Abstract
Elevated serum CD44, ICAM-1 and VCAM-1 have been linked to poor prognosis in aggressive lymphomas but their utility in low grade lymphomas remains undefined. We evaluated serum CD44, VCAM-1 and ICAM-1 levels in 100 patients with newly diagnosed indolent NHL. The median pre-treatment values of the markers were as follows: CD44: 540 ng/ml (range 102 – 1222), ICAM-1: 311 ng/ml (range 248 – 4779) and VCAM-1: 1165 ng/ml (range 248 – 4779). On univariate analysis, elevated sCD44, sICAM-1 and sVCAM-1 were significantly associated with worse overall (OS) and progression-free survival (PFS). In a subset analysis of stage IV patients, the effects of sCD44 and sICAM-1 on OS persisted (p<0.05), as did the effect of sCD44 on PFS (p<0.01). In a multivariate analysis that included conventional prognostic factors and the FLIPI model, sICAM-1 demonstrated prognostic value on OS and PFS. We conclude that serum CD44, ICAM-1, and VCAM-1 can potentially be prognostic in patients with indolent NHL. Though the FLIPI model remains the gold standard for prognosis, these quantitative serologic markers may be useful as adjunct tools in assessing disease risk.
Keywords: indolent lymphoma, biomarkers, CD44, ICAM-1, VCAM-1
INTRODUCTION
Indolent lymphomas make up 39% of all non-Hodgkin lymphomas (NHL) and include follicular lymphoma, small lymphocytic lymphoma (SLL), marginal zone lymphoma and mucosa-associated lymphoid tissue (MALT) lymphoma [1]. The clinical course of the indolent lymphomas can be highly variable but prognostic models can help to separate patients into high, intermediate and low risk groups. Useful models include the International Prognostic Index (IPI), which was originally developed for patients with all lymphoma subtypes, but can also be useful for indolent lymphomas [2]. The Follicular Lymphoma International Prognostic Index (FLIPI) was developed to predict outcomes for follicular lymphoma, the most common of the indolent NHL’s [3]. The FLIPI-2 is also useful for follicular lymphoma; notably the FLIPI-2 model incorporates a serologic marker, β2 microglobulin, that is reflective of the biological aggressiveness of certain follicular lymphomas [4].
Recently there has been a surge in the knowledge about the cellular characteristics and microenvironment of lymphomas [5,6]. This has produced a wealth of possibilities for biomarkers that may be surrogates for disease status and risk. Specifically in lymphoma, cell adhesion molecules have shown promise as predictors of clinical outcome. CD44, intercellular adhesion molecule 1 (ICAM-1, also known as CD54) and vascular cell adhesion molecule-1 (VCAM-1, also known as CD106) are present on cell surfaces and regulate cell-matrix interaction as well as cell migration. They are constantly shed into the circulation, and serum concentrations can be measured by commercially available enzyme-linked immunosorbent assays (ELISA).
CD44 is a transmembrane glycoprotein which is involved in lymphocyte homing, activation, adhesion and migration [7]. Alternative splicing of the gene, located on chromosome 11 (11p13) generates numerous isoforms [8]. These molecules interact with the hyaluronic acid component of the extracellular matrix to mediate leukocyte rolling [9]. Additionally, CD44 promotes cell proliferation and invasion [10] and its expression has been implicated in the metastatic potential of several malignancies [11,12].. Elevated serum CD44 (sCD44) levels have been associated with worsened overall survival in childhood lymphomas [13] as well as aggressive NHL in adults [14,15].
ICAM-1 is a cell adhesion molecule of the immunoglobulin superfamily. Though its role has traditionally been thought to be primarily for adhesion, it is now known that ICAM-1 on B lymphocytes interacts with leukocyte function-associated antigen 1 (LFA-1) on T lymphocytes to increase B cell activation [16]. Furthermore, binding of ICAM-1 to its various ligands may lead to intracellular signaling within the lymphocyte to enhance its invasive properties [17]. Elevated serum ICAM-1 (sICAM-1) levels have been shown to correlate with the degree of aggression in childhood lymphomas [18]. Increased sICAM-1, in comparison with normal controls, has also been shown in patients with newly diagnosed Hodgkin lymphoma; these levels subsequently decrease after chemotherapy, suggesting a role for this biomarker in monitoring disease burden [19]. In diffuse large B cell lymphoma, higher levels of sICAM-1 have been associated with shorter time to treatment failure and decreased overall survival [20].
VCAM-1 is a cell adhesion molecule that mediates lymphocyte binding to endothelium. Interaction between VCAM-1 on the endothelial cell and very late antigen-4 (VLA-4) on lymphocytes is essential for the latter’s migration through the vascular wall and into tissue [21]. Elevated levels of serum VCAM-1 (sVCAM-1) have been demonstrated in Hodgkin lymphoma, advanced NHL and pediatric acute lymphoblastic leukemia [19,22,23]. In addition, sVCAM-1 has demonstrated independent prognostic value in Hodgkin lymphoma [24].
Thus, each these 3 cell surface proteins appear to play a role in the disease course in various lymphomas. But while these molecules have been studied in Hodgkin lymphoma and aggressive NHL, their prognostic value in indolent NHL’s is not known. Here we present a study of serum CD44, ICAM-1 and VCAM-1 levels in 93 patients with indolent lymphoma. We show that elevated levels of these markers are associated with a poorer overall survival (OS) and progression-free survival (PFS).
PATIENTS and METHODS
Patients
One hundred patients with a new diagnosis of indolent lymphoma at MD Anderson Cancer Center (MDACC) were treated between 1990 and 1992. There were 78 patients with grade 1–2 follicular lymphoma, 13 with small lymphocytic lymphoma, and 9 patients had other histologies, including marginal zone lymphoma, MALT lymphoma and mantle cell lymphoma. Because the initial biopsies had been collected over 15 years ago, we were unable to re-review the pathology to re-confirm the lymphoma subtype for this analysis. Eighteen patients were Ann Arbor stage I disease at presentation, 13 patients were stage II, 1 patient was stage III, and 68 patients were stage IV. Additional baseline information, such as lactic dehydrogenase values, β2 microglobulin (β2M) levels, bone marrow infiltration, and size of lymphadenopathy were available and analyzed. Pre-treatment serum was collected at the time of diagnosis.
Treatment and Follow-Up
Patients were treated according to their stage, either on clinical protocols, or otherwise per the standard of care. Thirty-one patients with stage I–III were treated with cyclophosphamide, vincristine, prednisone (COP) and radiation therapy (XRT). One patient received prednisone, doxorubicin, cyclophosphamide, etoposide, cytarabine, bleomycin, vincristine, methotrexate, leucovorin (ProMACE-CytaBOM). Most patients with stage IV (n= 68) were treated on a protocol (MDACC 88-050) consisting of alternating triple chemotherapy: cyclophosphamide, doxorubicin, vincristine, prednisone- bleomycin (CHOP-Bleomycin), etoposide, methylprednisolone, cytarabine, cisplatin (ESHAP) and mitoxantrone, vincristine, procarbazine, prednisone (NOPP), every 3–4 weeks in alternating basis, for a total of 4 cycles for each combination (12 cycles total). The patients were followed every 3–4 months for the first 2–3 years, then every 4–6 months until year 5, and thereafter at the discretion of the provider, unless earlier evaluation was necessary. The follow-up evaluations included a, complete physical examination, chest-X-ray, computerized tomography of the abdomen and pelvis, and bone marrow aspiration and biopsy in patients with previous bone marrow disease.
Enzyme-Linked Immunosorbent Assay (ELISA) for sCD44, sICAM-1 and sVCAM-1
All serum was collected at initial presentation, before treatment. Samples were stored in the MDACC tissue bank and analyzed all at once, in triplicate, in 1995 with new ELISA kits. All of these ELISA kits are still available commercially and manufacturers’ instructions were followed. Serum CD44 levels were determined by an ELISA kit from Bender MedSystems, (Vienna, Austria, sensitivity = 0.016 ng/ml) [25]. Serum ICAM-1 levels were quantified by an ELISA kit from R&D Systems (Minneapolis, MN, sensitivity = 0.35 ng/ml). Serum VCAM-1 levels were determined using an ELISA kit from British Bio-technology Products Ltd (Abingdon U.K., sensitivity = 2 µg/L) [26].
Statistical Analysis
The serum marker values in continuous scores were first fit into Cox proportional hazards models (all p-values <=0.001). There were no obvious cutoff points identified by the Martingale residual plots, so median points were used to dichotomize the markers into high and low groups. For time-to-event analysis for overall survival (OS) and progression free survival (PFS) Kaplan-Meier curves were generated. Progression was defined as any new, biopsy-proven site of disease after prior response to therapy. The log-rank test was used to compare the difference in time-to-event outcomes between the prognostic factor subsets (such as stage IV, FLIPI ≥3 etc), and the serum marker high and low groups. Cox proportional hazards models were utilized for multivariate analysis; a stepwise selection method was used to select the final set of covariates. When possible, variables were analyzed as continuous (sCD44, SsICAM-1, sVCAM-1, β2M, LDH, per cent bone marrow involvement). The p values and hazard ratios with 95% confidence intervals are reported. All analyses were performed using SAS (Cary, NC) and Splus (TIBCO, Sommerville, MA) software. All tests were two-sided, and p-values less than 0.05 were considered statistically significant.
RESULTS
Patients
Of the 100 patients studied, 93 were considered evaluable. Excluded were 5 patients with mantle cell lymphoma, which is now recognized as an aggressive rather than indolent lymphoma. In addition, 2 patients died soon after initial diagnosis so that neither response nor progression could be analyzed. The median age at diagnosis was 54 years (range: 29–74). Patient characteristics are detailed in Table I. With a median follow-up time of 17.7 years (1.44 – 19.87) for the censored observations, 63% progressed and 57% died.
Table I.
Patient Characteristics, N=93
| Characteristic | Value |
|---|---|
| Male | 52 (56%) |
| Female | 41 (44%) |
| Median Age (Years) | 54 (29–74) |
| Cell Type | |
| Follicular, grade 1–2 | 74 (80%) |
| Small Lymphocytic Lymphoma | 13 (14%) |
| Other indolent lymphomas | 6 (6%) |
| Ann Arbor Stage | |
| I | 16 (17%) |
| II | 13 (14%) |
| III | 1(1%) |
| IV | 63 (68%) |
| FLIPI | |
| 0 | 18 (19.35%) |
| 1 | 35 (37.63%) |
| 2 | 30 (32.26%) |
| 3 | 9 (9.68%) |
| 4 | 1 (1.08%) |
| Median sCD44 | 540 ng/ml (156 – 1201) |
| Median sICAM-1 | 311 ng/ml (102 – 1222) |
| Median sVCAM-1 | 1165 ng/ml (248 – 4779) |
| Progressed | 59 (63%) |
| Deaths | 53 (57%) |
| Median Progression-Free Survival (years, 95% CI) | 6.8 (4.7,8.8) |
| Median Overall Survival | 15.6 (12.4, NA) |
Serum Marker Values and Associations With Established Prognostic Markers
The median values for the serum markers were 540 ng/ml (range: 156 – 1201) for sCD44, 311 ng/ml (range: 102 – 1222) for sICAM-1 and 1165 ng/ml (range: 248 – 4779) for sVCAM-1. Evaluated by chi-square or Fisher’s exact test, those patients with serum marker levels above the median, for each of the 3 markers were significantly more likely to have stage IV disease (Table II). In addition, patients with high serum levels of any of the adhesion molecules were more likely to be high-intermediate or high risk by IPI score and to have a FLIPI score of 3 or 4 (Table II). Serum marker levels were not significantly associated with histological subtype or age >60. Females tended to have lower sCD44 levels than males (p=0.0239) but this was not true for the other markers (data not shown). Finally, 29% of patients had all 3 markers in the “low” category while 34% had all 3 markers in the “high” category. When analyzed as continuous variables, there were significant correlations among the serum markers themselves (p < 0.0001) and also with β2M (p < 0.0001), as seen in Table III.
Table II.
Higher serum marker values are significantly associated with presence of Stage IV disease, intermediate-high or high IPI score and FLIPI score >3.
| Covariate | STAGE I, II, III |
STAGE IV |
p-value | IPI=L | IPI=LI | IPI=IH | p-value | FLIPI=0,1 | FLIPI=2 | FLIP≥3 | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sCD44 | low | 21(48.8%) | 22(51.2%) | .0008 | 34(77.3%) | 7(15.9%) | 3(6.8%) | .0265 | 36(81.8%) | 5(11.4%) | 3(6.8%) | <.0001 |
| high | 8(16.3%) | 41(83.7%) | 25(51%) | 19(38.8%) | 5(10.2%) | 17(34.7%) | 25(51%) | 7(14.3%) | ||||
| sICAM-1 | low | 20(46.5%) | 23(53.5%) | .0037 | 34(77.3%) | 10(22.7%) | 0(0%) | .0035 | 35(79.5%) | 8(18.2%) | 1(2.3%) | .0001 |
| high | 9(18.4%) | 40(81.6%) | 25(51%) | 16(32.7%) | 8(16.3%) | 18(36.7%) | 22(44.9%) | 9(18.4%) | ||||
| sVCAM-1 | low | 24(53.3%) | 21(46.7%) | <.0001 | 38(82.6%) | 8(17.4%) | 0(0%) | .0001 | 38(82.6%) | 7(15.2%) | 1(2.2%) | <.0001 |
| high | 5(10.6%) | 42(89.4%) | 21(44.7%) | 18(38.3%) | 8(17%) | 15(31.9%) | 23(48.9%) | 9(19.1%) | ||||
Table III.
Serum markers analyzed as continuous variables are significantly correlated with each other.
| Marker 1 | Marker 2 | Spearman Correlation Coefficient |
P value |
|---|---|---|---|
| sCD44 | sICAM-1 | 0.669 | <0.0001 |
| sCD44 | sVCAM-1 | 0.706 | <0.0001 |
| sICAM-1 | sVCAM-1 | 0.518 | <0.0001 |
| sCD44 | β2M | 0.602 | <0.0001 |
| sICAM-1 | β2M | 0.494 | <0.0001 |
| sVCAM-1 | β2M | 0.765 | <0.0001 |
Patients With Higher Serum Marker Values Have Worse Overall Survival and Worse Progression-Free Survival
For each marker, a higher value correlated with a significantly worse overall survival (Figure 1). The median overall survival time for high sCD44 was 11.5 years, for high sICAM-1, 12.3 years and for high sVCAM-1, 12.0 years. Median overall survival times for those patients in the low serum marker groups were not reached for any of the three markers. P-values from the log-rank test were <0.002 for each marker. In a subset analysis of the follicular lymphoma patients alone, the differences in OS between the high and low serum marker groups were maintained for sCD44 and sICAM-1 (p <0.05 for each marker, data not shown). In another subset analysis done for the 63 pts (68%) with stage IV disease, elevated levels of sCD44 and sICAM-1 were associated with significantly decreased OS (p < 0.05 for each marker, data not shown). There was a trend towards worsened OS for elevated sVCAM-1 in stage IV patients, but this did not reach statistical significance (p=0.0563).
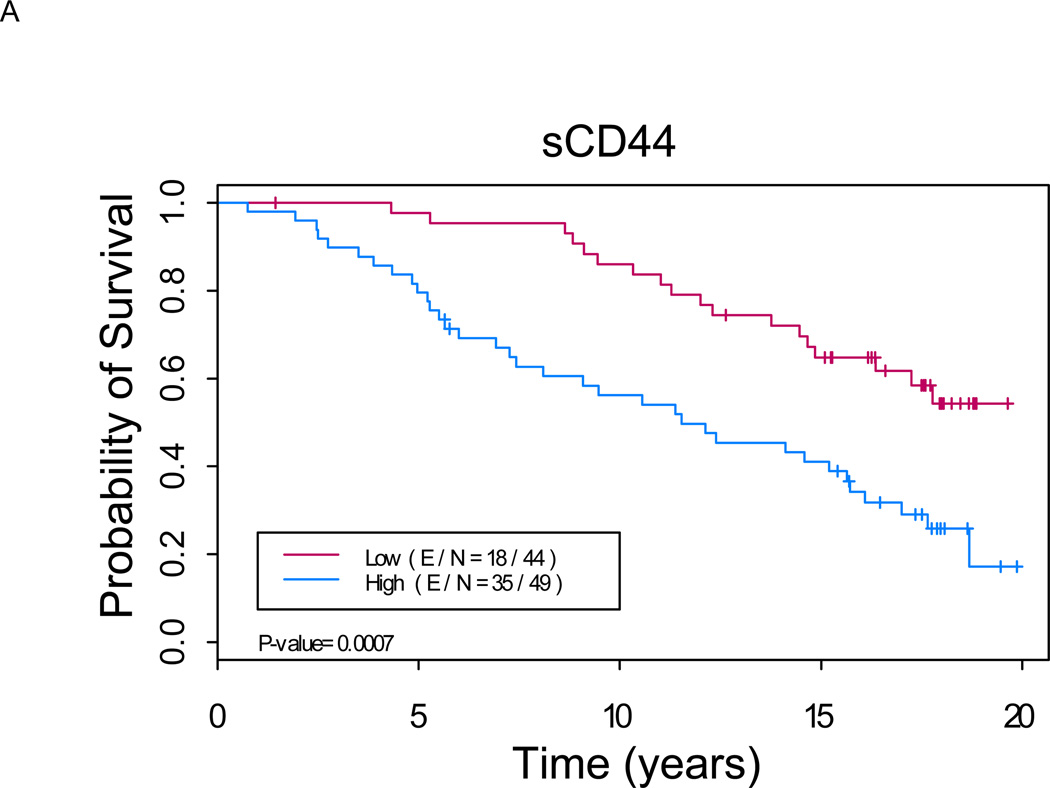
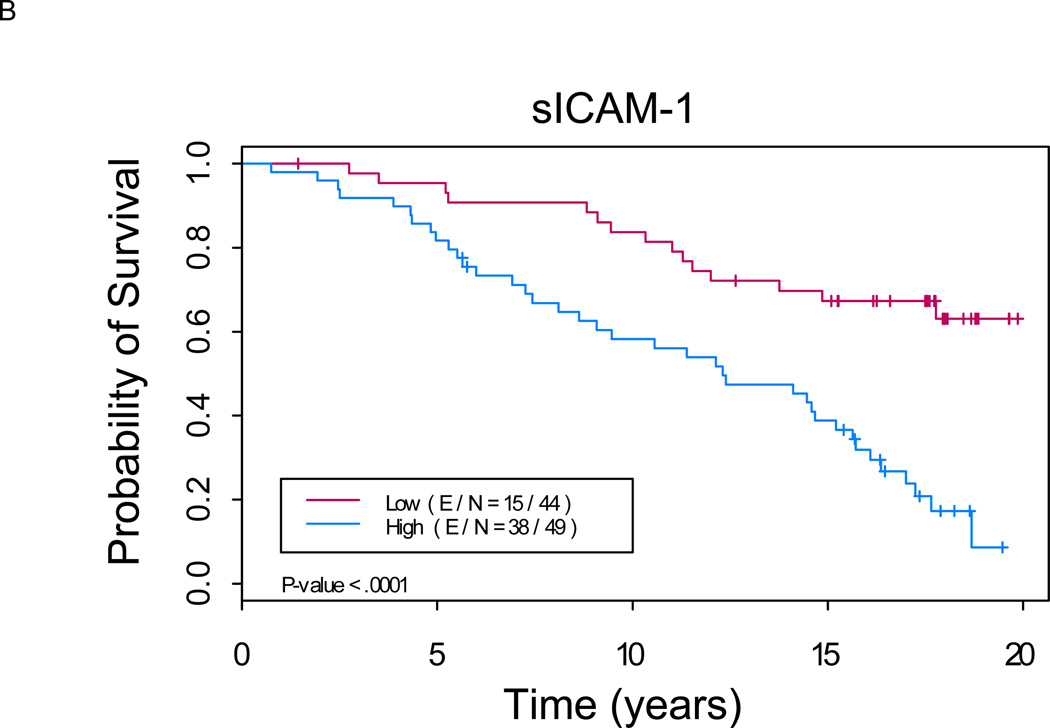
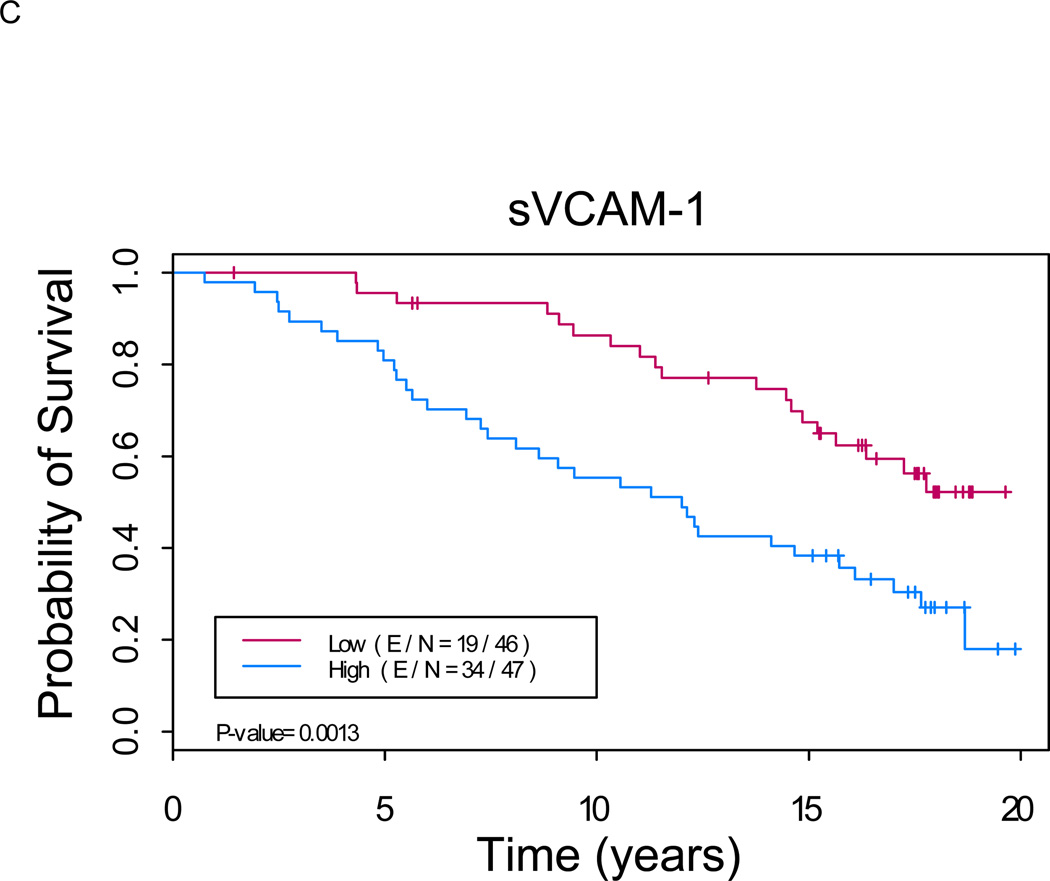
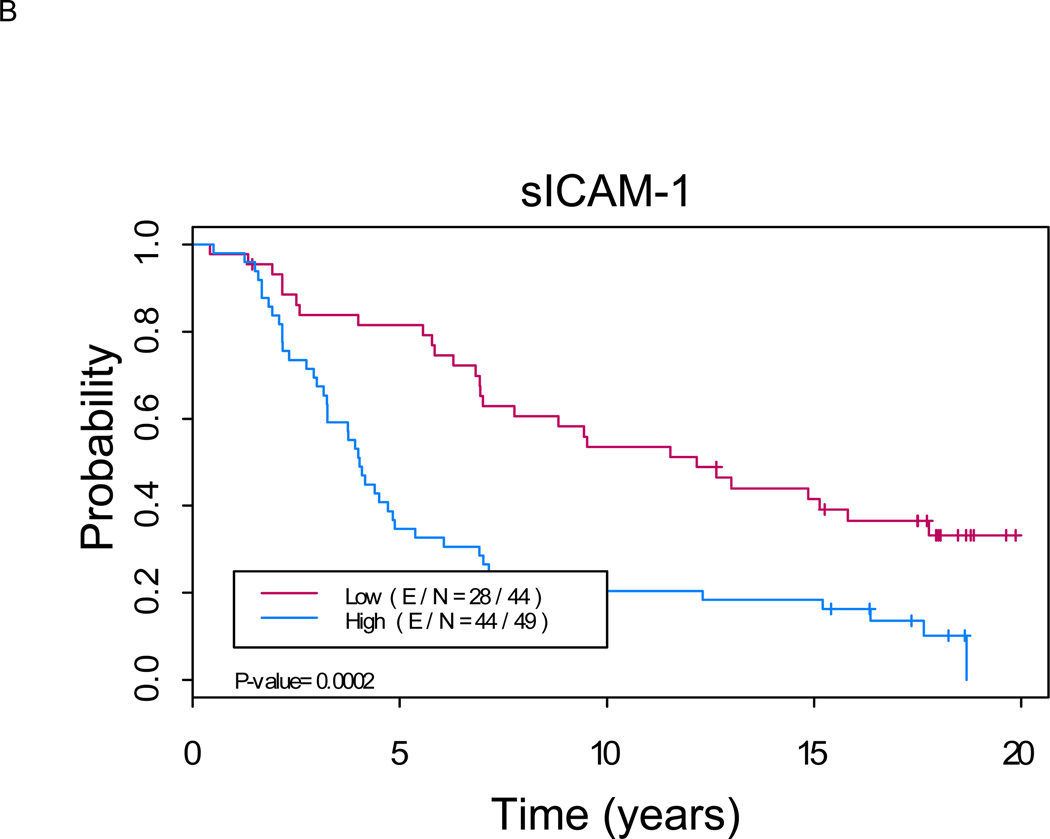
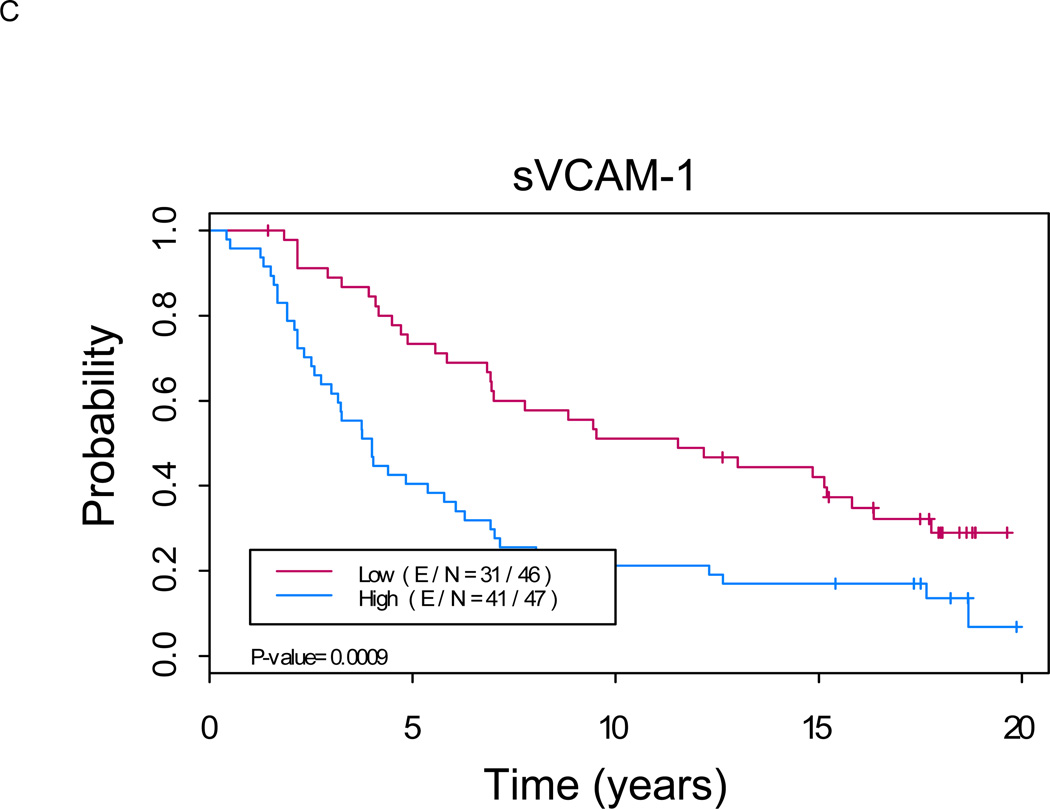
Figure 1. Kaplan-Meier analysis shows a significant difference in overall survival between patients with low versus high levels of serum markers (p < 0.01 for each marker).
A. sCD44: Median OS of 11.5 years (8.1, 16.1) in “sCD44 high” group versus not reached in “sCD44 low” group (p=0.0007). B. sICAM-1: Median OS of 12.3 years (9.1, 15.7) in “sICAM-1 high” group versus not reached in “sICAM-1 low” group (p<0.0001). C. sVCAM-1: Median OS of 12.0 years (8.1, 17.0) in “sVCAM-1 high” group versus not reached in “sVCAM-1 low” group (p<0.0001). (E/N = Events per number of patients in a particular group.)
Not surprisingly, several standard prognostic factors were also predictive of survival. Patients with stage IV disease had significantly worse median survival than those patients with stage I-III disease (12.3 vs. 18.7 years, p=0.0047). Patients with high β2M had a shorter median survival thank those with normal β2M (9.0 vs. 17.2 years, p=0.0154). Patients with a FLIPI score ≥2 also had a shorter median OS versus those with FLIPI < 2 (10.5 vs 17.8 years, p< 0.0001). In addition, age >60, presence of B symptoms and high-intermediate or high IPI were each associated with poorer OS in a univariate analysis. There were no significant differences in median survival times among the histological subtypes of indolent lymphoma (p=.4801).
Higher serum values of each of the serum markers were also associated with significantly worse PFS (Figure 2). The median PFS for high sCD44 was 3.7 years, for high sICAM-1, 4.0 years, and for high sVCAM-1, 4.0 years, compared with median PFS of 12.3, 12.2 and 11.5 years, respectively, for those patients with low serum marker levels (p <0.001 for each comparison). When the analysis was restricted to the follicular lymphoma subset, the differences in PFS between the high and low serum marker groups were maintained for sCD44 and sICAM-1 (p <0.05 for each marker, data not shown).
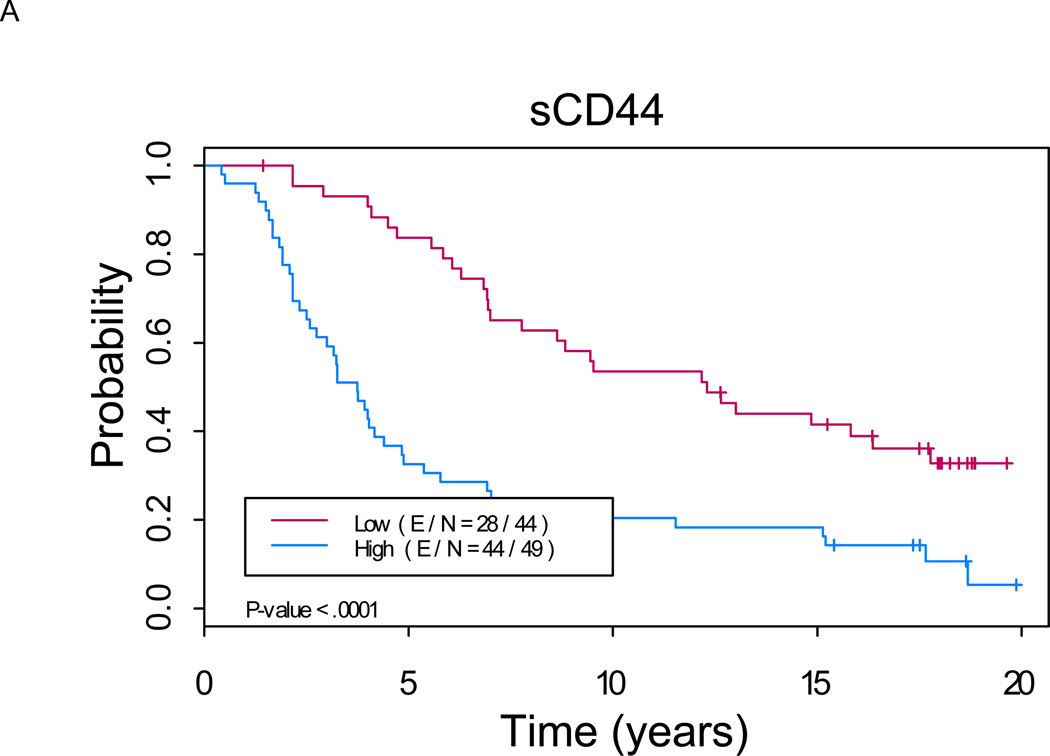
Figure 2. Kaplan-Meier analysis shows a significant difference in progression-free survival between patients with low versus high levels of serum markers (p < 0.01 for each marker).
A. sCD44: Median PFS of 3.7 years (2.7, 4.9) in “sCD44 high” group versus not reached in “sCD44 low” group (p<0.0001). B. sICAM-1: Median PFS of 4.0 years (3.3, 5.4) in “sICAM-1 high” group versus not reached in “sICAM-1 low” group (p=0.0002). C. sVCAM-1: Median PFS of 4.0 years (3.0, 6.3) in “sVCAM-1 high” group versus not reached in “sVCAM-1 low” group (p=0.0009).
Conventional prognostic factors were also predictive of PFS. Patients with stage IV disease had significantly worse median PFS than those with stage I-III disease (4.1 vs. 16.4 years, p<0.0001). Patients with high β2M had a shorter median PFS than those with normal β2M (3.6 vs. 7.0 years, p=0.0324). There were no significant differences in median PFS times among the histological subtypes of indolent lymphoma (p=.3091). Regarding PFS in stage IV patients, only higher sCD44 levels were associated with a significantly worse outcome (p < 0.01, data not shown).
Multivariate Analysis of Overall Survival and Progression-Free Survival
The final set of covariates in the analysis model for OS included FLIPI (0–1 vs. 2–4), presence of B symptoms (yes vs. no) and sICAM-1 (as a continuous variable). As shown in Table IV, with the adjustment of the variables FLIPI and B symptoms, the hazard of death was 14% higher in average with every 100 units of increase in sICAM-1(p=0.0217; hazard ratio (HR)=1.14, 95% confidence interval (CI): 1.02, 1.28). As expected, the FLIPI remained as a strong independent prognostic factor in this multivariate analysis (p= 0.0051).
Table IV.
Multivariate analysis of prognostic factors for overall survival
| Variable | p-value | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
|
|---|---|---|---|---|
| FLIPI: 0–1 vs. 2–4 | 0.0051 | 0.408 | 0.218 | 0.764 |
| B Symptoms: N vs. Y | 0.0524 | 0.420 | 0.174 | 1.009 |
| sICAM-1 | 0.0217 | 1.144 | 1.020 | 1.283 |
For PFS, the final set of covariates in the analysis included FLIPI (0–1 vs. 2–4), and sICAM-1. With the adjustment of FLIPI in the model, the hazard of an event was 19% higher on average with every 100 units of increase in sICAM-1 (p = 0.0016, Table V; HR=1.19, 95%CI: 1.07, 1.33). The effect of the FLIPI score on PFS was not significant at the 0.05 significance level (p=0.0540).
Table V.
Multivariate analysis of prognostic factors for progression-free survival
| Variable | Pr > ChiSq | Hazard Ratio |
95% Hazard Ratio Confidence Limits |
|
|---|---|---|---|---|
| FLIPI: 0–1 vs. 2–4 | 0.0540 | 0.576 | 0.329 | 1.009 |
| sICAM-1 | 0.0016 | 1.191 | 1.068 | 1.328 |
DISCUSSION
Cell adhesion molecules are essential for cellular activation and communication with the microenvironment. Specifically, CD44, ICAM-1 and VCAM-1 have been shown to play important roles in lymphocyte proliferation, migration, and survival. Therefore, these 3 molecules might potentially be surrogates for malignant lymphocyte activity. Numerous reports have shown of these markers’ value in predicting disease outcome in aggressive lymphomas. In this study we sought to elucidate the prognostic value of serum CD44, ICAM-1 and VCAM-1 levels in patients with indolent lymphoma.
Our results suggest that elevated levels of sCD44, sICAM-1 and sVCAM-1 are associated with adverse outcome. All 3 were associated with other poor prognostic factors, and elevation in each markers was significantly associated with a worse OS and a worse PFS. In addition, higher levels of sCD44 and sICAM-1 continued to confer a poorer prognosis with regards to OS in stage IV patients.
The reasoning behind the prognostic significance of these biomarkers is, at first glance simple: adhesion molecules facilitate cell migration, among other functions. However, there are emerging theories about each molecule which suggest a more complex mechanism. In the case of CD44, for example, data suggests that the presence of CD44 augments the DNA repair mechanism of lymphocytes, thereby rendering them more resistant to chemotherapy [27]. Even more interesting is that CD44 can have either pro-oncotic or tumor suppressive activity, depending on cell density [10]. Similarly, the rise in serum ICAM-1 was originally thought to be due to proteolytic release of the molecule from the malignant cell surface. More recently, however, regulatory T cells as well as natural killer and endothelial cells have been suggested as sources for the serum ICAM-1 elevation [28]. Thus serum ICAM-1 levels could reflect more the overall systemic drive towards higher risk disease versus merely the character of the malignant cell itself. Finally, in addition to promoting transendothelial migration of lymphocytes, VCAM-1 may also mediate resistance to chemotherapy in by the interaction between LFA-1 on lymphocytes and VCAM-1 on stromal tissue [29]. The stage IV subgroup analysis suggests that the elevated levels of theses markers may not simply be due to increased tumor mass but rather may reflect a qualitative difference between low and high risk disease.
Our multivariate analysis raises several issues. This is not the first study to report the independent prognostic value of ICAM-1in lymphoma [30,31], but this finding has not been reliably reproducible [32]. That said, one could hypothesize that this marker may hold the most prognostic value of the 3 serum markers studied. This would be interesting, given that, as mentioned above, ICAM-1 likely plays a role in both tumor behavior and host immune response. One could hypothesize that sICAM-1 levels reflect the deleterious contribution of both factors. This finding with sICAM-1 should be prospectively validated in a larger cohort before for any further conclusions can be made.
Otherwise, our finding that the other 2 markers are not predictive independent of the FLIPI and none are predictive independent of the IPI has several implications. Obviously these 2 indices are valuable and should continue to be used at diagnosis. However, this study is hypothesis generating. It may suggest that cellular adhesion molecules can be used in conjunction with the existing prognostic markers early on to help estimate risk. In addition, the establishment of these markers as a valid tool in initial prognosis could lay the groundwork for their development into dynamic biomarkers which may eventually be followed throughout the patient’s course, even after remission. In support of this is a study by Fukuda in which serum CD44 levels decreased significantly after therapy for NHL [33] and a study by Syrgrios et al in which serum ICAM-1 levels decreased after therapy for Hodgkin’s lymphoma [19]. Finally, Maenpaa et al have shown that elevated serum CD44 levels could be detected between 0.9 to 7.2 years before initial (de novo) diagnosis of NHL [34].
Though the measurement of serum markers is more laborious than calculating the IPI or the FLIPI, it is easier than gene expression profiling or direct tissue study by immunohistochemistry. In addition, it may be less time consuming for the patient than complete radiologic staging. However, as mentioned, our findings only begin to support the use of these biomarkers in initial prognosis; whether their course will parallel the patients’ clinical course remains to be studied.
The proposition of sCD44, ICAM-1 and VCAM-1 as prognostic factors naturally raises the question of their role in designing therapy. Interestingly, the idea of targeting these markers is not a new one and several pre-clinical and early phase clinical trials have examined this. This endeavor is most mature in the case of CD44, specifically for solid tumors, where it has been implicated in metastasis and angiogenesis [35,36] but efficacy has been limited. The potential for intervention with ICAM-1 is less clear, as it is obviously involved with tumor cell adhesion [37] but, when expressed on target tumor cells, may also promote effector NK or T cell cytotoxicity [38–40]. Finally, VCAM-1 may best be utilized not as a tumor target but a drug delivery target, as it is particularly present on tumor vasculature [41].
There are several limitations to this study. First, this is a retrospective study with a relatively small number of patients. With a larger cohort, the data may be different for different subtypes of indolent lymphoma, particularly since the subject population was comprised mainly of patients with follicular lymphoma. A larger number of patients might also help to clarify any true differences of biomarkers between genders to explain our results with sCD44. Second, the serum marker analysis was done on frozen samples which had various storage durations. Analysis of fresh samples would be necessary in future validation studies. In addition, we would need to run these samples against normal controls to better understand the distribution of each population and establish the validity of using median values to divide the group of patients. Finally, these patients were treated in the pre-rituximab era; the prognostic value of each of these markers would ideally need to be validated in the current era of targeted therapy.
We have focused on only 3 biomarkers, outside of the context of newer markers being studied in lymphoma. The literature on biomarkers in lymphoma is evolving quickly. Serum markers such as cytokines [42], free light chains [43] and other cell surface molecules hold promise as additional cogs in the prognostic machinery to optimize our evaluation of patients with lymphoma. In addition, data from gene expression profiling [44], immunhistochemistry [45] and proteomics [46] hold promise in building on the valuable platform of the IPI and FLIPI in order to better estimate prognosis and, eventually, apply more personalized therapies. Though this study offers only a few pieces in the prognostic puzzle, the proposed biomarkers are easily measureable, by relatively simple and commonly available assays that could be applied on a larger scale.
Future investigation should focus on prospectively validating the prognostic significance of these markers in a larger cohort of patients. In addition, serum CD44, ICAM-1 and VCAM-1 levels should be prospectively followed through the full course of indolent lymphomas. Each of the ELISAs mentioned in this paper are still available commercially. If measurement techniques can be standardized between institutions, a larger scale study would be possible. This would provide increased power to study the statistical interactions between these 3 markers in an effort to provide a more robust tool of increased prognostic value.
Footnotes
DECLARATION OF INTERESTS
The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Ansell SM, Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2005;80:1087–1097. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Guillermo A, Montserrat E, Bosch F, Terol MJ, Campo E, Rozman C. Applicability of the International Index for aggressive lymphomas to patients with low-grade lymphoma. J Clin Oncol. 1994;12:1343–1348. doi: 10.1200/JCO.1994.12.7.1343. [DOI] [PubMed] [Google Scholar]
- 3.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 4.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U, Pro B, Pileri S, Pulsoni A, Soubeyran P, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27:4555–4562. doi: 10.1200/JCO.2008.21.3991. [DOI] [PubMed] [Google Scholar]
- 5.Drillenburg P, Pals ST. Cell adhesion receptors in lymphoma dissemination. Blood. 2000;95:1900–1910. [PubMed] [Google Scholar]
- 6.Gribben JG. Implications of the tumor microenvironment on survival and disease response in follicular lymphoma. Curr Opin Oncol. 22:424–430. doi: 10.1097/CCO.0b013e32833d5938. [DOI] [PubMed] [Google Scholar]
- 7.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gal I, Lesley J, Ko W, Gonda A, Stoop R, Hyman R, Mikecz K. Role of the extracellular and cytoplasmic domains of CD44 in the rolling interaction of lymphoid cells with hyaluronan under physiologic flow. J Biol Chem. 2003;278:11150–11158. doi: 10.1074/jbc.M210661200. [DOI] [PubMed] [Google Scholar]
- 10.Herrlich P, Morrison H, Sleeman J, Orian-Rousseau V, Konig H, Weg-Remers S, Ponta H. CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. Ann N Y Acad Sci. 2000;910:106–118. doi: 10.1111/j.1749-6632.2000.tb06704.x. discussion 118-20. [DOI] [PubMed] [Google Scholar]
- 11.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
- 13.Tacyildiz N, Cavdar AO, Yavuz G, Gozdasoglu S, Unal E, Ertem U, Duru F, Ikinciogullari A, Babacan E, Kuzu I, et al. Serum levels and differential expression of CD44 in childhood leukemia and malignant lymphoma: correlation with prognostic criteria and survival. Pediatr Int. 2001;43:354–360. doi: 10.1046/j.1442-200x.2001.01415.x. [DOI] [PubMed] [Google Scholar]
- 14.Niitsu N, Iijima K. High serum soluble CD44 is correlated with a poor outcome of aggressive non-Hodgkin's lymphoma. Leuk Res. 2002;26:241–248. doi: 10.1016/s0145-2126(01)00122-9. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K, Niitsu N. Elevated serum levels of soluble CD44 variant 6 are correlated with shorter survival in aggressive non-Hodgkin's lymphoma. Eur J Haematol. 2000;65:195–202. doi: 10.1034/j.1600-0609.2000.065003195.x. [DOI] [PubMed] [Google Scholar]
- 16.Holland J, Owens T. Signaling through intercellular adhesion molecule 1 (ICAM-1) in a B cell lymphoma line. The activation of Lyn tyrosine kinase and the mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:9108–9112. doi: 10.1074/jbc.272.14.9108. [DOI] [PubMed] [Google Scholar]
- 17.Lalancette M, Aoudjit F, Potworowski EF, St-Pierre Y. Resistance of ICAM-1-deficient mice to metastasis overcome by increased aggressiveness of lymphoma cells. Blood. 2000;95:314–319. [PubMed] [Google Scholar]
- 18.Abdelrazik N, Fouda M, Zaghloul MH, Abbas D. Serum level of intercellular adhesion molecule-1 in children with malignant lymphoma. Med Princ Pract. 2008;17:233–238. doi: 10.1159/000117798. [DOI] [PubMed] [Google Scholar]
- 19.Syrigos KN, Salgami E, Karayiannakis AJ, Katirtzoglou N, Sekara E, Roussou P. Prognostic significance of soluble adhesion molecules in Hodgkin's disease. Anticancer Res. 2004;24:1243–1247. [PubMed] [Google Scholar]
- 20.Terol MJ, Tormo M, Martinez-Climent JA, Marugan I, Benet I, Ferrandez A, Teruel A, Ferrer R, Garcia-Conde J. Soluble intercellular adhesion molecule-1 (s-ICAM-1/s-CD54) in diffuse large B-cell lymphoma: association with clinical characteristics and outcome. Ann Oncol. 2003;14:467–474. doi: 10.1093/annonc/mdg057. [DOI] [PubMed] [Google Scholar]
- 21.Deem TL, Abdala-Valencia H, Cook-Mills JM. VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B. J Immunol. 2007;178:3865–3873. doi: 10.4049/jimmunol.178.6.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiansen I, Sundstrom C, Kalkner KM, Bring J, Totterman TH. Serum levels of soluble vascular cell adhesion molecule-1 (sVCAM-1) are elevated in advanced stages of non-Hodgkin's lymphomas. Eur J Haematol. 1999;62:202–209. doi: 10.1111/j.1600-0609.1999.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 23.Hatzistilianou M, Athanassiadou F, Agguridaki C, Catriu D. Circulating soluble adhesion molecule levels in children with acute lymphoblastic leukaemia. Eur J Pediatr. 1997;156:537–540. doi: 10.1007/s004310050657. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen I, Sundstrom C, Enblad G, Totterman TH. Soluble vascular cell adhesion molecule-1 (sVCAM-1) is an independent prognostic marker in Hodgkin's disease. Br J Haematol. 1998;102:701–709. doi: 10.1046/j.1365-2141.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 25.Loeffler-Ragg J, Germing U, Sperr WR, Herrmann H, Zwierzina H, Valent P, Ulmer H, Stauder R. Serum CD44 levels predict survival in patients with low-risk myelodysplastic syndromes. Crit Rev Oncol Hematol. doi: 10.1016/j.critrevonc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Fabris C, Falleti E, Pirisi M, Soardo G, Toniutto P, Vitulli D, Bortolotti N, Gonano F, Bartoli E. Non-specific increase of serum carbohydrate antigen 19-9 in patients with liver disease associated with increased circulating levels of adhesion molecules. Clin Chim Acta. 1995;243:25–33. doi: 10.1016/0009-8981(95)06150-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Chang MC, Hsieh RK, Chang YF, Lin J, Tsan KW. Activation of CD44 facilitates DNA repair in T-cell lymphoma but has differential effects on apoptosis induced by chemotherapeutic agents and ionizing radiation. Leuk Lymphoma. 2005;46:1785–1795. doi: 10.1080/10428190500232501. [DOI] [PubMed] [Google Scholar]
- 28.Aboul-Enein M, El-Sayed GM, El-Maghraby S, Abd-Elatif NA, Abd Elwahab GA, Elbasmy AA. Intercellular adhesion molecule-1(ICAM-1), CD44s expression and serum level of sICAM-1 in disseminated non-Hodgkin's lymphoma: correlation with overall survival. J Egypt Natl Canc Inst. 2004;16:244–251. [PubMed] [Google Scholar]
- 29.Weekes CD, Kuszynski CA, Sharp JG. VLA-4 mediated adhesion to bone marrow stromal cells confers chemoresistance to adherent lymphoma cells. Leuk Lymphoma. 2001;40:631–645. doi: 10.3109/10428190109097661. [DOI] [PubMed] [Google Scholar]
- 30.Christiansen I, Gidlof C, Wallgren AC, Simonsson B, Totterman TH. Serum levels of soluble intercellular adhesion molecule 1 are increased in chronic B-lymphocytic leukemia and correlate with clinical stage and prognostic markers. Blood. 1994;84:3010–3016. [PubMed] [Google Scholar]
- 31.Perez-Encinas M, Quintas A, Bendana A, Rabunal MJ, Bello JL. Correlation and prognostic value of serum soluble ICAM-1, beta-2 microglobulin, and IL-2alphaR levels in non-Hodgkin's lymphoma. Leuk Lymphoma. 1999;33:551–558. doi: 10.3109/10428199909058459. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen I, Enblad G, Kalkner KM, Gidlof C, Glimelius B, Totterman TH. Soluble ICAM-1 in Hodgkin's disease: a promising independent predictive marker for survival. Leuk Lymphoma. 1995;19:243–251. doi: 10.3109/10428199509107894. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda Y. Clinical significance of serum CD44 measurement in malignant lymphoma. Kurume Med J. 2001;48:65–69. doi: 10.2739/kurumemedj.48.65. [DOI] [PubMed] [Google Scholar]
- 34.Maenpaa H, Ristamaki R, Virtamo J, Franssila K, Albanes D, Joensuu H. Serum CD44 levels preceding the diagnosis of non-Hodgkin's lymphoma. Leuk Lymphoma. 2000;37:585–592. doi: 10.3109/10428190009058511. [DOI] [PubMed] [Google Scholar]
- 35.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 36.DeLisser HM. CD44: target for antiangiogenesis therapy. Blood. 2009;114:5114–5115. doi: 10.1182/blood-2009-10-246397. [DOI] [PubMed] [Google Scholar]
- 37.Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW. ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis. 2005;22:449–459. doi: 10.1007/s10585-005-2893-8. [DOI] [PubMed] [Google Scholar]
- 38.Textor S, Accardi R, Havlova T, Hussain I, Sylla BS, Gissmann L, Cerwenka A. NF-kappa B-dependent upregulation of ICAM-1 by HPV16-E6/E7 facilitates NK cell/target cell interaction. Int J Cancer. 128:1104–1113. doi: 10.1002/ijc.25442. [DOI] [PubMed] [Google Scholar]
- 39.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Slavin-Chiorini DC, Catalfamo M, Kudo-Saito C, Hodge JW, Schlom J, Sabzevari H. Amplification of the lytic potential of effector/memory CD8+ cells by vector-based enhancement of ICAM-1 (CD54) in target cells: implications for intratumoral vaccine therapy. Cancer Gene Ther. 2004;11:665–680. doi: 10.1038/sj.cgt.7700741. [DOI] [PubMed] [Google Scholar]
- 41.Gosk S, Moos T, Gottstein C, Bendas G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim Biophys Acta. 2008;1778:854–863. doi: 10.1016/j.bbamem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Morito T, Fujihara M, Asaoku H, Tari A, Sato Y, Ichimura K, Tanaka T, Takata K, Tamura M, Yoshino T. Serum soluble interleukin-2 receptor level and immunophenotype are prognostic factors for patients with diffuse large B-cell lymphoma. Cancer Sci. 2009;100:1255–1260. doi: 10.1111/j.1349-7006.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin W, Abraham R, Shanafelt T, Clark RJ, Bone N, Geyer SM, Katzmann JA, Bradwell A, Kay NE, Witzig TE. Serum-free light chain-a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res. 2007;149:231–235. doi: 10.1016/j.trsl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Iqbal J, Liu Z, Deffenbacher K, Chan WC. Gene expression profiling in lymphoma diagnosis and management. Best Pract Res Clin Haematol. 2009;22:191–210. doi: 10.1016/j.beha.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Seki R, Ohshima K, Fujisaki T, Uike N, Kawano F, Gondo H, Makino S, Eto T, Moriuchi Y, Taguchi F, et al. Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2009;100:1842–1847. doi: 10.1111/j.1349-7006.2009.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang MZ, Sun ZC, Fu XR, Nan FF, Fan QX, Wu XA, Geng L, Ma W, Wang RL. Analysis of serum proteome profiles of non-Hodgkin lymphoma for biomarker identification. J Proteomics. 2009;72:952–959. doi: 10.1016/j.jprot.2009.03.009. [DOI] [PubMed] [Google Scholar]