SUMMARY
SETTING
Gaborone, Botswana.
OBJECTIVE
To determine if starting anti-tuberculosis treatment at clinics in Gaborone without co-located human immunodeficiency virus (HIV) clinics would delay time to highly active antiretroviral therapy (HAART) initiation and be associated with lower survival compared to starting anti-tuberculosis treatment at clinics with on-site HIV clinics.
DESIGN
Retrospective cohort study. Subjects were HAART-naïve, aged ≥21 years with pulmonary tuberculosis (TB), HIV and CD4 counts ≤250 cells/mm3 initiating anti-tuberculosis treatment between 2005 and 2010. Survival at completion of anti-tuberculosis treatment or at 6 months post-treatment initiation and time to HAART after anti-tuberculosis treatment initiation were compared by clinic type.
RESULTS
Respectively 259 and 80 patients from clinics without and with on-site HIV facilities qualified for the study. Age, sex, CD4, baseline sputum smears and loss to follow-up rate were similar by clinic type. Mortality did not differ between clinics without or with on-site HIV clinics (20/250, 8.0% vs. 8/79, 10.1%, relative risk 0.79, 95%CI 0.36–1.72), nor did median time to HAART initiation (respectively 63 and 66 days, P = 0.53).
CONCLUSION
In urban areas where TB and HIV programs are separate, geographic co-location alone without further integration may not reduce mortality or time to HAART initiation among co-infected patients.
Keywords: care coordination, health systems, co-infection, antiretroviral therapy
TUBERCULOSIS (TB) is a leading cause of death in human immunodeficiency virus (HIV) infected patients.1-3 In Botswana, among those aged ≥18 months, HIV prevalence is estimated to be 17.6%, with TB the reported cause of death in approximately 40% of HIV-infected individuals.4,5 Recent trials offer strong evidence supporting prompt initiation of highly active antiretroviral therapy (HAART) in patients with HIV and TB, particularly those with advanced immunosuppression.6-8 These results are consistent with 2009 World Health Organization (WHO) guidelines advocating HAART initiation as soon as possible after starting anti-tuberculosis treatment for all patients with TB-HIV.9 A 2010 report found that only 45% of patients with HIV and TB in Botswana initiated HAART during anti-tuberculosis treatment.10
Greater distance to the clinic was associated with treatment discontinuation in Uganda,11 and was cited as a barrier to accessing HAART among HIV patients.12 In many resource-limited settings, HIV and TB programs are separate, non-integrated entities; patients with TB are referred to distinct HIV-specific clinics (which may or may not be co-located with the TB clinics) to obtain HAART.13 This system concentrates physical and human resources, but creates potential barriers to care; those at TB clinics without co-located HIV clinics may have greater delays in accessing HAART than patients who do not have to travel to separate facilities.
The relationship between clinic co-location and outcomes has not been systematically addressed. We hypothesized that HIV-infected adults who initiated anti-tuberculosis treatment at TB clinics without co-located HIV clinics would have higher mortality rates during anti-tuberculosis treatment and longer time to HAART initiation than patients who initiated anti-tuberculosis treatment at TB clinics with co-located HIV clinics.
METHODS
Study setting
This study was conducted in Gaborone, the capital of and largest city in Botswana, and the neighboring urbanized villages of Mogoditshane and Tlokweng. Botswana offers free anti-tuberculosis treatment to patients with active TB (6 months of directly observed combination therapy per WHO guidelines) and free HAART to citizens with qualifying CD4 counts or clinical conditions, including TB.14,15
Per Botswana’s 2008 national guidelines, co-infected patients with CD4 counts of <100 cells/mm3 should begin HAART 1–2 weeks after initiating anti-tuberculosis treatment, while those with CD4 counts between 100 and 250 cells/mm3 should begin HAART after 2–4 weeks. Per these guidelines, delays in the latter group are acceptable if the patient’s clinical condition is ‘fair or good’. HAART initiation was to occur after completion of anti-tuberculosis treatment in those with CD4 counts of >250 cells/mm3.16
In Botswana, all primary care clinics treat patients for TB. In greater Gaborone, 8 of 22 government clinics have an HIV clinic/pharmacy providing HAART located on the same site (seven of which were operating during the study period), while the remaining 14 clinics do not provide HAART. Although all patients require a referral for HIV care, those receiving anti-tuberculosis treatment at clinics with on-site HIV clinics are referred to the on-site HAART clinic; those receiving treatment at clinics without on-site HIV clinics must be referred to another site further away.
Study design
This was a retrospective cohort study where the primary exposure was receiving initial out-patient anti-tuberculosis treatment at a government clinic that did not have an on-site HIV treatment center, and the primary outcome was death before completion of anti-tuberculosis treatment. Patients were categorized as alive at the end of treatment if they were listed in the TB register as having completed treatment or if there was evidence that they were alive >180 days after starting anti-tuberculosis treatment. Evidence of being alive included any of the following: laboratory data, out-patient appointments attended, filled prescriptions or hospitalizations. A secondary composite variable of death or hospitalization for any reason during anti-tuberculosis treatment was also analyzed. Secondary objectives included comparing the two clinic types with respect to the interval between anti-tuberculosis treatment and HAART initiation and the proportion of patients starting HAART within 60 days of starting anti-tuberculosis treatment, given the importance of this timepoint to survival, as shown in recent trials.6-8
Study patients
Included patients were HIV-infected adults aged >21 years who presented to any of the 22 public clinics in greater Gaborone for out-patient anti-tuberculosis treatment for a new episode of pulmonary TB between 1 January 2006 and 31 January 2010, or those who initiated anti-tuberculosis treatment in 2005 but had complete data listed in the 2006 TB register. Patients had to have CD4 values of ≤250 cells/mm3 before or within 1 month of anti-tuberculosis treatment initiation and be HAART-n aïve at the time of anti-tuberculosis treatment initiation. The CD4 count cut-off was based on the 2008 national guidelines, which did not specify a need for HAART initiation during anti-tuberculosis treatment for those patients with CD4 counts of >250 cells/mm3.16 The starting date of the study period was determined by the rollout of new TB registers in 2006, which included data necessary for conducting the study. The end date of the study period was selected so as to allow for at least 6 months of observation of all study subjects from the time of data collection.
Patients were excluded if they were known to be pregnant, had a history of previous TB or defaulting on anti-tuberculosis treatment or had multidrug resistant TB,16 had no information in the electronic medical record, had no CD4 counts listed in the electronic medical record or if they transferred out of the Gaborone metropolitan area before either completing anti-tuberculosis treatment or taking 6 months of anti-tuberculosis treatment.
Data collection
TB registers from each clinic were used to identify patients with new cases of pulmonary TB and new or previously diagnosed HIV and to determine patient treatment outcome/death, anti-tuberculosis treatment initiation date, age, sex and sputum smear status. The electronic medical record was searched to obtain laboratory values, hospitalizations, out-patient appointments, pharmacy records and other records. Date of HAART initiation was determined by reviewing notes and prescribing records in the electronic medical record.
Site of out-patient anti-tuberculosis treatment initiation (in-patient/out-patient) was determined by the initiation date in the TB registers and hospitalization records in the electronic medical record; patients who initiated anti-tuberculosis treatment as in-patients had their clinic determined by the location of their first dose of out-patient anti-tuberculosis treatment.
Data analysis
Unadjusted analyses compared patient characteristics by clinic type. As TB is a curable disease, where the primary outcome of importance is death during anti-tuberculosis treatment and not necessarily duration of survival on anti-tuberculosis treatment, we considered the primary outcome as dichotomous. We calculated the proportion of deaths during follow-up for exposed and unexposed groups, and determined a relative risk (RR) with 95% confidence interval (CI).17 Logistic regression was used to evaluate potential confounders of the primary relationship, defined as characteristics associated with exposure or outcome at P ≤ 0.2. In the primary analysis for clinic type and outcome, patients lost to follow-up (LTFU) were excluded, but sensitivity analyses were performed counting LTFU patients as either all living or all dead. We tested for effect modification of the relationship between clinic type and outcome using interaction terms in logistic regression models (considered present if the interaction term’s P value was ≤0.05), examining baseline CD4, sputum smear, age and sex. For patients with available HAART initiation data, median time to HAART after anti-tuberculosis treatment initiation was compared by clinic type, as was the proportion of patients starting HAART within 60 days of starting anti-tuberculosis treatment, with the former as a continuous and the latter as a dichotomous variable.
The study was approved by the University of Pennsylvania Institutional Review Board and the Botswana Ministry of Health Human Resources Development Committee.
RESULTS
Patient characteristics
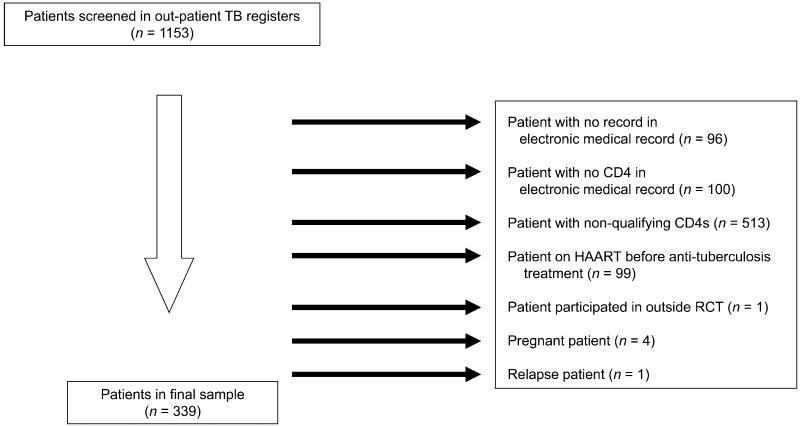
Overall, 1153 patients with TB-HIV were identified as potentially eligible for the study; however, nearly half had CD4 counts that were >250 cells/ml (Figure) and were excluded. Other reasons for exclusion are shown in the Figure. A total of 339 patients were included in the study, 152 (45%) of whom were females. The median CD4 cell count before or within 1 month of anti-tuberculosis treatment initiation was 95 cells/mm3 (interquartile range [IQR] 44–161); 98 (29%) patients had a CD4 count of <50 cells/mm3. Baseline sputum smears were positive in 153 (45%), negative in 74 (22%), and 112 (33%) had no test recorded. Eighty (24%) patients attended TB clinics with co-located HIV clinics, while 259 (76%) attended clinics without co-located HIV clinics.
Figure.
Reasons for patient exclusion. TB = tuberculosis; HAART = highly active antiretroviral therapy; RCT = randomized controlled trial.
Treatment outcomes by clinic type
Patient characteristics were highly similar between clinic types (Table 1). Overall, 28 (8.5%) of 329 patients died during follow-up; 10 (2.9%) patients were LTFU. Excluding patients who were LTFU, the proportion dying during follow-up was not significantly different among those who initiated at clinics without or with on-site HIV clinics (20/250, 8.0% vs. 8/79, 10.1%, RR 0.79, 95%CI 0.36–1.72). Among patient characteristics listed in Table 2, only baseline CD4 count was associated with increased death risk. Adjusting for CD4 count did not change the primary unadjusted relationship by more than 7%. There was no evidence of effect modification by age, baseline CD4 count or sputum smear status (data not shown); we did note a trend toward significance for sex as an effect modifier (P = 0.06), with women at clinics without attached HIV centers having an increased risk of mortality (RR 2.00, 95%CI 0.47–8.42), and men at clinics without attached HIV centers having a reduced risk of mortality (RR 0.38, 95%CI 0.13–1.06). The number of patients who were LTFU was similar between the clinic types (9/259, 4% patients at clinics without attached HIV clinics and 1/80, 1% patients at clinics with attached HIV clinics, P = 0.30). The primary relationship was essentially unchanged after including those LTFU as either all alive (RR 0.77, 95%CI 0.35–1.69) or all dead (RR 1.00, 95%CI 0.49–2.01). There was no difference between clinic types when a composite outcome of death or hospitalization during anti-tuberculosis treatment was used (52/251 [21%] in clinics without attached HIV clinics and 24/80 [30%] in clinics with attached HIV clinics, RR 0.69, 95%CI 0.46–1.04).
Table 1.
Baseline characteristics and outcomes of patients in attending clinics with and without on-site HIV clinics
| With on-site HIV clinics (n = 80) n (%) |
Without on-site HIV clinics (n = 259) n (%) |
P value | |
|---|---|---|---|
| Total (n = 339) | 80 (24) | 259 (76) | — |
| Female | 35 (44) | 117 (45) | 0.82* |
| Age, years, median [range] | 35.5 [24–56] | 35 [21–60] | 0.88† |
| <35 years | 37 (46) | 113 (44) | 0.68* |
| CD4, median [IQR] | 100 [45–154] | 91 [43–165] | 0.95† |
| Baseline CD4 categories, cells/mm3 |
|||
| <50 | 22 (28) | 76 (29) | |
| 50–100 | 18 (23) | 62 (24) | |
| 100–250 | 40 (50) | 121 (47) | 0.88* |
| Sputum results | |||
| Sputum-positive | 32 (40) | 121 (47) | |
| Sputum-negative | 21 (26) | 53 (21) | |
| No sputum | 27 (34) | 85 (33) | 0.46* |
| TB treatment initiation as in-patient |
10 (13) | 49 (19) | 0.19* |
χ2 test.
Wilcoxon rank-sum.
HIV = human immunodeficiency virus; IQR = interquartile range; TB = tuberculosis.
Table 2.
Outcomes by baseline patient characteristics
| Number/deaths (% dying within strata) n/N (%) |
RR (95%CI) | |
|---|---|---|
| Total mortality | 28/329 (9)* | — |
| Type of clinic | ||
| Without attached HIV clinic | 20/250 (8) | |
| With attached HIV clinic | 8/79 (10) | 0.79 (0.36–1.7) |
| Sex | ||
| Female | 15/149 (10) | |
| Male | 13/180 (7) | 1.4 (0.69–2.8) |
| Age, years | ||
| <35 | 10/144 (7) | |
| ≥35 | 18/185 (10) | 0.71 (0.34–1.50) |
| Baseline CD4 count, cells/mm3 | ||
| <100 | 23/173 (13) | |
| >100 | 5/1 56 (3) | 4.15 (1.62–10.65) |
| Baseline sputum status | ||
| Positive | 10/147 (7) | |
| Negative or not recorded | 18/182 (10) | 0.69 (0.33–1.44) |
| Initiated anti-tuberculosis treatment as in-patient |
||
| Yes | 6/57 (11) | |
| No | 22/272 (8) | 1.3 (0.55–3.06) |
| Initiated HAART within 60 days of starting anti-tuberculosis treatment |
||
| Yes | 1/86 (1) | |
| No | 3/97 (3) | 0.38 (0.04–3.55) |
As 10 patients were lost to follow-up, mortality calculations are based on a patient population of 329.
RR = relative risk; CI = confidence interval; HAART = highly active antiretroviral therapy.
Time to HAART initiation
A total of 185 patients (55%) had HAART initiation data available: 42/80 (53%) from clinics with co-located HIV clinics and 143/259 (55%) from clinics without co-located HIV clinics (P = 0.67; Table 3). Among those with and those without HAART initiation data, there was no significant difference in baseline CD4, sex or baseline sputum status; those with initiation data were slightly older (median age among those with and without HAART data 36 and 34.5 years, respectively, P = 0.013). Median time to HAART initiation after anti-tuberculosis treatment initiation was 64 days (IQR 36–106), and did not differ by clinic type (63 days [IQR 32–104] for clinics without attached HIV clinics and 66 days [IQR 37–124] for clinics with co-located HIV clinics, P = 0.53, rank-sum test). The proportions of these 185 patients starting HAART <60, 60–180 and >180 days after anti-tuberculosis treatment initiation were respectively 47%, 36% and 17%, and did not differ significantly between the clinic types (P = 0.53, χ2 test). Baseline CD4 count was the only factor associated with time to HAART initiation; patients with baseline CD4 ≤100 cells/mm3 initiated earlier than those with CD4 >100 cells/mm3 (median interval = 48 days [IQR 27–71] vs. median 76 days [IQR 59–157], P < 0.001, rank-sum test). Median time to HAART initiation did not differ by clinic type when examined in those with CD4 counts of ≤100 cells/mm3 (48 days [IQR 27–71] for clinics without attached HIV care centers and 50 days [IQR 25.5–83.5] for clinics with co-located HIV clinics, P = 0.83, rank-sum test). While those initiating anti-tuberculosis treatment after the 2008 treatment guidelines had a somewhat shorter median time to HAART initiation than those initiating treatment before the guidelines (60 vs. 66 days, respectively), the difference was not significant (P = 0.20).
Table 3.
Time to HAART by clinic type
| Outcome n/N (%) |
P value | |
|---|---|---|
| Days to HAART, median [IQR] (n = 185) | ||
| Clinics with attached HIV clinics | 66 [37–124] | |
| Clinics without attached HIV clinics | 63 [32–104] | 0.53* |
| Days to HAART initiation after anti-tuberculosis treatment initiation |
||
| ≤60 | 0.53† | |
| With HIV clinic | 17/42 (41) | |
| Without HIV clinic | 70/143 (49) | |
| >60 and ≤180 | ||
| With HIV clinic | 16/42 (38) | |
| Without HIV clinic | 51/143 (36) | |
| >180 | ||
| With HIV clinic | 9/42 (21) | |
| Without HIV clinic | 22/143 (15) |
Wilcoxon rank-sum.
χ2 test.
HAART = highly active antiretroviral therapy; IQR = interquartile range; HIV = human immunodeficiency virus.
DISCUSSION
In this study, conducted in urban Botswana among patients with TB-HIV and CD4 counts of <250 cell/mm3, we found no association between HIV and TB clinic co-location and either mortality or time to HAART initiation. Of the factors assessed, only CD4 count was associated with either exposure or outcomes, as more advanced immunosuppression was associated with an increased risk of mortality and a shorter time to ART initiation.
Strengths of the study include the distribution of HIV clinics within the primary care system, which enabled us to evaluate the relationship between clinic type and outcomes in a community-based setting. Furthermore, baseline similarities between the populations at the two clinic types allowed us to assess this relationship in the absence of obvious known confounding. This was, however, an observational study, and unequal distribution of unobserved confounders affecting mortality risk, such as undiagnosed HIV-associated comorbidities, presence of extra-pulmonary TB and WHO disease stage, could have biased our results.18,19 Generalizability is suggested by the fact that the observed mortality rates and treatment intervals in this study are consistent with outcomes of other studies as well as pooled data from a recent meta-analysis.20-23 The impact of HIV and TB clinic co-location likely depends on the ability of patients to afford travel, transportation options and the geographic distribution of the HIV clinics within the system. Gaborone benefits from being a relatively small city with numerous HIV clinics and relatively reliable and affordable transportation options. Our results may therefore not apply to other settings—particularly rural areas or very large cities—where clinics are dispersed across a broader geographic area and travel may be more difficult or costly. Another limitation of the analysis of time to HAART initiation is the substantial amount of missing data, which could have biased our results. The high degree of similarity between patients with and without missing data suggests, however, that severe bias due to missing data is unlikely.
The results of this study suggest that co-location alone as a policy to integrate TB-HIV care will not necessarily improve outcomes. These data can be interpreted as indicating that in urban settings where patients with TB-HIV are referred to specialized HIV clinics for HAART, lack of an on-site HIV treatment center does not necessarily result in delayed HAART initiation or increased mortality during anti-tuberculosis treatment. A possible explanation for this finding is that the referral process itself may be a source of delay affecting all individuals equally, regardless of where they initiate anti-tuberculosis treatment. In Botswana, even at sites with co-located HIV clinics, TB-HIV care is not fully integrated because all patients have to be referred to a separate system before they initiate HAART. Ideally, care would be integrated at the provider level, where a single provider coordinates TB-HIV treatment without referral.24 Assessments of patient outcomes in fully integrated systems—as well as the associated costs and logistical issues—should be performed.
Notably, fewer than half of patients initiated HAART during the first 60 days, and even fewer initiated within the first 2 weeks of anti-tuberculosis treatment. This is important, given recent clinical trial data documenting that HAART initiation within the first 2 weeks of anti-tuberculosis treatment improves survival among those with the most advanced HIV disease.7,8,25 Although the percentage of patients with TB-HIV initiating HAART appears to be increasing,26 and the delays between TB diagnosis and HAART initiation may be decreasing,27 further efforts to facilitate timely HAART initiation in those with TB-HIV are needed.
CONCLUSION
This study provides valuable data informing the lack of an association between co-location of HIV and TB clinics and outcomes in co-infected patients in an urban setting. To fulfill WHO guidelines for integration of HIV and TB care,13 co-location should be supplemented by coordination and communication between HIV and TB providers.
Acknowledgements
The authors thank C Snyman, R Lebelonyane, R R MacGregor, R Gross, S Barenbaum, P Sonenthal, J Cataldi, J Ludmir, S Danan and S Schwartz. This research was indirectly supported by the Doris Duke Charitable Foundation through its Clinical Research Fellowship for medical students (provided to then-medical student AS and mentor GB), although funding was not tied to any specific project.
Footnotes
Abstract presented at the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 7 May 2012.
Conflict of interest: none declared.
References
- 1.Van der Sande MA, van der Loeff MF, Bennett RC, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS. 2004;18:1933–1941. doi: 10.1097/00002030-200409240-00009. [DOI] [PubMed] [Google Scholar]
- 2.Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5:225–232. [PubMed] [Google Scholar]
- 3.Murray J, Sonnenberg P, Shearer SC, Godfrey-Faussett P. Human immunodeficiency virus and the outcome of treatment for new and recurrent pulmonary tuberculosis in African patients. Am J Respir Crit Care Med. 1999;159:733–740. doi: 10.1164/ajrccm.159.3.9804147. [DOI] [PubMed] [Google Scholar]
- 4.Republic of Botswana . Botswana AIDS Impact Survey III. Central Statistics Office, Republic of Botswana; Gaborone, Botswana: 2008. [Google Scholar]
- 5.Republic of Botswana . National TB Programme manual. 6th ed. Ministry of Health, Republic of Botswana; Gaborone, Botswana: 2007. [Google Scholar]
- 6.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during TB therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanc FX, Sok T, Laureillard D, et al. Early versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Final report. WHO; Geneva, Switzerland: 2009. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. [Google Scholar]
- 10.Republic of Botswana . Annual tuberculosis and leprosy report: 2009. Botswana National Tuberculosis Program, Ministry of Health, Republic of Botswana; Gaborone, Botswana: 2010. [Google Scholar]
- 11.Elbireer S, Guwatudde D, Mudiope P, Nabbuye-Sekandi J, Manabe YC. Tuberculosis treatment default among HIV-TB co-infected patients in urban Uganda. Trop Med Int Health. 2011;16:981–987. doi: 10.1111/j.1365-3156.2011.02800.x. [DOI] [PubMed] [Google Scholar]
- 12.Posse M, Meheus F, van Asten H, van der Ven A, Baltussen R. Barriers to access to antiretroviral treatment in developing countries: a review. Trop Med Int Health. 2008;13:904–913. doi: 10.1111/j.1365-3156.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Final report. WHO; Geneva, Switzerland: 2003. Guidelines for implementing collaborative TB and HIV programme activities. WHO/CDS/TB/2003.319. WHO/HIV/2003.01. [Google Scholar]
- 14.World Health Organization . Final report. WHO; Geneva, Switzerland: 2009. Treatment of tuberculosis: guidelines. WHO/HTM/TB/2009.420. [Google Scholar]
- 15.Avert . HIV & AIDS in Botswana. Avert; Horsham, UK: [Accessed July 2013]. 2011. http://www.avert.org/aids-botswana.htm [Google Scholar]
- 16.Republic of Botswana . Final report. Ministry of Health, Republic of Botswana; Gaborone, Botswana: 2008. Botswana national HIV/AIDS treatment guidelines: 2008 version. [Google Scholar]
- 17.Hosmer DW, Lemeshow L. Applied logistic regression. John Wiley & Sons; Hoboken, NJ, USA: 2000. [Google Scholar]
- 18.Whalen C, Horsburgh CR, Jr, Hom D, Lahart C, Simberkoff M, Ellner J. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS. 1997;11:455–460. doi: 10.1097/00002030-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann CJ, Fielding KL, Johnston V, et al. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr. 2011;58:269–276. doi: 10.1097/QAI.0b013e31823219d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaisson RE, Clermont HC, Holt EA, et al. Six-month supervised intermittent tuberculosis therapy in Haitian patients with and without HIV infection. Am J Respir Crit Care Med. 1996;154:1034–1038. doi: 10.1164/ajrccm.154.4.8887603. [DOI] [PubMed] [Google Scholar]
- 21.Letang E, Miró JM, Nhampossa T, et al. Incidence and predictors of immune reconstitution inflammatory syndrome in a rural area of Mozambique. PLoS ONE. 2011;6:e16946. doi: 10.1371/journal.pone.0016946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tansuphasawadikul S, Saito W, Kim J, et al. Outcomes in HIV-infected patients on antiretroviral therapy with tuberculosis. Southeast Asian J Trop Med Public Health. 2007;38:1053–1060. [PubMed] [Google Scholar]
- 23.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS ONE. 2011;6:e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard AA, El-Sadr WM. Integration of tuberculosis and HIV services in sub-Saharan Africa: lessons learned. Clin Infect Dis. 2010;50:s238–244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
- 25.Abdool-Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Campbell L, Kaplan R, et al. Time to initiation of antiretroviral therapy among patients with HIV-associated tuberculosis in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2011;57:136–140. doi: 10.1097/QAI.0b013e3182199ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]