Abstract
Establishment of humoral immunity against pathogens is dependent on events that occur in the germinal center and the subsequent induction of high-affinity neutralizing antibodies. Quantitative assays that allow monitoring of affinity maturation and duration of antibody responses can provide useful information regarding the efficacy of vaccines and adjuvants. Using an anthrax protective antigen (rPA) and alum model antigen/adjuvant system, we describe a methodology for monitoring antigen-specific serum antibody concentration and avidity by surface plasmon resonance during primary and secondary immune responses. Our analyses showed that following a priming dose in mice, rPA-specific antibody concentration and avidity increases over time and reaches a maximal response in about six weeks, but gradually declines in the absence of antigenic boost. Germinal center reactions were observed early with maximal development achieved during the primary response, which coincided with peak antibody avidity responses to primary immunization. Boosting with antigen resulted in a rapid increase in rPA-specific antibody concentration and five-fold increase in avidity, which was not dependent on sustained GC development. The described methodology couples surface plasmon resonance-based plasma avidity measurements with germinal center analysis and provides a novel way to monitor humoral responses that can play a role in facilitating vaccine and adjuvant development.
Keywords: surface plasmon resonance (SPR), protective antigen (PA), antibody avidity, germinal centers
1. Introduction
Generation of a high-avidity antigen-specific antibody response is crucial for efficacy of many vaccines (Pulendran, 2004). Success of a vaccine is dependent on the strength and duration of elicited protective immunity. Protective humoral immunity against many pathogens is dependent on the establishment of long-lived plasma cells that secrete high-affinity antibodies, which are a result of B cell selection events that occur in germinal centers (GC) within B cell follicles of reactive lymphoid tissues (Kelsoe, 1996; Allen et al., 2007a; Allen et al., 2007b; Cyster and Schwab, 2012; Victora and Nussenzweig, 2012). Development of germinal centers in response to antigens (Nieuwenhuis and Opstelten, 1984) occurs over a period of days with the formation of clusters of antigen-specific B cells that undergo proliferation and somatic hypermutation of the immunoglobulin V gene and thus give rise to memory and plasma B cells that secrete high-affinity antibodies (Eisen and Siskind, 1964; MacLennan, 1994). Within GCs, B cells undergo a selection process that involves clonal expansion, somatic hypermutation and class switching (Klein and Dalla-Favera, 2008). Development and maturation of high affinity antibody responses occurs in lymphoid tissue germinal centers, where high-affinity mature B cells are positively selected, proliferate, and differentiate into memory B cells or long-lived plasma cells (McHeyzer-Williams and McHeyzer-Williams, 2005). These developments within GCs give rise to memory B cells with high-avidity B cell receptors (BCR) and plasma cells that secrete high-avidity antibodies, maintain long-term antibody production, and protect the host during secondary challenge. Thus, for a vaccine that provides protective humoral immunity, it is critical to monitor the progressive development of the affinity maturation process and to quantitate the avidity of induced antibody responses following both a single or multiple (i.e. boosting) antigen immunization regimen.
The model antigen utilized in this study was recombinant Bacillus anthracis protective antigen (rPA), the predominant immunogenic component of the anthrax vaccine. Anthrax pathogenesis is mediated by two B. anthracis toxins: edema toxin and lethal toxin. Function of both toxins requires complex formation with PA. The current vaccine for anthrax, Anthrax Vaccine Adsorbed (AVA), is a cell-free filtrate of an attenuated B. anthracis culture adsorbed to alum. AVA contains PA as well as the other functional components of edema and lethal toxins (Friedlander et al., 2002), which may account for frequently reported adverse injection site reactions (Pittman et al., 2001; Wasserman et al., 2003; Sever et al., 2004). In addition to the occurrence of adverse reactions, anthrax vaccination also requires an inconvenient administration regimen of six doses over eighteen months followed by yearly boosters For these reasons, development of more effective vaccine/adjuvants and a more convenient regimen for administration are required. A recent study in rhesus macaques indicated that a 3-dose IM injection can induce sustained responses and long-term protection against inhalation anthrax (Quinn et al., 2012, Clin. Vaccine Immunol., 19(11):1730).
Successful vaccination regimens result in antibody responses that are robust in both quantity and quality. Avidity is an assessment of antibody quality that is influenced by antibody valency and affinity of antibody-antigen binding. High-avidity antibody responses to vaccination, measured by traditional avidity ELISA or surface plasmon resonance (SPR), correlate with improved antibody function, as assessed by in vitro neutralizing activity (Kasturi et al., 2011; Mouquet et al., 2012) or by protection from challenge in an in vivo model (Kasturi et al., 2011). Thus, antigen-specific antibody avidity following vaccination is a critical surrogate of protection that must be monitored in experimental vaccine studies (e.g. animal models and humans).
In the present study we have demonstrated that SPR technology can be readily used to measure antibody avidity and concentration in a large number of individual (not pooled) longitudinal murine serum samples using a small sample volume (1-10 μL). By simultaneously measuring plasma antibody avidity and histologically assessing germinal center development in draining lymph nodes, we have described a methodology for the evaluation of the antigen-specific response to experimental vaccines and adjuvants.
2. Materials and methods
2.1 Immunizations and serum isolation
Groups of eighteen (18) female C57Bl/6 (National Cancer Institute/Charles River Laboratories, Wilmington, MA) mice at 8-12 weeks of age were subcutaneously immunized with saline, 5 μg recombinant anthrax protective antigen (rPA; List Biological Laboratories, Inc., Campbell, CA) alone or with 1.3 mg alum (Alhydrogel; Sigma, St. Louis, MO). On day 71 post-immunization, three mice from each group were given a boost of rPA (no adjuvant) at the same dose as the primary immunization (see Fig. 1). All animal studies were performed in accordance with approved Duke IACUC protocols in the AAALAC-certified Duke Division of Laboratory Animal Resources vivarium (Durham, NC).
Fig. 1.
Groups of eighteen (18) mice were subcutaneously immunized on day 0 with saline (not indicated), rPA alone, or rPA with alum adjuvant. On day 71 post-immunization, three mice from each group were given a boost of rPA only at the same dose as the primary immunization. Serum samples were collected from three mice per group at the indicated days post-immunization. In addition, 2 mice per group were sacrificed for lymph node collection at times indicated by arrows.
Blood samples were collected from three mice from each group by submandibular puncture at indicated time points. Samples were coagulated at room temperature for 1-2 hours. Serum was collected after a 10 minute centrifugation at 3,000 rpm in a microcentrifuge and stored at −80°C until analysis. All serum samples were analyzed individually and were not pooled.
Four draining lymph nodes (brachial and inguinal) were harvested from two mice from each group at the indicated time points for immunohistochemistry. Mice were sacrificed according to approved Duke IACUC protocols.
2.2 SPR Binding measurements
Surface plasmon resonance binding and kinetic measurements were carried out on a BIAcore™ 3000 instrument. Data analysis was done using BIAevaluation version 4.1 software (BIAcore/GE Healthcare, Pittsburgh, PA). Assays were performed in the Duke Human Vaccine Institute Biomolecular Interaction Analysis Shared Resource Facility (Durham, NC).
CM5 sensor chip (BIAcore/GE Healthcare, Pittsburgh, PA) immobilization was carried out on the BIAcore 3000 in manual mode at a flow rate of 5 μL/minute with 0.22 μM filtered, degassed, sterile 1X PBS. Using amine coupling chemistry, flow cell (Fc) 1 was activated with a 50 μL solution of N-Hydroxysuccinimide/N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide (NHS/EDC), followed by an injection of approximately 10 μL recombinant anthrax lethal factor (rLF; List Biological Laboratories) at a concentration of 10 μg/mL in 10 mM sodium acetate buffer, pH 4.5. Flow cell 2 was activated in the same way, followed by a 35 μL injection of rPA at 10 μg/mL in sodium acetate, pH 5.0. This process was repeated for flow cells 3 and 4. After immobilization, 40 μL Ethanolamine-HCl was injected over all surfaces to block any remaining active sites on the sensor chip surface. Post-ethanolamine response for all flow cells was approximately 3500 response units (RU).
A method was written in the BIAcore control software to run all monoclonal antibody and serum samples. The flow rate was set to 10 μL/min over all flow cells. For each cycle, 20 μL of sample was KINjected, optimized injection for kinetic measurements. Report points were taken 5 seconds before injection start to establish baseline, 15 seconds after injection end for an early stability measurement, and 595 seconds later for a late stability measurement. After 600 seconds of dissociation time to monitor bound complex, the flow rate was changed to 50 μL/min. A 5 μL QUICKinject of 25 mM NaOH was run to regenerate chip surfaces. Flow rate was then decreased to 10 μL/min for an additional 450 seconds and another report point was taken at the end of the cycle.
Two previously tested anti-PA monoclonal antibodies, 27H11 and 3F11 (Staats et al., 2007) were run at 100 μg/mL to assess immobilized chip activity. Serum samples were diluted 1:50 and run by an automated method. 3F11 at a concentration of 100 μg/mL was run in-line with samples at an interval of every 15 cycles to correct for any degradation in sensor chip activity. After all samples were run, 3F11 was titrated 0.1-350 μg/mL to generate a standard curve to calculate equivalent concentration of rPA-specific antibody in experimental serum samples. Avidity score was determined using the ratio of binding response (response unit, RU) to dissociation rate (Avidity score = Binding response/kd , in RU.s). Using a serum sample from the PA+Alum group collected at day 28 as an example, the ratio of the maximum binding response of 528.8 RU to the dissociation rate of 8.06×10−3s−1 results in an avidity score of 6.56×104 RU·s.
2.3 Antigen-specific enzyme-linked immunosorbent assays (ELISA)
Two-fold serial dilutions of test sera were performed in 384-well plates that were coated with rPA and blocked for two hours at room temperature with carbonate bicarbonate buffer with 3% (w/v) non-fat dry milk. Plates were incubated overnight at 4°C then washed four times with phosphate-buffered saline plus 0.1% Tween-20. Horseradish peroxidase-conjugated goat anti-mouse total Ig polyclonal antibody (Southern Biotech, Birmingham, AL) was added to the plates at a 1:4,000 dilution. Plates were incubated at room temperature for two hours and washed four times. The substrate solution (TMB Microwell Peroxidase Substrate System, Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was prepared according to manufacturer’s instructions and added to the plates. After 10 minutes at room temperature, the peroxidase reaction was stopped by the addition of 10 μL/well of 2N sulfuric acid. Optical density (OD) values were read within 15 minutes at 450 nm using a Perkin Elmer Victor3 plate reader (Waltham, Massachusetts). Endpoint titer was determined to be the reciprocal of the highest serum dilution at which the OD value was equal to or greater than three times the average background OD value.
2.4 Anthrax toxin neutralization assay (TNA)
The anthrax toxin neutralization assay procedure was adapted from previously published methods (Staats et al., 2007) with the help of Dr. Herman Staats (Duke University) and Freyja Lynn (NIAID, NIH). Approximately 18 hours prior to the start of the assay, mouse macrophage J774A.1 cells were seeded at 5 × 105 cells/well in 384-well plates in phenol red-free DMEM plus 1.5 g/L sodium bicarbonate and 10% FBS. In a separate plate a standard curve of lethal toxin was prepared by making 1:1.2 dilutions in tissue culture medium of a solution containing 0.25 μg/mL of rPA and 0.20 μg/mL of rLF. Each plate contained a human rPA-immune reference serum (NR-719; BEI Resources, Manassas, VA) for assay validation. Two-fold serial dilutions of the reference serum and each test sera were prepared in tissue culture medium and incubated with 0.05 μg/mL rPA and 0.04 μg/mL rLF in a volume of 200 μL at 37°C for 30 minutes. The standard curve and test sera mixtures were then added to the cells and incubated at 37°C for four hours. Cell viability was assayed by adding 5 μL/well of CellTiter 96 Aqueous One Cell Proliferation Assay solution (Promega Corporation, Madison, WI) followed by a two-hour incubation at 37°C. After addition of 5 μL/well of 10% SDS, absorbance at 490 nm was read for each plate using a Perkin Elmer Victor3 plate reader.
Percent neutralization was calculated by subtracting background and then dividing the OD value for the test sera by the OD of the Cells Only control. The 50% neutralization titer (NT50) was calculated by linear regression analysis and is reported as the log of the reciprocal of the serum dilution that resulted in protection of 50% of the cells. In cases where the data did not fit a sigmoidal curve due to low or undetectable neutralization, the NT50 was not determined.
2.5 Immunohistochemistry of lymph nodes
Mouse lymph nodes were harvested, snap-frozen in OCT compound (Sakura Finetek USA, Inc., Torrance, CA) and stored at −80°C. Tissue sections of 5 μM thickness were thaw-mounted onto glass slides and air dried at room temperature. Slides were fixed in a 1:1 100% Ethanol/Acetone (histological grade) solution at −20°C for five minutes, followed by air drying. Sections were then blocked with 2.5% rat serum and 2.5% goat serum in 1X PBS for 30 minutes at room temperature, followed by an overnight incubation with primary antibodies at 4°C in a humid chamber. Following two washings with 1X PBS/0.1% Tween-20 and one wash with 1X PBS, slides were incubated with secondary reagents for approximately one hour at room temperature, followed by washing. B220 was detected with Biotin anti-mouse/human CD45R (B220; eBioscience, San Diego, CA) at a 1:500 dilution and streptavidin-AlexaFluor 350 (Invitrogen, Carlsbad, CA). Germinal center B cells were visualized with anti-mouse T and B cell activation antigen (GL-7) conjugated to FITC (BD Biosciences, San Jose, CA) at 1:200 and anti-FITC-AlexaFlour 488 (Invitrogen) at 1:500. CD4 was detected using Alexa Fluor® 647 anti-mouse CD4 (L3T4; 1:100) (eBioscience) at 1:200. Slides were mounted with Fluoromount-G (Southern Biotech, Birmingham, AL), coverslipped and imaged on a Nikon TE2000 deconvolution microscope (Melville, NY).
3. Results
3.1 Avidity measurement of rPA-specific antibody responses in post-immune sera
In this study, quantitative assessment of antigen-specific responses in individual serum samples from rPA +/− alum immunized mice was carried out by measuring binding responses and relative antibody concentration. Qualitative analysis was done by calculating the avidity score as defined by the product of binding responses in Response Units (RU) and the dissociation rate (kd). Two previously described monoclonal antibodies specific for rPA, 3F11 and 27H11 (Staats et al., 2007), were used to determine that the directly immobilized rPA and recombinant lethal factor (rLF) from B. anthracis were active on the CM-5 sensor surfaces and gave specific binding when compared to the control rLF surface (Supplementary Fig. 1). As previously described, on the immobilized rPA surface, we could measure binding of two mAbs with strikingly differing kinetics (3F11 vs 27H11, Supplementary Fig. 1), and thus demonstrating that the assay would allow monitoring of qualitative differences in antibody responses based on dissociation rates.
Serum samples were assayed for binding to immobilized rPA on the sensor surface. Given that the immunization cocktail did not include rLF, any binding to the rLF-coated surface was subtracted as nonspecific binding (Supplementary Fig. 1C) as described above. These rPA binding data were used to assess the magnitude of serum antibody response in terms of binding response units (RU) and equivalent concentration of rPA-specific antibodies.
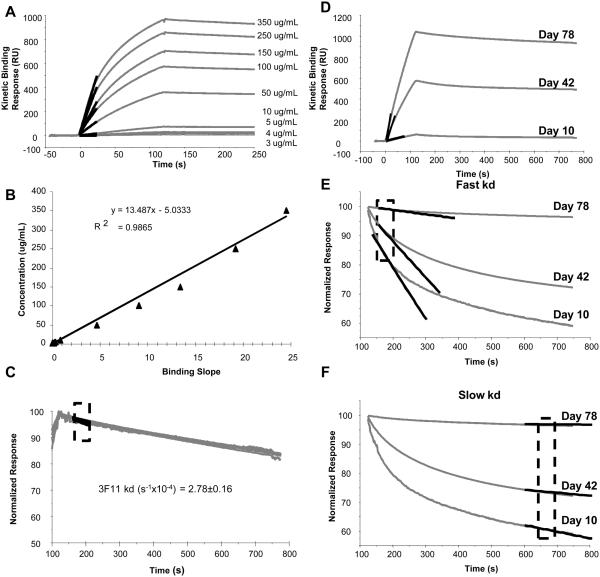
Antigen-specific antibody concentration was determined using the binding response measured during the early mass transport-limited binding phase of the SPR sensorgram (Karlsson et al., 1991). Binding rate (slope) measured during this phase reflects diffusion rates that are dependent on antibody concentration but not binding kinetics (Karlsson et al., 1991). This is evident in binding response curves from a titration of known concentrations of the 3F11 monoclonal antibody (Fig. 2A), which shows a linear relationship between binding slope and antibody concentration (Fig. 2B). The standard curve of known 3F11 anti-PA monoclonal antibody concentrations was used to calculate relative concentration (equivalent μg/mL) of rPA-specific antibodies in polyclonal serum samples from experimentally immunized mice.
Fig. 2.
Calculation of antibody concentration and dissociation rate. A, Anti-rPA monoclonal antibody 3F11 was run at known concentrations over r-PA immobilized sensor surface. The highlighted region shows the slope calculated from a 10-second window during the first 30 seconds of the injection, when binding is limited by mass transfer. B, Binding slopes derived from data shown in A were plotted against the known 3F11 concentrations to generate the standard curve. The standard curve was used to calculate equivalent concentration of rPA-specific antibody from binding response slopes measured in sera. C, Dissociation rate constants (kd) were measured in a 50-second window during the early phase (after injection), shown in the highlighted region. Mean and standard error of kd measurement of 3F11 was calculated from a range of 10-350 μg/mL and is shown to be independent of concentration. D, Representative binding curves of polyclonal sera from three different post immunization time. The measured slope of each of the binding response is highlighted. E Biphasic dissociation phase of each the polyclonal sera and the fitted slope for kd measurement of the fast component is shown. The kd of the fast component was calculated within the boxed region. F, The slower component of the dissociation phase is highlighted and the fitted curve for kd measuerment is shown. The measured kd for both the fast and the slow components are given in Table 1.
SPR binding response data was also used to assess the quality of rPA-specific antibody response in terms of avidity by measuring dissociation rate constants (kd). Since the dissociation phase of several binding responses was bi-phasic, we first compared the kd (dissociation rate constant) values obtained during the early (fast) and the late (slow) phases of the dissociation curves (Fig. 2C, 2D). The dissociation phase of each of the binding curves was normalized to maximum (100%) binding, and dissociation rates were calculated by curve-fitting analysis to a 1:1 dissociation model (Langmuir model). A better resolution between early (day 10) and late (day 78) time points was seen for the early dissociation phase compared to the late dissociation phase (compare Fig. 2C to 2D). Since the effect of the bivalency of IgG antibodies (the predominant class induced, data not shown) and other secondary binding effects like rebinding on avidity are more pronounced in the later phase of the curve, dissociation rate constants were determined using the early dissociation phase as shown in Fig. 2C.
3.2 SPR analysis of primary and secondary responses to rPA
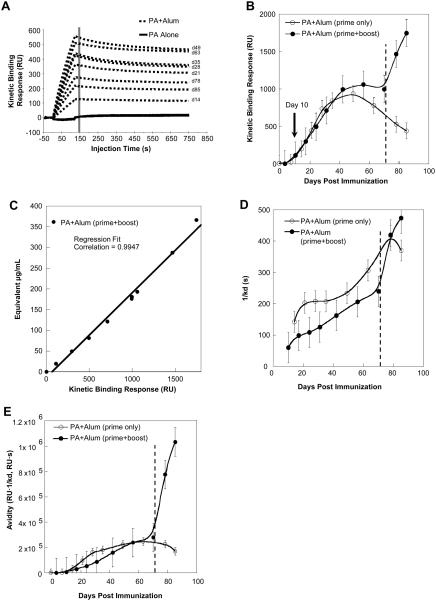
The methods described above enabled analysis of multiple parameters of rPA-specific antibody response in mice following primary immunization with rPA with or without alum and rPA only boost, which was given on day 71 post-immunization. A comparison of binding response curves for individual serum samples collected over time following rPA alone or rPA + alum immunization shows the effect of time and adjuvant on antibody response development (Fig. 3A). Post-immune sera from rPA + alumimmunized mice displayed higher binding responses compared to sera from mice immunized with rPA alone, indicating that antibody response in this group is of higher magnitude and avidity. Binding responses of the rPA alone group were too low to be used for further determination of antibody quantity and quality.
Fig. 3.
Primary and secondary antigen-specific antibody response over time following rPA + alum immunization. A, Binding response curves for individual serum samples collected over time following rPA alone or rPA + alum immunization show the effect of time and adjuvant on antibody response development. B, Maximum binding response (derived from data shown in A) of serum antibodies from mice given rPA + alum with or without rPA boost on day 71. C, Positive correlation of binding response and equivalent antibody concentration in rPA + alum + rPA boost mice over time. D, Dissociation rates of serum antibodies collected over time following rPA + alum immunization alone or with antigen boost on day 71. E, Serum antibody avidity score over time in mice given primary rPA + alum immunization only or those given primary immunization + rPA boost on day 71. Error bars indicate standard error of the mean of 3 (n=3).
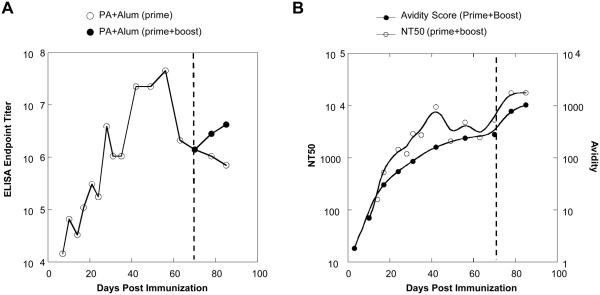
Maximum binding responses for the rPA + alum group were measured during a 10-second period at the end of a two-minute injection and recorded in RU, as indicated by the line in Fig. 3A. This value is plotted against time post-immunization in Fig. 3B to illustrate development of rPA-specific antibody responses. Although antigen-specific antibodies were detected during the first 10 days post-immunization, the magnitude of rPA-specific antibody binding response was relatively low (<100 RU). The rPA-specific response to a single immunization followed a predicted bell-shaped curve, with a peak response, approximately 10-fold higher RU than the earliest response, was reached around day 40 but gradually declined over time in the absence of boost (Fig. 3B). Following administration of antigen only boost however, binding response rapidly increased, indicating a strong secondary humoral response to the antigen. The highest detected binding response of the prime + boost group was at the last experimental time point, which was 14 days post-boost. At this point, maximum binding response was about two-fold higher than the peak primary response. In comparison, traditional rPA-specific endpoint titer data from ELISA (Fig. 4A) also indicated a two-fold increase following PA boost during the secondary response (serum dilution of 1:1.32×106 at day 70 to 1:3.33×106 on day 85; Fig. 4A, closed circles). These data demonstrated that the secondary antibody binding response was more robust in strength and temporal kinetics than the primary response.
Fig. 4.
Comparison of SPR to traditional methods. A, ELISA endpoint binding titers of rPA-specific serum antibodies collected over time following primary immunization with and without antigen boost on day 71. B, NT50 (50% neutralizing titer) and antibody avidity of sera collected over time following immunization with rPA + alum and antigen-only boost. Data represents the mean of three individual mice.
A strong positive correlation exists between the kinetic binding response and SPR-determined equivalent antibody concentration throughout the primary and secondary responses (Fig. 3C). This suggested that the increase in binding responses measured in SPR RU reflects predominantly the increase in antibody concentration. Thus taking into account both binding responses and dissociation rates would better represent the overall avidity of the rPA-specific antibody responses. Indeed serum antibodies produced during the primary response had lower dissociation rates (indicating weaker affinity binding) compared to secondary response antibodies (Fig. 3D), although these differences were small and not significant.
Measurements of avidity score, which is calculated using the ratio of the binding response to the dissociation rate (binding response in RU/kd, s-1), revealed that the earliest antibodies produced during the primary response in the PA + alum group have very weak avidity. The avidity gradually increased during the first 20 days thereafter (Fig. 3E). In the absence of boost, the avidity peak, once reached, was relatively steady during the course of the study, although antibody concentration did decline. However, following the boost there was a rapid increase in avidity (about five-fold, Fig. 3E), which was due to both improvement in dissociation rates and increase in concentration (Fig. 3C & 3D). The observed increase in antibody avidity during the secondary response was rapid when compared to the primary response (Fig. 3E). The lower average avidity observed during the primary response likely reflects a broader polyclonal B cell response, with expansion of B cells with BCRs of varying level of affinities.
3.3 Avidity of plasma antibody responses correlates with toxin neutralization
Antigen-specific antibodies of higher avidity are likely to have higher functionality in terms of neutralizing infection. Using in vitro anthrax lethal toxin neutralization assays, the pattern of avidity maturation as detected by SPR is shown to correlate with neutralizing activity in immunized sera (Fig. 4B). For each serum sample, the serum dilution that achieved in vitro neutralization of 50% of anthrax lethal toxin (NT50) was determined. The toxin neutralization activity in the sera mirrored the gradual increase in binding avidity. The peak neutralization activity during the primary response was at a time point when the plasma antibody avidity had reached a plateau (Fig. 4B). These data, therefore, suggested that the toxin neutralization is mediated by high affinity antibodies that were made around day 40 post- immunization and that the functional activity in sera mirrors the avidity maturation profile.
3.4 Lymph node germinal center response and plasma antibody avidity
Progression of the germinal center reaction in draining lymph nodes following primary immunization and antigen boost was observed using immunohistochemical staining. Histological development of the B cell response was visualized using fluorescent staining of B cell markers B220 and activated germinal center (GC) cell marker, GL7 (Fig. 5). Activated B cells express the GL7 epitope, a glycan moiety containing terminal sialic acid (Naito et al., 2007), but mature B cells do not, and therefore GL7 serves as a marker for germinal centers in immunized lymph nodes (Han et al., 1996; Pasare and Medzhitov, 2005). Localization of T cells around and within the B cell follicles was observed by staining for the CD4 T cell marker (Fig. 5).
Fig. 5.
Germinal center response reflects antibody avidity maturation. Draining lymph node tissue was harvested on days 10, 14, 28, 70, and 85 from 2 mice from groups immunized with rPA alone (A, C) or rPA + alum (B, D) and boosted with rPA alone on day 71. Tissue sections were stained for B220+ (blue) B-cells, B-cells, GL7+ (green) germinal center B-cells and CD4+ (red) T-cells. Representative images of lymph node B cell follicles from each of the time points are shown. Scale bars indicate 100 μm.
Immunofluorescent staining of the draining lymph nodes revealed several differences in the two immunization groups, rPA alone and rPA + alum. During post-immunization days 1-14, only B220+ cells and CD4+ T cells were observed in the cortical and medullary region of the lymph nodes of the rPA alone group (Fig. 5A). The earliest GL7+ cells to appear in the rPA group were observed in a single follicle on a representative day 10 section, where a small region of clustered GL7+ cells was present. A second follicle having much smaller and more diffused cluster was also detected. Germinal center development occurs over the following two weeks and is evident in day 28 sections, where multiple GL7+ follicles were observed (average of 3 in two lymph nodes). This response is greatly accelerated in the rPA + alum group (Fig. 5B).
Although diffused GL7+ cells were observed earlier, appearance of multiple robust GL7+ follicles took only 14 days (average of 3.5 in two lymph nodes). The appearance of these mature GC follicles in the rPA + alum group was at least a week earlier than those observed in the rPA alone group. This observation corresponds to the higher magnitude and avidity of the rPA-specific antibody response in the adjuvant group compared to antigen only group (Fig. 3A). These data suggest that the adjuvant alum expedites the B cell development process resulting in early appearance of GC cells.
Interactions between B and T cells drive B cell activation and selection of high-affinity antigen-specific B cell clones. The timing of the infiltration of CD4+ T cells into the B cell follicles was also not the same for the two immunization groups (Fig. 5C). The infiltration of T cells and subsequent events occurred rapidly in the rPA + alum group and thus can only be followed closely in the rPA alone group. Tissue sections from this group showed a progressive increase in infiltration of CD4+ T cells with time, with T cells appearing within the follicle before the detection of GL7+ cells. GL7+ clusters were also observed in close proximity to the infiltrated CD4+ T cells, suggesting B-T cell contact in this region and the resulting appearance of germinal center B cells. GC clusters became more compact by day 28 and fewer CD4+ T cells were present at this time. Such a slow progression of events was not clearly observed in the rPA + alum group, as GL7+ cells were observed much earlier. CD4+ T cells were detected within the early GC clusters of rPA + alum tissues and these cells continued to be present until day 14.
At day 70, although an increased number of T cells were observed, signs of dissipating GC clusters were evident in both groups. Staining of lymph nodes harvested at day 85, 14 days after administration of antigen only boost, shows redevelopment of GCs during the secondary response but continued GC dissipation in lymph nodes of unboosted animals (Fig. 5C). This pattern is supported by SPR data showing rapid increase in binding response during the secondary response in rPA-boosted animals (Fig. 3B). However, the robustness of GCs after boost is not as strong as that of the primary response. Therefore, the observed increase in serum antibody binding avidity and equivalent concentration during the secondary response measured using SPR (Fig. 3C) likely indicates the secretion of high affinity antibodies by long-lived plasma cells and memory B cells. Thus indicating that the secondary response is not dependent on new GC formation, which is consistent with the rapid increase in binding responses and avidity as measured by SPR analysis. Overall the avidity maturation profile as measured by SPR reflects the histological changes that occur in the draining lymph nodes following immunization with rPA+alum.
4. Discussion
Development of assays to monitor the quality and quantity of antibody responses is critical for vaccine development and for selecting effective adjuvants. A full qualitative analysis of an antigen-specific IgG pool in a polyclonal serum would require information on clonality, relative concentrations, and binding affinities. Recent advances in techniques to isolate antibodies following infection or vaccination have led to clonal lineage studies of novel antibodies and form the basis of infectious disease vaccine immunogen design (Verkoczy et al., 2011; Bonsignori et al., 2012). Furthermore, correlate of protection determination in vaccine trials (Haynes et al., 2012) is dependent on assays to determine titers and specificity of antibodies in vaccinated serum samples. Therefore, sensitive assays that monitor antibody responses in longitudinal samples and provide information on relative antibody concentrations and affinities would assist in selecting samples for further evaluation of the nature of induced antibodies and help determine the efficacy of vaccines and adjuvants.
Described herein is a novel approach to measuring characteristics of the antigen-specific antibody response to a neo-antigen (rPA), including avidity and relative antibody concentration, using surface plasmon resonance. The methodology described here has made possible high-throughput analysis of antibody avidity in a large number of serum samples from a comprehensive longitudinal murine study. Specifically, the relative concentration and avidity of an antigen-specific antibody response produced from 1 to 85 days after immunization with rPA with the common adjuvant alum and with or without antigen boost was assessed. Quantitative and qualitative measurements of humoral response were correlated with histological changes in lymphoid tissue germinal centers, which is the center of the maturation of B cell responses and toxin neutralizing functionality. Our described methodology couples antibody avidity measurements with histological analysis of GC development and offers a potentially useful tool for monitoring the development of high avidity antibody responses against experimental vaccines and adjuvants.
Surface plasmon resonance (SPR) measurements are routinely used to measure kinetic rate constants and dissociation constants (Kd) of bi-molecular interactions, including antibody-antigen binding (Alam et al., 2007; Alam et al., 2009; Alam et al., 2013). Although the majority of SPR studies use purified recombinant antibody and antigens, the application of the SPR platform in studying antibody responses in complex mixtures like hybridoma culture supernatants (Lullau et al., 1996; Canziani et al., 2004; Safsten et al., 2006; Leonard et al., 2007) or polyclonal Ig preparations from human sera samples have been described (Flynn, 2011; Kasturi et al., 2011; Haynes et al., 2012). Typically, serum antibody analysis has been done using traditional methods such as antigen-specific enzyme-linked immunosorbent assays (ELISA), which allow a researcher to determine antigen-specific antibody titer relative to a predetermined endpoint or EC50 values. Endpoint binding titer data is not always amenable to comparison between research groups, as the endpoint can be subjectively set and varies with research groups. Furthermore, traditional ELISA methods may fail to detect low affinity interactions with fast dissociation rates and often EC50 calculation is limited to a symmetrical sigmoid curve (using 4-parameter curve fitting) and data from non-symmetrical curves may be less reliable. Other indirect immunoassays for measuring binding strength include use of chaotropic or dissociating agents like diethylamine (Barkoff et al., 2012) or NH4SCN (Almanzar et al., 2013; Fried et al., 2013; Prelog et al., 2013). In this modified ELISA immmuoassay, a relative avidity index is calculated after dissociation of low avidity antibodies (Kneitz et al., 2004; Fried et al., 2013; Prelog et al., 2013). A notable caveat associated with assays that use dissociating agents is that antibodies that bind to conformational epitopes can be more easily dissociated from their bound complex due to changes in the epitope conformation. Conformational changes at a location remote from the binding site may also affect the stability of the complex and therefore the measured relative avidity might not correctly reflect the inherent binding strength. To also get better resolution of avidity differences, it may become necessary to measure avidity indices using varying amounts of the dissociating agents and therefore, prolonging the assay when a large number of samples are used.
We have adopted a methodology in which high throughput can be achieved and dissociation rate is measured in real time in the absence of any denaturing agent. We observed that for the majority of the samples, particularly those harvested early and likely of lower average affinities, the dissociation phase was biphasic (Fig. 2). A distinct faster component of the dissociation phase could be identified and resolved from the slower component. The slower dissociation component could not be resolved between samples taken early from those harvested at later time points, and are very likely a result of rebinding and avidity affects due to bivalency of the IgG molecule (Alam et al., 2007). However, calculation of kd during the early phase of the dissociation curve allowed us to discriminate between binding responses that showed dissociation rates that were 10-20 fold faster (Table 1). This method for calculating dissociation rates provides better resolution than the previously described method of binding decay by comparing stability late versus stability early in binding response units (Safsten et al., 2006). It should, however, be noted that the relative proportion of antibodies will impact the resolution of the dissociation phase and the assay at best, provides average dissociation rates of the most predominant antibodies. Therefore, to account for both the binding response level, which reflects both association rate and the concentration of antigen-specific antibodies in the sera, and the stability of the bound complex, we have calculated an avidity score as a measure of relative avidity of PA-specific antibodies in each serum sample (ratio of binding response to dissociation rate). Our methodology is a semi-quantitative scoring of relative avidities and is a modified form of affinity-based ranking previously described for measuring antibody responses in either hybridoma supernatant (Safsten et al., 2006) or in polyclonal sera samples from immunized experimental animals (Flynn, 2011; Kasturi et al., 2011).
Table 1.
Fast and slow dissociation rates of serum rPA-specific antibodies.
| Days Post-Immunization | Fast kd (s−1×10−4) |
Slow kd (s−1×10−4) |
|---|---|---|
| 10 | 20.l | 2.64 |
| 42 | 11.5 | 1.92 |
| 78 | l.l7 | 0.3l7 |
Study of the effect of adjuvants on the germinal center reaction as well as on the quality and quantity of antibody response is essential for development and evaluation of vaccine adjuvants. Kasturi and co-workers showed that inclusion of adjuvants MPL and R837, ligands for TLR7 and TLR4, respectively, in a nanoparticle-based immunization regimen induced generation of increased numbers of germinal centers in mouse spleen, compared to adjuvant alone (Kasturi et al., 2011). These observations corresponded with increased quantity of neutralizing antibody and higher affinity antibodies (Kasturi et al., 2011). Although the mechanism responsible for alum adjuvanticity is still unclear, recent studies suggest that activation of the NLRP3 inflammasome and subsequent release of IL-1β, IL-18, and IL-33 is involved (reviewed by Marrack et al., 2009). In T-dependent B cell responses, it takes about three weeks for the GC to appear following a single dose of antigen (Kraal et al., 1986; Liu et al., 1991). This is consistent with our observation that much of the GC development occurs during the primary response to antigen. The early GC response is oligoclonal (Jacob and Kelsoe, 1992; Kuppers et al., 1993) and as reported earlier we found that the GC persisted for about 4 weeks following immunization. It has also been reported that while repeated boosting results in the reappearance of GCs, their size gradually diminishes (Liu et al., 1991). Thus we observed that on day 85, following the antigen boost, both a reduction in number and size of the GC in the draining lymph nodes. Overall, the histological data showed that including alum in rPA immunization enhances B cell response to antigen by expediting the infiltration of CD4+ T cells and the development of activated germinal center B cells. It is evident from the images that the germinal center response with alum is quicker and more robust; by day 14 multiple GC clusters were observed (Fig. 5).
Timing of GC appearance and growth in size coincided with gradual increases in PA-specific avidity, thus this period defined the affinity maturation stage of the B-cell response to rPA. When GC size later diminished (day 70), there was no measurable change in antibody avidity; and the rapid increase in avidity following the boost was largely due to increase in antibody concentration and not dissociation rates. The increase in antibody production in response to antigen boost is therefore likely driven by high-affinity antibody secretion by memory and long-lived plasma cells. In the early phase of the primary response, developing B cells make antibodies of differing affinities, and later development is an affinity-driven selection process that takes place in the GC and results in generation of high affinity antibodies (Dal Porto et al., 2002; Shih et al., 2002). Thus it is observed that serum antibodies produced during the primary response to immunization are of lower affinity but undergo maturation over time (Eisen and Siskind, 1964; Siskind and Benacerraf, 1969). Overall, our SPR-based avidity measurements provide an assessment of the gradual increase in antibody avidity driven by GC response, as well as a procedure to qualitatively differentiate between primary and secondary responses to immunization. SPR-based avidity measurements, together with histological analysis of GC development in the draining lymph nodes, provide two important parameters to monitor antibody responses following immunization. The methodology described here can complement currently used conventional assays and potentially be a useful tool in the development of new vaccines and adjuvants.
Supplementary Material
Supplemental Fig. 1. Binding of anti-rPA monoclonal antibodies 3F11 and 27H11 to immobilized rPA and rLF. A, Nonspecific binding of antibody to the negative control rLF surface was subtracted and, B, the resultant curves demonstrate a difference in binding kinetics. 27H11 shows fast association and dissociation, while the kinetics of 3F11 are relatively slower, indicating higher avidity. C, One representative serum sample run in a similar manner with subtraction of nonspecific binding to the rLF surface.
Acknowledgments
The authors wish to acknowledge the expert animal husbandry of Ms. Kristina Riebe. This study was funded in part by NIH HHSN266200500019C and UC6-AI58607. The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Human anti-AVA Reference Serum AVR801 (Lot #2), NR-719. Work was performed in the Duke Human Vaccine Institute’s Immune Reconstitution & Biomarker Analysis Shared Resource Facility (Durham, NC), under the direction of Dr. Gregory D. Sempowski, and the Biomolecular Interaction Analysis Shared Resource Facility (Durham, NC), under the direction of Dr. S. Munir Alam.
References
- Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Jr., Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. Journal of virology. 2013;87:1554–68. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. Journal of immunology. 2007;178:4424–35. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20234–9. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007a;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007b;315:528–31. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Almanzar G, Ottensmeier B, Liese J, Prelog M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme-linked immunosorbent assay (ELISA) using ammonium thiocyanate. Journal of immunological methods. 2013;387:36–42. doi: 10.1016/j.jim.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Barkoff AM, Grondahl-Yli-Hannuksela K, Vuononvirta J, Mertsola J, Kallonen T, He Q. Differences in avidity of IgG antibodies to pertussis toxin after acellular pertussis booster vaccination and natural infection. Vaccine. 2012;30:6897–902. doi: 10.1016/j.vaccine.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends in microbiology. 2012;20:532–9. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canziani GA, Klakamp S, Myszka DG. Kinetic screening of antibodies from crude hybridoma samples using Biacore. Anal Biochem. 2004;325:301–7. doi: 10.1016/j.ab.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annual review of immunology. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195:1215–21. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen HN, Siskind GW. Variations in Affinities of Antibodies during the Immune Response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Flynn BJ, Kastenmuller K, Willie-Reece U, Tomaras GD, Alam SM, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, Esteban M, Park CG, Trumpfheller C, Keler T, Panteleo G, Steinman RM, Seder R. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York Vaccinia virus induces ribust T-cell immunity in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried AJ, Altrich ML, Liu H, Halsey JF, Bonilla FA. Correlation of Pneumococcal Antibody Concentration and Avidity with Patient Clinical and Immunologic Characteristics. Journal of clinical immunology. 2013 doi: 10.1007/s10875-013-9870-9. [DOI] [PubMed] [Google Scholar]
- Friedlander AM, Welkos SL, Ivins BE. Anthrax vaccines. Current topics in microbiology and immunology. 2002;271:33–60. doi: 10.1007/978-3-662-05767-4_3. [DOI] [PubMed] [Google Scholar]
- Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–7. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–87. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. Journal of immunological methods. 1991;145:229–40. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–11. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Kneitz RH, Schubert J, Tollmann F, Zens W, Hedman K, Weissbrich B. A new method for determination of varicella-zoster virus immunoglobulin G avidity in serum and cerebrospinal fluid. BMC infectious diseases. 2004;4:33. doi: 10.1186/1471-2334-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G, Hardy RR, Gallatin WM, Weissman IL, Butcher EC. Antigen-induced changes in B cell subsets in lymph nodes: analysis by dual fluorescence flow cytofluorometry. Eur J Immunol. 1986;16:829–34. doi: 10.1002/eji.1830160718. [DOI] [PubMed] [Google Scholar]
- Kuppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993;12:4955–67. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard P, Safsten P, Hearty S, McDonnell B, Finlay W, O'Kennedy R. High throughput ranking of recombinant avian scFv antibody fragments from crude lysates using the Biacore A100. Journal of immunological methods. 2007;323:172–9. doi: 10.1016/j.jim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Cairns JA, Holder MJ, Abbot SD, Jansen KU, Bonnefoy JY, Gordon J, MacLennan IC. Recombinant 25-kDa CD23 and interleukin 1 alpha promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur J Immunol. 1991;21:1107–14. doi: 10.1002/eji.1830210504. [DOI] [PubMed] [Google Scholar]
- Lullau E, Heyse S, Vogel H, Marison I, vonStockar U, Kraehenbuhl JP, Corthesy B. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J. Biol. Chem. 1996;271:16300–16309. doi: 10.1074/jbc.271.27.16300. [DOI] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annual review of immunology. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:875–80. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, Sugai M, Okuno Y, Tsujimoto G, Yamaji T, Hashimoto Y, Itohara S, Kawasaki T, Suzuki A, Kozutsumi Y. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Molecular and cellular biology. 2007;27:3008–22. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P, Opstelten D. Functional anatomy of germinal centers. The American journal of anatomy. 1984;170:421–35. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–8. doi: 10.1016/s0264-410x(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Prelog M, Almanzar G, Rieber N, Ottensmeier B, Zlamy M, Liese J. Differences of IgG antibody avidity after an acellular pertussis (aP) booster in adolescents after a whole cell (wcP) or aP primary vaccination. Vaccine. 2013;31:387–93. doi: 10.1016/j.vaccine.2012.10.105. [DOI] [PubMed] [Google Scholar]
- Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunological reviews. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- Safsten P, Klakamp SL, Drake AW, Karlsson R, Myszka DG. Screening antibody-antigen interactions in parallel using Biacore A100. Anal. Biochem. 2006;353:181–190. doi: 10.1016/j.ab.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Sever JL, Brenner AI, Gale AD, Lyle JM, Moulton LH, Ward BJ, West DJ. Safety of anthrax vaccine: an expanded review and evaluation of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) Pharmacoepidemiology and Drug Safety. 2004;13:825–840. doi: 10.1002/pds.936. [DOI] [PubMed] [Google Scholar]
- Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- Siskind GW, Benacerraf B. Cell selection by antigen in the immune response. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- Staats HF, Alam SM, Scearce RM, Kirwan SM, Zhang JX, Gwinn WM, Haynes BF. In vitro and in vivo characterization of anthrax anti-protective antigen and anti-lethal factor monoclonal antibodies after passive transfer in a mouse lethal toxin challenge model to define correlates of immunity. Infection and immunity. 2007;75:5443–52. doi: 10.1128/IAI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Kelsoe G, Moody MA, Haynes BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Current opinion in immunology. 2011;23:383–90. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Wasserman GM, Grabenstein JD, Pittman PR, Rubertone MV, Gibbs PP, Wang LZ, Golder LG. Analysis of adverse events after anthrax immunization in US Army medical personnel. Journal of Occupational and Environmental Medicine. 2003;45:222–233. doi: 10.1097/01.jom.0000058345.05741.6b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Binding of anti-rPA monoclonal antibodies 3F11 and 27H11 to immobilized rPA and rLF. A, Nonspecific binding of antibody to the negative control rLF surface was subtracted and, B, the resultant curves demonstrate a difference in binding kinetics. 27H11 shows fast association and dissociation, while the kinetics of 3F11 are relatively slower, indicating higher avidity. C, One representative serum sample run in a similar manner with subtraction of nonspecific binding to the rLF surface.