Abstract
Fragile X syndrome (FXS) is a monogenic disease caused by mutations in the FMR1 gene. The Fmr1 knockout (KO) mice show many aspects of FXS-related phenotypes, and have been used as a major pre-clinical model for FXS. Although FXS occurs in both male and female patients, most studies on the mouse model use male animals. Few studies test whether gender affects the face validity of the mouse model. Here, we examined multiple behavioral phenotypes with male hemizygous and female homozygous Fmr1 KO mice on C57BL/6 background. For each behavioral paradigm, we examined multiple cohorts from different litters. We found that both male and female Fmr1 KO mice displayed significant audiogenic seizures, hyperactivity in the open field test, deficits in passive avoidance and contextual fear memory, and significant enhancement of PPI at low stimulus intensity. Male and female Fmr1 KO mice also showed more transitional movement between the lit and dark chambers in the light-dark tests. The lack of gender effects suggests that the Fmr1 KO mouse is a reasonable tool to test the efficacy of potential FXS therapies.
Keywords: Fragile X syndrome, Fmr1 knockout mice, Genetic background, Gender, Behavioral phenotypes
1. Introduction
Fragile X syndrome (FXS), the most common inherited form of intellectual disability (ID), is caused by mutations in the FMR1 (Fragile X mental retardation) gene resulting in lack of FMRP (Fragile X mental retardation protein) expression [1-3]. In addition to ID, FXS patients also display other symptoms including hyperactivity, higher susceptibility to seizures, hyperarousal, and autism-related behavior. Cellular and molecular investigations have implicated that FMRP interacts with mRNA [4-6] as well as micro-RNA [7, 8] at the synapses and suppresses protein synthesis [9]. Lack of FMRP also causes alteration of neurotransmitter receptor function and intracellular signaling. Such functions of FMRP have suggested that inhibiting the translation of certain FMRP targets [10-12] or signaling molecules (such as Gq-mediated signaling cascade) [13-16] may be therapeutic.
Among the different animal models of FXS, the Fmr1 knockout (KO) mouse is the most utilized to understand the etiology of FXS and develop therapeutic strategies [1]. In general, the Fmr1 KO mouse recapitulates the main CNS (central nervous system) and peripheral symptoms of FXS [17]. It is interesting to note that, although FXS affects both men and women, behavioral phenotypes in female Fmr1 KO mice have not been thoroughly investigated. In this study, we concurrently examined male and female Fmr1 KO mice on C57BL/6 background. We measured multiple FXS-related behavioral phenotypes including audiogenic seizures (AGS), hyperactivity, defective memory, and defective prepulse inhibition (PPI). Testing an array of FXS symptoms with mice in both genders will lead to better understanding of the face validity of this specific FXS animal model. We chose to examine mice on the C57BL/6 background, because this specific genetic background is widely used in preclinical studies. We expect that the outcome of this study will provide valuable behavioral indexes for the evaluation of therapeutic efficacies and treatments.
2. Methods
2.1. Animals
WT and Fmr1 KO (hemizygouse male and homozygouse female) mice on C57BL/6 background were maintained in the University Laboratory Animal Resource under 12-h light and 12-h dark condition. The breeding strategy and genotyping methods were according to previous studies. Breeding of hemizygous male and homozygous female Fmr1 KO mice on C57BL/6 background were used to generate mutant mice. Breeding of WT male and WT female mice on C57BL/6 background were used to generate WT mice. The animals had free access to food and water. All procedure used in this study were approved by the Institutional Animal Care and Use Committee at Michigan State University. Except for audiogenic seizures, all animals used were between 2 to 3 months of age.
2.2. Behavioral analysis
To minimize the effects of incidental environmental variations, each behavioral analysis was done with animals from at least 2 different litters. To avoid carry-over effects, animals were only used for one behavioral paradigm. The only exception was that the mice used for light-dark test were previously examined with open field activity (the interval between these two tests was 1 week).
2.2.1. Audiogenic seizures
The ages of all WT and Fmr1 knockout animals used in the experiments were between 21 to 24 days. The apparatus used was a plastic chamber (30 cm L, 17 cm W. and 12 cm H) equipped with an alarm (Streetwise, item #SWPDAL), which was attached to the ceiling of the chamber and produced sound at 120 dB. The animals were placed individually in the chamber and allowed to explore for 5 min, following which the alarm was sounded for 2min. The behavior of the animal was recorded in three categories: wild running, tonic/clonic seizures, and death.
2.2.2. Open field exploration test
Mouse was placed in an open field chamber (Coulbourn Instruments) and allowed to explore freely for 2 hrs. The locomotor activity (such as travel distance) in the whole chamber as well as in the center area was monitored and analyzed by an automated system (the TruScan Activity System, Coulbourn Instruments).
2.2.3. Light/dark test
The light/dark test set-up consisted of two connected chambers (a lit chamber and a dark chamber) with a trap door in the separation wall. Mouse was first placed in the dark chamber for 2 min, after which the trap door was opened and the animal was allowed to freely travel between the two chambers. The number of transitions between the two compartments and the amount of time spent in the lit chamber were recorded for 5 min.
2.2.4. Contextual fear conditioning
Contextual fear conditioning was performed as described in our previous study [18]. Briefly, mouse was placed in the contextual chamber (Coulbourn Instruments), and allowed to move freely for 2 min before a mild foot shock (0.7 mA, 2 sec) was delivered. The mouse remained in the chamber for 1 min, and was returned to its home cage. Twenty-four hours later, the trained mouse was re-introduced to the contextual chamber for 2 min, during which its freezing behavior (i.e. immobility) was scored.
2.2.5. Passive avoidance test
Training and testing of passive avoidance memory were performed as described [19]. Briefly, mouse was introduced to the lit half of the passive avoidance chamber (Coulbourn Instruments) and allowed to explore for 1 min before the trap door was opened. Immediately after the mouse entered the dark compartment, the trap door was closed and a mild foot shock (0.7 mA, 2 sec) was delivered. The mouse was remained in the dark compartment for 30 sec before being returned to its home cage. The test session was performed 24 h later. The trained mouse was re-introduced to the lit chamber. After the trap door was opened, the time that the mouse remained in the lit chamber was scored as crossover latency. If the mouse stayed in the lit chamber for more than 10 min without crossing over to the dark chamber, it would be manually removed and 10 min was registered as its crossover latency.
2.2.6. Prepulse inhibition
Prepulse inhibition (PPI) was examined with the SR-LAB apparatus (San Diego Instruments). Individual mouse was placed and acclimated in the startle chamber for 5 min, followed by 5 trials of 120 dB startle stimulation. Subsequently, the animal was subjected to 10 blocks of test trials. Each trial consisted of no stimulation, pulse alone (120 dB), prepulse alone at various intensities (68, 71, 77 and 82 dB) and prepulse plus pulse. The duration of the prepulse and pulse stimuli was 20 ms and 40 ms, respectively. In the “prepulse plus pulse” trial, the time between the prepulse and pulse was 100 ms. The inter-trial interval was pseudorandom ranging from 5 to 20 s. The percentage of PPI was defined as (startle response in the pulse alone trial – startle response in the prepulse-plus-pulse trial) / startle response in the pulse alone trial × 100%.
2.3. Statistical analysis
The results from the light/dark test, contextual fear conditioning and passive avoidance test were analyzed with two-way ANOVA followed by pairwise comparison. Locomotor activity in open field test and acoustic startle response data were analyzed by three-way ANOVA with repeated measures. The statistic analysis was done with the SPSS software.
3. Results
3.1. Juvenile Male and female Fmr1 KO mice show significant audiogenitc seizures
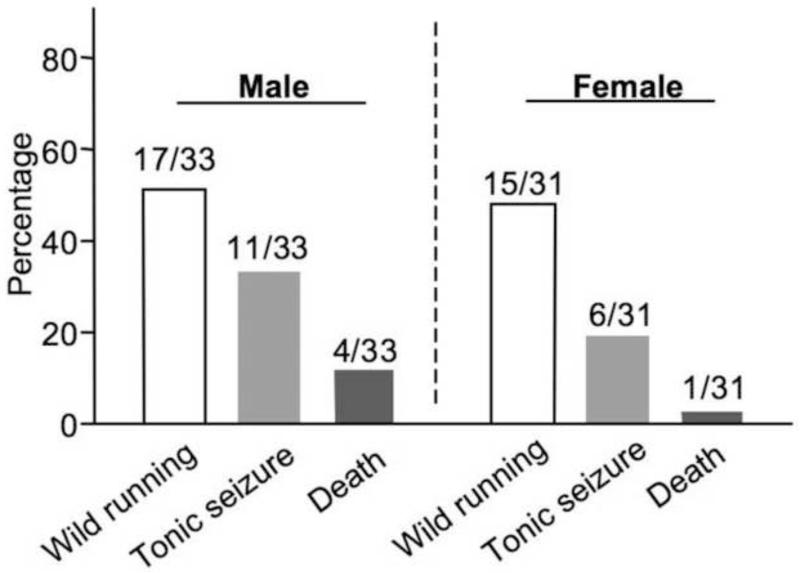
One of the secondary autism-related symptoms in FXS is the higher susceptibility to seizures. As a major animal model of FXS, Fmr1 KO mouse shows robust audiogenic seizure (AGS). While AGS is observed in a wider development window in mice on the FVB background [20], AGS in C57BL/6 Fmr1 KO mice can only be observed around 22 days of age [21, 22]. Here, we confirmed that juvenile male Fmr1 KO mice displayed significant wild running, seizures and death (Fig. 1). Female Fmr1 KO mice also showed AGS comparable to the males (Fig. 1). In contrast, wild type (WT) C57BL6 mice of both genders (10 males and 10 females) did not show measurable responses in the AGS test (data not shown).
Fig. 1.
Male and female C57BL/6 Fmr1 knockout mice show significant audiogenic seizures. 21- to 24-day old mice (n = 33 for male and n = 31 for female, as indicated) were used. These mice were exposed to sound at 120 dB for 2 min, during which wild running, tonic seizures, and death were scored.
3.2. Male and female Fmr1 KO mice show hyperactivity in the open field and the light-dark test
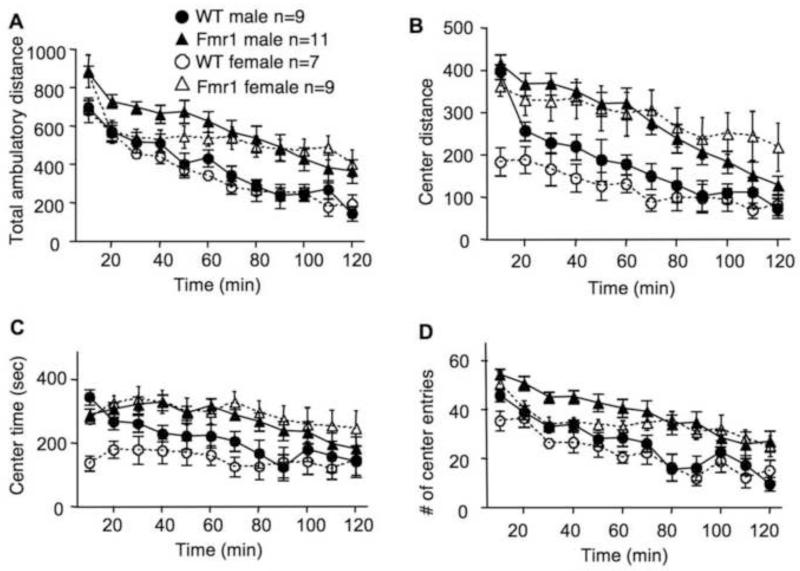
Human FXS patients are hyperactive. To determine locomotor activity, we subjected Fmr1 KO mice to an open field arena, and monitored their total travel distance, distance traveled in the center area of the arena, time spent in the center area, and the number of entries to the center area. We found statistically significant difference between two genotypes but not genders in all parameters monitored. As shown in Figure 2A, both male and female Fmr1 KO mice showed significantly more movement than their WT controls (Fgenotype(1,32) = 19.081, p < 0.005; Fgender(1,32) = 0.795, p = 0.379, Fgenotype × gender(1,32) = 0.024, p = 0.879). Fmr1 KO mice also showed more movement (Fig 2B; Fgenotype(1,32) = 23.395, p < 0.005; Fgender(1,32) = 0.595, p = 0.446, Fgenotype × gender(1,32) = 1.543, p = 0.223), occupancy time (Fig. 2C; Fgenotype(1,32) = 18.544, p < 0.005; Fgender(1,32) = 0.637, p = 0.431, Fgenotype × gender(1,32) = 2.959, p = 0.095), and entry number (Fig. 2D; Fgenotype(1,32) = 24.919, p < 0.005; Fgender(1,32) = 2.904, p = 0.098, Fgenotype × gender(1,32) = 0.012, p = 0.913) in the center area of the open field arena.
Fig. 2.
Male and female C57BL/6 Fmr1 knockout mice show significant hyperactivity in the open field test. WT and Fmr1 KO mice at 2 to 3 months of age were placed in an open field arena, and allowed to freely explore for 2 hours. The total movement distance (A), center distance (B), time spent in the center (C), and entry number to the center (D) were scored. Data presented as mean ± SEM were analyzed by three-way (genotype × gender × time) ANOVA with repeated measures. Both male and female mutants are hyperactive than the corresponding WT controls (p < 0.005)
Analysis on locomotion during the first 10 min of the open field test demonstrated that the novelty-induced exploratory locomotion was also higher in Fmr1 KO mice. Fmr1 KO mice in both genders showed higher total travel distance (Fgenotype(1,32) = 11.857, p < 0.005; Fgender(1,32) = 0.018, p = 0.894, Fgenotype × gender(1,32) = 0.024, p = 0.877). For the locomotor activity in the center area, there was also a significant main effect of genotype, as well as gender and genotype-gender interaction (Fgenotype(1,32) = 16.621, p < 0.005; Fgender(1,32) = 28.993, p < 0.005, Fgenotype × gender(1,32) = 11.092, p < 0.005). However, pairwise comparison indicated that only female (Fgenotype(1,32) = 24.628, p < 0.005) but not male (Fgenotype(1,32) = 0.314, p = 0.579) Fmr1 KO animals showed significant higher center activity during the first 10 min in the open field.
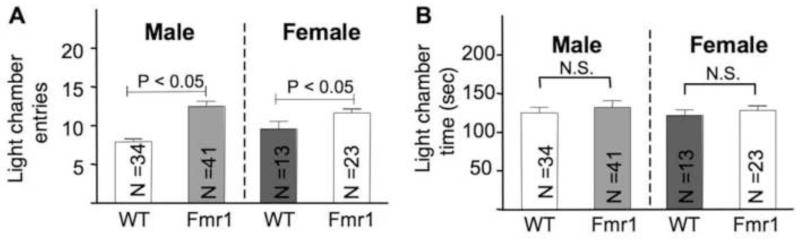
In the light-dark test, both male and female Fmr1 KO mice made more transitions between the lit and dark chambers than WT animals (Fig. 3A; Fgenotype(1,107) = 32.353, p < 0.005; Fgender(1,107) = 1.747, p = 0.189, Fgenotype × gender(1,107) = 4.167, p = 0.044). WT female animals apparently show more transitions than WT males (Fgender(1,107) = 4.632, p <0.05), however, this gender difference was not observed in Fmr1 KO animals (Fgender(1,107) = 0.332, p = 0.565), as suggested by pairwise comparison followed two-way ANOVA analysis. There was no effect of Fmr1 gene deletion on the time spent in the light chamber (Fig. 3B; Fgenotype(1,107) = 2.229, p = 0.138; Fgender(1,107) = 0.006, p = 0.938, Fgenotype × gender(1,107) = 0.158, p = 0.692). The results from these two tests demonstrate that there is no gender effect on hyperactivity in this particular FXS animal model.
Fig. 3.
Male and female C57BL/6 Fmr1 knockout mice show significant hyperactivity in the light-dark test. The number of entries to the lit chamber is presented in A. Total time spent in the lit chamber is presented in B. Data are presented as mean ± SEM. Two-way ANOVA followed by pairwise comparison analysis indicated that the Fmr1 mutants made more entries than the corresponding WT controls (p < 0.05).
3.3. Male and female Fmr1 KO mice show impaired performance in contextual fear conditioning and passive avoidance
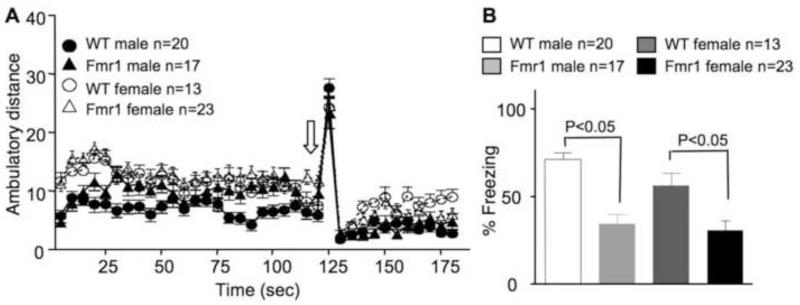
We examined Fmr1 KO mice with two hippocampus-dependent learning paradigms. During the contextual fear conditioning training, delivery of a mild electric foot shock (as indicated by the block arrow in Fig. 4A) elicited similar reactions in both WT and Fmr1 KO mice. Both male and female animals showed significant increase of movement during the shock, indicating that all groups had similar shock sensitivity. During testing, an overall main effect of genotype but not gender was observed (Fgenotype(1,69) = 32.101, p < 0.005; Fgender(1,69) = 2.791, p = 0.099, Fgenotype × gender(1,69) = 1.120, p = 0.294). Both male and female Fmr1 mutants displayed less freezing than their WT controls (p < 0.005) (Fig. 4B), indicating impaired contextual memory formation.
Fig. 4.
Male and female C57BL/6 Fmr1 knockout mice show significant deficits in contextual memory. Animals at 2 to 3 months of age were trained by contextual fear conditioning, during which a mild electric foot shock (as indicated by the block arrow in A) was delivered. The ambulatory distance the animals traveled during the 3-min training is shown in A. During the testing (24 hours after training), the percentage of freezing (immobility behavior) was scored (B). Data presented as mean ± SEM were analyzed by two-way ANOVA followed by pairwise comparison analysis, which indicates significant less freezing in both male and female Fmr1 knockout animals comparing to the corresponding WT controls.
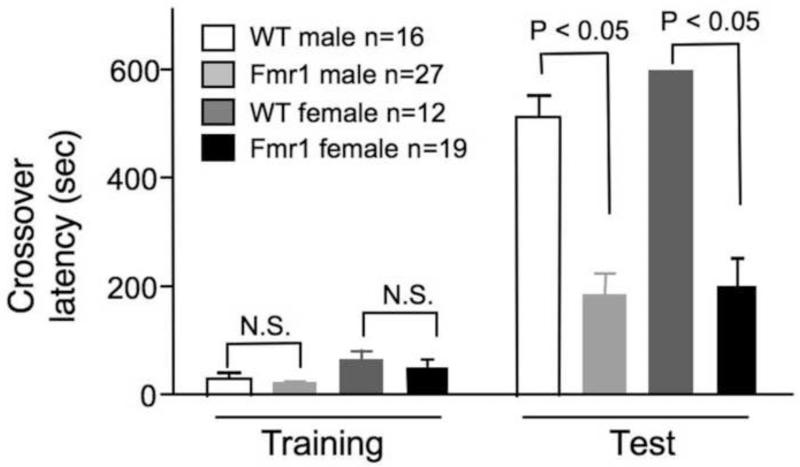
In the passive avoidance examination, Fmr1 KO mice showed normal crossover latency during training. However, both male and female mutants had weaker memory, as demonstrated by the shorter crossover latency during testing (Fig. 5; Fgenotype(1,70) = 68.340, p < 0.005; Fgender(1,70) = 1.330, p = 0.253, Fgenotype × gender(1,70) = 0.625, p = 0.432).
Fig. 5.
Male and female C57BL/6 Fmr1 knockout mice show significant deficits in passive avoidance memory. The crossover latency during training and testing (24 hours after training) was recorded. Data presented as mean ± SEM were analyzed by two-way ANOVA followed by pairwise comparison analysis, which indicates no significant difference in crossover latency during the training session but significant difference during the testing session (p < 0.005) between the Fmr1 mutants and the WT controls.
3.4. Male and female Fmr1 KO mice show mild defect in prepulse inhibition
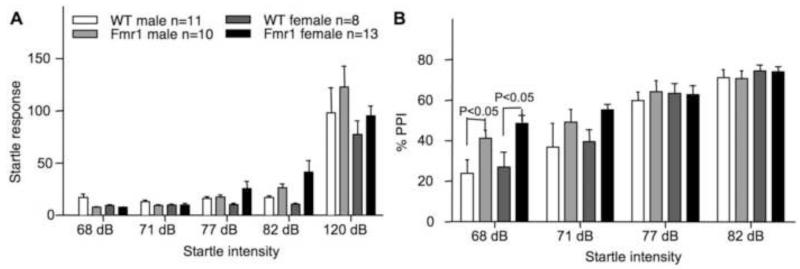
Although human FXS patients are hypersensitive and show decreased prepulse inhibition (PPI), results from studies with Fmr1 KO mice indicated that the phenotype may depend on genetic backgrounds [23, 24]. Here, we show that there is no significant main effect of genotype or gender in acoustic startle response between WT and Fmr1 KO mice (Fig. 6A; Fgenotype(1,38) = 3.017, p = 0.091; Fgender(1,38) = 0.916, p = 0.344, Fgenotype × gender(1,38) = 0.689, p = 0.412). However, there is a significant main difference in PPI between WT and Fmr1 KO mice but not between genders (Fig. 6B; Fgenotype(1,38) = 4.126, p < 0.05; Fgender(1,38) = 0 .657, p = 0.423, Fgenotype × gender(1,38) = 0.004, p = 0.949). Both male and female Fmr1 mutants showed a statistically significant increased PPI at 68 dB (p < 0.05).
Fig. 6.
Male and female C57BL/6 Fmr1 knockout mice show increased PPI. The responses to different levels of acoustic startle are presented in A. The values of PPI are presented in B. Data are presented as mean ± SEM. Three-way (genotype × gender × tone intensity) ANOVA with repeated measures indicated no significant difference in acoustic startle response but significant difference in PPI between genotypes. Pairwise comparison indicates significantly enhanced PPI in both male and female Fmr1 mutants with the 68 dB tone as the prepulse stimulation.
4. Discussion
The Fmr1 KO mice have provided valuable tools to study the function of FMRP and the pathophysiologies associated with FXS. The majority of biochemical, physiological, behavioral and therapeutic investigations with Fmr1 KO mice have been carried out on C57BL/6, FVB, C57BL/6-FVB hybrid, and C57 albino backgrounds. Although FXS affects both men and women, behavioral studies have been mostly carried out with male Fmr1 KO mice. To our knowledge, there are only two early studies that examined gender differences in multiple behaviors with Fmr1 KO on FVB [25] and C57 albino background [24]. Here, we report that male and female Fmr1 KO mice on C57BL/6 background display robust AGS, hyperactivity, cognitive impairment in passive avoidance and contextual memory, and a significant enhancement in PPI. For each behavioral paradigm in this study, the result was obtained by concurrently examining males and females using more than one cohort from different litters. To avoid carry-over effects, animals were not repeatedly examined with multiple behavioral tests. The only exception was that the same animals were used for open field test followed by the light-dark test (see Methods).
AGS, which may reflect that FXS patients have higher susceptibility to seizures, is the most confirmed phenotype in Fmr1 KO mice on multiple genetic backgrounds. It has been shown that the degree of AGS in male Fmr1 KO mice is the highest on FVB followed by C57BL/6-FVB hybrid and C57BL/6 background [20]. Here, we found that the absence of Fmr1 expression caused AGS in both male and female mice on C57BL/6 background. Consistently, Qin et al. also reported AGS in female FVB Fmr1 KO mice [25]. It is important to note that, while AGS is observed with both juvenile and adult FVB mutants [20, 25], AGS is only exhibited in C57BL/6 mutants at 20-24 days of age [21, 22].
The open field test has been widely used to examine the hyperactivity phenotype in Fmr1 KO mice. Depending on the experimental procedure and genetic background, previous studies have shown different results. Yan et al. reported that male Fmr1 KO mice on the C57BL/6-FVB mixed background showed normal overall locomotion but spent more time in the center area during the 5 min open filed test [20, 26]. Considering that novelty may induce exploration activity in both WT and Fmr1 KO mice, examinations longer than 5 min have been performed. Three reports from the same group consistently showed that male Fmr1 KO mice on the FVB background are hyperactive and spend more time in the center area of the open field arena during a 30 min test [25, 27, 28]. Interestingly, they also found that WT and Fmr1 KO mice showed comparable activity during the first 12 min (measurements for the first and the second 6 min were presented) [25], indicating effects of novelty-induced exploration on the hyperactivity phenotype. Female Fmr1 KO mice on the FVB background travel more than WT females, but they do not spend more time in the center [25]. Another study, which concurrently examined male and female Fmr1 KO mice on the C57 albino background, did not observe any significant hyperactivity but found that Fmr1 mutants in both genders spent more time in the center during a 30 min test [24]. Higher locomotor activity in C57BL/6 male Fmr1 KO mice has been reported previously [29, 30]. Interestingly, studies by Veeraragavan et al. failed to observe significant higher locomotion and center activity in C57BL/6 male Fmr1 KO mice during a 30 min open field test [21, 31]. Because some of the mice were first examined by marble burying test, it is not clear whether there were some carry-over effects. In this study, we extended the examination time to 2 hours, which may cover both the novelty-induced exploration phase (e.g. the first 10 min of the open field test) and the habituation phase that allows the examination of activity in a familiar environment. Both male and female Fmr1 KO mice on C57BL/6 background showed a general increase in locomotion and center activity.
The light/dark box test has been mainly used to examine anxiety-related behavior. WT and Fmr1 KO mice spent comparable time in the light chamber, indicating similar level of anxiety-related behavior. This is in contrast to that Fmr1 KO mice showed more activity in the center area of the open field arena, which implicates lower level of anxiety. It is important to note that Fmr1 KO mice display higher anxiety-related behavior in a modified open field test. When the open field arena is surrounded by mirrored walls, Fmr1 KO mice spend less time in the center [32]. The results suggest that the anxiety phenotype associated with FXS may depend on certain environmental cues. Interestingly, we found that both male and female C57BL/6 Fmr1 KO mice showed more transition between the lit and dark chamber, indicating hyperactivity and repetitive behavior. Veeraragavan et al. also found that male Fmr1 KO mice on a mixed background of C57BL/6J and C56BL/6NTac had more transition in the light/dark box test. The time spent in the light chamber was not reported [33]. Using older C57BL/6 mice (at 6-11 months of age) that were first examined with open field, marble burying, elevated plus maze, social choice and social dominance, Goebel-Goody et al. found that Fmr1 KO mice showed normal transition but spent more time in the light chamber [22]. It is likely that age and previous experience may affect behavior in the light/dark box test.
FXS patients show significant mental retardation. Here, we used passive avoidance and contextual fear conditioning as complementary approaches to examine fear memory. Both male and female C57BL/6 Fmr1 KO mice displayed defective memory. Previous studies using slightly different training protocols have reported variable results, but mainly implicated defective passive avoidance memory in Fmr1 KO mice. By using male C57BL/6 mice at 3 months of age, Yuskaitis et al. reported that Fmr1 KO mice had shorter crossover latency than WT mice [29]. By using a weaker training, during which the animals received a 1-sec shock at 0.2 mA, Qin et al. showed that both male and female Fmr1 KO on FVB background displayed shorter crossover latency than WT mice [25, 27]. It is interesting that previous experience may affect memory formation. Male C57BL/6 WT and Fmr1 KO mice, which have been previously examined by multiple behavioral tests (such as marble burying, open filed, and PPI [31]; or open field, light-dark, marble-burying, PPI, and rotarod [33]), did not show significant difference in crossover latency [31, 33]. Such experience-dependent variation may reflect certain roles of stress. Qin et al. reported that stress (e.g., chronic immobilization) had more influence on anxiety-like behavior in WT than Fmr1 KO mice [34].
To our knowledge, contextual fear memory in female Fmr1 KO mice has not been reported. Our study demonstrated that C57BL/6 Fmr1 KO mice, regardless of gender, are impaired. In contrast to our protocol, which involved one 2-sec mild foot shock, methods used in most of the previous studies applied two 2-sec shocks. Paradee et al. reported that, when the intensity of 0.35 mA (2 shocks) was used during training, male C56BL/6 Fmr1 KO mice showed defective contextual memory [35]. Interestingly, when the shock intensity was increased to 0.7 mA (2 or 3 shocks) during training, Fmr1 KO mice showed similar memory (as indicated by comparable freezing behavior) to that in WT mice [36-38]. Thus, the impaired fear memory in Fmr1 KO mice was observed after receiving weaker trainings (1 shock at 0.7 mA, this study; 2 shocks at 0.35 mA in the study by Paradee et al.). Stronger trainings (multiple shocks at 0.7 or 0.75 mA) may cause ceiling effects on fear memory formation. It is interesting to note that the FXS phenotype in long-term potentiation (LTP), which is an activity-dependent increase in synaptic efficacy and considered as a potential cellular mechanism underlying memory formation, depends on the intensity of electric induction protocol. While Fmr1 KO neurons show normal LTP following a strong induction protocol [35], LTP in Fmr1 KO mice is significantly impaired when a weaker induction protocol is used [39]. It is also important to note that the “no impairment” contextual memory was reported when the mice were repeatedly used for multiple other tests. The fear conditioning was performed following Morris water maze [36], or following open filed, light-dark test, marble-burying, rotarod test, tail suspension, PPI, and acoustic startle habituation [38], or following open field, light-dark test, marble-burying, and PPI [37].
Although human FXS patients are more sensitive to startle and show lower PPI, several studies have found that Fmr1 KO mice display higher PPI and various startle responses depending on the intensity of acoustic stimulation. Interestingly, while most of the studies using male C57BL/6 Fmr1 KO animals documented increased PPI [23, 37, 38, 40, 41], there is no significant PPI phenotype in Fmr1 KO mice on the C57BL/6-FVB hybrid background [40]. As for the acoustic startle responses in male C57BL/6 Fmr1 KO mice, Nielsen et al. reported higher responses to tones at 70 and 80 dB and reduced responses to the 120 dB tone [40]. Thomas et al. also found reduced responses to the 120 dB tone [37]. However, Pietropaolo et al. reported normal startle responses [23]. Most of these previous studies have examined the animals with other behavioral paradigms (such as open field [37, 38, 40], elevated plus maze [40], social interaction and Y-maze [23], tail suspension test [38], light-dark test [37, 38], and marble-burying [37, 38]) before the PPI test. Thus, it is not clear whether there is significant carry over effect. Qin et al. have examined male and female Fmr1 KO mice on the FVB background, and reported lower startle response only in male mutants. They did not report on PPI [25]. Here, we found no statistical differences in acoustic startle response in either male or female C57BL/6 Fmr1 KO mice but higher PPI when the 68 dB tone was used as the prepulse stimulations.
5. Conclusion
In the present study, we investigated the effects of gender on multiple behavioral alterations that are related to FXS in an animal model. We have chosen the Fmr1 KO mice on C57BL/6 background, which have been extensively used for therapeutic development. The major finding is that both male and female Fmr1 KO mice show significant AGS, hyperactivity, and memory deficits. Thus, our study further supports that the Fmr1 KO mice can be used as a reasonable FXS animal model. However, it is important to note that we examined homozygous female mice, and human woman patients are normally heterozygous and show milder symptoms.
HIGHLIGHTS.
We examined multiple behavioral phenotypes in Fmr1 KO mice on the C57BL/6 background.
Both male and female Fmr1 KO mice displayed significant audiogenic seizures and hyperactivity in the open field and light-dark test.
Both male and female Fmr1 KO mice showed impaired contextual and passive avoidance memory and altered PPI.
Acknowledgement
This work was supported by research grants from the National Institute of Health (MH093445) and FRAXA Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- [1].Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122:4314–22. doi: 10.1172/JCI63141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–22. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- [3].Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- [4].Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–87. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- [5].Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ascano M, Jr., Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–6. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–7. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- [8].Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–84. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–45. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- [10].Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–6. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–38. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–27. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron. 2012;76:325–37. doi: 10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sethna F, Moon C, Wang H. From FMRP Function to Potential Therapies for Fragile X Syndrome. Neurochem Res. 2014 doi: 10.1007/s11064-013-1229-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–42. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- [19].Zhang M, Moon C, Chan GC, Yang L, Zheng F, Conti AC, et al. Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory. J Neurosci. 2008;28:4736–44. doi: 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004;3:337–59. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- [21].Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Carpenter RL, Paylor R. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome. Psychopharmacology (Berl) 2011;217:143–51. doi: 10.1007/s00213-011-2276-6. [DOI] [PubMed] [Google Scholar]
- [22].Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11:586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baker KB, Wray SP, Ritter R, Mason S, Lanthorn TH, Savelieva KV. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9:562–74. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- [25].Qin M, Kang J, Smith CB. A null mutation for Fmr1 in female mice: effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience. 2005;135:999–1009. doi: 10.1016/j.neuroscience.2005.06.081. [DOI] [PubMed] [Google Scholar]
- [26].Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–66. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [27].Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:15758–63. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2011;14:618–30. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem Pharmacol. 2010;79:632–46. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- [31].Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav Neurosci. 2011;125:783–90. doi: 10.1037/a0025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–30. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- [33].Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS) Behav Brain Res. 2012;228:1–8. doi: 10.1016/j.bbr.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qin M, Xia Z, Huang T, Smith CB. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 2011;194:282–90. doi: 10.1016/j.neuroscience.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–92. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- [36].Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–9. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- [37].Thomas AM, Bui N, Graham D, Perkins JR, Yuva-Paylor LA, Paylor R. Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome. Behav Brain Res. 2011;223:310–21. doi: 10.1016/j.bbr.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, et al. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–94. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res. 2002;927:8–17. doi: 10.1016/s0006-8993(01)03309-1. [DOI] [PubMed] [Google Scholar]
- [41].Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–25. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]