Abstract
Background
African Americans have the highest incidence and mortality from colorectal cancer (CRC). Despite guidelines to initiate screening with colonoscopy at age 45 in African Americans, CRC incidence remains high in this group.
Objective
To examine rates and predictors of CRC screening uptake as well as time-toscreening in a population of African Americans and non-African Americans in a healthcare system that minimizes variations in insurance and access.
Design
Retrospective cohort study.
Setting
Greater Los Angeles Veterans Affairs (VA) Healthcare System.
Patients
Random sample (N=357) of patients eligible for initial CRC screening.
Interventions
NA.
Main Outcome Measurements
Uptake of any screening method, uptake of colonoscopy in particular, predictors of screening, and time-to-screening in African Americans and non-African Americans.
Results
The overall screening rate by any method was 50%. Adjusted rates for any screening were lower among African Americans than non-African Americans (42%v.58%; OR=0.49,95%CI=0.31–0.77). Colonoscopic screening was also lower in African Americans (11%v.23%; adjusted OR=0.43,95%CI=0.24–0.77). In addition to race, homelessness, lower service connectedness, taking more prescription drugs, and not seeing a primary care provider within two years of screening eligibility predicted lower uptake of screening. Time-to-screening colonoscopy screening was longer in African Americans (adjusted HR=0.43,95%CI=0.25–0.75).
Limitations
The sample may not be generalizeable.
Conclusions
We found marked disparities in CRC screening despite similar access to care across races. Despite current guidelines aimed to increase screening in African Americans, participation in screening remained low and use of colonoscopy was infrequent.
Keywords: African American, colorectal cancer, screening, disparities, colonoscopy, healthcare utilization
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States [1]. Although colorectal cancer affects all racial and ethnic groups, African Americans carry an excessive burden of disease with the highest overall incidence, highest incidence of advanced stage at disease presentation, highest attributable mortality, and lowest survival rates after diagnosis when compared to any other ethnic or racial group [1, 2]. Further, although CRC incidence has decreased by 10% in Caucasians in the past 30 years, rates have remained stable in African Americans over the same period of time [3].
The specific causes for CRC outcome inequalities in African Americans are not fully characterized. Biologic susceptibility, a dietary proclivity to fats and red meats, increased smoking, social and economic disparities, and low utilization of screening methods have been implicated [2, 4, 5]. Particular attention has been paid to low adherence to screening guidelines among African Americans, and multiple studies demonstrate that African Americans are less likely to engage in CRC screening than non-African Americans [2, 6]. In 2009, in response to evidence supporting a younger age of presentation and a high prevalence of proximal tumors in African Americans, the American College of Gastroenterology (ACG) updated CRC screening guidelines, suggesting initiation of CRC screening at age 45 and use of colonoscopy as the preferred screening modality in African Americans [7].
The 2002 Institute of Medicine (IOM) report, Unequal Treatment: Confounding Racial and Ethnic Disparities in Health Care, attributed health care disparities to the interplay between patient characteristics, provider practices, and attributes of the health care system [8]. Patient-level characteristics are the demographic and health characteristics unique to an individual that may predict or act as barriers to screening. Provider-related factors include specific practices or preferences that may determine whether screening is recommended by a clinician. System-level attributes are the aspects of the health care system that affect a patient’s ability to obtain CRC screening.
The Veterans Affairs (VA) health system presents an ideal model to test whether patient and provider factors impact CRC screening after controlling for system-level factors. Because access inequalities are minimized in the VA, and given recent studies indicating fewer disparities in CRC treatment in VA settings, it is possible that CRC screening rates are equal between races in the VA population. However, the extent to which disparities in screening adherence currently exist in the VA population is unknown [9].
We aimed to determine rates of screening uptake and time to screening uptake in African American and non-African American Veterans in a large VA Healthcare System database. In addition, we sought to identify modifiable predictors of CRC screening in non- African American and African American Veterans using a conceptual framework accounting for a wide range of clinical and demographic characteristics.
METHODS
Study Population and Data Collection
This study was reviewed and approved by the Institutional Review Board. We sought patients seeking care in the VA Greater Los Angeles Healthcare System, an integrated network of 12 sites serving a racially- and ethnically-diverse population in Southern California. We used a random number generator to identify African Americans over age 45 years and non-African Americans over age 50 years. We then extracted data from the VA electronic medical records, the Computerized Patient Record System (CPRS). Included subjects were eligible for CRC screening between January 1996 and October 2012. Before January 2009, all subjects were considered screening eligible after a 50th birthday. Due to new screening recommendations for African Americans in the 2009 ACG CRC screening guidelines, we also included African Americans who turned 45 after 2009.
We excluded subjects with one or more of the following: (1) no VA CPRS chart notes within two years of his/her age of CRC screening eligibility; (2) a history of colon, rectal, or colorectal cancer diagnosed before his/her age of eligibility; (3) a colectomy performed before age of eligibility; (4) a recorded family history of colon, rectal, or colorectal cancer; (5) a history of ulcerative colitis or Crohn’s disease; or (6) CRC screening at any time before his/her age of screening eligibility (colonoscopy, flexible sigmoidoscopy, colonography, barium enema, Fecal Immunochemical Testing (FIT) or Fecal Occult Blood Test (FOBT)).
Outcome Variables
The primary outcome was uptake of any CRC screening procedure after the age of screening eligibility (age ≥50 for non-African Americans and African Americans before 2009 and age ≥45 for African Americans after 2009). Included screening methods were those listed in the 2009 ACG guidelines: colonoscopy, flexible sigmoidoscopy, colonography, barium enema imaging, FIT, or FOBT. If multiple screening methods were employed, we used the first screening test as the endpoint. Secondary outcomes were uptake of colonoscopic screening and time-to-screening, which we calculated by subtracting the date of CRC screening from the date of screening eligibility.
Predictor Variables
Before data collection, we created a conceptual model to delineate potential predictors and barriers to screening uptake. The conceptual framework was informed by the IOM disparities report and includes predisposing patient characteristics, provider practices, and system-level factors that predict or act as barriers to screening [8]. Subject demographic characteristics in the model included gender, race/ethnicity, zip code, level of service connectedness to the VA, history of combat experience, marital status, employment, and housing status at age of eligibility. We also extracted health information from the age of screening eligibility for each subject, including physiological and psychological comorbidities, number of primary care physician visits, substance abuse history (alcohol, tobacco, and illicit drugs), and use of prescription medications, psychiatric medications, narcotics, NSAIDs and aspirin. We computed a Charlson Comorbidity Index score for each subject, where higher scores reflect greater disease burden [10].
Specific information about subject socioeconomic status (SES) is not available in CPRS. As a proxy for SES, we used subject zip code and data on median income by zip code to compute a SES income variable for each subject. Data on median incomes were obtained from the American Community Survey [11]. Estimates are based on five years of data collection within each zip code and are subdivided by race.
Service connectedness is a system-level variable for patients treated at VA hospitals based on the degree to which a given injury or condition can be attributed to military service experiences. In our analysis, service connectedness was a three-level variable, where patients with no service connectedness (none) were compared to those with 10–50% service connectedness (low) and those with more than 50% service connectedness (high). In the VA system, individuals with high service connectedness do not bear financial responsibility for screening services, whereas those with low service connectedness bear some financial responsibility. In addition to service connectedness, we documented if each subject had medical insurance and/or care outside the VA healthcare network.
Statistical Analyses
Our objective was to measure the independent effect of African American race on screening uptake while controlling for relevant confounders. To ensure the sample size was large enough to detect significant differences between African Americans and non-African Americans, we performed a power calculation using a two-sided test with 80% power. Based on prior literature estimates of a 41% screening rate in African Americans and a 56% screening rate in non-African Americans, we determined that at least 174 individuals would be needed for each group [2].
For race, we used a dichotomous variable – African American versus all other racial/ethnic groups. We performed independent sample t-tests and chi-squared tests to examine differences in demographic variables between African American and non-African American subjects. To examine predictors of screening utilization, we conducted both univariate and multivariate logistic regression analyses for screening by any method or by colonoscopy. We included predictors in multivariate models if univariate odds ratios had p-values <0.05 and adjusted correlated predictors to reduce overlapping variance. We then utilized Cox proportional hazard regression analyses to examine the effect of each predictor variable on time-to-screening uptake. Further, we explored predictors of screening in the overall study sample and in the subgroup of African Americans using the same approach. To examine differences in time-to-screening between African American and non-African Americans, we performed Kaplan-Meier survival analyses with log-rank testing. We applied censoring when a subject died before screening or if a subject remained unscreened on October 15th, 2012, the end of the study. As less than 3% of the sample had missing data, we did not impute missing values.
We performed two sensitivity analyses: First, we compared time-to-screening for African Americans over age 45 to time-to-screening for non-African Americans over age 50 in order to assess for an independent effect of the African Americans between ages 45 and 50 considered non-adherent to screening guidelines when guidelines changed in 2009. Then we excluded the small subset of non-African American minorities (Hispanic, Asian, and other non- Caucasians) from the non-African American sample to compare screening uptake between African Africans and Caucasians. All analyses were conducted using SAS for Windows, version 9.3 (SAS Institute, Inc.; Cary, NC, USA).
RESULTS
Descriptive Characteristics and Screening Uptake
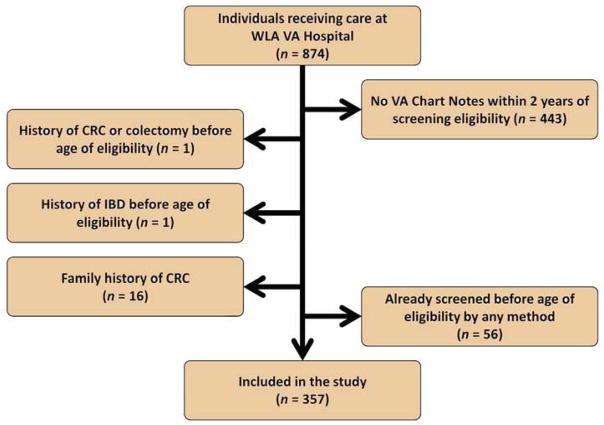
Figure 1 details the inclusion and exclusion criteria that yielded our final sample of 357 subjects. As shown in Table 1, African Americans were significantly different from non-African Americans in frequency of homelessness (p<0.01), median income (p<0.01), number of primary care visits within two years of age of screening eligibility (p<0.01), and number of prescriptions at age of eligibility for screening (p=0.03).
Figure 1.
Flowchart of Exclusion Criteria for CRC Disparities Study.
Table 1.
Baseline characteristics of subjects eligible for CRC screening by race.
| Total n = 357 |
African Americans n = 178 |
Non African Americans n = 179 |
p Value | |
|---|---|---|---|---|
| Male Gender, n (%) | 332 (93%) | 169 (95%) | 163 (91%) | 0.27 |
| Married, n (%) | 80/260 (31%) | 38/132 (29%) | 42/128 (33%) | 0.48 |
| Employed, n (%) | 126/276 (46%) | 67/147 (46%) | 59/129 (46%) | 0.98 |
| Homeless, n (%) | 82 (22%) | 55 (31%) | 27 (15%) | <0.01 |
| Median Income, Mean (SD) | 50990.06 (21603.78) | 41310.84 (21413.67) | 56537.22 (19727.52) | <0.01 |
| Combat Experience, n (%) | 21 (6%) | 9 (5%) | 12 (7%) | 0.54 |
| Service Connectedness, Mean Percentage (SD) | 22.75 (33.65) | 21.01 (31.77) | 24.47 (35.45) | 0.33 |
| PCP Visit within 2 Years of Age of Eligibility, n (%) | 309 (87%) | 144 (81%) | 165 (93%) | <0.01 |
| Subject receives health services from non-VA provider, n (%) | 48/242 (25%) | 22/129 (27%) | 26/113 (23%) | 0.25 |
| Number of Prescriptions at Age of Eligibility, Mean (SD) | 4.22 (4.05) | 4.70 (4.39) | 3.75 (3.63) | 0.03 |
| History of alcohol/drug abuse, n (%) | 41 (11%) | 20 (11%) | 21 (12%) | 0.88 |
| Charlson Comorbidity Index, Mean (SD) | 0.39 (1.09) | 0.40 (1.10) | 0.37 (1.08) | 0.83 |
Note: Means were compared by t
Test; Frequencies were compared using χ2 or Fisher’s Exact Test.
A total of 179 (50%) subjects received CRC screening by at least one method. Forty-two percent of African Americans were screened by any method, compared to 58% of non-African Americans. In adjusted logistic regression analysis, African Americans were 51% less likely to receive screening by any method when compared to non-African Americans (adjusted OR=0.49, 95%CI=0.31–0.77). Colonoscopy uptake also varied by race. Overall, 17% of VA subjects were screened by colonoscopy. Eleven percent of African Americans were screened by colonoscopy, compared to 23% of non-African American subjects (adjusted OR=0.43, 95%CI=0.24–0.77).
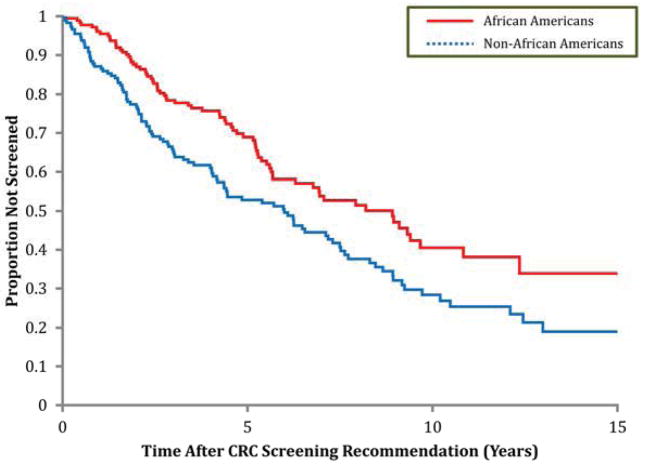
Figure 2 demonstrates that time to screening by any method was significantly longer in African Americans vs. non-African Americans (adjusted HR = 0.63; 95%CI = 0.46–0.85). When we restricted analyses to uptake of screening colonoscopy, time-to-screening in African Americans compared to non-African Americans was similarly longer (adjusted HR=0.43; 95%CI=0.25–0.75).
Figure 2.
Kaplan-Meier survival analysis for probability of CRC screening by any method in eligible subjects.
Predictors of CRC Screening Uptake
In addition to race, we identified other predictors of CRC screening status. Both unadjusted and adjusted odds ratios for key predictors of screening by any method are shown in Table 2. African American race (OR=0.49, 95%CI=0.31–0.77), homelessness (OR=0.43, 95%CI=0.25–0.77), and greater use of prescription drugs (OR=0.75, 95%CI=0.61–0.93) were independent predictors of lack of screening by any method. On the other hand, having a primary care visit within two years of screening eligibility (OR=4.86, 95%CI=2.10–11.26) and higher service connectedness (OR=1.33, 95%CI=1.01–1.77) were independent predictors of screening uptake by any method.
Table 2.
Univariate and multivariate global predictors of CRC screening by any method (n = 357).
| Predictor | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Race/Ethnicity (African-American versus all other groups) | 0.53 (0.35 – 0.80) | 0.49 (0.31 – 0.77) |
| Male Gender | 1.10 (0.49 – 2.46) | -- |
| Married | 1.30 (0.77 – 2.20) | -- |
| Employed | 1.47 (0.91 – 2.37) | -- |
| Homeless | 0.37 (0.22 – 0.62) | 0.43 (0.25 – 0.77) |
| Median Income | 1.49 (0.97 – 2.29) | -- |
| Combat Experience | 2.62 (0.99 – 6.92) | -- |
| Service Connectedness | 1.31 (1.02 – 1.69) | 1.33 (1.01 – 1.77) |
| PCP Visit within Two Years of Eligibility for Screening | 3.69 (1.81 – 7.54) | 4.86 (2.10 – 11.26) |
| Subject receives health services from non-VA provider | 1.42 (0.76 – 2.68) | -- |
| Number of Prescriptions at Age of Eligibility | 0.81 (0.67 – 0.98) | 0.75 (0.61 – 0.93) |
| History of Alcohol Abuse | 0.99 (0.42 – 2.36) | -- |
| History of Drug Abuse | 0.54 (0.23 – 1.25) | |
| Charlson Comorbidity Index Score | 0.89 (0.73 – 1.08) | -- |
Note: Non-significant univariate predictors were removed from multivariate analysis.
When examining predictors of CRC screening by colonoscopy, African American race (OR=0.43, 95%CI=0.24–0.77) and lower service connectedness (OR=1.46, 95%CI=1.06–2.02) were predictive of lower screening uptake in adjusted multivariate models. When we examined time-to-screening, we found that both race (HR=0.63, 95%CI=0.46–0.85) and lack of primary care visits within two years of screening eligibility (HR=3.26, 95%CI=1.75–6.07) were significant in multivariate analyses, predicting greater time-to-screening by any method.
Race-Specific Predictors of CRC Screening
After determining an association between race and screening uptake, we sought predictors of screening within the subset of African Americans. Not visiting a primary care provider within two years of screening eligibility (adjusted OR=3.09, 95% CI=1.25–7.64) and homelessness (adjusted OR=0.47, 95% CI=0.24–0.95) were associated with lower screening uptake by any method for African Americans. No variables predicted uptake of screening by colonoscopy in African Americans.
Sensitivity Analyses: ACG Guideline Changes and Other Minority Groups
To study the impact of the 2009 ACG CRC screening guideline changes, we performed a sensitivity analysis in which we compared screening uptake in African Americans vs. non-African Americans over the age of 50: uptake was 42.4% vs. 58.1%, respectively (p=0.003). Differences in uptake of colonoscopic screening also remained significant (11.1% vs. 22.9%; p=0.003). Notably, only 6 additional African Americans became eligible for screening when the 2009 guideline change was applied.
In order to assess for possible bias from the inclusion of non-African American minority groups, we also conducted a sensitivity analysis that excluded this subgroup from the dataset. When comparing African Americans to Whites, uptake of any screening was 42.1% vs. 58.1% (p=.005) whereas uptake of colonoscopic screening was 11.2% vs. 22.8% (p=.006).
DISCUSSION
We found that previously described disparities in uptake of CRC screening in African Americans were also evident in a sample of randomly selected patients in a large, urban VA healthcare network that minimizes inequalities to insurance and access to care. African Americans were 51% less likely to uptake CRC screening by any method. Further, despite ACG recommendations for screening by colonoscopy in African Americans, uptake of screening colonoscopy was 46% lower in African American Veterans than in non-African American Veterans in our adjusted models. These screening uptake patterns persisted over time in survival analysis.
When adjusting for race, homelessness, low service connectedness, greater use of prescription drugs, and lack of a primary care visit within two years of CRC screening eligibility independently predicted low screening. Notably, individuals with a primary care visit within two years of CRC screening eligibility were nearly four times more likely to have had at least one CRC screening test. For screening by colonoscopy, race and service connectedness again emerged as significant predictors. Further, when we examined the subset of African American Veterans, we found that a primary care visit within two years of screening eligibility and having a place of residence were associated with an increased likelihood of colonoscopy.
The 16% difference in overall screening uptake between African Americans and non- African Americans is within the range of the 6% to 18% discrepancy seen in the non-VA published literature [2, 12, 13]. Our finding of an 11% uptake of colonoscopy among African Americans is also consistent with prior research [14, 15]. Uptake of colonoscopy among non- African Americans was also low in our cohort, which may represent some preference for non-colonoscopic CRC screening methods among VA patients and providers or/and VA system challenges, including long wait times for colonoscopy. Although racial differences in CRC screening resolve after adjusting for SES in some studies, we did not identify an independent role of SES when adjusting for zip-code-based income [16]; this may reflect smaller variations in SES status among VA patients versus other healthcare settings, or may reflect partial omitted variable bias in the use of zip-code-based income as a proxy for SES [17, 18].
Use of primary care services as a predictor of CRC screening has been demonstrated in both African Americans and non African Americans in the literature [18, 19]. Similarly, there are known associations between having a usual place of medical care and uptake of prostate cancer screening in African American males as well as with uptake of breast and cervical cancer screening in African American women [20, 21]. The common relationship across various screening programs emphasizes the role that regular use of medical care plays in uptake of preventative services [22]. Perhaps more striking, our study demonstrates that even in a healthcare network where all Veterans have similar access to services, those subjects who see a primary care provider near the age of CRC screening eligibility are most likely to be enrolled in a screening program.
Our study has several strengths. First, we employed a highly granular dataset in order to examine the relationship between race and screening uptake with greater precision than previously achieve. By abstracting detailed, patient- and provider-level information from VA records, we minimized the effect of system variation in utilization of health care services while collecting granular data to inform a conceptual model. The VA provides medical services to all Veterans, regardless of race, personal income, ability to purchase insurance, education, occupation, and health status. In addition, individuals within the network have access to identical system-level benefits. By investigating the effect of patient, provider, and system-related characteristics on uptake of CRC screening in this population, we attempted to minimize confounding by issues of access to insurance and socioeconomic status that complicate studies of health care inequalities. Second, the study population was selected to include only those subjects with a documented interaction with the healthcare network near the time of CRC screening eligibility. We sought to limit the analysis to individuals actually interacting with the system to minimize the likelihood that VA patients with no mechanism for preventative services or with established care outside the VA system were included in the study. Our sensitivity analyses suggest that even when we assume low-uptake of the 2009 ACG guidelines for earlier and more aggressive screening in African Americans, CRC screening rates within our VA network remain underutilized in African Americans. In addition, our sensitivity analysis suggests a relationship between African American race and screening uptake, even when limited the study sample to African Americans over age 50. Lastly, incorporation of survival analysis for time-to-screening application of the 2009 ACG guidelines, and focus on a system that attempts to minimize access to care disparities are strengths of our analysis and novel contributions to the literature.
Although use of a VA patient population was beneficial for further understanding of the factors that predict CRC screening, the population also presents limitations. First, it is difficult to extrapolate our findings outside of the Greater Los Angeles VA. In other healthcare networks and in more diverse patient populations, there are system barriers to screening that are not encountered in the VA health care system – this is both a weakness and a strength that provides rationale for investigating this question in the VA. Lack of insurance coverage, lack of a usual source of healthcare, poor access to gastroenterologists, and difficulty navigating the health care system prevent some African Americans from getting screened in traditional settings [23–25]. Further, given that the VA population is predominately male, we were unable to investigate the role of gender in adherence to CRC screening uptake. Previous literature suggests that CRC screening is higher in females; however, the small number of females in our study population precluded this analysis[2].
The results of our study may inform health care providers, patients, and designers of health policy. Although modifications in CRC screening guidelines aim to assure that primary care providers and gastroenterologists employ best screening practices, disparities in health will persist unless we understand why guidelines are underused in some populations. Poor adherence to guidelines may be due to inadequate provider knowledge, failure of providers to recommend screening to African Americans, ineffective communication between providers and patients, or patient refusal of recommendations. As evidence supports guideline knowledge deficits among primary care providers and gastroenterologists, at least some of the disparities may be reversed with formal education about guidelines and quality assessment of adherence to guidelines among providers [26]. Empowering African American patients with information about their health risks and the benefits of screening is also imperative to improve screening uptake.
Efforts to improve provider and patient knowledge are fundamental now as the health care system in the United States evolves. With the advent of the Affordable Care Act and universal insurance, all Americans will have access to regular and continuous care. Our findings support the conclusions of other researchers that individuals with regular primary care demonstrate increased uptake of preventative services. Access without health knowledge does not guarantee patient or provider adherence, however. The incidence and mortality from CRC in African Americans can only improve if we identify pathways to increase awareness about screening benefits and underuse among both providers and patients.
In conclusion, our analysis suggests that disparities in CRC screening between African Americans and non-African Americans exist in a large, urban VA healthcare network. These inequities in health exist in a patient population that has the same source of health care and despite 2009 guidelines aimed to increase screening efforts in African Americans, confirming that there is still a need for focused and targeted efforts to address barriers to screening and screening colonoscopy in African Americans. Our findings also highlight that established primary care at the time of screening eligibility plays a significant role in screening uptake. As insurance coverage is extended to all Americans, it will be important to emphasize regular use of health care services in middle-aged adults and knowledge about the benefits of screening in order to increase CRC screening in African Americans and overall.
TAKE HOME MESSAGE.
African Americans have the highest incidence of and mortality from colorectal cancer (CRC). In a patient population where variations in access to insurance and a usual place of care are minimized, we found marked disparities in CRC screening among African Americans compared with non- African Americans. These disparities exist despite American College of Gastroenterology guidelines recommending initiation of CRC screening at age 45 in African Americans with colonoscopy as the preferred method.
The findings confirm that there is still a need for targeted efforts to identify and address barriers to CRC screening and screening colonoscopy in African Americans.
Acknowledgments
Grant Support: This research was supported by the NIH Training grant (T32DK07180— 34) for Dr. May.
Abbreviations
- CRC
Colorectal Cancer
- VA
Veterans Affairs
- ACA
Affordable Care Act
Footnotes
Previous Research Presentation: Presented as an abstract at Digestive Disease Week 2013, Orlando, Florida
Disclaimer: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veteran Affairs.
Disclosures: None.
Writing Assistance: None.
Author Contributions:
Folasade May: study concept and design, acquisition of data, conceptual model, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript.
Erica Bromley: acquisition of data, analysis and interpretation of data, drafting of the manuscript.
Mark Reid: statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript.
Michael Baek: acquisition of data, analysis and interpretation of data.
Jessica Yoon: acquisition of data.
Erica Cohen: acquisition of data.
Aaron Lee: acquisition of data.
Martijn van Oijen: study concept and design, conceptual model, analysis and interpretation of data, critical revision of the manuscript.
Brennan Spiegel: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, overall study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Colorectal cancer facts & figures 2011–2013. Atlanta, GA: [Google Scholar]
- 2.Agrawal S, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100(3):515–23. doi: 10.1111/j.1572-0241.2005.41829.x. discussion 514. [DOI] [PubMed] [Google Scholar]
- 3.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15(30):3734–43. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baquet CR, Commiskey P. Colorectal cancer epidemiology in minorities: a review. J Assoc Acad Minor Phys. 1999;10(3):51–8. [PubMed] [Google Scholar]
- 5.Butler LM, et al. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. 2003;157(5):434–45. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 6.Breen N, et al. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–13. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 8.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care 2003. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 9.Sabounchi S, Keihanian S, Anand BS. Impact of race on colorectal cancer. Clin Colorectal Cancer. 2012;11(1):66–70. doi: 10.1016/j.clcc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Charlson M, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Bureau, U.S.C. 2011 American Community Survey 5-Year Estimates. 2011. S1903 - Median Income in the past 12 months (in 2011 Inflation-Adjusted Dollars) [Data] [Google Scholar]
- 12.Ananthakrishnan AN, et al. Disparities in colon cancer screening in the Medicare population. Arch Intern Med. 2007;167(3):258–64. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 13.United States Department of Health Services. Percentage of Adults Who Receive Recommended Colorectal Cancer Screening by Race. 2012. [Google Scholar]
- 14.Shih YC, Zhao L, Elting LS. Does Medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff (Millwood) 2006;25(4):1153–62. doi: 10.1377/hlthaff.25.4.1153. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100(2):418–24. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley AS, et al. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005;165(18):2129–35. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 17.Doubeni CA, et al. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–5. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeff LC, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 19.Zapka JG, et al. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23(1):28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 20.Selvin E, Brett KM. Breast and cervical cancer screening: sociodemographic predictors among White, Black, and Hispanic women. Am J Public Health. 2003;93(4):618–23. doi: 10.2105/ajph.93.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross LE, Taylor YJ, Howard DL. Trends in prostate-specific antigen test use, 2000–2005. Public Health Rep. 2011;126(2):228–39. doi: 10.1177/003335491112600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronan TA, et al. Predictors of mammography screening among ethnically diverse low-income women. J Womens Health (Larchmt) 2008;17(4):527–37. doi: 10.1089/jwh.2007.0331. [DOI] [PubMed] [Google Scholar]
- 23.James AS, Daley CM, Greiner KA. Knowledge and attitudes about colon cancer screening among African Americans. Am J Health Behav. 2011;35(4):393–401. doi: 10.5993/ajhb.35.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt CL, et al. Use of focus group data to develop recommendations for demographically segmented colorectal cancer educational strategies. Health Educ Res. 2009;24(5):876–89. doi: 10.1093/her/cyp024. [DOI] [PubMed] [Google Scholar]
- 25.Patel K, et al. Factors influencing colorectal cancer screening in low-income African Americans in Tennessee. J Community Health. 2012;37(3):673–9. doi: 10.1007/s10900-011-9498-8. [DOI] [PubMed] [Google Scholar]
- 26.White PM, et al. Colorectal cancer screening of high-risk populations: A national survey of physicians. BMC Res Notes. 2012;5:64. doi: 10.1186/1756-0500-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]