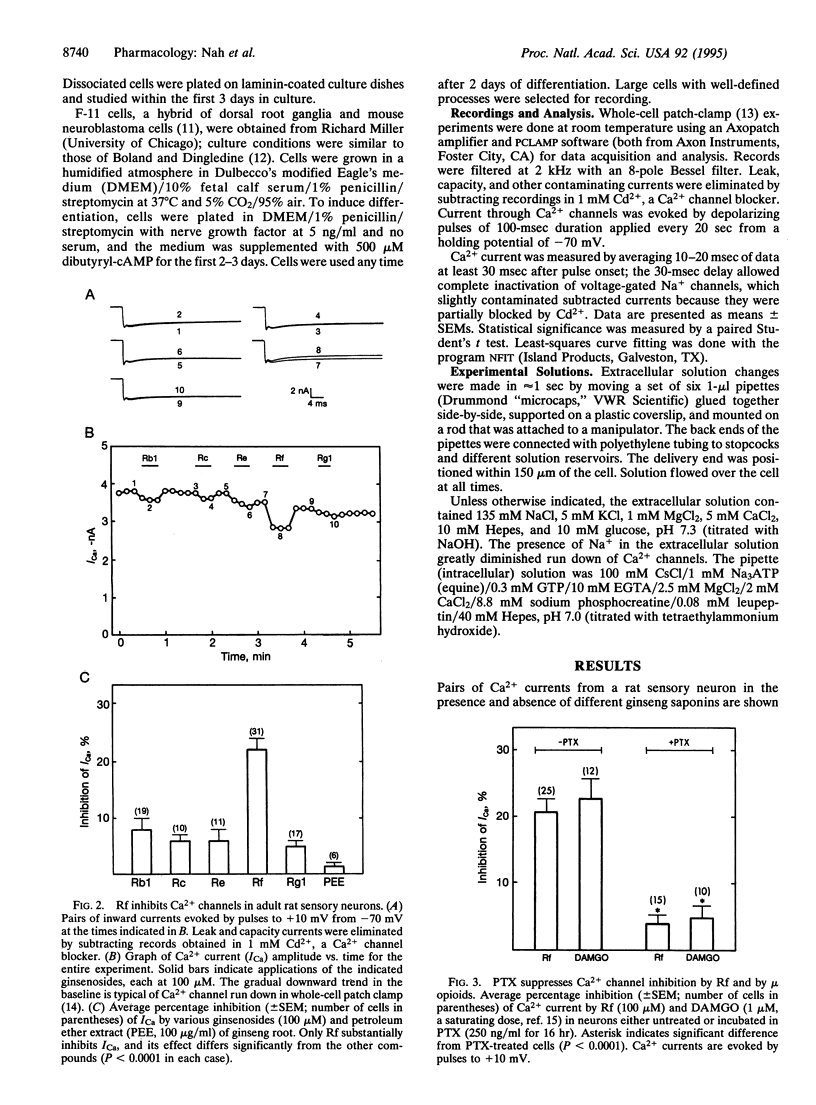
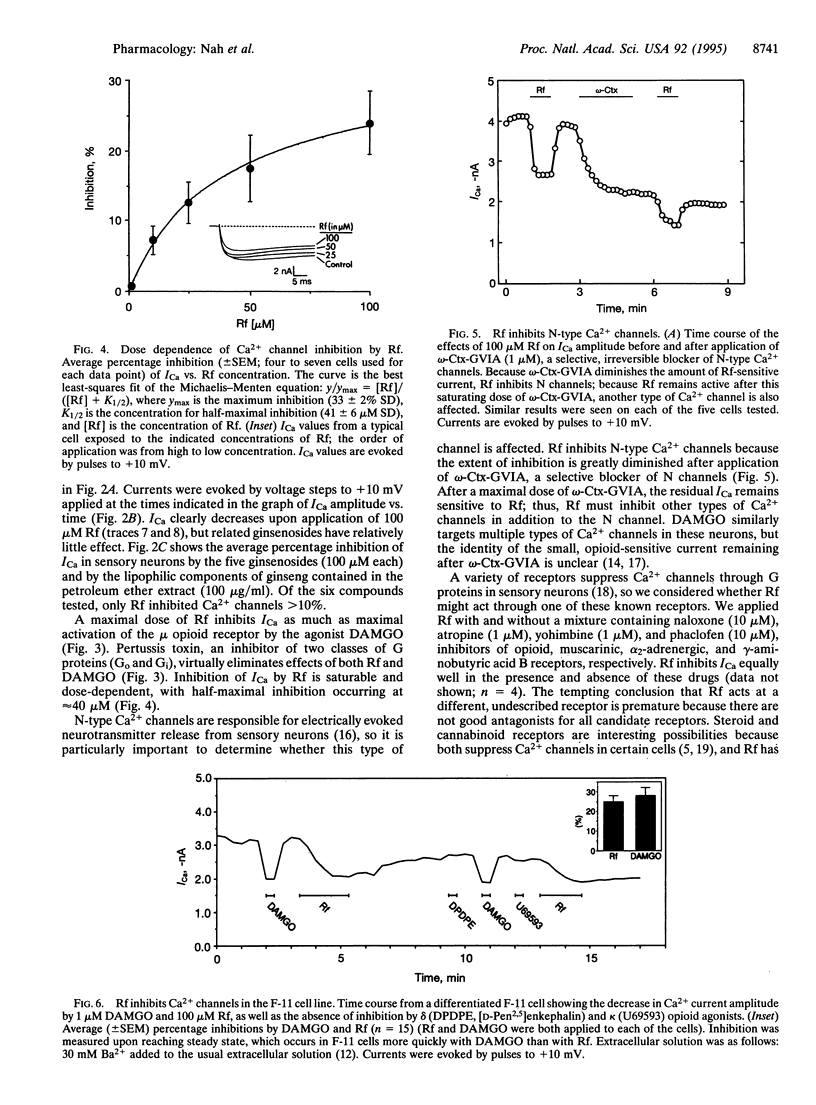
Abstract
A crude extract from ginseng root inhibits high-threshold, voltage-dependent Ca2+ channels through an unknown receptor linked to a pertussis toxin-sensitive G protein. We now have found the particular compound that seems responsible for the effect: it is a saponin, called ginsenoside Rf (Rf), that is present in only trace amounts within ginseng. At saturating concentrations, Rf rapidly and reversibly inhibits N-type, and other high-threshold, Ca2+ channels in rat sensory neurons to the same degree as a maximal dose of opioids. The effect is dose-dependent (half-maximal inhibition: 40 microM) and it is virtually eliminated by pretreatment of the neurons with pertussis toxin, an inhibitor of G(o) and Gi GTP-binding proteins. Other ginseng saponins--ginsenosides Rb1, Rc, Re, and Rg1--caused relatively little inhibition of Ca2+ channels, and lipophilic components of ginseng root had no effect. Antagonists of a variety of neurotransmitter receptors that inhibit Ca2+ channels fail to alter the effect of Rf, raising the possibility that Rf acts through another G protein-linked receptor. Rf also inhibits Ca2+ channels in the hybrid F-11 cell line, which might, therefore, be useful for molecular characterization of the putative receptor for Rf. Because it is not a peptide and it shares important cellular and molecular targets with opioids, Rf might be useful in itself or as a template for designing additional modulators of neuronal Ca2+ channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins P. T., McCleskey E. W. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993 Oct;56(3):759–769. doi: 10.1016/0306-4522(93)90372-m. [DOI] [PubMed] [Google Scholar]
- Bhargava H. N., Ramarao P. The effect of Panax ginseng on the development of tolerance to the pharmacological actions of morphine in the rat. Gen Pharmacol. 1991;22(3):521–525. doi: 10.1016/0306-3623(91)90017-z. [DOI] [PubMed] [Google Scholar]
- Boland L. M., Dingledine R. Multiple components of both transient and sustained barium currents in a rat dorsal root ganglion cell line. J Physiol. 1990 Jan;420:223–245. doi: 10.1113/jphysiol.1990.sp017909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C. G protein modulation of calcium currents in neurons. Annu Rev Physiol. 1990;52:243–255. doi: 10.1146/annurev.ph.52.030190.001331. [DOI] [PubMed] [Google Scholar]
- Go V. L., Yaksh T. L. Release of substance P from the cat spinal cord. J Physiol. 1987 Oct;391:141–167. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., Stevens C. F. Two components of transmitter release at a central synapse. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L. Dynorphin A selectively reduces a large transient (N-type) calcium current of mouse dorsal root ganglion neurons in cell culture. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5469–5473. doi: 10.1073/pnas.84.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Dunlap K., Kream R. M. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988 Feb;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Kaku T., Miyata T., Uruno T., Sako I., Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. I. Chemical part. Arzneimittelforschung. 1975 Mar;25(3):343–347. [PubMed] [Google Scholar]
- Kaku T., Miyata T., Uruno T., Sako I., Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975 Apr;25(4):539–547. [PubMed] [Google Scholar]
- Kim H. S., Jang C. G., Lee M. K. Antinarcotic effects of the standardized ginseng extract G115 on morphine. Planta Med. 1990 Apr;56(2):158–163. doi: 10.1055/s-2006-960915. [DOI] [PubMed] [Google Scholar]
- Liu C. X., Xiao P. G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992 Feb;36(1):27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Harris G. H., Giangiacomo K. M., Feigenbaum P., Reuben J. P., Addy M. E., Burka J. F., Kaczorowski G. J., Garcia M. L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993 Jun 22;32(24):6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- Moises H. C., Rusin K. I., Macdonald R. L. Mu- and kappa-opioid receptors selectively reduce the same transient components of high-threshold calcium current in rat dorsal root ganglion sensory neurons. J Neurosci. 1994 Oct;14(10):5903–5916. doi: 10.1523/JNEUROSCI.14-10-05903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabata H., Saito H., Takagi K. Pharmacological studies of neutral saponins (GNS) of Panax Ginseng root. Jpn J Pharmacol. 1973 Feb;23(1):29–41. doi: 10.1254/jjp.23.29. [DOI] [PubMed] [Google Scholar]
- Nah S. Y., McCleskey E. W. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as mu-type opioids. J Ethnopharmacol. 1994 Mar;42(1):45–51. doi: 10.1016/0378-8741(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Penington N. J., Kelly J. S., Fox A. P. Action potential waveforms reveal simultaneous changes in ICa and IK produced by 5-HT in rat dorsal raphe neurons. Proc Biol Sci. 1992 May 22;248(1322):171–179. doi: 10.1098/rspb.1992.0059. [DOI] [PubMed] [Google Scholar]
- Platika D., Boulos M. H., Baizer L., Fishman M. C. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao P., Bhargava H. N. Antagonism of the acute pharmacological actions of morphine by panax ginseng extract. Gen Pharmacol. 1990;21(6):877–880. doi: 10.1016/0306-3623(90)90448-u. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Morita M., Takagi K. Pharmacological studies of Panax Ginseng leaves. Jpn J Pharmacol. 1973 Feb;23(1):43–56. doi: 10.1254/jjp.23.43. [DOI] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Mamo M., McCleskey E. W. Two components of high-threshold Ca2+ current inactivate by different mechanisms. Neuron. 1990 Oct;5(4):445–452. doi: 10.1016/0896-6273(90)90083-r. [DOI] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Zheng D., McCleskey E. W. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991 Jan;6(1):13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- Seward E., Hammond C., Henderson G. Mu-opioid-receptor-mediated inhibition of the N-type calcium-channel current. Proc Biol Sci. 1991 May 22;244(1310):129–135. doi: 10.1098/rspb.1991.0061. [DOI] [PubMed] [Google Scholar]
- Shefner S. A., North R. A., Zukin R. S. Opiate effects on rabbit vagus nerve: electrophysiology and radioligand binding. Brain Res. 1981 Sep 21;221(1):109–116. doi: 10.1016/0006-8993(81)91066-0. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Oh K. W., Kim H. S., Takahashi M., Kaneto H. A non-opioid mechanism in the inhibitory effect of ginseng saponins on electrically evoked contractions of guinea-pig ileum and mouse vas deferens. J Pharmacobiodyn. 1988 Jun;11(6):453–458. doi: 10.1248/bpb1978.11.453. [DOI] [PubMed] [Google Scholar]
- Williams J., Zieglgänsberger W. Mature spinal ganglion cells are not sensitive to opiate receptor mediated actions. Neurosci Lett. 1981 Jan 20;21(2):211–216. doi: 10.1016/0304-3940(81)90384-0. [DOI] [PubMed] [Google Scholar]
- Womack M. D., McCleskey E. W. Interaction of opioids and membrane potential to modulate Ca2+ channels in rat dorsal root ganglion neurons. J Neurophysiol. 1995 May;73(5):1793–1798. doi: 10.1152/jn.1995.73.5.1793. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. The spinal pharmacology of acutely and chronically administered opioids. J Pain Symptom Manage. 1992 Aug;7(6):356–361. doi: 10.1016/0885-3924(92)90089-z. [DOI] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Danks P., Spence K. T. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J Neurosci. 1994 Apr;14(4):1963–1977. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


