1016/j.jcmg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Iglehart JK. Health insurers and medical-imaging policy--a work in progress. N Engl J Med. 2009;360:1030–7. doi: 10.1056/NEJMhpr0808703. [DOI] [PubMed] [Google Scholar]
- 32.Jessup M, Brozena SC. Guidelines for the management of heart failure: differences in guideline perspectives. Cardiol Clin. 2007;25:497–506. v. doi: 10.1016/j.ccl.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Patel MR, White RD, Abbara S, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. Journal of the American College of Cardiology. 2013;61:2207–31. doi: 10.1016/j.jacc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Matulevicius SA, Rohatgi A, Das SR, Price AL, Deluna A, Reimold SC. Appropriate use and clinical impact of transthoracic echocardiography. JAMA internal medicine. 2013;173:1600–7. doi: 10.1001/jamainternmed.2013.8972. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca C, Morais H, Mota T, et al. The diagnosis of heart failure in primary care: value of symptoms and signs. European journal of heart failure. 2004;6:795–800. 821–2. doi: 10.1016/j.ejheart.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Quinones MA, Greenberg BH, Kopelen HA, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction Journal of the American College of Cardiology. 2000;35:1237–44. doi: 10.1016/s0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 37.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. Journal of the American College of Cardiology. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 38.Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography: a Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229–67. doi: 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brindis RG, Douglas PS, Hendel RC, et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol. 2005;46:1587–605. doi: 10.1016/j.jacc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Centers for medicare & Medicaid Services. [Accessed November 4, 2013];EHR Incentive Programs. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/index.html?redirect=/ehrincentiveprograms.
- 42.Centers for Medicare & Medicaid Services. [Accessed november 4, 2013];Details for Demonstration Project Name: Medicare medical Home Demonstration. http://www.cms.gov/Medicare/Demonstration-Projects/DemoProjectsEvalRpts/Medicare-Demonstrations-Items/CMS1199247.html.
- 43.Centers for Medicare & Medicaid Services. [Accessed November 4, 2013];Accountable Care Organizations (ACOs): General Information. http://innovation.cms.gov/initiatives/aco.