Abstract
Purpose
To determine whether neuroretinal function differs in healthy adult males and females under and over the age of 50 years.
Methods
This study included one eye from each of 50 normal subjects (29 females and 21 males). Neuroretinal function was assessed using first-order P1 implicit times (IT) and N1-P1 amplitudes (AMP) obtained from photopic multifocal electroretinograms (mfERG). To assess local differences, retinal maps of local IT and (separately) AMP averages were constructed for each subject group. To examine global differences, each subject's 103 ITs and (separately) AMPs were also averaged to create whole eye averages. Subsequently, retinal maps and whole eye averages of one subject group were compared to those of another.
Results
In subjects <50 years old, neuroretinal function differed significantly between the males and females: local ITs were significantly shorter at 83 of the 103 tested retinal locations, and whole eye IT averages were shorter (P=0.015) in the females compared to the males. In contrast, no analysis indicated that the males and females >50 years old were significantly different. A sub-analysis showed that the females who reported a hysterectomy (n=5) had the longest whole eye ITs of all subject groups (P≤0.0013). In the females who did not report a hysterectomy, neuroretinal function was worse in the females >50 years old compared to the females <50 years old: local ITs were significantly longer at 62 of the 103 retinal locations tested, and whole eye IT averages tended to be greater (P=0.04). Conversely, ITs were not statistically different between the younger and older males. AMP did not differ between the sexes.
Conclusions
mfERG IT differs between males and females, depending on age group and hysterectomy status.
Keywords: electrophysiology, electroretinogram, multifocal electroretinogram, male-female differences, sex, gender
Neuroretinal function in healthy males and females is known to differ. Conventional flash electroretinograms (ERGs) have repeatedly demonstrated larger b-wave amplitudes in females compared to males.1-3 This difference manifests under scotopic conditions, mainly in subjects 31 to 60 years in age, but it is not present in subjects 20 to 30 years of age, nor in subjects 61 to 80 years of age.3
Implicit times (ITs) of conventional and multifocal ERGs (mfERGs) are not thought to differ for healthy males and females. However, results of our previous study4 suggested that IT of the control males and females might be different. In that study, we demonstrated that females possess some factor that protects them from the neurodegenerative changes (local mfERG IT delays) associated with type 2 diabetes. However, the magnitude of those differences between the diabetic males and females depended on how Z-scores were calculated. Despite similar IT averages in the normative data for the males and females, gender-specific Z-scores (normative data from males to calculate Z-scores for diabetic males and normative data from females to calculate Z-scores for diabetic females) demonstrated greater differences compared to conventional Z-scores (normative data from males and females combined to calculate Z-scores for each sex).
To our knowledge, there has been only one ERG study that has reported an IT difference between the sexes. Under photopic conditions, oscillatory potentials of conventional ERGs occurred significantly earlier in healthy females compared to males.5 That study was unusual because the subjects were young; their average age was approximately 24 years.
The latter finding suggests that some factor may enhance neuroretinal function in young females relative to their male counterparts. To be consistent with the literature, that effect must then decline or disappear with age in the females. Otherwise, studies using the ERG or the mfERG with typically older subject age ranges would have found IT differences between healthy males and females.
To determine whether younger or older males and females differ in neuroretinal function, the present study subdivided healthy males and females into those who were under 50 years of age and those who were over 50 years of age. ITs and amplitudes (AMPs) of the mfERG were then compared among the four subject groups.
METHODS
Subjects
Fifty healthy subjects, who had served as controls for previous studies,6, 7 were included in this study. They were divided into 4 groups: males <50 years (n=11), males >50 years (n=10), females <50 years (n=13), and females >50 years (n=16). Fifty years of age was used to subdivide each sex for two reasons: it allowed the four groups to be similar in size; and the age at which menopause occurs in the United States is approximately 50 years.8, 9 The average ages of the four groups (± standard deviations) were 35.6 ± 7.7, 56.5 ± 3.9, 34.6 ± 8.8, and 57.6 ± 5.0 years, respectively. Age was neither significantly different between the males and females <50 years (younger subjects), nor between the males and females >50 years (older subjects).
The female subjects denied taking birth control pills or hormone replacements. Five of the 16 females (who were >50 years, average = 56.0 ± 5.1 years) reported having had a hysterectomy. The average age of the females with hysterectomies was not significantly different (P=0.43) from the other females >50 years (58.3 years), nor was it different (P=0.85) from the males >50 years.
During recruitment, a subject was excluded if his or her refractive error was outside the range of −6.00 to +4.00 diopters (spherical equivalent); if best-corrected visual acuity was worse than 20/20; or if ocular history was remarkable for a media opacity, glaucoma, or other pathology that might affect the results. The University of California Committee for Protection of Human Subjects approved the research, and all subjects provided written consent.
Multifocal Electoretinogram
Bearse et al have previously described the mfERG methods.10 In short, a Burian-Allen bipolar contact lens electrode (Hansen Ophthalmic Development Laboratory, Coralville, IA) filled with 1% carboxymethylcellulose acquired the retinal signals. A ground electrode was clipped to the right ear lobe, and a Visual Evoked Response Imaging System (VERIS Science 4.3, EDI, San Mateo, CA, USA) recorded the mfERGs. The stimulus was a scaled 103-element hexagonal array that subtended an angle of approximately 45 degrees. The hexagons alternated between black (< 2 cd/m2) and white (200 cd/m2). The frame rate of the stimulus was 75 Hz, and the retinal signals were filtered from 10-100 Hz.
To obtain the photopic mfERGs, eyes were anesthetized with 0.5% proparacaine, and pupils were dilated with 2.5% phenylephrine and 1.0% tropicamide. While responses were recorded from one eye, the fellow eye was occluded with light pressure to prevent blinking. Only data obtained from the eye tested first was used.
The Hood and Li template-scaling method was applied to all exported waveforms.11 The waveform template was constructed from the data of the fifty subjects included in this study. The time from the onset of the local flash to the peak P1 voltage was considered the IT, and the difference between the peak P1 voltage and the preceding N1 trough was deemed the AMP.
Statistical Analysis
To create a global index of retinal function for each eye, each subject's 103 ITs and (separately) AMPs were averaged to create whole eye averages. Subsequently, a two-tailed Student's t-test was used to compare whole eye averages from one subject group to those of another. Since each analysis involved seven comparisons, P-values < 0.007 were considered significant.
To localize potential differences in IT and AMP between subject groups, retinal maps of local IT and (separately) AMP averages were constructed for each subject group. To create these maps, the average IT and AMP at each of the 103 stimulus locations were calculated for each subject group. Then, the map with 103 local means of one group was compared (location by location) to the map of another group using 95% confidence intervals calculated with the appropriate t-statistic. To make comparisons between subject groups conservative, the larger of the two groups’ confidence intervals was used to define significant local differences.
RESULTS
IT Analysis Comparing Males to Females
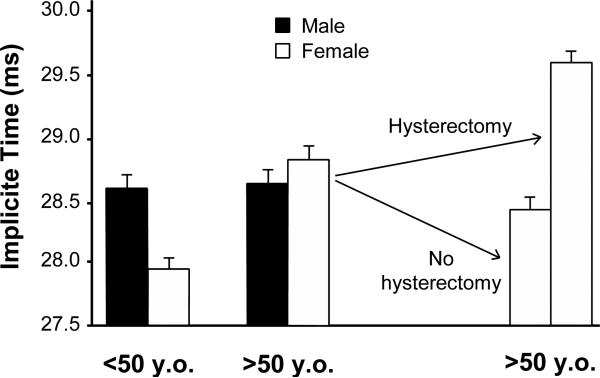
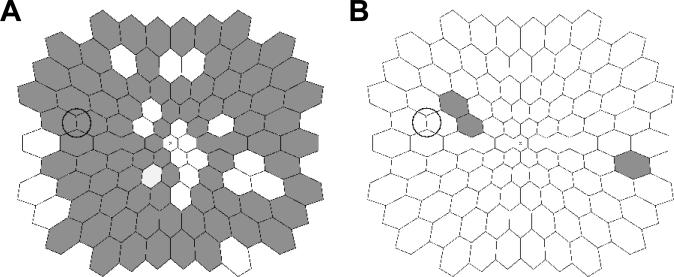
Whole eye ITs for the younger females were shorter (P=0.015) compared to the younger males (27.9 ± 0.2 ms versus 28.8 ± 0.2 ms, respectively, average ± standard error). See Figure 1. Local ITs of the females <50 years (younger females) were significantly shorter than the males <50 years (younger males) in 83 of the 103 tested retinal locations (80.6%). See Figure 2A. Furthermore, no local ITs of the females were significantly longer compared to the corresponding ITs of their male counterparts in any retinal location.
Figure 1.
Mean whole eye mfERG IT of each subject group. The younger females had the shortest whole eye ITs, and the females who had reported a hysterectomy had the longest ones. Whole eye ITs of the males were similar between the younger and older subject groups. The older females (excluding those with hysterectomies) tended to have longer whole eye ITs compared to the younger females (P=0.04). Error bars indicate 1 SEM.
Figure 2.
(A) Comparison of local ITs between the younger males and females. Dark hexagons indicate retinal locations where local ITs of the younger females were significantly shorter than the corresponding ITs of the younger males. (B) Comparison of local ITs between the older males and females (excluding those with hysterectomies). Shaded hexagons indicate retinal locations where local ITs of the older females were significantly shorter than the corresponding ITs of the older males. Diagrams show retinal view in left-eye format, the circle indicates the optic disc, and “x” indicates the foveola.
In contrast to the younger subjects, whole eye ITs were similar (P=0.58) between the older males and females (>50 years): 28.7 ± 0.2 ms versus 28.8 ± 0.2 ms, respectively. See Figure 1. Five females reported a hysterectomy (all of whom were >50 years). A sub-analysis of these eyes revealed that their whole eye ITs were significantly longer (P=0.0013) than the older males (29.7 ± 0.1 ms versus 28.7 ± 0.2 ms, respectively). After the exclusion of females with a reported history of a hysterectomy, whole eye ITs between the older males and the remaining older females remained similar (28.7 ± 0.2 versus 28.4 ± 0.2 ms, respectively, P=0.41).
Local IT differences between the older males and females were noticeably absent. In contrast to the results of the younger groups, no corresponding retinal locations in the older males and older females had significantly different local ITs. When the females who had reported a hysterectomy were excluded, local ITs of the older males and females remained similar; only three corresponding retinal locations in the older females (essentially chance) had significantly shorter ITs than the older males (Figure 2B).
IT Analysis Comparing Younger to Older Subjects
Whereas whole eye ITs of the younger males (28.6 ± 0.2 ms) and older males (28.7 ± 0.2 ms) were similar (P=0.83), whole eye ITs of the younger females were significantly shorter (P=0.0011) than the older females (27.9 ± 0.2 ms versus 28.8 ± 0.2 ms, respectively). See Figure 1. The five females who reported a hysterectomy had significantly longer whole eye ITs (P<0.001) than the other 11 older females who did not report a hysterectomy (29.7 ± 0.1 ms versus 28.4 ± 0.2 ms, respectively). See Figure 1. The older females who did not report a hysterectomy tended to have longer (P=0.04) whole eye ITs than the younger females (28.4 ± 0.2 ms versus 27.9 ± 0.2 ms, respectively), but this difference was not significant after Bonferroni correction for multiple comparisons.
Local mfERG ITs for the younger females were significantly shorter than the older females at every retinal location. When the females who reported a hysterectomy were excluded, the ITs at 62 corresponding retinal locations (60.2%) were significantly shorter in the younger females than those of the remaining older females. See Figure 3A. The most susceptible retinal locations for developing longer ITs appeared to coincide with the vascular arcades. No local IT of the younger females was significantly longer than the older females at any corresponding retinal location. Unlike the females, local ITs of the younger and older males were not significantly different at any corresponding retinal location (Figure 3B).
Figure 3.
Comparison of local ITs between the younger and older subjects within each sex. (A) Dark hexagons indicate retinal locations where ITs of the younger females were significantly shorter than the corresponding ITs in the older females with no reported hysterectomies. (B) Local ITs of the younger and older males were not significantly different in any corresponding retinal location. Format of the diagrams is as in Figure 1.
AMP Analysis Comparing Males to Females
No significant differences in global or local AMPs were found between the males and females. In fact, whether or not the females who reported a hysterectomy were excluded, all subject groups had similar AMPs.
DISCUSSION
When assessed with the mfERG IT, neuroretinal function was significantly different, both locally and globally, in the younger (<50 years old) females compared to the younger males. In contrast, the older males and females (>50 years old) did not differ. Notably, the females who had reported a history of hysterectomy had the longest whole eye ITs of all subject groups.
The exclusion of the females with hysterectomies affected the mfERG IT results in only one substantive way. It made the global difference between the older and younger females smaller, but the remaining local differences were still significant. Neuroretinal function in the older females was clearly worse than that in the younger females. In striking contrast, neuroretinal function did not differ between the younger and older males in any IT analysis.
The AMP results were completely different from the major IT findings. No AMP differences were detected between the younger females and males; aging changes were not observed between the younger and older females; and finally, there was no apparent association between AMP and hysterectomies.
The IT differences demonstrated in our study suggest that electrophysiological studies, particularly those involving the mfERG, should consider age and sex of subjects as potential confounding factors. ERG studies, including those that do not investigate sex-based differences, should attempt to match subject groups for age and gender. They should also consider excluding females with hysterectomies to avoid potentially erroneous conclusions.
Although, by convention, Z-scores have been calculated by combining male and female normative data, the results of this study also suggest that Z-scores may need to be calculated with gender-specific normative data. Furthermore, the female normative data may need to be age-specific, whereas in the males, such a precaution may not be as important. Our own experience4 demonstrates dramatically different (occasionally contradictory) results depending on how Z-scores are calculated.
Some may wonder why results such as ours have not been reported previously. Many groups have investigated age-related changes of neuroretinal function with the mfERG.12-20 Of these studies, however, only one considered sex.16 That study did not find any interaction between age, sex and IT using an analysis of covariance. Given our results, an analysis of covariance would have had difficulty detecting an interaction between those three factors for two reasons. First, IT does not appear to vary with age in males, and second, IT may not vary linearly with age in females.
All of the aforementioned studies, except for one18, reported that mfERG P1 ITs increase with age. If our male and female data had been combined, it would have demonstrated a general increase of 0.24 ms/decade with age. This aging trend is similar to three studies that reported ITs increased at a rate of 0.3 ms/decade14, 15 and 0.28 ms/decade.16
A logical but speculative explanation for our results involves age-related change in average estradiol level. Estradiol is the predominant form of estrogen from menarche to menopause. Its concentration is higher in young females compared to young males, and the average levels in females decline with age, regardless of the day within a woman's cycle21, and independent of menopause status22. In males, however, serum levels of total estradiol either remain constant23, or they decline marginally24, 25 throughout life. Furthermore, although bioavailable estradiol levels decrease with age in males, there may be a threshold level that maintains retinal function, as appears to be the case in rates of bone loss among males.26
The IT results of our study appear to vary inversely with the presumptive levels of total estradiol in both sexes: shorter ITs in younger females compared to males; similar ITs in younger and older males, and the longest ITs in the females with hysterectomies. Furthermore, the retina not only possesses estradiol receptors, it also demonstrates the ability to synthesize estradiol.27 Moreover, estradiol can affect the viability of retinal cells through mechanisms that do not require known estrogen receptors.28
Since the menstrual cycle is not known to affect photopic ERGs,5 we hypothesize that longer-term exposure, or potentiation by, estradiol is associated with neuroretinal function rather than short-term fluctuations. This idea is akin to the “timing” hypothesis in cardiovascular disease and the effect of menopausal hormonal therapy.29 In addition, time since menopause, rather than the type of menopause (natural or surgical) is a major factor in subclinical atherosclerosis,30 and the mechanisms underlying this phenomenon may also apply to retinal function in females.
A limitation of this study was the small sample sizes. However, we were able to replicate this study's results using a separate normative data set obtained with a different mfERG apparatus. A larger study needs to be performed to confirm our findings, and to determine the impact of IT differences found in the two sexes. Such a study also needs to establish whether Z-scores calculated on a gender-specific basis yield significantly different results than Z-scores generated conventionally (using young and old, males and females combined).
A second limitation is the retrospective nature of our study. Specifically, the details of the hysterectomy (type, reason for procedure, date of surgery, etc) and whether hormonal contraception was used are unknown. In addition, the menopause status and the phase of the menstrual cycle in the pre-menopausal women were not determined. Moreover, estradiol levels of our subjects were also not measured. To show a causal relationship between estradiol and neuroretinal function, estradiol levels would need to be systematically manipulated. For this reason, animal studies may be preferred to determine the effect of estrogen on neuroretinal function. Such experiments would allow retinal function of ovariectomized animals, and ovariectomized animals with estradiol supplementation, to be compared.
The results of this study raise interesting questions. Presumably, younger females with diabetes have higher estradiol levels than younger males with diabetes. Does neuroretinal function of younger diabetic males and females differ? In an earlier study, we showed that there are neurodegenerative differences between males and females with type 2 diabetes, but we did not subdivide the sexes into younger and older groups.4 It has been reported that diabetes erases the female advantage in NOS-dependent reactivity of peripheral blood vessels31 and deaths associated with coronary artery disease32. Does diabetes neutralize the advantage in neuroretinal function for younger females that was demonstrated in the present study? The answer to this question awaits further studies.
Sex differences in neuroretinal function.
In this study, the authors demonstrate that multifocal electroretinogram implicit time (IT) differs between males and females, depending on age group and hysterectomy status. Furthermore, the IT results appear to vary inversely with the presumptive levels of estradiol in both sexes: shorter ITs in younger females compared to males; similar ITs in younger and older males; similar ITs in older females and males; and the longest ITs in the females with hysterectomies. Both sex and age might be important considerations when establishing normative values for multifocal electroretinogram IT, a sensitive measure of local neuroretinal function.
ACKNOWLEDGMENTS
This research was funded by NEI EY02271 (AJA), JDRF 8-2008-0823 (MAB) and NIH EY021811 (MES).
REFERENCES
- 1.Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–6. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- 2.Vainio-Mattila B. The clinical electroretinogram; II. The difference between the electroretinogram in men and in women. Acta Ophthalmol (Copenh) 1951;29:25–32. doi: 10.1111/j.1755-3768.1951.tb07612.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeidler I. The clinical electroretinogram. IX. The normal electroretinogram. Value of the b-potential in different age groups and its differenes in men and women. Acta Ophthalmol (Copenh) 1959;37:294–301. doi: 10.1111/j.1755-3768.1959.tb03437.x. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa GY, Bearse MA, Jr., Bronson-Castain KW, Harrison WW, Schneck ME, Barez S, Adams AJ. Neurodegenerative differences in the retinas of male and female patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:3040–6. doi: 10.1167/iovs.11-8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brule J, Lavoie MP, Casanova C, Lachapelle P, Hebert M. Evidence of a possible impact of the menstrual cycle on the reproducibility of scotopic ERGs in women. Doc Ophthalmol. 2007;114:125–34. doi: 10.1007/s10633-007-9045-1. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Bearse MA, Jr., Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:948–54. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 7.Harrison WW, Bearse MA, Jr., Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52:772–7. doi: 10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Harlow SD, Elliott MR. Distinguishing 6 population subgroups by timing and characteristics of the menopausal transition. Am J Epidemiol. 2012;175:74–83. doi: 10.1093/aje/kwr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, Rodriguez G, Simon J, Pincus S. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–91. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 10.Bearse MA, Jr., Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–48. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood DC, Li J. A technique for measuring individual multifocal ERG records. In: Yager D, editor. Non-invasive Assessment of the Visual System. Trends in Optics and Photonics. Vol. 11. Optical Society of America; Washington, DC: 1997. pp. 33–41. [Google Scholar]
- 12.Langrova H, Zrenner E, Kurtenbach A, Seeliger MW. Age-related changes in retinal functional topography. Invest Ophthalmol Vis Sci. 2008;49:5024–32. doi: 10.1167/iovs.07-1309. [DOI] [PubMed] [Google Scholar]
- 13.Tam WK, Chan H, Brown B, Leung KW, Woo V, Yap M. Aging and mfERG topography. Eye (Lond) 2006;20:18–24. doi: 10.1038/sj.eye.6701777. [DOI] [PubMed] [Google Scholar]
- 14.Tzekov RT, Gerth C, Werner JS. Senescence of human multifocal electroretinogram components: a localized approach. Graefes Arch Clin Exp Ophthalmol. 2004;242:549–60. doi: 10.1007/s00417-004-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiple W, Vajaranant TS, Szlyk JP, Clemens C, Holopigian K, Paliga J, Badawi D, Carr RE. Multifocal electroretinography as a function of age: the importance of normative values for older adults. Invest Ophthalmol Vis Sci. 2003;44:1783–92. doi: 10.1167/iovs.02-0518. [DOI] [PubMed] [Google Scholar]
- 16.Gerth C, Garcia SM, Ma L, Keltner JL, Werner JS. Multifocal electroretinogram: age-related changes for different luminance levels. Graefes Arch Clin Exp Ophthalmol. 2002;240:202–8. doi: 10.1007/s00417-002-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortune B, Johnson CA. Decline of photopic multifocal electroretinogram responses with age is due primarily to preretinal optical factors. J Opt Soc Am (A) 2002;19:173–84. doi: 10.1364/josaa.19.000173. [DOI] [PubMed] [Google Scholar]
- 18.Nabeshima T, Tazawa Y, Mita M, Sano M. Effects of aging on the first and second-order kernels of multifocal electroretinogram. Jpn J Ophthalmol. 2002;46:261–9. doi: 10.1016/s0021-5155(02)00475-6. [DOI] [PubMed] [Google Scholar]
- 19.Mohidin N, Yap MK, Jacobs RJ. Influence of age on the multifocal electroretinography. Ophthalmic Physiol Opt. 1999;19:481–8. doi: 10.1046/j.1475-1313.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 20.Jackson GR, Ortega J, Girkin C, Rosenstiel CE, Owsley C. Aging-related changes in the multifocal electroretinogram. J Opt Soc Am (A) 2002;19:185–9. doi: 10.1364/josaa.19.000185. [DOI] [PubMed] [Google Scholar]
- 21.Key TJ, Chen J, Wang DY, Pike MC, Boreham J. Sex hormones in women in rural China and in Britain. Br J Cancer. 1990;62:631–6. doi: 10.1038/bjc.1990.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph JF, Jr., Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–61. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Yamada T. Association of bioavailable estradiol levels and testosterone levels with serum albumin levels in elderly men. Aging Male. 2008;11:63–70. doi: 10.1080/13685530701779234. [DOI] [PubMed] [Google Scholar]
- 24.Venkat K, Desai M, Arora MM, Singh P, Khatkhatay MI. Age-related changes in sex steroid levels influence bone mineral density in healthy Indian men. Osteoporos Int. 2009;20:955–62. doi: 10.1007/s00198-008-0765-1. [DOI] [PubMed] [Google Scholar]
- 25.Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–95. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 26.Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012;41:629–41. doi: 10.1016/j.ecl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cascio C, Russo D, Drago G, Galizzi G, Passantino R, Guarneri R, Guarneri P. 17beta-estradiol synthesis in the adult male rat retina. Exp Eye Res. 2007;85:166–72. doi: 10.1016/j.exer.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Nixon E, Simpkins JW. Neuroprotective effects of nonfeminizing estrogens in retinal photoreceptor neurons. Invest Ophthalmol Vis Sci. 2012;53:4739–47. doi: 10.1167/iovs.12-9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reslan OM, Khalil RA. Vascular effects of estrogenic menopausal hormone therapy. Rev Recent Clin Trials. 2012;7:47–70. doi: 10.2174/157488712799363253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004;82:391–7. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation. 2000;101:2040–6. doi: 10.1161/01.cir.101.17.2040. [DOI] [PubMed] [Google Scholar]
- 32.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–8. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]