Abstract
Among patients with epilepsy, atypical (rightward) language lateralization has been associated with left-handedness, a left seizure focus, an early age at seizure onset, and familial sinistrality, although these associations are not consistently observed. No study has examined all of these factors in relation to language lateralization in the same epilepsy sample, let alone in a sample comprised only of patients with temporal lobe epilepsy. Moreover, little consideration has been given in previous studies to how language lateralization might be influenced by the interplay between different factors, or how much unique variance in language lateralization is explained by each factor. The primary aim of this study was to examine the combined influences of handedness, side of seizure focus, age at seizure onset, and familial sinistrality on language lateralization in temporal lobe epilepsy patients. A secondary aim was to determine which factors uniquely contribute to the prediction of language lateralization. 162 patients with intractable temporal lobe epilepsy underwent functional MRI language mapping, from which language lateralization indexes were derived. Degree of handedness was measured via the Edinburgh Handedness Inventory. Main and 2-way interaction effects on language lateralization indexes were examined via linear regressions and Fisher exact tests. Significant effects were next examined in multiple regressions to identify unique predictors of language lateralization indexes. When examined in isolation in regressions, only left-handedness and a left seizure focus predicted atypical (rightward) language lateralization. These results, however, were qualified by interaction effects demonstrating that stronger left hand preference was associated with greater atypical language lateralization only among patients with a left seizure focus, an early or intermediate age at seizure onset, or no familial sinistrality. In follow-up multiple regressions, the interaction terms accounted for a significant amount of variance in language lateralization indexes above and beyond main effects. Additionally, side of seizure focus and its interaction with handedness uniquely predicted language lateralization indexes. Results indicate that degree of left-handedness is a marker of greater atypical (rightward) language lateralization in temporal lobe epilepsy but only in the context of seizure characteristics that have the potential to drive joint reorganization of language and hand preference (i.e., left seizure focus, or early or intermediate age at seizure onset) or in the absence of a genetic predisposition for left-handedness (i.e., no familial sinistrality). This study advances existing knowledge by illustrating how different factors combine to jointly affect language lateralization, and by identifying side of seizure focus and its interaction with handedness as unique predictors of language lateralization in temporal lobe epilepsy.
Keywords: epilepsy, cerebral dominance, language lateralization, reorganization, functional MRI
Introduction
Language functions in neurologically normal individuals are mediated mainly by the left hemisphere of the brain in approximately 95% of right-handers (Pujol et al., 1999; Springer et al., 1999; Knecht et al., 2000) and approximately 80% of individuals who are mixed- or left-handed (Szaflarski et al., 2002). Symmetric or predominantly right hemisphere language representation is relatively more frequent among patients with epilepsy. In the context of evaluation for epilepsy surgery, determining which patients exhibit “atypical” (i.e., symmetric or right-lateralized) language representation is critical because surgical intervention on the dominant temporal lobe can result in verbal memory and naming decline (Sabsevitz et al., 2003; Binder et al., 2008).
Some, but not all, studies of patients with epilepsy have associated atypical language lateralization with left-handedness (Springer et al., 1999; Isaacs et al., 2006; Sveller et al., 2006), a left seizure focus (Isaacs et al., 2006), early age at seizure onset (Springer et al., 1999; Swanson et al., 2002), and familial sinistrality (Isaacs et al., 2006) (for null results pertaining to side of seizure focus and familial sinistrality, see Springer et al., 1999; for a null result pertaining to age at seizure onset, see Sveller et al., 2006). No study, however, has simultaneously examined all of these factors in the same sample of epilepsy patients. It therefore remains to be determined which of these factors are most strongly correlated with language lateralization and whether any of them provide unique information about language lateralization. Furthermore, the apparent lack of replication across studies, although in part due to the fact that some study samples were restricted to patients who were right-handed (Springer et al., 1999) or had a left seizure focus (Swanson et al., 2002; Sveller et al., 2006), is easily misinterpreted. Findings might be misinterpreted as spurious when, in fact, they are valid but apply only to a subgroup of epilepsy patients. Alternatively, findings that are specific to one epilepsy subgroup might be inappropriately generalized to a different subgroup in which the original findings do not hold. As a case in point, atypical language lateralization is less prevalent among patients with temporal lobe epilepsy (TLE) versus extra-temporal epilepsy (Woermann et al., 2003), yet most studies did not specify if their samples were comprised of patients with TLE, extra-temporal epilepsy, or other epilepsy syndromes (e.g., Rasmussen and Milner, 1977; Isaacs et al., 2006; Sveller et al., 2006). Consequently, it is unclear to which epilepsy subgroups the results of these studies apply. Clarification of the rates and correlates of typical versus atypical language lateralization within specific epilepsy syndromes is needed, most notably in TLE, the most common form of adult epilepsy and the epilepsy syndrome most amenable to surgery (Tellez-Zenteno et al., 2005).
A related limitation of the literature is a focus on the effect of single factors in isolation, without consideration of the conjoint effect of multiple factors on language lateralization. A noteworthy exception to this is Rasmussen and Milner’s (1977) classic article. In that study, the conjoint effect of handedness, side of seizure focus, and age at precipitating event was examined in relation to language lateralization, as measured by the intracarotid amobarbital (Wada) test, in 396 epilepsy patients. Among patients with early left hemisphere lesions, atypical language lateralization was observed in 72% of non-right-handers (i.e., individuals who are mixed- or left-handed) versus 19% of right-handers. By comparison, among those without early left hemisphere lesions, atypical language lateralization was observed in 30% of non-right-handers and 4% of right-handers. The markedly elevated rate of atypical language lateralization observed among non-right-handers with early left hemisphere lesions was interpreted as “pathological” left-handedness – that is, a shift from right- to left-handedness that occurs as a result of early left hemisphere injury and is often accompanied by a shift from left to right cerebral language dominance (Satz et al., 1985; Loring, 1999). Notably, had Rasmussen and Milner examined the effect of handedness irrespective of side of seizure focus and age of precipitating event, the close correspondence between hand preference and language reorganization would have been grossly underappreciated because the majority of non-right-handers in the overall sample were left language dominant (52%1). Moreover, the rate of atypical language lateralization in their sample would have been underestimated by 24% in non-right-handers with early left hemisphere lesions and overestimated by 18% in non-right-handers without early left hemisphere lesions.
While pioneering, the Rasmussen and Milner study is limited by a lack of inferential statistical analyses and by the treatment of language lateralization, handedness, and age of precipitating event as categorical variables. Regarding the latter, the importance of measuring language lateralization and handedness along continua is demonstrated by studies associating degree of left-handedness with degree of rightward language lateralization (Springer et al., 1999; Isaacs et al., 2006; Sveller et al., 2006). The clinical utility of such an approach is demonstrated in studies associating degree of left language lateralization with degree of verbal memory and naming decline following left anterior temporal lobectomy (Sabsevitz et al., 2003; Binder et al., 2008). More broadly, there is a need to compare Rasmussen and Milner’s Wada-based findings to functional MRI (fMRI)-based findings, given the increasing use of fMRI language lateralization in epilepsy centers worldwide (Baxendale et al., 2008).
To address these issues, the present study examined fMRI language lateralization in relation to handedness, side of seizure focus, age at seizure onset, and familial sinistrality in a large, clinically diverse sample comprised only of TLE surgery candidates. Where possible, variables were treated as both categorical and continuous measures in parallel analyses, with an emphasis on the conjoint effect between factors on language lateralization. The present study also examined which factors, if any, are uniquely associated with language lateralization.
Materials and methods
Participants
Participants were 162 patients with intractable TLE who underwent evaluation at the Medical College of Wisconsin Comprehensive Epilepsy Program to determine their candidacy for epilepsy surgery. Participants met the following criteria: (1) English was their primary language; (2) their full scale IQ was ≥ 70 (Wechsler, 1997, 2009); and (3) they demonstrated above chance behavioral performance on fMRI tasks. The sample included a mixture of patients without and with hippocampal sclerosis: 92 patients were without hippocampal sclerosis, 30 had right hippocampal sclerosis, 36 had left hippocampal sclerosis, and 4 had bilateral hippocampal sclerosis. The sample also included 23 patients with other pathologies: 14 had vascular abnormalities (small arteriovenous malformation or cavernoma), 8 had low-grade brain tumors (astrocytoma, dysembryoplastic neuroepithelial tumor), and 1 had a focal cortical dysplasia. Degree of handedness was measured via the Edinburgh Handedness Inventory (EHI) (Oldfield, 1971). The EHI scale varies continuously from -100 (strong left-hand preference) to 0 (symmetric handedness) to 100 (strong right-hand preference). Patients with EHI scores ≥ 50 were classified as right-handed, and those with scores < 50 were classified as non-right-handed (i.e., having mixed handedness or predominant left-hand preference). Side of seizure focus was determined by the side of anterior temporal lobe resection in patients who later underwent resective surgery (71 left, 65 right). Patients who did not undergo surgery were included in the study if they were determined to have a unilateral temporal seizure focus following review of long-term video EEG monitoring (20 left, 6 right). Age at seizure onset was defined as the age at which the patient first developed recurring seizures. Familial sinistrality was defined as having at least one left-handed first-degree relative. EHI score (but not hand preference) was missing for one patient, and age at seizure onset and familial sinistrality were missing for two patients. Written informed consent was obtained from all patients prior to fMRI using a protocol approved by the Medical College of Wisconsin Institutional Review Board.
Demographic information, fMRI task performance, and seizure characteristics for the right-handed group and non-right-handed group are presented in Table 1. The right- and non-right-handed groups did not differ in terms of demographics or seizure characteristics with the following exceptions: non-right-handers had lower EHI scores (per their group classification) and had attained a slightly higher level of education. Performance on the fMRI tasks was well above chance and did not differ between right- and non-right-handers (Table 1).
Table 1.
Sample characteristics
|
Right-handed (n = 126) Mean (SE) |
Non-right-handed (n = 36) Mean (SE) |
p | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 38.3 (1.05) | 39.5 (1.72) | .59 |
| Gender (% male/female) | 48/52 | 42/58 | .47 |
| Education (years) | 13.2 (0.22) | 14.3 (0.49) | .02 |
| Full-scale IQ* | 93.5 (1.13) | 91.3 (2.41) | .38 |
| EHI score | 89.6 (1.19) | −50.5 (8.06) | <.001 |
| Familial sinistrality (% with/without) | 44/56 | 56/44 | .20 |
| (n with/without) | 54/70 | 20/16 | -- |
| fMRI task performance | |||
| Tone decision task (% correct) | 91.9 (0.92) | 89.8 (2.03) | .32 |
| Semantic decision task (% correct) | 81.2 (0.84) | 80.9 (1.48) | .83 |
| Seizure characteristics | |||
| Side of seizure focus (% right/left) | 44/56 | 44/56 | .93 |
| (n right/left) | 55/71 | 16/20 | -- |
| Age at onset of intractable seizures (years) | 17.7 (1.14) | 15.8 (1.82) | .41 |
| (% early/intermediate/late) | 34/32/34 | 34/40/26 | .62 |
| (n early/intermediate/late) | 42/41/42 | 12/14/9 | -- |
| Hippocampal sclerosis (% right/left/bilateral/none)** | 20/19/3/56 | 14/33/0/50 | .35 |
| (n right/left/bilateral/none) | 25/24/4/70 | 5/12/0/18 | -- |
| Later had epilepsy surgery (%) | 85 | 81 | .53 |
| (n) | 107 | 29 | -- |
Note. EHI = Edinburgh Handedness Inventory;
Based on Wechsler Adult Intelligence Scale – III or IV;
Hippocampal sclerosis data were missing for three right-handed patients and one left-handed patient.
MRI acquisition
Imaging was conducted on either a 1.5 T GE Signa scanner (119 patients) or a 3 T GE Excite scanner (43 patients) (General Electric, Waukesha, WI, USA.). High-resolution, T1-weighted anatomical reference images of the entire brain were acquired with a spoiled-gradient-echo (“SPGR”) sequence. Whole brain functional imaging used a T2*-weighted gradient-echo, echoplanar sequence with the following parameters at 1.5 T: TE = 40 ms, TR = 3000 ms, field of view = 240 mm, pixel matrix = 64 × 64, 19 sagittal slices, voxel size = 3.75 × 3.75 × 7 mm. Echoplanar imaging parameters at 3 T were as follows: TE = 25 ms, TR = 3000 ms, field of view = 224 mm, pixel matrix = 64 × 64, 34 axial slices, voxel size = 3.5 mm3.
fMRI activation tasks
The fMRI protocol used a block design that alternated between a semantic decision task and a tone decision task. In the semantic decision task, patients heard animal names and indicated via button press whether each animal is both “found in the United States” and “used by humans”. In the tone decision task, patients heard brief trains of high and low tones and indicated via button press whether the train contained two high tones. Two functional imaging time series were collected, each comprised of 8 task alternations, providing 16 blocks of each task. Each block contained 8 trials, providing 128 trials for each task. Participants performed the tasks for about 15 minutes in total, and the entire protocol (with anatomical scans) was completed in about 30 minutes. These tasks, their rational, and the typical patterns of activation and lateralization have been well described (Binder et al., 1997; Frost et al., 1999; Springer et al., 1999; Szaflarski et al., 2002). In brief, the contrast between the tasks highlights speech perception and lexical-semantic processes engaged during the semantic decision task while controlling for early auditory, attention, executive, and motor response processes with the tone decision task. In healthy individuals, this contrast produces reliable and strongly left-lateralized activation in areas previously implicated in language processing, including frontal, temporal, and parietal association cortices (Binder et al., 1997; Frost et al., 1999; Springer et al., 1999) and in the left hippocampus and the surrounding medial temporal lobe (Binder et al., 1997; Bellgowan et al., 1998).
The validity of this fMRI protocol is supported by studies demonstrating its high concordance with Wada test results (Janecek, Swanson, Sabsevitz, Hammeke, Raghavan, Rozman et al., 2013) and by studies showing that fMRI lateralization indexes (LIs) calculated based on activation during these tasks predict verbal memory and language decline following left anterior temporal lobectomy, over and above noninvasive predictors of cognitive outcome (e.g., preoperative neuropsychological test performance, age at seizure onset), and relatively better than Wada testing (Sabsevitz et al., 2003; Binder et al., 2008; Binder et al., 2010). In another recent study, this fMRI protocol predicted postsurgical naming outcome with relatively better accuracy compared to the Wada test in patients with discordant Wada and fMRI language lateralization (Janecek et al., 2013).
fMRI data analysis
Image processing and statistical analyses were conducted with AFNI (http://afni.nimh.nih.gov/afni) using automated scripts, and all analyses were performed blind to any clinical data, including Wada test results. All analyses were performed at the individual subject level. Image alignment was used to reduce the effects of head movement. Task-related changes in MRI signal were identified using multiple regression. This method compares the time series of MRI signal values in each image voxel with an idealized hemodynamic response to the task alternation. The idealized response was modeled by convolving a gamma function with a time series of impulses representing each task trial. The regression model also included six movement vectors (computed during image registration) to further reduce any effects of head movement on estimation of the task response, and first- and second-order covariates to model any linear or quadratic baseline shifts. Unsmoothed individual statistical parametric maps were thresholded at voxelwise p < .01 followed by removal of clusters < 250 μl in size, resulting in a whole-brain family-wise corrected alpha of p < .05.
A region of interest (ROI) volume consisting of the left lateral hemisphere was defined based on an average left hemisphere activation map from 80 normal, right-handed participants (Frost et al., 1999; Szaflarski et al., 2002). This ROI included all activated regions in the lateral convexity of the frontal, temporal, and parietal lobes and excluded medial hemispheric regions. A mirror-image right lateral hemisphere ROI was created by reflecting the left lateral hemisphere ROI symmetrically across the midline. Significantly activated voxels were counted for each patient in the left and right lateral hemisphere ROIs. LIs reflecting the interhemispheric difference between voxel counts in left and right ROIs were calculated using the formula: LI = [Lv – Rv]/[Lv + Rv] × 100, where Lv and Rv are the number of voxels activated above threshold in the left and right hemispheres (Binder et al., 1996; Springer et al., 1999; Sabsevitz et al., 2003). This approach yields LIs ranging from -100 (strong right hemisphere dominance) to 100 (strong left hemisphere dominance). LIs were also categorized as “typical” (i.e., left) hemisphere language dominance (LI > 20) versus “atypical” (i.e., symmetrical or right) language representation (LI ≤ 20). These LI cutoffs were selected in accord with previous fMRI language lateralization studies (Springer et al., 1999; Szaflarski et al., 2002; Sveller et al., 2006).
Of note, LIs were not related to behavioral performance on the fMRI tasks or scanner field strength. Correlations between LIs and fMRI task performance (i.e., percent correct) did not approach significance (all p’s > .24). Mean LIs acquired at 1.5 T and 3 T did not differ (1.5 T = 41.6; 3 T = 43.9; t-test: p = .71), nor did the rate of atypical language lateralization (20 of 119 patients (17%) at 1.5 T, 5 of 43 patients (12%) at 3 T; Fisher exact test: p = .30).
Although this LI measure focuses on overall lateralization, the phenomenon of crossed lateralization in frontal and temporal ROIs was examined (i.e., an LI > 20 in one region and an LI < −20 in the other region). This pattern was observed in only 7 of the 162 patients, or 4% of the sample. Five of these patients showed left dominance in the frontal lobe and right dominance in the temporal lobe, whereas the remaining 2 patients showed the converse pattern. These 7 patients did not differ notably from the rest of the sample in terms of seizure focus (4 right, 3 left), age at seizure onset (4 early, 2 intermediate, 1 late), handedness (6 right, 1 left), or familial sinistrality (4 with, 3 without). Crossed lateralization was not examined further because it was rare and not systematically related to predictor variables.
In addition, to determine whether study results were robust to different fMRI activation thresholds, LIs were subsequently recomputed at two alternate thresholds, both of which were stricter than the original threshold at the voxel-level (alternate threshold #1: voxelwise p < .005, removal of clusters < 170 μl in size, whole-brain family-wise corrected alpha of p < .05; alternate threshold #2: voxelwise p < .001, removal of clusters < 80 μl in size, whole-brain family-wise corrected alpha of p < .05). The results reported below pertain to LIs calculated at the original threshold (i.e., voxelwise p < .01, removal of clusters < 250 μl in size, whole-brain family-wise corrected alpha of p < .05) unless explicitly stated.
Statistical analyses
Simple linear regression analyses were performed as a first step to examine relationships between LIs and the four factors of interest: EHI score, side of seizure focus, age at seizure onset, and history of familial sinistrality. Next, a series of multiple linear regression analyses were performed to examine 2-way interactions. In each multiple regression model, main effects of two of the four factors were examined by entering those two factors in Step 1 (e.g., EHI score, side of seizure focus). The factors’ 2-way interaction was examined by entering the corresponding cross-product term in Step 2 (e.g., EHI x side of seizure focus). All 2-way interactions between the four factors were tested in this manner, resulting in a total of six multiple regression models. Significant interactions were followed by simple slope analyses examining the effect of one factor across levels of the other factor (Aiken & West, 1991). Simple slope analyses determined whether the slopes of the simple regression lines differed from zero. If indicated, age at seizure onset was to be divided into tertiles (i.e., the distribution of age at seizure onset was divided into three parts, each containing a third of the overall sample) in simple slope analyses. Patients with recurrent medically intractable seizures starting before or at age 11 represented the first tertile and were labeled as having an “early” age at seizure onset (n = 54). The second tertile was comprised of patients with seizures starting from age 12 to 20 and was labeled “intermediate” age at seizure onset (n = 55). Patients in the third tertile started having seizures at or after age 21 and were labeled “late” age at seizure onset (n = 51). It should be emphasized that tertile cutoffs were statistically determined, and that tertile labels reference age at seizure onset in relative terms. This approach differs from other studies that have used quasi-arbitrary cutoffs (e.g., “before or at age 5”) to distinguish between early versus late age at seizure onset.
Parallel to the regression approach, one-tailed Fisher exact tests were conducted to examine rates of “typical” (i.e., left) hemisphere language dominance (LI > 20) versus “atypical” (i.e., symmetrical or right) language representation (LI ≤ 20) in relation to handedness, side of seizure focus, age at seizure onset, and familial sinistrality. One-tailed Fisher exact tests were justified due to strong a priori hypotheses about the direction of effects, as illustrated in some previous studies. The Freeman-Halton extension of the Fisher exact test was administered to examine the rate of typical versus atypical language representation across the early, intermediate, and late age at seizure onset groups. These analyses are the nonparametric equivalent to the simple regressions and simple slope analyses described above. They also provide descriptive information that is not captured in the multiple regression approach.
A final set of multiple linear regression models were to be conducted in the event that multiple main or interaction effects were observed in the previous regression analyses. The purpose of these “combined” models was to determine whether any significant effects provided unique information about LIs. To determine whether any interaction effects were unique predictors of LIs, main effects of the four factors under investigation were to be entered in Step 1, and any significant interaction effects from the previous regression analyses were to be entered in Step 2. Next, to determine whether any main effects were unique predictors of LIs, modified versions of the initial “combined” regression model were to be created. These modified models would be identical to the initial “combined” model, except that the main effect of interest would be removed from Step 1 and added to a new Step 3.
In addition, in replication analyses, all simple regressions, multiple regressions and follow-up simple slope regressions, and “combined” multiple regressions were re-run using LIs derived from the two alternate thresholds.
Results
Patterns of language laterality indexes
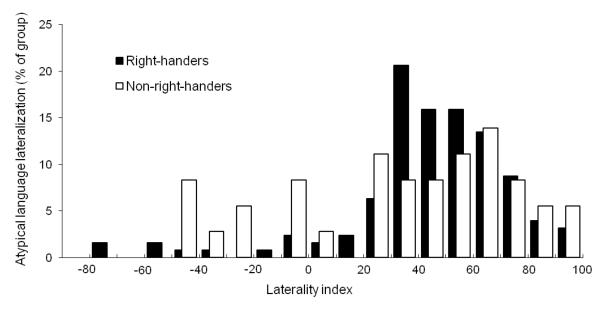
LIs varied continuously from strong left hemisphere lateralization to strong right hemisphere lateralization in both the right-handed and non-right-handed groups (Fig. 1). The majority of right-handers (88%) and non-right-handers (72%) were classified as left hemisphere dominant (i.e., LI > 20). However, left dominant patients still had, on average, 23% of activated voxels in the right hemisphere, demonstrating the continuous nature of LIs. Of note, LIs derived from the original fMRI activation threshold and the two alternate thresholds were highly correlated with one another (all r’s > .98; all p’s < .001) and, on average, differed only by roughly 1 LI unit.
Figure 1.
Histogram of language laterality indexes in right-handed and non-right-handed patients with epilepsy.
Predictors of language laterality indexes
In simple regressions, lower LIs were predicted by lower EHI scores (β = .23, R2 = .05, p < .01) and a left seizure focus (β = −.24, R2 = .06, p < .01). That is, greater rightward language lateralization was predicted by stronger left-hand preference and by a left seizure focus. A trend linking lower LIs (greater rightward language lateralization) with an earlier age at seizure onset was also observed (β = .13, R2 = .02, p = .09). LIs were not predicted by familial sinistrality (β = .04, R2 = .002, p = .60). Parallel Fisher exact tests yielded consistent main effect findings. Atypical language lateralization (i.e., symmetric representation or right language lateralization) was more common among non-right-handers (28%) versus right-handers (11%) (p = .02) and among patients with a left seizure focus (22%) versus a right seizure focus (7%) (p < .01). Additionally, rates of atypical language lateralization were higher among patients with a seizure onset in the early (20%) or intermediate (18%) tertiles compared to those in the late tertile (6%) (Freeman-Halton extension: p = .06; follow-up Fisher exact tests: early versus intermediate, p = .48; early versus late, p = .03; intermediate versus late, p = .05). The rate of atypicality did not differ between patients with familial sinistrality (12%) compared to those without familial sinistrality (19%) (p = .18).
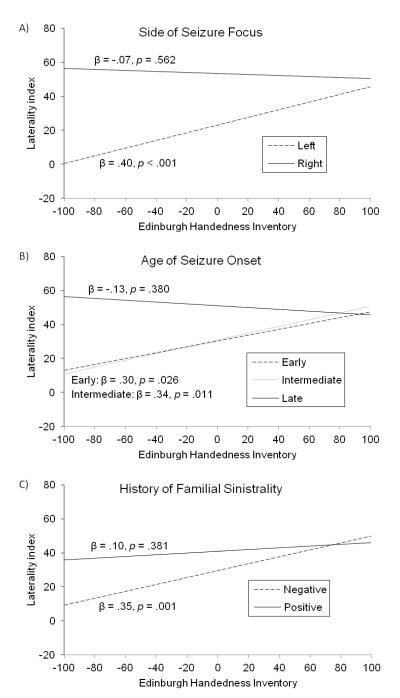
Critically, results from the simple regressions were qualified by interaction effects, as three of the six multiple regression models yielded significant or trending 2-way interaction effects, and all of these significant interactions involved handedness (Table 2). First, the EHI x side of seizure focus interaction showed a strong relationship with LIs, accounting for an additional 6% of variance in LIs above and beyond the main effects of EHI score and side of seizure focus (p < .001) (Table 2, Model A). As illustrated in Fig. 2, Panel A, lower EHI scores were associated with lower LIs among patients with a left seizure focus. That is, stronger left-hand preference was associated with greater rightward language lateralization in patients with a left seizure focus. By contrast, EHI scores were not related to LIs among patients with a right seizure focus. Analogous Fisher exact tests yielded consistent findings (Table 3, Model A). Atypical language lateralization was over three times more common among non-right-handed patients with a left seizure focus compared to their right-handed counterparts (p < .01). By contrast, over 90% of patients with a right seizure focus were left language dominant, regardless of handedness (p = .69).
Table 2.
Results of models regressing laterality indexes on main effects and corresponding interaction effects
| B | SE | β | R2 | ΔR2 | ΔF | |
|---|---|---|---|---|---|---|
| Model A(n = 161) | ||||||
| Step 1 | .104 | – | 9.13*** | |||
| EHI | 0.12 | 0.04 | 0.22** | |||
| Side of seizure focus | −15.39 | 5.09 | −0.23** | |||
| Step 2 | .161 | .058 | 10.82*** | |||
| EHI × side of seizure focus | 0.26 | 0.08 | 0.37*** | |||
| Model B (n= 159) | ||||||
| Step 1 | .059 | – | 4.86** | |||
| EHI | 0.11 | 0.04 | 0.20** | |||
| Age at onset | 0.32 | 0.21 | 0.12 | |||
| Step 2 | .113 | .054 | 9.44** | |||
| EHI × age at onset | −0.01 | 0.01 | −0.23** | |||
| Model C (n= 159) | ||||||
| Step 1 | .054 | – | 4.48* | |||
| EHI | 0.12 | 0.04 | 0.23** | |||
| Familial sinistrality | 2.46 | 5.29 | 0.04 | |||
| Step 2 | .075 | .021 | 3.51† | |||
| EHI × familial sinistrality | −0.15 | 0.08 | −0.21† | |||
| Model D (n = 160) | ||||||
| Step 1 | .071 | – | 6.01** | |||
| Side of seizure focus | −15.95 | 5.29 | −0.23** | |||
| Age at onset | 0.35 | 0.21 | 0.13 | |||
| Step 2 | .073 | .002 | 0.35 | |||
| Side of seizure focus × age at onset | 0.26 | 0.43 | 0.07 | |||
| Model E (n = 160) | ||||||
| Step 1 | .059 | – | 4.90** | |||
| Side of seizure focus | −16.76 | 5.40 | −0.24** | |||
| Familial sinistrality | 0.25 | 5.37 | 0.01 | |||
| Step 2 | .059 | .001 | 0.02 | |||
| Side of seizure focus × familial sinistrality |
−1.44 | 10.85 | −0.02 | |||
| Model F (n = 158) | ||||||
| Step 1 | .020 | – | 1.58 | |||
| Age at onset | 0.37 | 0.22 | 0.13 | |||
| Familial sinistrality | 3.70 | 5.49 | 0.05 | |||
| Step 2 | .020 | .001 | 0.01 | |||
| Age at onset × familial sinistrality | −0.02 | 0.45 | −0.01 |
Note. EHI = Edinburgh Handedness Inventory;
p < .05;
p < .01;
p < .001;
p = .06
Figure 2.
Language laterality indexes regressed on side of seizure focus (Panel A, top), age at seizure onset (Panel B, middle), and familial sinistrality (Panel C, bottom). Early, intermediate, and late age at seizure onset correspond to the first tertile (age at seizure onset ≤ 11 years old), second tertile (age at seizure onset from 12 to 20 years old), and third tertile (age at seizure onset ≥ 21 years old), respectively.
Table 3.
Results of the Fisher exact tests of laterality indices
| Language lateralization | p | ||
|---|---|---|---|
| Typical | Atypical | ||
| Model A | |||
| Left seizure focus | .006 | ||
| Right-handed (n = 70) | 86% | 14% | |
| Non-right-handed (n = 20) | 55% | 45% | |
| Right seizure focus | .686 | ||
| Right-handed (n = 55) | 93% | 7% | |
| Non-right-handed (n = 16) | 94% | 6% | |
| Model B | |||
| Early onset (age ≤ 11) | .192 | ||
| Right-handed (n = 42) | 83% | 17% | |
| Non-right-handed (n = 12) | 67% | 33% | |
| Intermediate onset (ages 12 to 20) | .164 | ||
| Right-handed (n = 40) | 88% | 12% | |
| Non-right-handed (n = 14) | 71% | 29% | |
| Late onset (age ≥ 21) | .449 | ||
| Right-handed (n = 42) | 95% | 5% | |
| Non-right-handed (n = 9) | 89% | 11% | |
| Model C | |||
| No familial sinistrality | .006 | ||
| Right-handed (n = 69) | 88% | 12% | |
| Non-right-handed (n = 16) | 56% | 44% | |
| Familial sinistrality | .459 | ||
| Right-handed (n = 54) | 89% | 11% | |
| Non-right-handed (n = 20) | 85% | 15% | |
| Model D | |||
| Left seizure focus | .069* | ||
| Early onset (n = 30) | 70% | 30% | |
| Intermediate onset (n = 31) | 74% | 26% | |
| Late onset (n = 28) | 93% | 7% | |
| Right seizure focus | 1.00* | ||
| Early onset (n = 24) | 92% | 8% | |
| Intermediate onset (n = 24) | 92% | 8% | |
| Late onset (n = 23) | 96% | 4% | |
| Model E | |||
| Left seizure focus | .358 | ||
| No familial sinistrality (n = 53) | 76% | 24% | |
| Familial sinistrality (n = 37) | 81% | 19% | |
| Right seizure focus | .445 | ||
| No familial sinistrality (n = 33) | 91% | 9% | |
| Familial sinistrality (n = 37) | 95% | 5% | |
| Model F | |||
| Early onset | .273 | ||
| No familial sinistrality (n = 27) | 74% | 26% | |
| Familial sinistrality (n = 26) | 85% | 15% | |
| Intermediate onset | .115 | ||
| No familial sinistrality (n = 32) | 75% | 25% | |
| Familial sinistrality (n = 23) | 91% | 9% | |
| Late onset | .439 | ||
| No familial sinistrality (n = 27) | 96% | 4% | |
| Familial sinistrality (n = 23) | 91% | 9% | |
Note. p-value associated with the Freeman-Halton extension of the Fisher exact test.
Second, the interaction of EHI x age at seizure onset showed a strong association with LIs, explaining an additional 5% of variance in LIs after accounting for the main effects of EHI score and age at seizure onset (p = .003) (Table 2, Model B). Follow-up analyses revealed that lower EHI scores (stronger left-hand preference) were associated with lower LIs (greater rightward language lateralization) among patients with an early seizure onset (age at seizure onset ≤ 11 years old) or an intermediate seizure onset (age at seizure onset from 12 to 20 years old) (Fig. 2, Panel B). However, EHI scores were not associated with LIs in patients with a late seizure onset (age at seizure onset ≥ 21 years old). These results were not replicated in Fisher exact tests (all p’s > .16), even though atypical language lateralization was approximately twice as common in non-right-handed patients compared to right-handed patients in each age at seizure onset group (Table 3, Model B).
Third, the EHI x familial sinistrality interaction trended towards statistical significance, accounting for 2% of variance in LIs above and beyond the main effects of EHI scores and familial left-handedness (p = .06) (Table 2, Model C). Simple regressions revealed that lower EHI scores (stronger left-hand preference) were associated with lower LIs (greater rightward language lateralization) among patients without a history of familial sinistrality (Fig. 2, Panel C). EHI scores were not related to LIs among patients with familial sinistrality. Fisher exact tests revealed analogous findings (Table 3, Model C). Non-right-handed patients without a family history of left-handedness were over three times more likely to demonstrate atypical language lateralization than their right-handed counterparts (p < .01). By contrast, a handedness effect was not observed in patients with a family history of left-handedness (p = .46).
Regression models examining the remaining 2-way interaction terms between side of seizure focus, age at seizure onset, and familial left-handedness were not significant (all p’s > .56) (Table 2, Models D, E, and F). Corresponding Fisher exact tests revealed consistent findings (Table 3, Models D, E, and F), with the exception that patients with left-sided seizures in the early or intermediate onset tertiles tended to have higher rates of atypicality compared to their late-onset counterparts (Freeman-Halton extension: p = .07; follow-up Fisher exact tests: early versus intermediate, p = .47; early versus late, p = .03; intermediate versus late, p = .06). By contrast, age at seizure onset was not related to atypicality in patients with a right seizure focus (Freeman-Halton extension: p = 1.0).
In the “combined” regression models, LIs were first regressed on the three significant interactions involving the EHI term and their corresponding main effects (Table 4). The full model accounted for 18% of the variance in LIs (p = .001). Main effects of handedness (EHI) and side of seizure focus were observed in Step 1 (see Table 4). Over and above the main effects, the interaction terms (Step 2), together, accounted for an additional 7% of unique variance in LIs (p = .005), with the EHI x side of seizure focus term alone accounting for 3% of unique variance in LIs (p = .02). As a follow-up, to determine whether the main effects of handedness or side of seizure focus were unique predictors of LIs in their own right, two modified versions of the initial “combined” regression model were created. The first of these modified models was identical to the initial “combined” model, except that the handedness term (EHI) was removed from Step 1 and added to a new Step 3. Similarly, the second model was identical to the initial “combined” model, except that side of seizure focus was transferred from Step 1 to Step 3. Results of these analyses demonstrated that side of seizure focus was a unique predictor, accounting for an additional 4% of variance in LIs (β = −.21, p < .01), but handedness was not (β = .12, p = .40).
Table 4.
Results of “combined” model examining laterality indices
| B | SE | β | R2 | ΔR2 | ΔF | |
|---|---|---|---|---|---|---|
| Model (n= 157) | ||||||
| Step 1 | .109 | – | 4.66** | |||
| EHI | 0.11 | 0.04 | 0.21** | |||
| Side of seizure focus | −14.79 | 5.24 | −0.22** | |||
| Age at seizure onset | 0.31 | 0.21 | 0.12 | |||
| History of familial sinistrality | 1.73 | 5.24 | 0.03 | |||
| Step 2 | .182 | .073 | 4.43** | |||
| EHI × side of seizure focus | 0.21 | 0.09 | 0.30* | |||
| EHI × age at onset | −0.01 | 0.01 | −0.07 | |||
| EHI × history of familial sinistrality | −0.14 | 0.09 | −0.18 |
Note. EHI = Edinburgh Handedness Inventory;
p < .05;
p < .01
All of the aforementioned regressions were re-run using LIs derived from the two alternate thresholds. All findings reported above were replicated at the alternate thresholds, with the minor exception that the EHI x familial sinistrality interaction, which previously showed a strong trend in predicting LIs (p = .06), reached statistical significance at both alternate thresholds (p’s = .04).
Discussion
In main effects analyses, greater rightward language lateralization was predicted by stronger left-hand preference, a left seizure focus, and an early or intermediate age at seizure onset, but not by familial sinistrality. These results, however, were qualified by interactions demonstrating that stronger left-hand preference was only associated with greater atypical (rightward) language lateralization in TLE patients with a left seizure focus, an early or intermediate seizure onset (i.e., onset ≤ 20 years old), or the absence of familial sinistrality, and not in patients with the opposing characteristics – that is, patients with a right seizure focus, a late seizure onset (onset ≥ 21 years old), or a history of familial sinistrality. Additionally, in nonparametric analyses, atypicality was associated with an early or intermediate age at seizure onset in patients with left-sided seizures but not in patients with right-sided seizures. Follow-up “combined” multiple regression models demonstrated that the prediction of LIs was markedly improved after accounting for interaction terms involving handedness, and that side of seizure focus and its interaction with handedness were unique predictors of LIs. The interpretation of these findings is that stronger left-hand preference serves as a marker of atypical language lateralization in TLE but only in the context of disease characteristics that have the potential to drive co-reorganization of language and hand preference (e.g., a left seizure focus or an early or intermediate seizure onset) or in the absence of a genetic predisposition for “familial” left-handedness (i.e., no history of familial sinistrality). Not only is this the first study to illustrate how disparate factors interact to influence language lateralization, it is the first to identify left seizure focus and its interaction with left-handedness as unique predictors of atypical (rightward) language lateralization in TLE.
The current study replicates previous findings demonstrating that left hemisphere seizures can result in co-reorganization of language and hand preference and, to our knowledge, is the first study to report on the incidence of atypical language dominance in a group of patients identified specifically as having left TLE. Previously, Sveller et al. (2006), using a verb generation fMRI task, found that stronger left-hand preference was predictive of greater atypical (rightward) language lateralization in 74 patients with left hemisphere seizures. Similarly, Isaacs et al. (2006) demonstrated that the rate of atypical language dominance, determined via Wada testing, increased with stronger left-hand preference in 106 patients with left hemisphere seizures (rate of atypical dominance: 15% of strong right-handers, 36% of moderate right-handers, 33% of ambidextrics, 63% of moderate left-handers, and 91% of strong left-handers). Comparing across studies, the overall rate of atypical language lateralization among patients with left seizures was higher in Sveller et al. (32% of right-handers, 69% of non-right-handers) and Isaacs et al. (21% of right-handers, 68% of non-right-handers2) than in the current study (14% of right-handers, 45% of non-right-handers). Notably, however, neither Sveller et al. nor Isaacs et al. reported on seizure localization in their sample. Consequently, the higher rate of atypical language lateralization found in those studies might be due to the presence of patients with extra-temporal epilepsy, who demonstrate higher rates of atypicality compared to TLE (Woermann et al., 2003). Although the absolute rate of atypicality differed across studies, the ratio of atypical language lateralization in right- versus non-right-handers was comparable (Sveller et al. = 1: 2.2; Isaacs et al. = 1: 3.2; current study = 1: 3.2). Together, these studies suggest that atypical language lateralization is approximately 3 times more common among non-right-handed patients with left-sided seizures compared to their right-handed counterparts.
The current study also clarifies the relationship between handedness and language lateralization in patients with right hemisphere seizures. This topic is understudied, as previous research has often focused exclusively on patients with left hemisphere seizures or lesions (e.g., Rasmussen & Milner, 1977; Sveller et al., 2006). Isaacs et al. (2006) did examine language lateralization in 68 patients with right hemisphere seizures and reported the following rates of atypical language dominance: 0% of strong right-handers, 13% of moderate right-handers, 60% of ambidextrics, 33% of moderate left-handers, and 20% of strong left-handers. The authors concluded that “smaller cell sizes are likely the reason why the linear pattern observed previously [in the left hemisphere seizure group, discussed above] no longer existed; however, the overall trend was generally the same” (p. 1585). Critically, a correlation coefficient was not calculated in that study because handedness and language lateralization were measured along ordinal and nominal scales, respectively. In the current study, the correlation between handedness and language lateralization did not approach statistical significance in right TLE patients (β = −.07, p = .56), nor did the rate of atypical language lateralization differ between right- and non-right-handers in this group (7% and 6%, respectively; p = .69). Thus, in contrast to Isaacs et al.’s interpretation, which was not based on inferential statistics, the null correlation of the current study indicates that the relationship between handedness and language lateralization is, at a minimum, markedly weaker in right TLE patients compared to left TLE patients (and potentially nonexistent in right TLE patients). This likely reflects the infrequency of leftward language and handedness co-reorganization, a process that theoretically would only occur in extremely rare cases, such as a patient who is genetically programmed to be right language dominant and left-handed (i.e., reverse lateralization) and has right hemisphere seizures.
In addition to the findings pertaining to side of seizure focus, this study’s novel treatment of age at seizure onset helps explain inconsistencies in the literature about the effect of this variable on language lateralization. Earlier age at seizure onset was correlated with rightward language lateralization in patients with left hemisphere seizures in a previous study (Swanson et al., 2002) but not in another (Sveller et al., 2006). In the current study, the joint effect of age at seizure onset and side of seizure focus was not predictive of language lateralization in regression models (Table 2, Model D). Nonparametric analyses, however, revealed trending or significant effects, as atypicality was associated with an early or intermediate age at seizure onset in patients with left-sided seizures but not in patients with right-sided seizures (Table 3, Model D). To further investigate the null regression result, we performed simple slope analyses restricted to patients with left hemisphere seizures. We found that age at seizure onset did not predict LIs in this subsample (β =.15, p = .15); however, a strong trend was observed after a square-root transformation was applied to age at seizure onset (β =.17, p = .06), which has the effect of adjusting for nonlinearities at the low and high ends of this scale, as well as overcrowding at the low end. This result, although only a trend, may account for inconsistent findings in the literature, as it suggests that the effect of age at seizure onset on language lateralization is nonlinear.
By comparison, the association between age at seizure onset and LIs was more robust when viewed in the context of handedness (Tables 2 and 3, Model C), as stronger left-hand preference was associated with atypical (rightward) language lateralization among patients in the early seizure onset group (age at seizure onset ≤ 11 years old) and, surprisingly, among patients in the intermediate seizure onset group (age at seizure onset from 12 to 20 years old). By contrast, this same effect was not observed in the late seizure onset group (age at seizure onset ≥ 21 years old) (Fig. 2, Panel B). These results raise the possibility that language and handedness co-reorganization can occur in patients up until young adulthood, well after the typically defined critical window for reorganization. However, an alternative explanation is that seizures may have developed following a relatively early precipitating neurological event in the early and intermediate seizure onset groups, whereas precipitating events occurred much later in the late seizure onset group (Salazar et al., 1985). To investigate this possibility, age at precipitating neurological event was examined in a subset of patients in whom such events had been identified (n = 68). Age at precipitating neurological event was defined as the age of the earliest known neurological event thought to precipitate epilepsy (e.g., birth trauma, head trauma, bout of meningitis or encephalitis), not counting febrile seizures. Results supported the alternative explanation: precipitating events typically occurred perinatally or during childhood in the early and intermediate seizure onset groups (mean age at precipitating event: 3.89 and 6.78 years, respectively; early versus intermediate onset groups t-test: p = .30), whereas precipitating events typically occurred well into adulthood in the late seizure onset group (mean age at precipitating event: 24.45 years; early versus late onset groups, intermediate versus late onset groups t-tests: all p’s < .001). Thus, the relationship between handedness and language lateralization among patients with an intermediate age at seizure onset appears to be partially explained by relatively early precipitating events, at least in those patients in whom such an event can be identified.
This situation illustrates the limitations of “age at seizure onset” and “age at precipitating event” as they relate to the onset of cerebral dysfunction and associated language and handedness co-reorganization. “Age at seizure onset” is relatively easily identified in patients with epilepsy but may not correspond closely to the onset of cerebral dysfunction (e.g., in patients with congenital cerebral abnormalities who suffer their first seizure in adolescence). Conversely, “age at precipitating event” better corresponds with the onset of cerebral dysfunction; however, many patients do not have identifiable precipitating events (e.g., patients who develop seizures idiopathically). A third alternative is to define age at onset as age at onset of recurrent seizures or age at precipitating event, whichever was earlier (Swanson et al., 2002). While this approach is limited in that it combines two different measures of “onset”, it better captures the effects of perinatal and congenital lesions that alter cerebral organization prior to the onset of seizures. In clinical practice, a reasonable rule when estimating the onset of cerebral dysfunction and associated reorganization is to use age at precipitating event whenever possible.
In addition to the interactions of handedness with side of seizure focus and age at seizure onset, the current study identifies a previously unknown interaction between handedness and familial sinistrality in epilepsy patients (Fig. 2, Panel C). Notably, our results directly contradict those of Isaacs et al. (2006), in which it was reported that “participants with a familial history of sinistrality generally had a higher incidence of atypical language dominance” (p. 1857). In the current study, the main effect of familial sinistrality on language lateralization not only failed to approach significance, but atypical language lateralization was more common among patients without familial sinistrality.
This result complements previous work from our group examining the effects of familial sinistrality and handedness on language lateralization in healthy individuals. Using the same fMRI language mapping protocol, we previously found that language lateralization in 100 healthy right-handers did not differ between those with familial sinistrality (median LI = 63) compared to those without (median LI = 66) (Springer et al., 1999). By contrast, among 50 healthy non-right-handers, familial sinistrality was associated with a greater degree of atypical (rightward) activation (mean LI = 32) compared to those without familial sinistrality (mean LI = 51) (Szaflarski et al., 2002). Taken together, these studies indicate that stronger left-hand preference is associated with a greater degree of rightward language lateralization in healthy individuals with familial sinistrality (median LI of right-handers = 63; mean LI of non-right-handers = 32), whereas this effect appears to be much weaker in healthy individuals without familial sinistrality (median LI of right-handers = 66; mean LI of non-right-handers = 51). These data suggest that a common genetic factor contributes to the hemispheric specialization of both language and handedness in healthy individuals (Szaflarski et al., 2002). By contrast, the converse pattern was observed in the current sample of TLE patients: stronger left-hand preference was associated with a greater degree of rightward language lateralization among patients without familial sinistrality (mean LI of right-handers = 47; mean LI of non-right-handers = 20); however, this association was not observed among patients with familial sinistrality (mean LI of right-handers = 43; mean LI of non-right-handers = 46). This converse pattern presumably reflects the presence of distinct subgroups of non-right-handers across the two familial sinistrality groups. Specifically, the non-familial group was likely comprised primarily of “pathologic” left-handers (left-hand dominant, right language dominant) and typically organized patients (right-hand dominant, left language dominant), resulting in the observed positive correlation between EHI scores and LIs. Similarly, the familial sinistrality group was likely comprised of “pathologic” left-handers and typically organized patients; however, this group also likely included “familial” left-handers, who are generally left-hand dominant and predominately left language dominant (Szaflarski et al., 2002). The presence of these “familial” left-handers, then, may have masked the EHI-LI correlation observed in the non-familial group. These findings suggest that disease characteristics are the primary determinants of atypical co-lateralization of language and handedness in TLE patients, overriding the genetic contributions that play a prominent role in atypical co-lateralization in healthy individuals.
Because multiple main and interaction effects were observed, the next logical question was which, if any, of these effects provided unique information about LIs. This question was addressed in “combined” multiple regressions, which revealed two primary findings. First, the addition of 2-way interaction terms (i.e., handedness x side of seizure focus, handedness x age at seizure onset, and handedness x familial sinistrality) significantly enhanced the prediction of LIs, over and above main effects. Second, side of seizure focus and its interaction with handedness were unique predictors of LIs. The finding that side of seizure focus remained uniquely predictive of LIs is important because it demonstrates that language and handedness reorganization, although typically correlated, are potentially dissociable processes. For example, in the current sample, 15% of right-handers with a left seizure focus exhibited atypical (rightward) language lateralization (by comparison, only approximately 5% of neurologically normal right-handers exhibit atypical language lateralization; see Pujol et al., 1999; Springer et al., 1999; Knecht et al., 2000). Also noteworthy, the two unique predictors (i.e., side of seizure focus and handedness x side of seizure focus), together, accounted for 16% of the total variance in LIs (p < .001), or 89% of the variance explained by the full model.
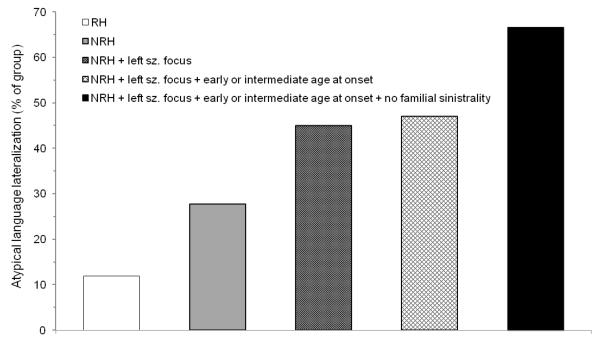
Limitations and strengths of this study should be recognized. One limitation is that 3-way interactions were not investigated statistically, as the necessary partitioning of data would have resulted in unstable regression models even with this study’s large sample size. Of most interest is the conjoint effect of handedness, side of seizure focus, and age at seizure onset on language lateralization. In their classic article, Rasmussen and Milner (1977) provided descriptive information (but no inferential analysis) about the effect of handedness in patients with early damage to the left hemisphere, reporting atypical language lateralization in 19% of right-handers (8/42 cases) and 72% of non-right-handers (66/92 cases). The same effect appears to be present, though less robust, in the current sample of patients with left-sided seizures and early or intermediate seizure onsets, as atypical (right or symmetric) language lateralization was observed in 22% of right-handers (10/46 cases) and 47% of non-right-handers (8/17 cases). The relatively lower rate of atypical language lateralization observed among non-right-handers likely reflects the fact that the current sample was restricted to TLE patients, who may demonstrate lower rates of atypical language lateralization than patients with extra-temporal epilepsy (Woermann et al., 2003), and to patients with full scale IQs greater than 70, which may have limited the number of patients who sustained severe early left hemisphere brain insults. Of note, the rate of atypicality in the current sample approached that reported in Rasmussen and Milner after accounting for familial sinistrality, as 66% (6/9 cases) of non-right-handers with left-sided seizures, an early or intermediate seizure onset, and no history of familial sinistrality exhibited atypical language lateralization (Fig. 3). Another limitation of this study is that language lateralization was based on a single fMRI language contrast and was not based on the assessment of multiple language domains. Although we cannot rule out the possibility of interhemispheric dissociations between expressive versus receptive language functions in individual patients, such dissociations are extremely rare, occurring in less than 1% of epilepsy surgery candidates (Risse et al., 1997), and are of unknown significance in terms of postsurgical cognitive outcome. A third limitation of the current study is its focus on correlation rather than causation. Future research should aim to identify genetic or environmental factors that prospectively differentiate those patients who undergo language and handedness co-reorganization from those who do not (Bishop, 2013; Pahs et al., 2013; Willems, Van der Haegen, Fisher, & Francks, 2014).
Figure 3.
Percentage of patients with atypical language lateralization among all right-handers (leftmost bar; 15/126 cases); among all non-right-handers (second bar from left; 10/36 cases); among non-right-handers with a left seizure focus (third bar from left; 9/20 cases); among non-right-handers with a left seizure focus and an early or intermediate age at seizure onset (fourth bar from left; 8/17 cases); and among non-right-handers with a left seizure focus, an early or intermediate age at seizure onset, and no history of familial sinistrality (rightmost bar; 6/9 cases). Note: RH = right-handed, NRH = non-right-handed, sz. = seizure.
Strengths of the present study include a large, well-characterized sample consisting exclusively of TLE patients, the simultaneous examination of a wide range of factors relevant to language lateralization, replication of key findings using LIs derived from different fMRI activation thresholds, and parallel parametric and nonparametric statistical approaches. Elaborating on the latter, to our knowledge, this is one of the only studies to treat handedness, age at seizure onset, and language lateralization as continuous variables and the first study to formally test interaction effects on language lateralization. In doing so, this study makes a substantial contribution to the existing literature, identifying which factors combine to influence language lateralization, and designating left seizure focus and its interaction with left-handedness as unique predictors of atypical (rightward) language lateralization in TLE. This study also has clear clinical implications. For example, it is often the case that patients undergo neurological and neuropsychological examinations prior to language mapping. While the current data are in no way a substitute for language mapping itself (the full “combined” regression model accounted for a highly significant, yet modest, 18% of the variance in LIs), this study provides clinicians with a heuristic to identify patients in whom atypical language lateralization should be suspected – namely, those patients who are mixed or left-handed and have one of the following characteristics: left hemisphere seizures, seizure onset prior to age 21 (roughly) or precipitating events during childhood, or no history of familial sinistrality. Also of clinical relevance, whereas handedness is typically treated as a dichotomous variable in clinical settings, the current data compellingly illustrate the utility of formal measures that treat handedness as a continuous variable. Measures such as the EHI are widely available, easily administered, and should be a routine component of presurgical epilepsy evaluations.
Highlights.
Interaction effects on language lateralization in epilepsy are poorly understood.
In this study, several main effects on language lateralization were observed.
These results, however, were qualified by highly significant interaction effects.
Side of seizure focus and its interaction with handedness uniquely predict atypicality.
Results paint a new, more complex picture of language lateralization in epilepsy.
Acknowledgements
The authors thank Linda Allen, Thomas Hammeke, Dongwook Lee, George Morris, Wade Mueller, Conrad Nievera, Edward Possing, Manoj Raghavan, Jane Springer, and Scott Winstanley for assistance with patient recruitment and collecting and coding the data.
Funding This study was supported by the National Institute of Neurological Diseases and Stroke (R01 NS35929), National Institutes of Health General Clinical Research Center M01 RR00058, a National Research Service Award Fellowship (F32 MH11921), and the Dana Foundation.
Abbreviations
- EHI
Edinburgh Handedness Inventory
- fMRI
functional MRI
- LI
laterality index
- ROI
region of interest
- TLE
temporal lobe epilepsy
Footnotes
Drs. Stewart, Swanson, Sabsevitz, Janecek, and Binder, as well as Ms. Rozman, report no conflicts of interest related to the content of this manuscript.
The rate of typical and atypical language lateralization among all non-right-handers was extrapolated from data presented in Rasmussen and Milner (1977) and found to be 52% and 48%, respecitvely.
These rates were extrapolated from data presented in Isaacs et al. (2006).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Sage Publications, Inc.; Thousand Oaks: 1991. [Google Scholar]
- Baxendale S, Thompson PJ, Duncan JS. The role of the Wada test in the surgical treatment of temporal lobe epilepsy: an international survey. Epilepsia. 2008;49(4):715–720. doi: 10.1111/j.1528-1167.2007.01515_1.x. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Binder JR, Swanson SJ, Hammeke TA, Springer JA, Frost JA, et al. Side of seizure focus predicts left medial temporal lobe activation during verbal encoding. Neurology. 1998;51(2):479–484. doi: 10.1212/wnl.51.2.479. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49(8):1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46(4):978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49(12):1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Mueller WM. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy: language lateralization versus hippocampal activation asymmetry. Epilepsia. 2010;51(4):618–626. doi: 10.1111/j.1528-1167.2009.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013;340 doi: 10.1126/science.1230531. DOI: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, et al. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122(Pt 2):199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Isaacs KL, Barr WB, Nelson PK, Devinsky O. Degree of handedness and cerebral dominance. Neurology. 2006;66(12):1855–1858. doi: 10.1212/01.wnl.0000219623.28769.74. [DOI] [PubMed] [Google Scholar]
- Janecek JK, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Mueller W, et al. Naming outcome prediction in patients with discordant Wada and fMRI language lateralization. Epilepsy & Behavior. 2013;27(2):399–403. doi: 10.1016/j.yebeh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecek JK, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Rozman M, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–22. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Loring DW, editor. INS dictionary of neuropsychology. Oxford University Press, Inc.; New York: 1999. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pahs G, Rankin P, Helen Cross J, Croft L, Northam GB, Liegeois F, et al. Asymmetry of planum temporale constrains interhemispheric language plasticity in children with focal epilepsy. Brain. 2013;136:3163–75. doi: 10.1093/brain/awt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52(5):1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, 3rd, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35(10):1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain Cogn. 1985;4(1):27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Sveller C, Briellmann RS, Saling MM, Lillywhite L, Abbott DF, Masterton RA, et al. Relationship between language lateralization and handedness in left-hemispheric partial epilepsy. Neurology. 2006;67(10):1813–1817. doi: 10.1212/01.wnl.0000244465.74707.42. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Binder JR, Possing ET, Hammeke TA, Sabsevitz DS, Spanaki M, et al. FMRI language laterality during a semantic decision task: age of onset and side of seizure focus effects. Paper presented at the Annual Meeting of the International Neuropsychological Society; Toronto, Canada. 2002. [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(Pt 5):1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition. Pearson; San Antonio: 2008. [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Willems RM, Van der Haegen L, Fisher SE, Francks C. On the other hand: including left-handers in cognitive neuroscience and neurogenetics. Nature Reviews Neuroscience. 2014;15(3):193–201. doi: 10.1038/nrn3679. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]