Abstract
Background
Epidemiological and genetic studies suggest a role for enteric flora in the pathogenesis of Crohn's disease (CD). CD-associated Escherichia coli (CDEC) are characterized by their ability to invade epithelial cells, and survive and induce high concentration of TNF-α from infected macrophages. However, the molecular mechanisms of CDEC survival in infected macrophages are not completely understood.
Methods
Intracellular survival of CDEC strain LF82 isolated from inflamed ileum tissue, 13I isolated from inflamed colonic tissue, and control E. coli strains were tested in the murine macrophage cell line, J774A.1 by Gentamicin protection assay. Modulation of intracellular cell signaling pathways by the E. coli strains were assessed by western blot analysis and confocal microscopy.
Results
13I demonstrated increased survival in macrophages with 2.6-fold higher intracellular bacteria compared to LF82, yet both strains induced comparable levels of TNF-α. LF82 and 13I differentially modulated key Mitogen-activated protein kinase (MAPK) pathways during the acute phase of infection; LF82 activated all three MAPK pathways, whereas 13I activated ERK1/2 pathway but not p38 and JNK pathways. Both 13I and LF82 suppressed nuclear translocation of NFκB compared to non-invasive E. coli strains during the acute phase of infection. However, unlike non-invasive E. coli strains, 13I and LF82 infection resulted in chronic activation of NFκB during the later phase of infection.
Conclusions
Our results showed that CDEC survive in macrophages by initially suppressing NFκB activation. However, persistence of bacterial within macrophages induces chronic activation of NFκB, which correlates with increased TNF-α secretion from infected macrophages.
Keywords: Crohn’s disease, CD-associated Escherichia coli, Macrophages, NFκB
INTRODUCTION
Crohn’s disease (CD) is a form of inflammatory bowel disease characterized by patchy, transmural, granulomatous inflammation commonly involving the terminal ileum1. As a result, patients with CD suffer from abdominal cramps and pain, diarrhea, tenesmus, and weight loss. The etiology of CD is multifactorial, and includes environmental and bacterial triggers in a genetically susceptible host. Genome-wide association studies have suggested that mutations in genes involved in intracellular bacterial detection and clearance predispose to CD2, 3. Furthermore, presence of intramucosal bacteria in CD lesions, and the impaired ability of CD patients to clear intracellular bacteria due to defective innate immune responses implicate bacteria in the initiation and pathogenesis of CD4–6. These studies suggest that defective processing of intracellular bacteria plays an important role in CD pathogenesis7–9.
The concept that Escherichia coli are involved in the pathogenesis of CD is supported by multiple studies demonstrating intramucosal or mucosa-associated E. coli in CD patients7. This hypothesis is further strengthened by culture-independent metagenomic analyses of gut microbiota showing increased abundance of E. coli in IBD patients10–12. One of the best characterized CD-associated E. coli (CDEC) strain, LF82 can adhere to and invade intestinal epithelial cells, infect and replicate in macrophages, and induce high amounts of TNF-α from infected macrophages13. Further studies showed that LF82 can also invade Peyer’s patches, translocate across M cells, and infect and replicate in neutrophils13–15.
CDEC strain LF82 survive and replicate in the acidic environment of active phagolysosomes in macrophages without causing cell death and surprisingly, induce secretion of TNF-α9, 16, 17. In contrast to the survival strategy of LF82 in macrophages, LF82 blocks autophagosome maturation and triggers death in neutrophils through NETosis15. Survival of LF82 in macrophages and neutrophils, first line of innate immune defense against invading bacteria, suggest that LF82 has the ability to modulate pro-inflammatory signaling pathways to avoid clearance by the professional phagocytes. The molecular mechanism of LF82 survival in macrophages and neutrophils are not fully elucidated, however, in human epithelial cells, LF82 is shown to suppress inflammatory responses by preventing IFN-γ mediated STAT1 activation18. Another CDEC strain, 13I, isolated in our laboratory, suppresses IL-8 expression in infected epithelial cells through yet an unknown mechanism19. Similarly to LF82, 13I survives in macrophages and induces high concentrations of TNF-α19. Given the inherent ability of CDEC strains to survive in professional phagocytes, it will be of paramount interest to study the molecular mechanisms of LF82 and 13I mediated effects on pro-inflammatory signaling in professional phagocytes of the innate immune system.
Therefore, to understand the molecular mechanism of CDEC survival in macrophages, we investigated the ability of CDEC strains LF82 and 13I to modulate cell signaling pathways in a previously established murine macrophage cell model16, 19. Here we show that the CDEC strains initially suppress NFκB activation, but induce chronic activation of NFκB in the later phase of infection, which correlates with increased TNF-α secretion from infected macrophages. We speculate that these data have implications for the pathophysiology of CD, allowing invading bacteria to gain a foothold in the host to induce chronic inflammation in the susceptible host.
MATERIALS AND METHODES
Bacterial strains
The CD-associated E. coli strain 13I was isolated from biopsy samples taken from macroscopically inflamed colonic tissue of a CD patient. The UC associated E. coli strain 30A was isolated from normal appearing UC tissue. The isolation of E. coli from the biopsy tissue was described previously19. The CD-associated E. coli strain LF82, isolated from inflamed ileum tissue, was a gift from Arlette Darfeuille–Michaud, Institut Universitaire de Technologie, Genie Biologique, Aubiere, France. The non-pathogenic E. coli strain EFC1, isolated from a healthy female volunteer was a gift from M.S. Donnenberg, University of Maryland, Baltimore, MD.
Cell Culture
The murine macrophage cell line, J774A.1 and the human epithelial colorectal adenocarcinoma cell line, Caco-2 (ATCC, Manassas, VA) were maintained at 37°C in a humidified 5% CO2 atmosphere in DMEM (Invitrogen, Carlsbad, CA) supplemented with 50 µg/ml Gentamicin (Invitrogen) and 10% FCS (Gemini, Calabasas, CA). Macrophages and Caco-2 cells were used between passages 4–12 and 21–30, respectively.
Bacterial Invasion Assay
Bacterial invasion of macrophages and epithelial cells was measured as described previously19. The macrophages and Caco-2 cells were infected with overnight cultures of aerobically grown bacteria at a multiplicity of infection (MOI) of 10 bacteria per macrophage or Caco2 cell. After 3 h incubation at 37°C, infected macrophages and Caco-2 cells were washed three times in PBS and fresh DMEM supplemented with 100 µg/ml Gentamicin was added to kill extracellular bacteria. Cells were incubated for an additional hour, washed three times with PBS, lysed in 1% Triton-X-100/PBS and serial dilutions were plated on LB plates. The initial inoculum (I/O) was determined by plating serial dilutions of bacterial cultures used for infection on LB agar plates and counting the number of colonies the next day. Invasion was considered significant if a minimum of 1% of I/O could be recovered from the infected cells. Data are reported as mean ± SD.
Cytokine Analysis
For cytokine analysis, J774A.1 macrophages were cultured on a 96 well plate and infected in quadruplicate at MOI 10:1 of an overnight culture of different E. coli strains for 3 h. Infected macrophages were washed three times with PBS, and cultured overnight in DMEM supplemented with 50 µg/ml Gentamicin and 10% FCS. Culture supernatants were harvested after 24 h and were analyzed by ELISA (eBioscience, San Diego, CA) in quadruplicate for TNF-α, IL-6, and IL-10 according to manufacturer’s specifications. Data were presented as mean ± SD per 1 × 105 cells.
Immunofluorescence Confocal Microscopy
J774A.1 macrophages cultured on coverslips were infected at MOI 10:1 for 30 min and 1 h, and cells were fixed with 3.7% paraformaldehyde in PBS, permeabilized with 0.2% Triton X100 (Sigma), and blocked in 3% bovine serum albumin in PBS. Cells were probed with NFκB p65 (Santa Cruz, CA) at 1:1000 dilution and Alexa Fluor 546 conjugated secondary goat antibody diluted at 1:1000 was used for detection. F-actin was labeled with phalloidin conjugated Alexa Fluor 488 (1:1000; Invitrogen) and nucleus was labeled with Topro-3 (Life Technologies, Grand Island, NY). Stained cells were mounted in Prolong Gold (Invitrogen) and visualized with an Axioskope 2 plus scope (Zeiss, Jena, Germany). For the 6 h infection experiments, macrophages cultured on coverslips were infected at MOI 10:1 for 3 h. Infected macrophages were washed three times with PBS and treated with 100 µg/mL Gentamicin for one hour to kill extracellular bacteria. Macrophages were then washed three times with PBS and cultured for an additional 2 h in DMEM supplemented with 50 µg/mL Gentamicin and 10% FCS.
Western blot analysis and densitometry
For western blot analysis J774A.1 macrophages were infected with various E. coli strains as described above. After infection, total protein extract were subjected to western blots analysis. Blots were quantified by densitometry using Image J software20. Experiments were replicated three times, and representative blots are shown.
Statistical Analysis
All data were analyzed by Student’s t test with the significance level set at 0.05.
Ethical Considerations
No human or animal subjects were used for this study.
RESULTS
CD-associated Escherichia coli invade and replicate in macrophages and epithelial cells without inducing apoptosis
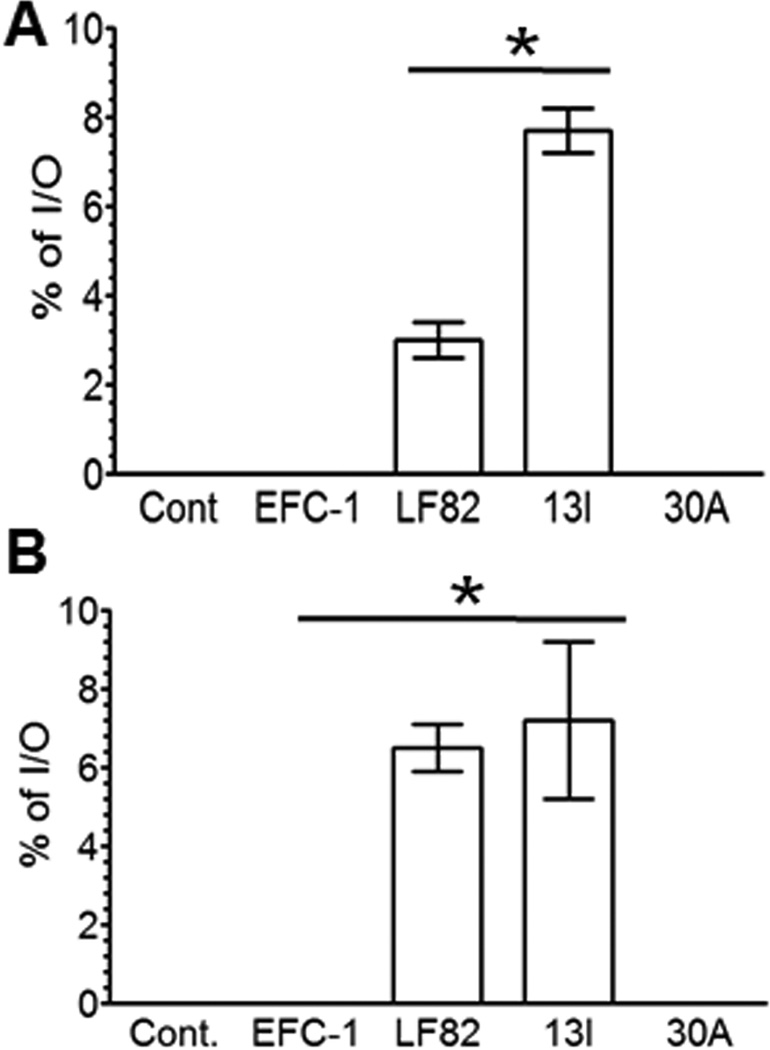
We have previously demonstrated that 13I induces high concentrations of TNF-α in infected macrophage cultures19. Since the ability of CDEC to invade macrophages correlates positively with TNF-α secretion9, we sought to determine if 13I invades macrophages by Gentamicin protection assay. Invasion of macrophage cultures by 13I was determined in comparison to CDEC reference strain LF82, non-pathogenic E coli isolate EFC-1, and UC-associated E coli strain 30A. As shown in Fig. 1A, at three hours post-infection, significantly higher percentage of initial inoculum (I/O) was recovered from the 13I infected macrophages (8% ± 0.5; mean ± SD) compared to LF82 infected macrophages (2.5% ± 0.4; p>0.05). In contrast, no intracellular bacteria were isolated from macrophages infected with control strains EFC-1 and 30A.
Fig. 1. Crohn's disease-associated Escherichia coli (CDEC) strain, 13I invades and replicates in macrophages and epithelial cells.
(A) J774.A1 macrophages and (B) Caco2 cells were infected for 3 hrs with CD-associated E. coli strains LF82 and 13I, and intracellular CFU was measured by Gentamicin protection assay, and expressed as percentage of initial inoculum (I/O). Non-pathogenic E coli strain, EFC-1 and UC-associated E coli strain, 30A were used as controls. Data are mean ± S.D. Statistical significance was determined by student's t test.
In parallel experiments, we determined if 13I invades epithelial cells. For this analysis we used a model intestinal epithelial cell line, Caco2. Infection of differentiated Caco-2 epithelial cells with 13I and LF82 resulted in the recovery of 7% (± 2) and 6% (± 0.6) of I/O, respectively, whereas no intracellular EFC-1 and 30A were recovered from infected Caco-2 cells (Fig. 1B). Taken together, these results show that CDEC strains 13I and LF82 effectively invade macrophages and epithelial cells in vitro.
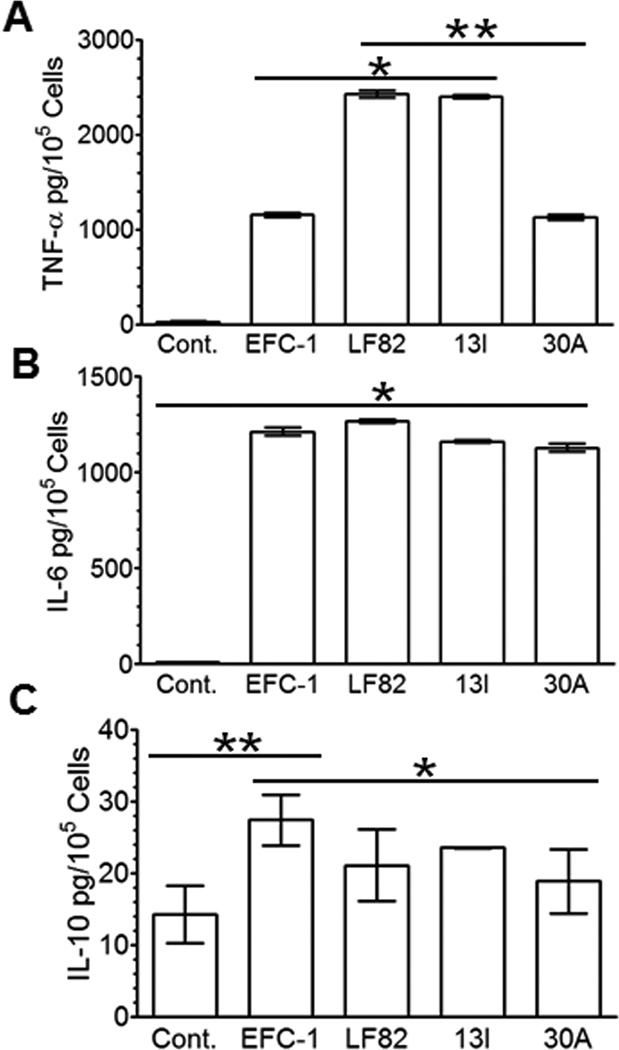
CD associated E. coli induce high concentrations of TNF-α and IL-6 in macrophage cultures
We and others have previously reported that CD associated E. coli regulate cytokine expression; specifically increase concentration of TNF-α in infected macrophage cultures9, 19. TNF-α is central to the pathogenesis of CD and neutralization with anti-TNF-α antibodies has been successfully used to treat active disease21–24. Our goal was to expand on previous observations and better define key cytokine expression in response to infection with CDEC. In agreement with our previous results19, both LF82 and 13I induced a significant 2.5-fold increase in TNF-α secretion from infected macrophages compared to non-pathogenic EFC-1 and 30A isolated from UC patient (Fig. 2A). In contrast to TNF-α expression, IL-6 concentrations were increased above background by EFC-1, LF82, 13I, and 30A, which were not significantly different among all four strains tested (Fig. 2B). Next, we sought to determine the effect of CDEC on anti-inflammatory cytokine IL-10. Interestingly, commensal strain EFC-1 induced significantly higher concentrations of IL-10 in macrophage cultures compared to LF82, 13I, and UC strain 30A (Fig. 2C). These findings indicate that CD-associated E. coli induce an imbalance of pro- and anti-inflammatory cytokine expression in infected macrophage cultures.
Fig. 2. Crohn's disease-associated Escherichia coli (CDEC) infected macrophages secrete high level of TNFα.
Cytokines secreted by J774.A1 macrophages infected with E. coli strains were measured by ELISA at 24 hrs post infection. (A) TNFα (B) IL-6 (C) IL-10. Data are mean ± S.D. Statistical significance was determined by student's t test.
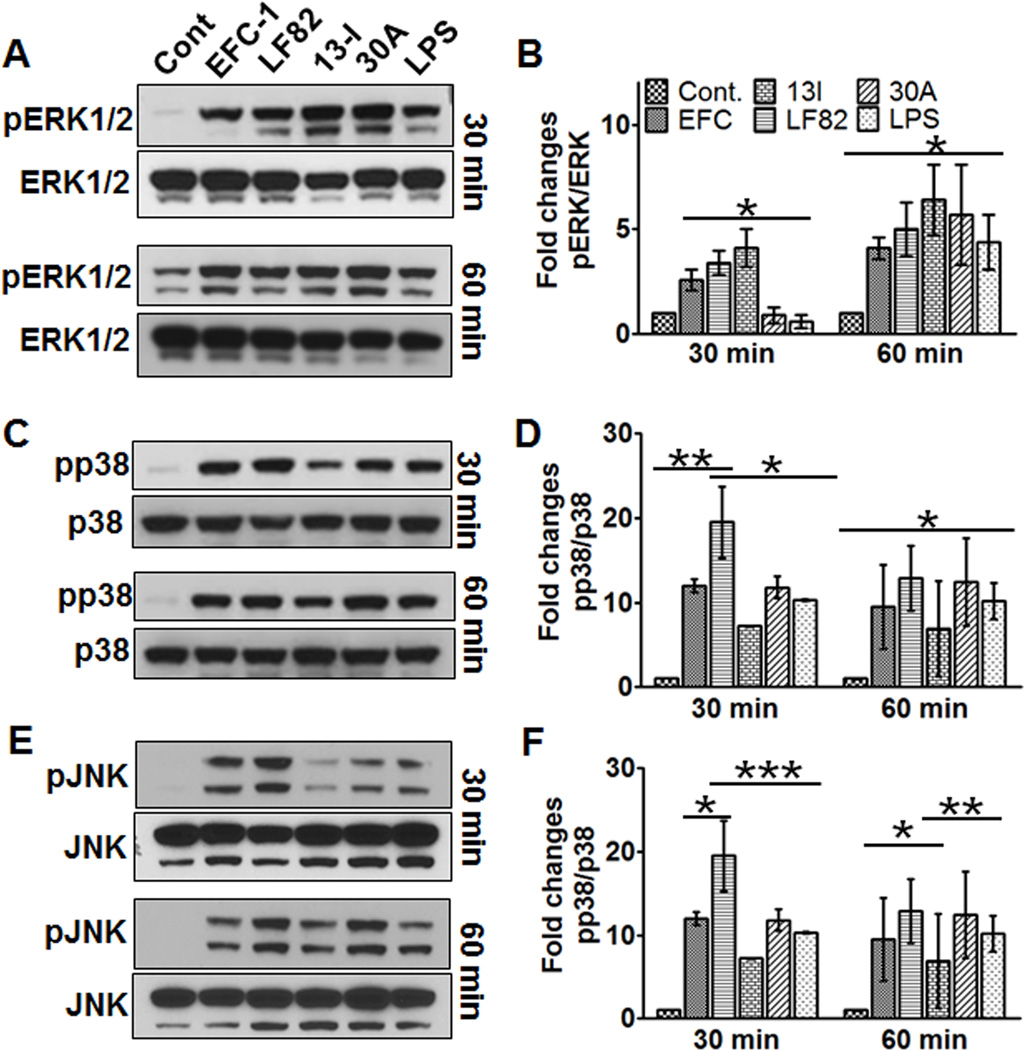
13I activates ERK1/2, but not p38 and JNK pathways
Mitogen activated protein kinases (MAPK) mediate pro-inflammatory signaling in response to bacterial effector proteins to aid in cellular survival25. To investigate whether CD strain 13I modulates the MAPK pathway, activation of Extracellular Signal-Regulated protein kinases 1 and 2 (ERK1/2), p38 MAPK (p38), and c-Jun NH(2)-terminal kinase (JNK) were determined during the early phase of infection. Infection of macrophage cultures with all E. coli strains resulted in a significant increase in ERK1/2 phosphorylation at 30 min and at 60 min post infection (Fig. 3A/B). Highest level of ERK1/2 activation was observed for 13I infected macrophages at 30 min post infection; a significant 2-fold increase in ERK1/2 phosphorylation compared to non-pathogenic E. coli strain EFC-1 and a significant 4-fold increase compared to UC strain 30A and LPS. Interestingly, level of ERK1/2 phosphorylation in LF82 infected macrophages was not significantly different from EFC-1 infected cells. By 60 min post infection, ERK1/2 activation increased in all macrophage cultures regardless of E. coli strain.
Fig. 3. Crohn's disease-associated Escherichia coli (CDEC) strain, 13I activates ERK1/2 but not p38 and JNK pathways.
(A, C, E) Representative western blots of cell lysates from J774.A1 macrophages infected with E. coli strains for 30 min or 1 hr and (B, D, F) densitometric analyses of the western blots of three independent experiments. Data are mean ± S.D. Statistical significance was determined by student's t test.
Next, we sought to determine activation status of MAPK p38 in response to infection of macrophage cultures with EFC-1, LF82, 13I, and 30A (Fig. 3 C/D). In comparison to control cultures, phosphorylation of p38 was significantly increased in all E. coli strains and LPS at both, 30 min and 60 min post infection. LF82 infection resulted in significant 2-fold increase in p38 activation compared to 13I, 30A, and LPS at 30 min, but no significant differences in p38 phosphorylation were observed at 60 min post infection. Although 13I infection resulted in significant 7-fold increase in p38 activation compared to negative control, activation of p38 in 13I infected macrophages was significantly lower than EFC-1, LF82, and 30A at both time points investigated.
As for JNK, all E. coli strains tested, in addition to LPS, increased phosphorylation of JNK compared to negative control at 30 min and 60 min post infection (Fig.3 E/F). At 30 min post infection, both EFC-1 (15-fold) and LF82 (25-fold) infection of macrophage cultures resulted in a significant increase in JNK phosphorylation in comparison to 13I (4-fold) and 30A (6-fold). Interestingly, JNK activation in 13I infected macrophages was even lower than LPS (6-fold) treatment. At 60 min post infection, JNK phosphorylation increased further in 30A (23-fold) infected and LPS (19-fold) treated macrophages. Lack of p38 and JNK activation in 13I infected macrophages suggest that 13I may regulate cytokine expression through activation of ERK1/2. In contrast, LF82 elicits a stimulatory effect on MAPK’s activation predominantly through p38 and JNK.
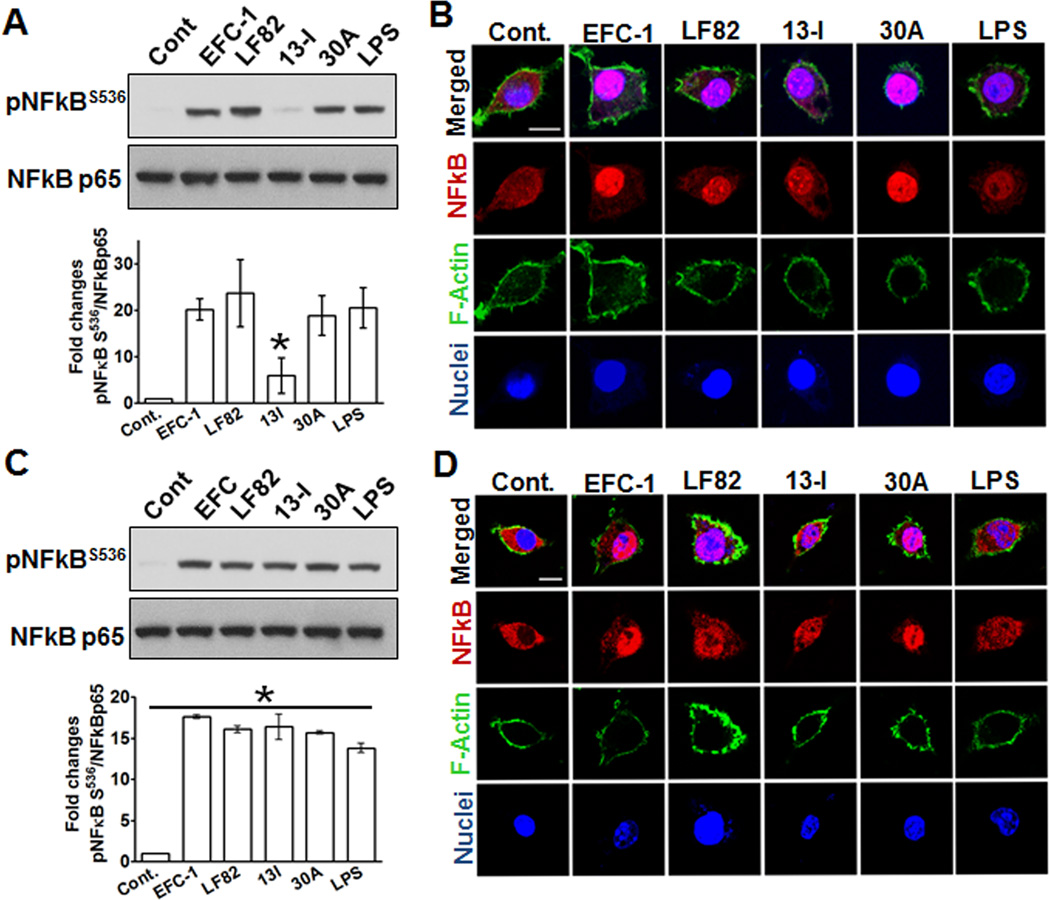
13I suppresses activation and nuclear translocation of NFκB p65 during the initial phase of infection
To determine how CD-associated strain 13I induces increased concentrations of TNF-α in macrophage culture supernatants, but not apoptosis (data not shown), we examined activation of NFκB; a master regulator of transcriptional program governing proliferation and inflammation25. We used an antibody against phosphorylated serine536 (S536) to measure activation of NFκB p65 by immunoblotting. As seen in Fig 4A, at 30 min post infection, with the exception of 13I (5-fold), E. coli strains EFC-1(21-fold), LF82 (28-fold), and 30A (19-fold) significantly increased phosphorylation of NFκB p65 at serine 536. To further visualize the effect of infection with Gram negative bacteria on macrophage cultures, cells were stained with anti-NFκB p65 at 30 min post infection. As shown in Fig 4B, infection with both, EFC-1 and 30A, caused increased translocation of NFκB p65 into the nucleus compared to negative control and LPS treated cells. In contrast, LF82 and 13I infection resulted in a moderate increase in NFκB p65 translocation. However, the lack of NFκB S536 phosphorylation in 13I and LF82 infected macrophages was transient and at 60 min post infection, none of the E. coli infected or LPS treated macrophages showed differences in NFκB S536 phosphorylation. Interestingly, nuclear translocation of NFκB p65 was still higher in EFC-1 and 30A infected macrophages compared to cells infected with LF82 and 13I. The decreased nuclear translocation of NFκB p65 in LF82 and 13I infected macrophages compared to EFC-1 and 30A at 30 min suggests either a lack of activation or active suppression of NFκB p65.
Fig. 4. Crohn's disease-associated Escherichia coli (CDEC) suppress activation and nuclear translocation of NFκB during the initial phase of infection.
(A, C) J774.A1 macrophages were infected with E. coli strains for (A) 30 min or (C) 1 hr and cell lysates were analyzed by western blotting. The western blots are representative of three independent experiments and the histograms represent densitometric analyses of western blots from two independent experiments. (B, D) Macrophages grown on coverslips were infected with E. coli strains for (B) 30 min or (D) 1 hr and were subjected to confocal imaging. Scale 10 µM.
CD associated E. coli strains persistently activate NFκB p65 during the later phase of infection
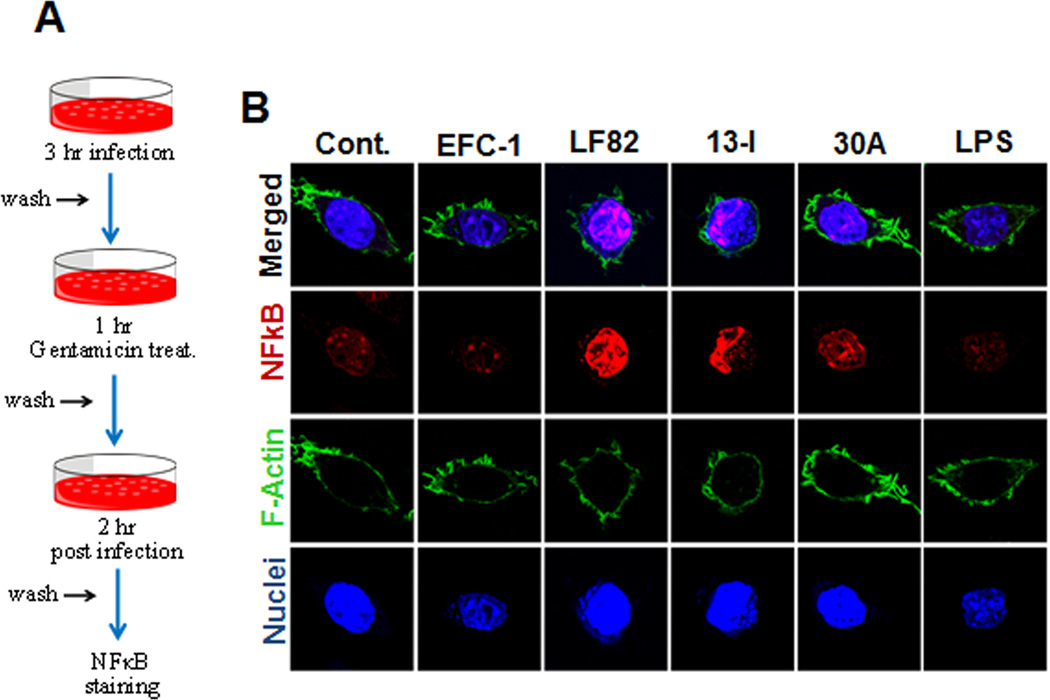
To further assess whether the lack of nuclear translocation of NFκB p65 in LF82 and 13I infected macrophages is sustained during the later phase of infection, nuclear translocation of NFκB was determined at 6 hrs post infection. In the absence of extracellular bacteria, at 6 hrs post infection, nuclear translocation of NFκB p65 was terminated in EFC-1 and 30A infected macrophages. Surprisingly, nuclear translocation of NFκB was still apparent in CD strains LF82 and 13I infected cells suggesting that ongoing intracellular replication of LF82 and 13I, and/or persistent action of bacterial effector proteins results in persistent activation of NFκB in the infected macrophages.
DISCUSSION
Escherichia coli strains associated with CD are characterized by their ability to invade immune and non-immune cells and induce high concentrations of TNF-α9, 17, 19. We recently reported a CDEC strain, 13I, that exhibits the characteristics of CDEC reference strain LF82 in its ability to adhere and infect epithelial cells and macrophages, and to induce high amounts of TNF-α from infected macrophages19. Further analysis of the strain 13I showed that 13I is significantly more invasive than reference strain LF82, but nevertheless induces similar level of TNF-α from infected macrophages. Here, we used a previously validated mouse macrophage cell line and the E. coli strains LF82 and 13I to show that these strains differentially modulate MAPK pathways but similarly suppress NFκB pathway during the acute phage of infection16, 19. However, persistence of CD-associated E. coli strains in macrophages induces chronic activation of NFκB, which may contribute to survival of the infected macrophages.
Recognition of bacteria or bacterial products by the pattern recognition receptors leads to activation of MAPK pathways that regulate both pro-survival and pro-inflammatory response pathways25. The differential modulation of MAPK pathways by LF82 and 13I points to the diversity in the pathogenicity of CDEC strains. It is also worth noting that LF82 was isolated from inflamed ileum of a CD patient whereas 13I was isolated from inflamed colonic tissue of a CD patient. The anatomical location of these two strains at the time of isolation might explain the differences in their ability to modulate MAPK pathways and is due to aberrant host mucosal recognition of bacteria26, tissue tropism27, or local dysbiosis12. During the early phase of infection, while LF82 activated all three MAPK pathways, 13I infection activated ERK1/2 pathway but not p38 and JNK pathways. Given the role of ERK1/2 as a pro-survival pathway that contributes to the regulation of cell proliferation and differentiation28, activation of ERK1/2 pathway by the CDEC strains fits well with the lack of apoptosis in the LF82 and 13I infected macrophages. Unlike ERK1/2 pathway, the p38 and JNK pathways regulate apoptotic cell death, and therefore the lack of p38 and JNK activation during the acute phase of infection may result in increased survival of 13I in macrophages29, 30. Furthermore, p38 MAPK pathway negatively regulates the fusion of phagosomes with lysosomes, and Mycobacterium tuberculosis exploits this pathway to inhibit fusion of phagosomes with lysosomes to ensure survival of bacillus in the phagosomes31. In contrast to M. tuberculosis, AIEC reference strain LF82 survives in phagolysosomes and the acidic environment of the phagolysosome is favorable for its replication17. It is possible that 13I may suppress p38 pathway to increase phagosome maturation to create a favorable environment for its replication. Further experiments are needed to identify the effector proteins used by the CDEC strains to modulate MAPK pathways.
Manipulation of NFκB pathway is a common strategy employed by many pathogenic bacteria to suppress host immune responses for their survival25, 32. The lack of NFκB activation during the acute phage of LF82 and 13I infection points to the possibility that CDEC strains initially modulate NFκB pathway to suppress acute phase pro-inflammatory responses. However, during the later phase of infection, survival of CDEC in infected macrophages results in persistent activation of NFκB, which may account for the high level of TNF-α induced by CDEC infected macrophages. Alternatively, high level of TNF-α secreted by infected macrophages may in turn result in persistent activation of NFκB33, 34. TNF-α is a pro-inflammatory cytokine that can either induce apoptosis by activating caspase-8 and -1035, 36, or inhibit apoptosis by activating NFκB37–40. The survival of infected macrophages in the presence of high amounts of TNF-α in LF82 and 13I infected macrophages suggest that LF82 and 13I strains may exploit the anti-apoptotic property of TNF-α to survive in infected macrophages. It been shown that the intra-macrophagic replication of the AIEC strain LF82 is dependent on the concentration of TNF-α as neutralization of this cytokine decreases while addition of exogenous TNF-α increases intra-macrophagic replication of LF829. It is possible that CDEC strains suppress initial burst of pro-inflammatory responses by suppressing NFκB activation, but later allow for activation of NFκB through TNF-α to induce anti-apoptotic pathways. Although a number of different bacterial effector proteins that can either inhibit or activate NFκB signaling have been characterized, regulation of NFκB signaling through TNF-α would be the first report of a mechanism where a pathogenic bacteria hijacks a host pro-inflammatory cytokine for its survival. More work is needed to understand the mechanisms underlying this pathway in order to design therapies to decrease bacterial persistence, and resulting chronic inflammation in CD patients.
Fig. 5. Intracellular replication of Crohn's disease-associated Escherichia coli (CDEC) results in activation of NFκB during the chronic phase of infection.
(A) J774.A1 macrophages grown on coverslips were infected with E. coli strains for 3 hr. After 3 hrs, cells were washed and again incubated for 3 hrs in complete medium supplemented with 10% serum and 50 ug/mL Gentamicin. Scale 10 µM.
Table 1.
Strains used in this study
Acknowledgements
KR, MS, JK supported by VA Merit Award 1BX000697-02, AN supported by NIH DK 55679, DK 59888.
REFERENCES
- 1.Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48:116–132. doi: 10.1111/j.1365-2559.2005.02248.x. [DOI] [PubMed] [Google Scholar]
- 2.Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 3.Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- 4.Segal AW, Loewi G. Neutrophil dysfunction in Crohn's disease. Lancet. 1976;2:219–221. doi: 10.1016/s0140-6736(76)91024-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 7.Strober W. Adherent-invasive E. coli in Crohn disease: bacterial"agent provocateur". J Clin Invest. 2011;121:841–844. doi: 10.1172/JCI46333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fava F, Danese S. Crohn's disease: bacterial clearance in Crohn's disease pathogenesis. Nat Rev Gastroenterol Hepatol. 2010;7:126–128. doi: 10.1038/nrgastro.2010.1. [DOI] [PubMed] [Google Scholar]
- 9.Bringer MA, Billard E, Glasser AL, et al. Replication of Crohn's disease-associated AIEC within macrophages is dependent on TNF-alpha secretion. Lab Invest. 2012;92:411–419. doi: 10.1038/labinvest.2011.156. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland L, Singleton J, Sessions J, et al. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 13.Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chassaing B, Rolhion N, de Vallee A, et al. Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J Clin Invest. 2011;121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chargui A, Cesaro A, Mimouna S, et al. Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death. PLoS One. 2012;7:e51727. doi: 10.1371/journal.pone.0051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasser AL, Boudeau J, Barnich N, et al. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringer MA, Glasser AL, Tung CH, et al. The Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol. 2006;8:471–484. doi: 10.1111/j.1462-5822.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 18.Ossa JC, Ho NK, Wine E, et al. Adherent-invasive Escherichia coli blocks interferon-gamma-induced signal transducer and activator of transcription (STAT)-1 in human intestinal epithelial cells. Cell Microbiol. 2013;15:446–457. doi: 10.1111/cmi.12048. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki M, Sitaraman SV, Babbin BA, et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007;87:1042–1054. doi: 10.1038/labinvest.3700661. [DOI] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowers Y, Allez M. Efficacy of anti-TNF in Crohn's disease: how does it work? Curr Drug Targets. 2010;11:138–142. doi: 10.2174/138945010790309876. [DOI] [PubMed] [Google Scholar]
- 22.Schinzari F, Armuzzi A, De Pascalis B, et al. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70–76. doi: 10.1038/sj.clpt.6100229. [DOI] [PubMed] [Google Scholar]
- 23.Marini M, Bamias G, Rivera-Nieves J, et al. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 25.Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol. 2011;195:1083–1092. doi: 10.1083/jcb.201107132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards LA, Lucas M, Edwards EA, et al. Aberrant response to commensal Bacteroides thetaiotaomicron in Crohn's disease: an ex vivo human organ culture study. Inflamm Bowel Dis. 2011;17:1201–1208. doi: 10.1002/ibd.21501. [DOI] [PubMed] [Google Scholar]
- 27.Chong Y, Fitzhenry R, Heuschkel R, et al. Human intestinal tissue tropism in Escherichia coli O157 : H7--initial colonization of terminal ileum and Peyer's patches and minimal colonic adhesion ex vivo. Microbiology. 2007;153:794–802. doi: 10.1099/mic.0.2006/003178-0. [DOI] [PubMed] [Google Scholar]
- 28.Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 29.Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 30.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 31.Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- 32.Alto NM, Orth K. Subversion of cell signaling by pathogens. Cold Spring Harb Perspect Biol. 2012;4:a006114. doi: 10.1101/cshperspect.a006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakhov AN, Collart MA, Vassalli P, et al. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 35.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 36.Hsu H, Shu HB, Pan MG, et al. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 37.Van Antwerp DJ, Martin SJ, Kafri T, et al. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 38.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 39.Wu M, Lee H, Bellas RE, et al. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donnell MA, Ting AT. Chronicles of a death foretold: dual sequential cell death checkpoints in TNF signaling. Cell Cycle. 2010;9:1065–1071. doi: 10.4161/cc.9.6.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobley HL, Green DM, Trifillis AL, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]