SUMMARY
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway integrates environmental clues to regulate cell growth and survival. We showed previously that depriving cells of a single essential amino acid rapidly and reversibly arrests purine synthesis. Here we demonstrate that amino acids via mTORC2 and IκB kinase regulate Akt activity, and Akt association and phosphorylation of transketolase (TKT), a key enzyme of the non-oxidative pentose phosphate pathway (PPP). Akt phosphorylates TKT on Thr382, markedly enhancing enzyme activity and increasing carbon flow through the non-oxidative PPP, thereby increasing purine synthesis. Mice fed a lysine-deficient diet for two days show decreased Akt activity, TKT activity, and purine synthesis in multiple organs. These results provide a new mechanism whereby Akt coordinates amino acid availability with glucose utilization, purine synthesis, and RNA and DNA synthesis.
INTRODUCTION
Cell proliferation depends on the availability of extracellular nutrients and growth factors, including amino acids. The latter regulate the PI3K/Akt signaling module and the mammalian target of rapamycin (mTOR) in both mTORC1 and mTORC2 complexes (Jewell et al., 2013; Laplante and Sabatini, 2012; Tato et al., 2011). Amino acids activate mTORC1 via Rag family GTPases in conjunction with a “ragulator” complex (Han et al., 2012; Zoncu et al., 2011), and may also act via Rheb, which is regulated by the tuberous sclerosis complex (TSC1/2) in response to PI3K/Akt phosphorylation (Gao et al., 2002; McManus and Alessi, 2002; Smith et al., 2005). mTORC2 is activated via PI3K-induced association with ribosomes, and is thus positioned to sense amino acid availability, but amino acid activation of mTORC2 has not been a consistent finding (Comb et al., 2012; Gulati et al., 2008; Tato et al., 2011; Zinzalla et al., 2011; Zoncu et al., 2011). Amino acids also regulate PI3K through the inhibitor of nuclear factor-κB kinase (IκB kinase), thereby regulating phospholipid-dependent protein kinase (PDK)-1 (Comb et al., 2012; Tato et al., 2011). PDK-1 and mTORC2 phosphorylate Akt, leading to full Akt activation and phosphorylation of proteins that promote cell growth and survival (Engelman et al., 2006; Manning and Cantley, 2007; Sarbassov et al., 2005).
Few studies have addressed downstream consequences of amino acid deprivation. We showed previously that depriving human lymphoblasts for a single essential amino acid rapidly and markedly reduces purine synthesis via the de novo and salvage pathways (Boss and Erbe, 1982). The decrease in purine synthesis was from reduced production of phosphoribosylpyrophosphate (PRPP), a key and rate-limiting substrate for both purine synthetic pathways (Boss, 1984; Boss and Pilz, 1985). PRPP is produced by the oxidative and non-oxidative branches of the pentose phosphate pathway (PPP), and we showed that almost all PRPP for purine synthesis comes from the non-oxidative PPP in lymphoblasts (Boss and Pilz, 1985). In synchronized human cancer cells, we demonstrated a marked increase in purine synthesis as cells progress from G1 into S phase driven by a corresponding increase in PRPP production from the non-oxidative PPP (Fridman et al., 2013). Other workers have also found the non-oxidative PPP to be the major provider of PRPP, with >70% of nucleic acid ribose in multiple human cancer cell lines derived through the transketolase (TKT), transaldolase, and triose phosphate isomerase reactions (Cascante et al., 2000; Comin-Anduix et al., 2001; Raivio et al., 1981).
TKT is a ubiquitous enzyme that catalyzes the reversible transfer of two-carbon ketol units between ketose and aldose phosphates (Schenk et al., 1998). It governs carbon flow through the non-oxidative branch of the PPP, with high metabolic flux control coefficients reported for TKT in several cell types (Cascante et al., 2000; Comin-Anduix et al., 2001). Though these data suggest TKT may control purine synthesis—and thereby RNA and DNA synthesis and represent a potential target for cancer therapy—astonishingly little is known about regulation of TKT. It is highly expressed in proliferating tumor cells, and TKT transcription appears to be under control of hypoxia-inducible factor-α, which is de-regulated in many cancers (Zhao et al., 2010). Proteomic and classical biochemical data suggest TKT may be modified post-translationally (Schenk et al., 1998), but, to our knowledge, post-translational regulation of TKT activity has not been demonstrated.
We found previously that growth factor stimulation of Akt regulates purine synthesis at several steps, including PRPP production (Wang et al., 2009). Here we show that amino acids regulate Akt phosphorylation of TKT Thr382, thereby regulating TKT activity, PRPP production, and purine nucleotide synthesis. Amino acid regulation of the Akt/TKT module is physiologically relevant, because depriving mice of lysine for only two days decreased Akt and TKT activity and reduced purine synthesis in liver, spleen, and kidneys. These data provide an underlying mechanism for amino acid deprivation as a therapeutic strategy for cancer.
RESULTS
Amino Acids Regulate de novo Purine Synthesis via Akt
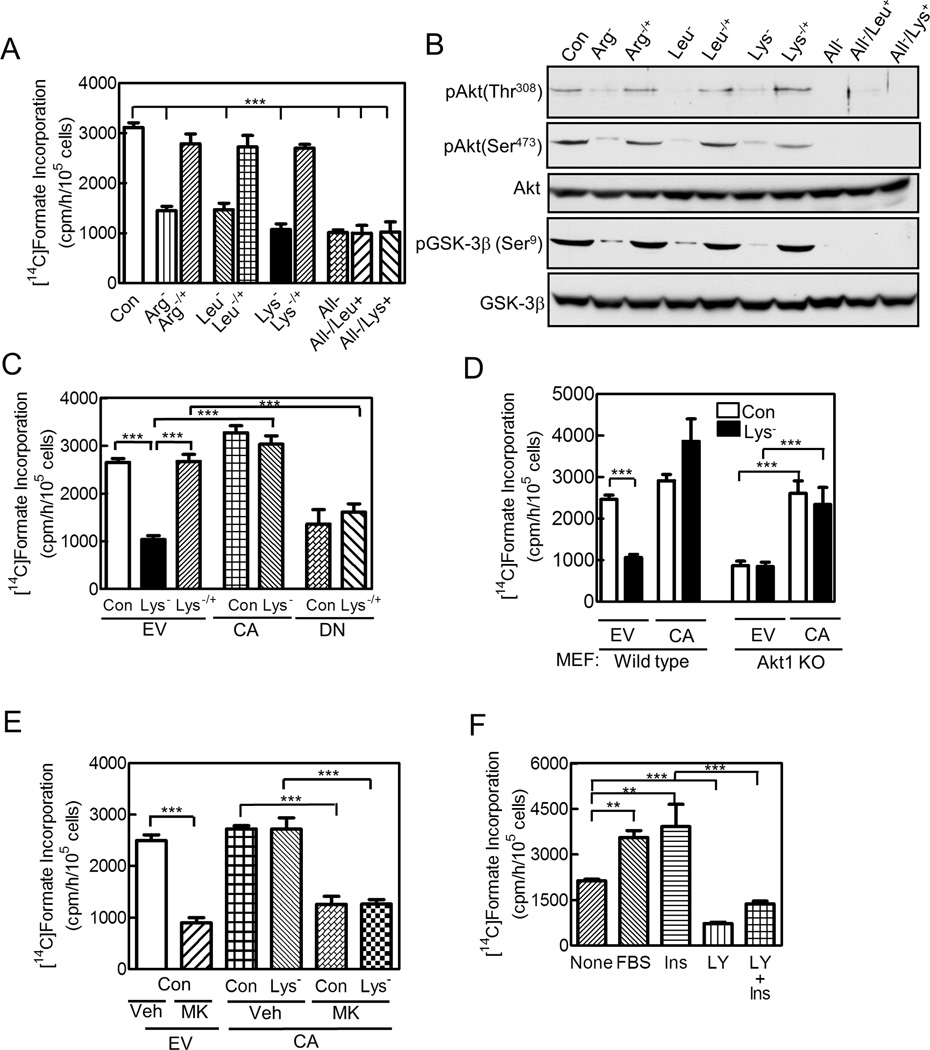
Depriving mammalian cells of a single essential amino acid for 3 h reduced de novo purine synthesis by ~ 60%, as measured by [14C]formate incorporation into purine nucleotides [Figures 1A,S1A show HeLa cells deprived of arginine, isoleucine, leucine, lysine, tyrosine, or valine, and Figure S1B shows HCT116 human colon adenocarcinoma cells deprived of arginine, leucine, or lysine. Fig 1D shows mouse embryonic fibroblasts (MEFs) deprived of lysine (described fully in next paragraph)]. Amino acid deprivation simultaneously reduced Akt activation by > 70%, as measured by Akt Thr308 and Ser473 phosphorylation and/or phosphorylation of the Akt substrate GSK-3β (Figures 1B,S1C–F,I). Similar results occurred on depriving HeLa cells of all essential amino acids (Figures 1A,B,S1C–E). Adding back the missing amino acid for 1 h fully recovered Akt activation and purine synthesis, while adding one amino acid to cells deprived of all essential amino acids had no effect (Figures 1A,B,S1B–F). In most subsequent experiments, we concentrated on depriving cells of lysine.
Figure 1. Amino Acids Regulate de novo Purine Synthesis via Akt.
(A,B) HeLa cells were cultured for 3 h in full DMEM (Con) or the same medium lacking arginine (Arg−), leucine (Leu−), lysine (Lys−), or all essential amino acids (All−); the deficient amino acid was then added for 1 h to some samples deprived of one amino acid (−/+), or leucine or lysine were added to some samples deprived of all amino acids (Leu+, Lys+). (C) HeLa cells were transfected for 24 h with empty vector (EV), or constitutively-active (CA) or dominant-negative (DN) Akt; some cells were deprived of lysine for 3 h, with lysine returned to some cells for 1 h (−/+). (D) Wild type and Akt1 knock-out (KO) MEFs were cultured in full or lysine-deficient medium. (E) HeLa ells were transfected with empty vector or the CA-Akt 24 h prior to lysine deprivation. In some cells, 1 µM MK2206 (MK) or vehicle (Veh, 0.1% DMSO) were present during the 3 h culture and 1 h labeling periods. (F) HeLa cells were cultured for 16 h in DMEM containing 0.1% FBS, and then 10% FBS, 10 nM insulin (Ins), and/or 20 µM LY294002 (LY) were added 3, 1, or 2 h respectively, before measuring purine synthesis. None denotes no additions to the serum-deficient medium. (A,C–F) Rates of de novo purine synthesis were measured. (B) Akt activation was assessed by immunoblotting using Akt(phospho-Thr308)-, Akt(phospho-Ser473)- and GSK-3β(phospho-Ser9)-specific antibodies; total Akt and GSK-3β were also assessed. Bar graphs show means ± SE of 3 independent experiments performed in duplicate (**, p<0.01; ***, p<0.001); immunoblots are representative of 3 independent experiments. See Figure S1.
Expressing a myristoylated constitutively-active (CA) Akt prevented the decrease of purine synthesis in lysine-deprived HeLa cells, and expressing a kinase-dead dominant-negative (DN) Akt prevented the return of purine synthesis on re-adding lysine [Figure 1C; expression of the CA- and DN-Akt and their effect on cellular Akt activity were confirmed by immunoblotting and GSK-3β phosphorylation, respectively (Figure S1G). The CA-Akt remained fully active during lysine deprivation as assessed by Thr308 and Ser473 phosphorylation and GSK-3β phosphorylation (Figure S1H)]. Purine synthesis was less in Akt1−/− than wild type MEFs, and, during lysine deprivation, decreased in the wild type but not the Akt1−/− cells (Figure 1D; Akt immunoblot is shown in Figure S1I). When expressed at amounts similar to endogenous Akt, the CA-Akt restored purine synthesis in the Akt1−/− cells to that of wild type MEFs, and prevented the decrease by lysine deprivation (Figures 1D,S1I). In the Akt1−/− cells, GSK-3β phosphorylation was less than in wild type cells and not regulated by lysine availability (Figure S1J).
The allosteric Akt inhibitor MK2206 reduced Akt, GSK-3β, S6 kinase, and 4E–BP1 phosphorylation by ~80% and purine synthesis by 73% in cells expressing endogenous Akt (Figures 1E,S1K) (Lindsley et al., 2008). It also inhibited CA-Akt activity as assessed by marked reduction in Thr308 and GSK-3β phosphorylation and a lesser reduction in Ser473 phosphorylation, and it blocked CA-Akt’s recovery of purine synthesis in lysine-deprived cells (Figures 1E,S1H). In serum-starved cells, Akt activity and rates of purine synthesis were low, and adding serum or insulin re-activated Akt and increased purine synthesis (Figures 1F,S1L). The PI3K inhibitor LY294002 inhibited Akt activity and purine synthesis, and prevented insulin from fully activating Akt and restoring purine synthesis (Figures 1F,S1L). We conclude that amino acids and growth factors regulate purine synthesis via Akt.
Amino Acids Regulate Akt and Purine Synthesis via mTORC2 and IκB Kinase
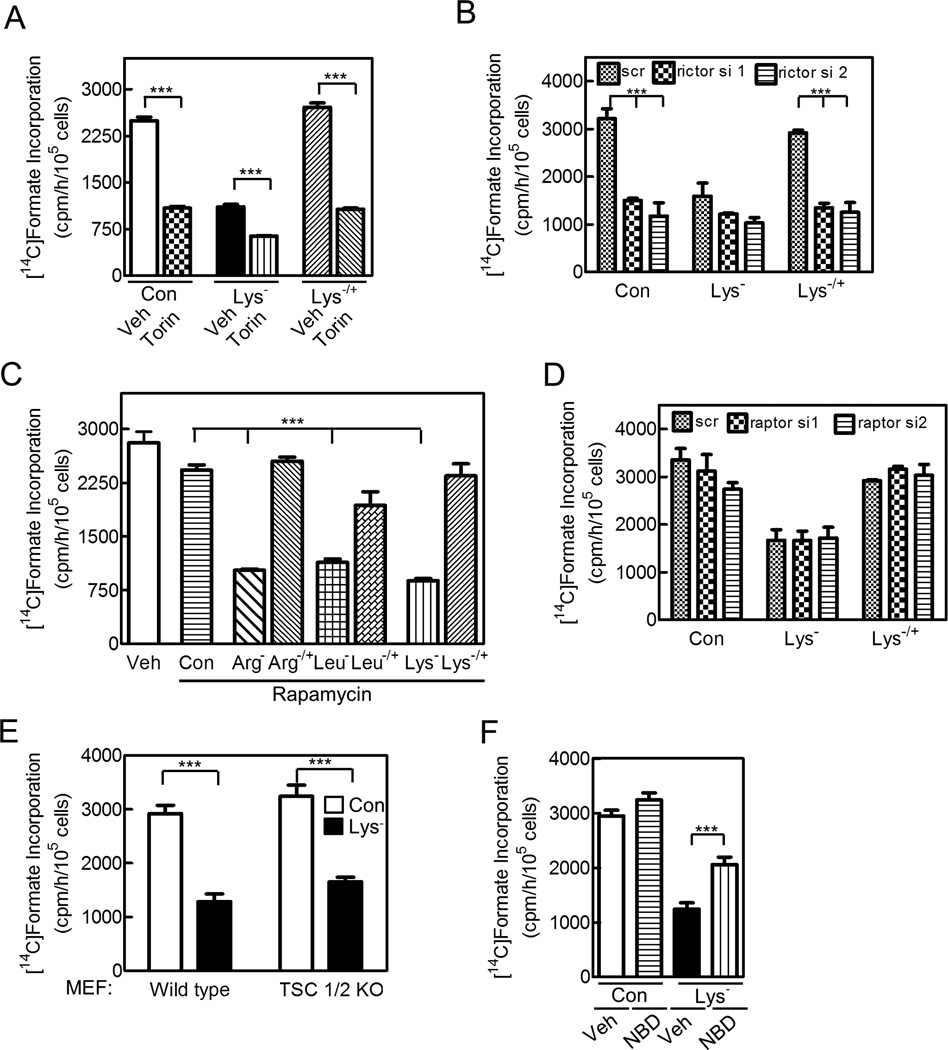
To determine if mTOR was involved in amino acid regulation of Akt and purine synthesis, we used Torin 1, a mTOR inhibitor in both mTORC1 and mTORC2 complexes (Thoreen et al., 2009); as expected, it inhibited S6 kinase, 4E–BP1, and Akt Ser473 phosphorylation (Figures S2A,B). Torin 1 reduced purine synthesis to a similar extent as lysine deprivation, further reduced purine synthesis when combined with lysine deprivation, and prevented recovery of purine synthesis on reconstituting lysine (Figure 2A). These effects correlated with its inhibition of Akt Ser473 phosphorylation (Figure S2B). Thus, amino acids regulate Akt and purine synthesis, at least in part, through mTOR. We found that two different rictor siRNAs, which reduced rictor expression and Akt Ser473 phosphorylation, reduced purine synthesis in control cells and prevented purine synthesis recovery on amino acid reconstitution (Figures 2B,S2C). These data indicate that amino acids regulate purine synthesis through mTORC2, and to determine if mTORC1 was also involved, we performed several experiments. Rapamycin, an mTORC1-specific inhibitor, inhibited S6 kinase and 4E-BP1 phosphorylation, but had no effect on purine synthesis in control cells or cells expressing CA-Akt, or on recovery of purine synthesis in amino acid-deprived cells (Figures 2C,S2D,E show HeLa cells, and Figure S2F shows HCT116 cells). Similarly, two different raptor siRNAs, which reduced raptor expression and S6 kinase and 4E-BP1 phosphorylation, did not significantly affect purine synthesis in control cells or on returning lysine to lysine-deprived cells (Figures 2D,S2G). And finally, lysine deprivation of TSC1/2-deficient cells, which show constitutive Rheb and mTORC1 activation (Zhang et al., 2003), reduced purine synthesis as much as in wild type cells (Figure 2E). Thus, amino acids did not appear to regulate purine synthesis through mTORC1, but they did regulate mTORC1 activity, because lysine deprivation reduced S6 kinase and 4E-BP1 phosphorylation in Akt1−/− cells (Figure S2H).
Figure 2. Amino Acids Regulate Akt and Purine Synthesis via mTORC2 and IκB Kinase.
Cells were cultured in deficient medium and purine synthesis was measured. (A,C,F) HeLa cells received (A) 250 nM Torin-1 (Torin), (C) 10 nM rapamycin, or (F) 100 µM NBD peptide during the 3 h culture and 1 h labeling periods as indicated. (B,D) HeLa cells were transfected with two different siRNAs targeting (B) rictor or (D) raptor 72 h before measuring purine synthesis; scr indicates scrambled siRNA. (E) Wild type or TSC1/2-deficient MEFs were studied. Data are the means ± SE of 3 independent experiments performed in duplicate (***, p<0.001). See Figure S2.
To determine if IκB kinase played a role in amino acid regulation of purine synthesis, we used NBD peptide, an IκB kinase inhibitor (Comb et al., 2012). We found that the NBD peptide did not affect purine synthesis in control cells, but that it partly prevented the reduction in purine synthesis during amino acid deprivation (Figure 2F; Figure S2I shows that NBD partly restores Akt phosphorylation during amino acid deprivation). We conclude that amino acids regulate Akt and purine synthesis through mTORC2 and IκB kinase.
Amino Acids Regulate PRPP Production by the Non-oxidative PPP via Akt
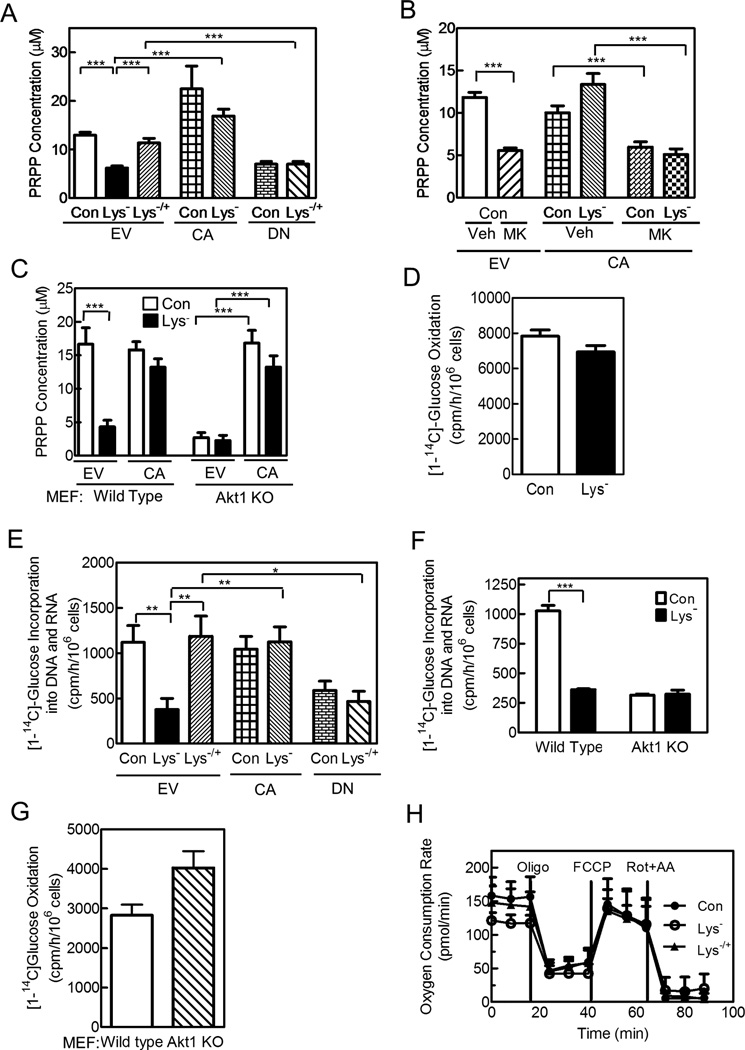
PRPP is a key substrate of purine synthesis (Becker et al., 1987), and we showed previously that depriving human lymphoblasts for an essential amino acid reduces cellular PRPP production (Boss, 1984). We now asked whether amino acids regulate PRPP production via Akt. Depriving HeLa cells of lysine for 3 h reduced intracellular PRPP production and the intracellular PRPP concentration by 42% and 52%, respectively, and adding back lysine restored both measures of PRPP [Figures 3A,S3A; the PRPP concentration was based on a mean intracellular volume of 1.76 pL for HeLa cells, and is similar to that reported in other cell types (Becker et al., 1987)]. Expressing CA-Akt prevented the decrease in PRPP production and concentration in lysine-deprived cells, and the DN-Akt prevented the return of PRPP on lysine reconstitution (Figures 3A,S3A). Inhibiting Akt with MK2206 decreased the PRPP concentration similarly to lysine deprivation, and blocked the effect of the CA-Akt, while rapamycin had no effect on the PRPP concentration in cells expressing the CA-Akt (Figures 3B,S3B). PRPP production and the PRPP concentration were considerably less in Akt1−/− MEFs compared to wild type MEFs, and lysine deprivation decreased both PRPP measures in wild type MEFs, but had no effect on PRPP in Akt1−/− MEFs (Figures 3C,S3C). The CA-Akt prevented the decrease in PRPP concentration in wild type MEFs and returned the PRPP concentration in Akt1−/− MEFs to that of wild type MEFs (Figure 3C). In all experiments, the PRPP concentration correlated well with rates of purine synthesis (compare Figures 3A and 1C, 3B and 1E, and 3C and 1D), and since PRPP is rate-limiting to purine synthesis, the changes in PRPP concentration likely explain the changes in purine synthesis (Becker et al., 1987).
Figure 3. Amino Acids Regulate PRPP Production by the Non-oxidative PPP via Akt.
(A,B,E) HeLa cells were transfected and cultured as described in Figure 1. (C,F,G) Wild type and Akt1 knock-out MEFs were cultured in full or lysine-deficient medium as indicated; in C, cells were transfected with empty vector or CA-Akt 24 h prior to study. (D,H) HeLa cells were deprived of lysine with lysine added back as indicated. (A–C) The intracellular PRPP concentration was measured in cell extracts by following [8-14C]hypoxanthine conversion to IMP in the presence of HPRT. Carbon flow through the oxidative (D,G) and non-oxidative (E, F) PPP was measured. (H) Oxygen consumption rates were measured with the following agents added as indicated: 1 µM oligomycin A (Oligo)—a complex V inhibitor, 1 µM carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP)—an uncoupler of electron transport that yields maximal respiratory capacity, and 1 µM rotenone (Rot)—a complex I inhibitor—with 1 µM antimycin A (AA)—a complex III inhibitor. Data are the means ± SE of three independent experiments performed in duplicate; *, **, and *** indicate p< 0.05, 0.01, and 0.001, respectively. See Figure S3.
PRPP can be produced by either the oxidative or non-oxidative PPP (Figure S3D), but we found no change in oxidative PPP activity in lysine-deprived HeLa cells (Figure 3D). Carbon flow through the non-oxidative PPP is difficult to measure, given the reversibility of reactions, but we and others have followed [1-14C]glucose incorporation into purine nucleotides and/or into DNA and RNA (Figure S3D) (Boss and Pilz, 1985; Brand and Deckner, 1970). Using this method, we found a 67 % reduction in carbon flow through the non-oxidative pathway in lysine-deprived HeLa cells, which largely reversed on returning lysine to the cells (Figure 3E). Because no change occurred in carbon flow through the oxidative pathway during amino acid deprivation, we could also follow [6-14C]glucose incorporation into purine nucleotides and DNA and RNA, and we found similar results as for [1-14C]glucose incorporation (Figure S3E). Expression of CA-or DN-Akt prevented the reduction in carbon flow during amino acid deprivation or reversal by amino acid return, respectively (Figure 3E). Carbon flow through the non-oxidative PPP was lower in Akt1−/− MEFs compared to their wild type counterparts, and was reduced by amino acid availability in the wild type but not Akt1−/− cells (Figure 3F); carbon flow through the oxidative pathway tended to be higher in Akt1−/− cells, suggesting a compensatory increase (Figure 3G).
To assess if amino acid deprivation caused general metabolic changes, we measured cellular oxygen consumption and found that lysine deprivation had no significant effect on basal or maximal oxygen consumption (Figure 3H). We also performed an unbiased screen of 534 metabolites (150 identified and 384 unidentified), and found only nine, including lysine, that changed significantly after 3 h of lysine deprivation: ribose, three purines (hypoxanthine, guanosine, and inosine), three pyrimidines (uracil, uridine, and xanthine), and a pyridine (nicotinamide) (Table S1). These metabolites could be expected to change based on decreased carbon flow through the non-oxidative PPP and decreased PRPP production, and they returned to control values on reconstituting lysine for 1 h (Table S1). We conclude that amino acids regulate PRPP production through the non-oxidative PPP via Akt, and that depriving cells briefly of an essential amino acid does not alter general cellular metabolism and causes reversible changes in PRPP-derived metabolites. The rapid reversible change in metabolites on amino acid reconstitution is consistent with the rapid reversible changes in PRPP production and purine synthesis (Figures 1A,S1B,3A).
Amino Acids Regulate TKT Activity through an Akt- dependent Mechanism; TKT Activity Is Regulated by Phosphorylation
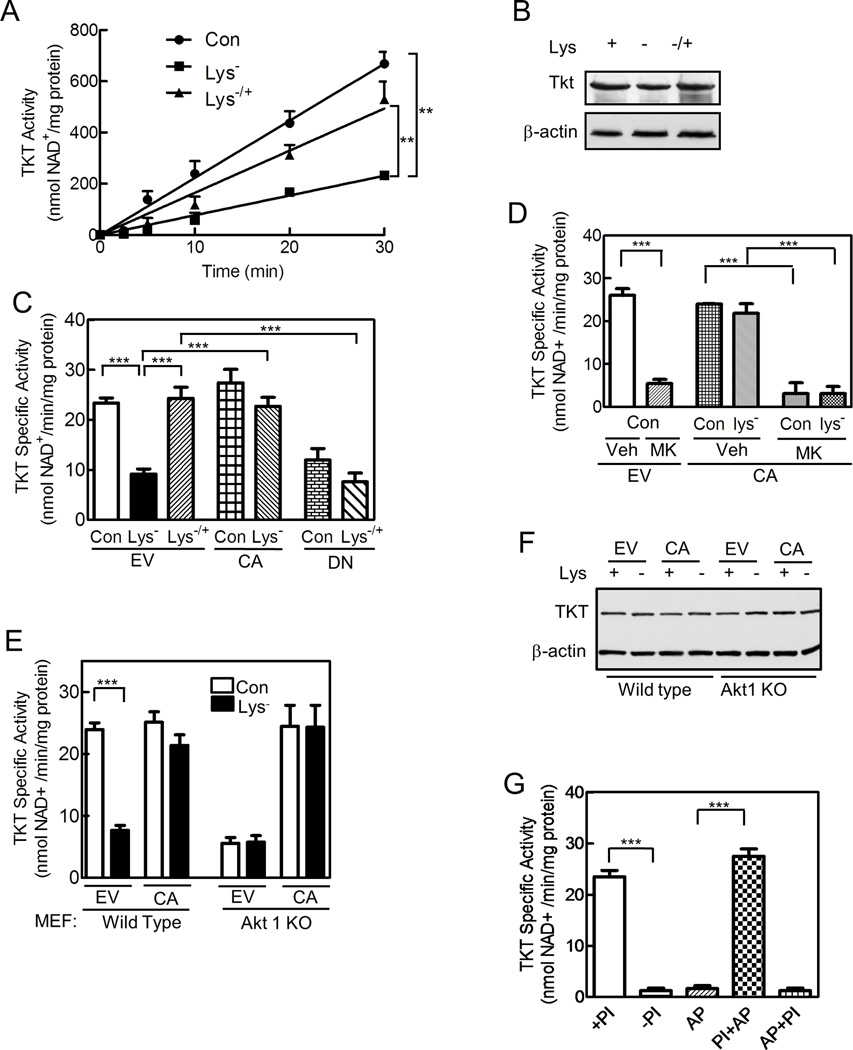
Since TKT is thought to control carbon flow through the non-oxidative PPP (Cascante et al., 2000), we measured its activity, and found that 3 h of deprivation for lysine or other essential amino acids reduced TKT activity by >60%; adding lysine back for 1 h recovered enzyme activity in lysine-deprived cells (Figures 4A,S4A). The changes in TKT activity were not from changes in the amount of TKT mRNA or protein (Figures 4B,S4B). Expressing CA-Akt prevented the decrease in enzyme activity during lysine deprivation, whereas expressing DN-Akt prevented the increase in enzyme activity on lysine return (Figure 4C). Consistent with these results, the Akt inhibitor MK2206 reduced TKT activity by >80%, and prevented the return of enzyme activity by the CA-Akt in lysine-deprived cells; rapamycin had no effect on TKT activity in cells expressing the CA-Akt (Figures 4D,S4C). Akt1−/− MEFs had significantly less TKT activity than their wild type counterparts, although they expressed similar amounts of TKT protein (Figures 4E,F). Lysine deprivation reduced enzyme activity by 65% in wild type MEFs, but had no effect in the Akt1−/− cells; expressing the CA-Akt prevented the decrease in enzyme activity in lysine-deprived wild type MEFs and returned TKT activity in the Akt1−/− cells to that of the wild type cells, without changing TKT expression (Figures 4E,F). In neither HeLa cells nor MEFs did lysine deprivation affect the activity of glucose 6-phosphate dehydrogenase, the rate-limiting enzyme of the oxidative PPP (Figures S4D,E). Together, these results suggest TKT activity is regulated by an Akt-dependent post-translational mechanism.
Figure 4. Amino Acids Regulate TKT Activity through an Akt-dependent Mechanism; TKT Activity Is Regulated by Phosphorylation.
HeLa cells (A–D,G) and wild type and Akt1 knock-out MEFs (E,F) were cultured and transfected as described in Figure 1. (A,C–E,G) Cells were extracted by sonication, and TKT activity was measured. In A, linearity of the assay is shown for cells that had been incubated in full (Con) or lysine-deficient medium (Lys−), or after lysine reconstitution (Lys−/+). (B,F) TKT protein and β-actin were assessed by immunoblotting. (G) HeLa cells were extracted with or without phosphatase inhibitors (+PI and −PI, respectively); some of the extracts were incubated with alkaline phosphatase bound to agarose beads (AP), and in some cases phosphatase inhibitors were added prior to (PI+AP) or after (AP+PI) incubation with alkaline phosphatase. In all cases, the alkaline phosphatase was removed, prior to measuring TKT activity. Graphs represent means ± SE of 3 independent experiments performed in duplicate; immunoblots are representative of at least 3 independent experiments (**, p<0.01;***, p< 0.001). See Figure S4.
For measuring TKT activity, we included phosphatase inhibitors in the extract buffer. Excluding the inhibitors and incubating extracts at room temperature for 30 min prior to measuring enzyme activity markedly reduced TKT activity; adding alkaline phosphatase, with its removal prior to measuring TKT activity, did not further reduce TKT activity (Figure 4G). Adding phosphatase inhibitors before alkaline phosphatase recovered full TKT activity, whereas adding them after alkaline phosphatase had no effect (Figure 4G). Thus, the inhibitors effectively blocked alkaline phosphatase activity, but did not increase TKT activity by affecting the coupled enzymatic reaction, which contained ribose 5-phosphate, xylulose 5-phosphate, and thiamine diphosphate. These results suggest that TKT is phosphorylated and that dephosphorylation largely inactivates the enzyme.
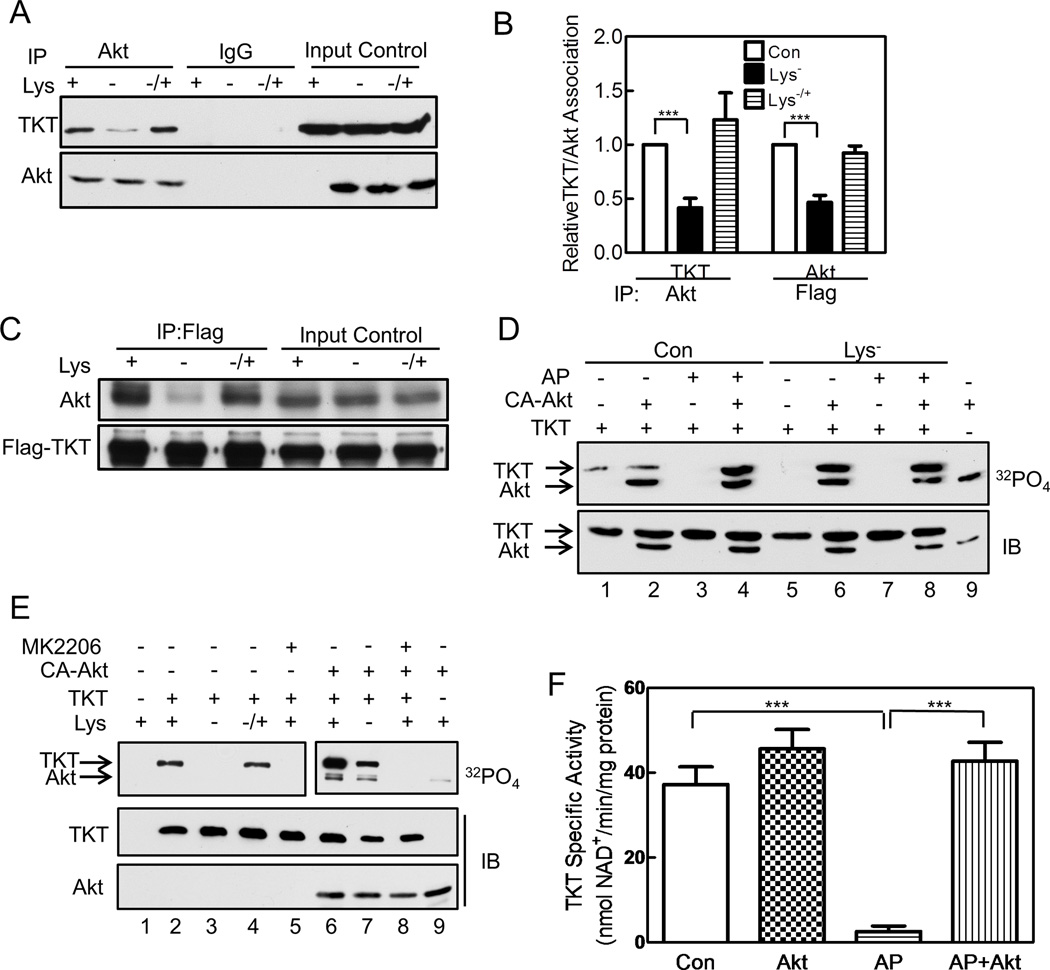
Akt and TKT Associate, and Akt Phosphorylates TKT, Regulating Its Activity
To assess if Akt phosphorylates TKT, we performed four sets of experiments. First, we found that Akt and TKT associate intracellularly by demonstrating TKT in Akt immunoprecipitates; the amount of TKT was significantly less in immunoprecipitates from lysine-deprived cells but returned to that of control cells on reconstituting lysine (Figures 5A,B). To perform the reciprocal experiment, we transfected HeLa cells with Flag-tagged TKT, because TKT antibodies are non-immunoprecipitating, and found Akt in anti-Flag immunoprecipitates; the amount of Akt was considerably less in immunoprecipitates from lysine-deprived cells and returned to that of control cells on lysine reconstitution (Figures 5B,C). Thus, TKT associates with Akt, and the association is regulated by amino acid availability.
Figure 5. Akt and TKT Associate, and Akt Phosphorylates TKT, Regulating Its Activity.
HeLa cells were cultured as described in Figure 1. (A) Cells were extracted and an Akt-specific antibody or control IgG was added to cell extracts to generate immunoprecipitates, which were analyzed by immunoblotting with anti-TKT and anti-Akt antibodies; input control represents 10% of the cell extract. (B) Immunoblots from A and C were scanned and the relative amounts of TKT present in Akt immunoprecipitates (left, n=2) or Akt present in TKT immunoprecipitates (right, n=3) are shown (mean ± SE, *p<0.05). (C) Similar experiment as in A, except the cells were transfected with empty vector (EV) or Flag-tagged TKT, and subjected to immunoprecipitation with anti-Flag antibody; blots were developed with anti-Akt or anti-Flag antibodies. (D) Flag-tagged TKT was isolated from cells and incubated with [γ-32P]ATP in vitro in the absence or presence of CA-Akt. In some cases, TKT was pre-incubated with alkaline phosphatase (AP)-coated agarose beads, which were removed by centrifugation prior to incubation with Akt. Lane 9 shows CA-Akt incubated without TKT. Upper panel shows an autoradiograph and lower panel an immunoblot. (E) HeLa cells were co-transfected with Flag-tagged TKT and empty vector (lanes 1–5) or CA-Akt (non-tagged, lanes 6–9). Cells were cultured for 3 h in control or lysine-deficient phosphate-free medium containing 32PO4; some of the cultures were reconstituted with lysine (lane 4) or received 1 µM MK2206 (lanes 5 and 8). TKT (anti-Flag) immunoprecipitates were analyzed by SDS-PAGE/autoradiography and immunoblotting. Two autoradiograph exposures are shown: 96 h for the left panel and 24 h for the right panel. (F) Flag-tagged TKT was isolated from amino acid-replete HeLa cells, and enzyme activity was measured as described in Figure 4. Some samples were incubated with Akt plus ATP, and some were pre-incubated with alkaline phosphatase, which was removed prior to adding Akt (n=3, mean ± SE, ***p<0.001). Panels C, D, and E are representative of three and panel A of two independent experiments. See Figure S5.
Second, to assess if Akt phosphorylates TKT in vitro, we isolated Flag-tagged TKT and CA-Akt from different sets of HeLa cells, and incubated the enzymes separately or together with [γ-32P]ATP. We found a small amount of TKT phosphorylation in the absence of added Akt, presumably from endogenous Akt associated with TKT (Figure 5D, lane 1). The CA-Akt underwent autophosphorylation and increased TKT phosphorylation, but only if TKT had been incubated previously with alkaline phosphatase (Figure 5D, compare lanes 2 and 4; Figure S5A shows activity of purified CA-Akt with GSK-3β). These data suggest that TKT was highly phosphorylated under normal, amino acid-replete conditions, preventing additional in vitro phosphorylation. When TKT was isolated from lysine-deprived cells, no phosphorylation was detected without exogenous Akt, but adding CA-Akt resulted in robust TKT phosphorylation, which was not increased after alkaline phosphatase treatment (Figure 5D, lanes 5–8). Thus, TKT isolated from lysine-deprived cells is poorly phosphorylated, allowing for efficient Akt phosphorylation in vitro. Akt also efficiently phosphorylated TKT purified from bacteria, in the absence, but not in the presence of MK2206 (Figure 6E, compare lanes 2 and 4).
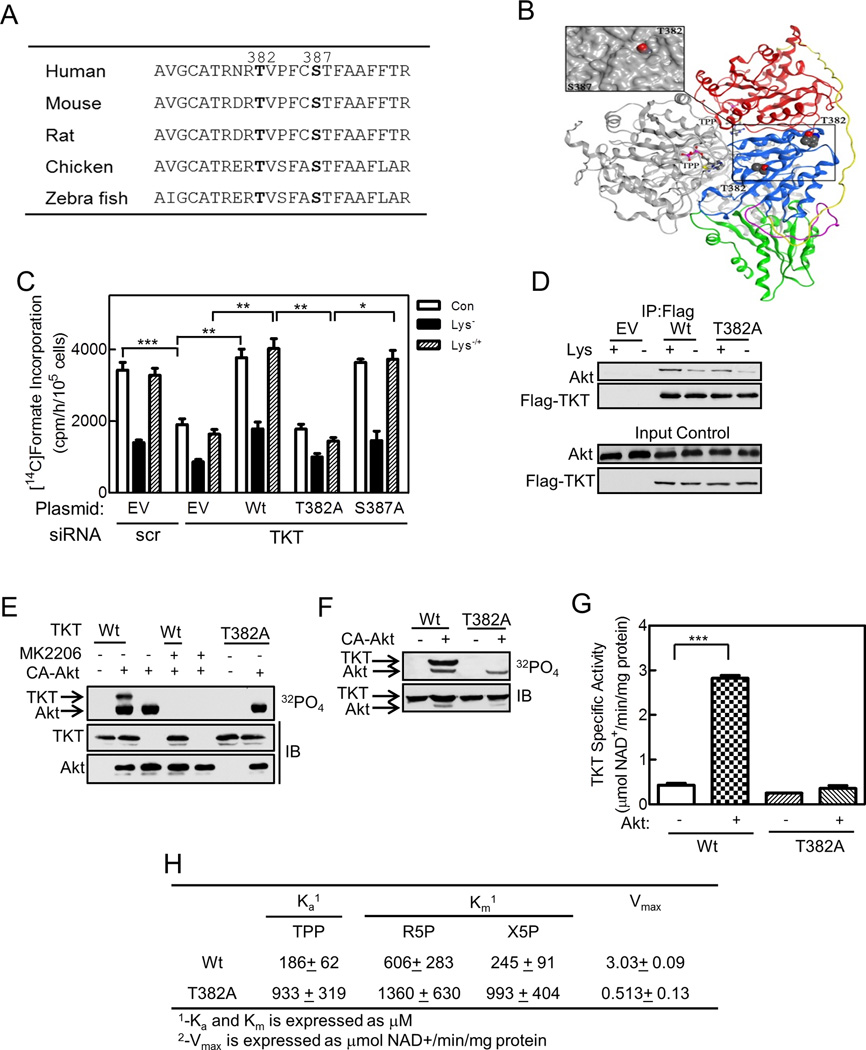
Figure 6. TKT Thr382 Is an Akt Phosphorylation Site Critical to Enzyme Activity and Purine Synthesis.
(A) TKT sequence corresponding to amino acids 373 to 395 of the human enzyme is shown for five species, with Thr382 and Ser387 in bold, since they were identified as phosphorylated residues by LC-MS/MS. (B) Structure of the TKT dimer (pdb 3MOS) is shown as ribbon representation (Mitschke et al, 2010). One monomer is shown in grey, while the other monomer is colored by domains: N-terminal pyrophosphate-binding domain (PP) in red, aminopyrimidine-binding domain (Pyr) in blue connected by a flexible linker in yellow, and C-terminal domain in green connected by a flexible linker in purple. Inset. Close up view of Thr382 and Ser387 on the Pyr domain with a molecular surface applied (grey) demonstrating solvent accessibility of Thr382. The figure was generated using MOE 2001.10 (Chemical Computing Group, Canada). (C) HeLa cells were transfected with siRNA targeting TKT or a scrambled (scr) control siRNA, and 72 h later were transfected with empty vector (EV), wild type TKT, or T382A or S387A mutant TKT. After 24 h, cells were cultured in full (Con) or lysine–deficient medium with lysine restoration as indicated. Purine synthesis was measured as described in Figure 1. (D) HeLa cells were transfected with empty vector (EV), Flag-tagged TKT wild type (Wt), or mutant (T382A). Anti-Flag immunoprecipitates were analyzed by immunoblotting with anti-Akt and anti-Flag antibodies; the lower two panels show 10% input controls. (E) Bacterially-produced wild type (Wt) or T382A mutant TKT were incubated with [γ-32P]ATP in the absence or presence of CA-Akt and 1 µM MK2206. An autoradiogram (upper panel) and immunoblots (lower panels) are shown. (F) HeLa cells were transfected with Flag-tagged wild type (Wt) or T382A mutant TKT, and additionally received empty vector or CA-Akt. Cells were incubated with 32PO4 for 3 h. Anti-Flag immunoprecipitates were analyzed by SDS-PAGE. An autoradiograph (top panel) and immunoblot (lower panel) are shown. (G,H) Bacterially-produced wild type (Wt) or T382A mutant TKT were incubated (G) in the absence or presence of CA-Akt plus ATP, or (H) with varying concentrations of thiamine pyrophosphate (TPP), ribose 5-phosphate (R5P), or xylulose 5-phosphate (X5P), and TKT activity was measured. In (H), Ka and Km were obtained from standard Lineweaver-Burke transformations of Michaelis-Menten plots; Vmax was measured at 10 times the Km concentration. Panels D and E are representative of three and panel F of two independent experiments. In panels C, G and H, data are mean ± SE. *, **, and *** indicate p< 0.05, 0.01, and 0.001, respectively. See Figure S6.
Third, to assess if Akt phosphorylates TKT in intact cells, we transfected HeLa cells with Flag-tagged TKT in the absence or presence of CA-Akt, incubated the cells with 32PO4 in full or lysine-deficient medium, and isolated TKT by immunoprecipitation. TKT was phosphorylated in cells incubated in full medium in the absence, but not in the presence of MK2206 (Figure 5E, lanes 2 and 5; the autoradiograph was exposed for 96 h). TKT phosphorylation was not observed in lysine-deprived cells, but reconstituting lysine for 1 h fully restored TKT phosphorylation [Figure 5E, lanes 3 and 4; TKT phosphorylation was also not detected in cells deprived of all essential amino acids and reconstituting one amino acid had no effect (Figure S5B)]. Co-transfecting CA-Akt increased TKT phosphorylation in cells in full medium and now revealed TKT phosphorylation in lysine-deprived cells; adding MK2206 to the cells prevented phosphorylation in the presence of CA-Akt (Figure 5E, lanes 6–9; the autoradiograph was exposed for 24 h).
And fourth, to assess if Akt phosphorylation of TKT affected enzyme activity, we immunoprecipitated Flag-tagged TKT from HeLa cells cultured in full medium, incubated the TKT with CA-Akt and/or alkaline phosphatase, and measured TKT activity. Incubation with Akt increased TKT activity a small non-significant amount, consistent with TKT being almost fully phosphorylated in amino acid-replete cells (Figure 5F). Incubation with alkaline phosphatase almost completely abolished TKT activity, but incubation with alkaline phosphatase followed by Akt fully restored enzyme activity, indicating that Akt phosphorylation of TKT regulated the enzyme’s activity (Figure 5F).
TKT Thr382 Is an Akt Phosphorylation Site Critical to Enzyme Activity and Purine Synthesis
To define TKT phosphorylation sites, we analyzed TKT purified from HeLa cells by mass spectrometry, and found phosphorylation of two sites—Thr382 and Ser387—under normal and lysine-deprived conditions. Phosphorylation of the sites under lysine-deprived conditions is consistent with residual TKT activity of ~ 35% in amino acid-deprived cells (Figures 4A,S4A); fully dephosphorylated TKT is almost completely devoid of activity (Figure 4G). Thr382 and Ser387 and their surrounding sequences are conserved evolutionarily to zebra fish (Figure 6A), with Thr382 showing greater surface exposure and hence accessibility to a protein kinase (Figure 6B). Ser387 lacks characteristics of an Akt consensus site, but Thr382 has Arg at position −1 and −3, a hydrophobic residue at +1 (Val), and a Pro at +2 that can form a tight turn, suggesting a potential Akt recognition site (Obata et al., 2000). While the classic Akt consensus sequence also has Arg at the −5 position, several well-documented Akt substrates lack this feature (Table S2, discussed later).
To study the potential importance of Thr382 and Ser387, we mutated them to alanine, and expressed the mutants in cells where TKT had been knocked down using a siRNA approach (TKT protein is shown in Figures S6A,B, and was decreased 66% by the siRNA). In cells transfected with a scrambled siRNA, we found normal rates of purine synthesis and normal regulation by amino acid deprivation (Figure 6C). However, transfecting cells with a TKT siRNA reduced rates of purine synthesis by ~ 45%, and depriving the cells of an amino acid further reduced purine synthesis; reconstituting the amino acid recovered only that portion of purine synthesis attributable to the amino acid effect (Figure 6C). The latter data indicate that the residual TKT protein was still subject to regulation by amino acid availability. Reconstituting wild type TKT or the S387A mutant to TKT knock-down cells fully restored rates of purine synthesis, whereas reconstituting the T382A mutant was without effect (Figure 6C). We conclude that the amount of TKT in HeLa cells is limiting for purine synthesis, and that Thr382, but not Ser387, is crucial for full rates of purine synthesis.
In subsequent experiments, we asked if Akt phosphorylates Thr382, performing some key experiments with Ser387. First, we found that similarly to wild type TKT, Akt associated with the T382A mutant TKT, and that the association was decreased by lysine deprivation (Figure 6D). Second, we found that both CA-Akt and wild type Akt isolated from HeLa cells phosphorylated wild type but not the T382A mutant TKT purified from bacteria [Figures 6E,S6C; Akt phosphorylated the S387A mutant similarly to the wild type protein]. Third, we found that Akt phosphorylated wild type TKT, but not the T382A mutant in intact cells (Figure 6F). Fourth, we found that the T382A mutant TKT isolated from amino acid-replete HeLa cells had significantly less activity than the wild type enzyme, with similar amounts of TKT protein recovered (Figures S6D,E; the S387A mutant had similar activity as the wild type enzyme). In contrast, wild type and T382A mutant TKT purified from bacteria exhibited similar specific activities, consistent with an unphosphorylated state of the bacterially-produced enzyme; adding Akt and ATP dramatically increased activity of the wild type, but not the mutant enzyme (Fig. 6G; expression and purity of both enzymes are shown in Figure S6F). These data indicate that Akt phosphorylates TKT on Thr382, and that this phosphorylation regulates TKT activity.
To study the basis for the decreased activity of the T382A mutant, we performed kinetic studies on the mutant and wild type enzymes purified from bacteria and incubated with Akt. We found that affinity of the mutant enzyme for the substrates ribose 5-phosphate and xylulose 5-phosphate was about one-half and one-fourth that of the wild type enzyme, respectively, while affinity for the essential co-factor thiamine pyrophosphate (TPP) and the Vmax were about one-fifth and one-sixth that of the wild type enzyme, respectively (Figure 6H).
Lysine Deprivation Decreases Akt and TKT Activity, and Purine De Novo Synthesis in Mice
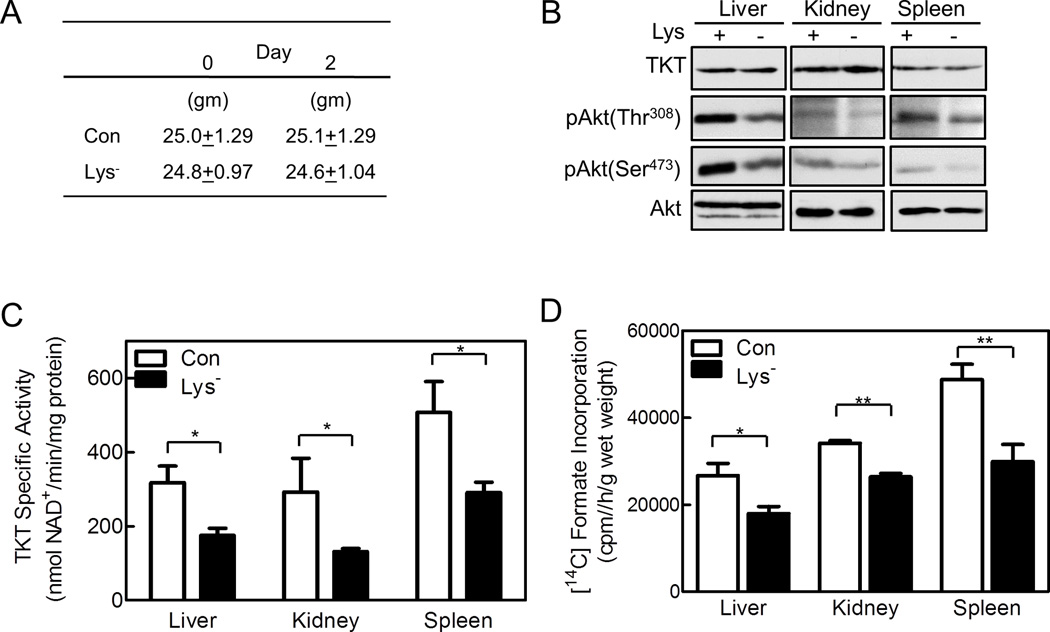
To assess the effects of removing a single essential amino acid from an animal’s diet for brief periods, we deprived mice of lysine for two days and measured Akt phosphorylation, TKT activity, and de novo purine synthesis. Other than lacking lysine, the diet was identical to standard mouse chow, including total calories. The animals’ total body weights and organ weights did not change over the two days (Figure 7A; Table S3), but plasma lysine concentrations decreased significantly, while other amino acids were unchanged (Table S4). We found no change in the amount of TKT or Akt protein in the liver, kidneys, and spleen of lysine-deprived animals, but Akt phosphorylation on Thr308 and Ser473 was decreased, indicating decreased Akt activation (Figure 7B). Concordant with reduced Akt activity, TKT activity and purine synthesis decreased an overall 46% and 28%, respectively in the organs of the lysine-deprived mice (Figures 7C,D). Thus, a brief period of amino acid deprivation had major effects on Akt and TKT activity and de novo purine synthesis in vivo.
Figure 7. Lysine Deprivation Decreases Akt and TKT Activity, and Purine De Novo Synthesis in Mice.
Eight week old C57BL6 male mice were fed a full diet (Con) or the same diet lacking lysine (Lys-) for two days. (A) The mice were weighed prior to starting the diet and on day two (n=6 per group). (B,C) The mice were euthanized on day two, and their livers, kidneys, and spleens were harvested. (B) Organ extracts were analyzed by SDS-PAGE/immunoblotting using anti-TKT and anti-phospho-Akt antibodies (n=2–3 per group), or (C) TKT activity in the extracts was measured. (D) On day two, mice were given an intra-peritoneal injection of 3 μCi [14C]formate, and 2 h later were euthanized. Livers, kidneys, and spleens were harvested and weighed; and [14C]formate incorporation into all cellular purines was measured. Panels C and D show means ± SE with 6 animals per group (*p<0.05, **p<0.01). See Tables S3, S4.
DISCUSSION
We have uncovered a mechanism whereby availability of a single essential amino acid controls production of PRPP, a crucial and rate-limiting substrate for purine, pyrimidine, and pyridine nucleotide synthesis. We have shown previously that decreased purine synthesis leads to decreased RNA and DNA synthesis, and cells must double their purine nucleotide pools to double their DNA content (Boss and Erbe, 1982). Thus, our results link amino acid availability to carbohydrate, purine, pyrimidine, and pyridine metabolism, RNA/DNA synthesis, and cell division. Recent work shows that mTORC1 regulates pyrimidine synthesis via S6 kinase phosphorylation of the enzyme catalyzing the first steps of pyrimidine synthesis (Ben-Sahra et al., 2013).
Amino acids positively regulate mTORC1 and mTORC2, and negatively regulate IκB kinase, although mTORC2 activation has not been observed in all studies (Comb et al., 2012; Gulati et al., 2008; Tato et al., 2011; Zoncu et al., 2011). We found regulation of all three enzyme complexes by amino acids, but only mTORC2 and IκB played a role in regulating purine synthesis. We deprived cells briefly for only a single essential amino acid in otherwise normal culture medium, whereas most investigators deprive cells of all amino acids for up to 24 h— sometimes using a balanced salt solution—and then reconstitute with amino acid mixtures (Comb et al., 2012; Gulati et al., 2008; Tato et al., 2011; Zoncu et al., 2011).
We found that Akt phosphorylates TKT Thr382, thereby activating the enzyme. Based on a crystal structure of human TKT, Thr382 is on the surface of the aminopyrimidine-binding (Pyr) domain, opposite the homodimerization interface of the two enzyme subunits [Figures 6B,S6G; (Mitschke et al., 2010)]. Residues from both the Pyr and pyrophosphate-binding (PP) domains form the TPP and substrate-binding sites at the dimer interface (Figures 6B,Figure S7). The Pyr domain is a parallel six-stranded β-sheet with three helices on each side—Thr382 is located on the turn preceding the β8 strand. At the end of the β8 strand is a connecting 3/10 helix containing Phe389 and Phe392, which bind the aminopyrimidine ring of TPP through π-stacking. We propose that Thr382 phosphorylation changes the conformation of the Pyr domain, thereby altering the dynamic state of the Pyr-PP dimer interface and increasing binding affinities for TPP and substrates. Supporting this hypothesis are recent sub-angstrom resolution crystallographic studies that show TKT activity to be particularly sensitive to conformational changes in the cofactor and substrate binding channels (Ludtke et al., 2013).
The sequence surrounding TKT Thr382 deviates from the classic Akt consensus site R-X-R-X-X-pS/pT, since Arg is at the −1 and 3, but not −5 position (Manning and Cantley, 2007; Obata et al., 2000). Peptide screening identified the −3 arginine as essential, but peptides lacking the −5 arginine are still phosphorylated—albeit with a decreased VMax/KM—and several studies show convincingly that Akt phosphorylates proteins lacking the −5 arginine (Table S2). Akt prefers hydrophobic amino acids at +1 and a proline at +2, as found in TKT (Obata et al., 2000).
In mice, TKT haploinsufficiency causes severe growth retardation and decreased fertility, and TKT-null embryos die at or before the morula stage (Xu et al., 2002). Two TKT-like proteins exist (TKTL1 and L2), but TKTL1 may be devoid of TKT activity and TKTL1 knockout mice have a mild phenotype (Schneider et al., 2012). TKT expression is up-regulated in various human cancers, and its activity correlates highly with tumor growth rate; TKT inhibitors reduce cancer cell proliferation in vitro and in vivo (Cascante et al., 2000; Comin-Anduix et al., 2001; Rais et al., 1999).
Akt stimulates glycolysis through multiple mechanisms, including increased glucose transport and stimulation of hexokinase and 6-phosphofructo-2-kinase (PFK) activity (DeBerardinis et al., 2008). Glycolytic flux is decreased in amino acid-deprived cells due to decreased PFK activity and restored on amino acid return (Novellasdemunt et al., 2013). These data, together with our results, suggest amino acids coordinately regulate glycolysis and non-oxidative PPP activity through Akt regulation of PFK and TKT, respectively.
Three Akt isoforms are encoded by separate genes, with Akt1 and 2 expressed ubiquitously and Akt3 restricted in expression (Gonzalez and McGraw, 2009). The three isoforms are functionally distinct, with isoform-specific substrate selectivity likely controlled by subcellular localization and binding partner association (Chen et al., 2001; Gonzalez and McGraw, 2009). We found that Akt1 knockout MEFs had low rates of purine synthesis not regulated by amino acids, suggesting Akt1 may preferentially target TKT.
Caloric restriction in humans down-regulates the PI3K/Akt pathway in skeletal muscle, possibly from protein/amino acid deprivation (Mercken et al., 2013). Refeeding fasting mice activates Akt in the liver of wild type animals, but not in liver-specific rictor-knockout mice, demonstrating that nutrient-induced Akt activation is mTORC2 dependent, similar to our results (Hagiwara et al., 2012). A 30–50% decrease in caloric intake decreases the incidence and growth of various cancers in many species including man, while obesity leads to high plasma concentrations of glucose and amino acids and increased cancer risk (Guo et al., 1993; Tato et al., 2011). The tumorigenic and metastatic potential of murine tumors increases as glycolysis, PPP activity, and nucleotide biosynthesis increase (Lu et al., 2010). Some human cancer xenografts in mice are extremely sensitive to the anti-proliferative effects of dietary restriction, whereas others with constitutive PI3K/Akt activation are completely resistant (Kalaany and Sabatini, 2009). Amino acid-deficient diets show some promise as nutritional therapy for cancer, and enzymatic arginine destruction has anti-tumor activity in patients with melanoma and hepatocellular carcinoma (Baracos and Mackenzie, 2006; Feun et al., 2008). Our data provide a mechanistic basis for these observations, since cancers with normal PI3K/Akt activity should be sensitive to amino acid deprivation whereas those with constitutively high PI3K/Akt activity should be resistant. Amino acid deprivation could still be useful in the latter group by rendering normal cells quiescent, thereby setting up the proliferating cancer cells to be killed by drugs that target dividing cells (Lee et al, 2012).
EXPERIMENTAL PROCEDURES
Materials and some of the methods are in Supplemental Information.
Cell Culture and Transfection
HeLa cells were obtained from the American Tissue Culture Collection. Akt-1 and tuberin-1/2 (TSC1/2) deficient MEFs were kindly provided by N. Hay and D.J. Kwiatkowski, respectively. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were transfected with plasmids for 24 h or siRNA for 72 h using Lipofectamine-2000 or Dharmafect I, respectively. Transfection efficiency was > 50% as assessed by GFP expression.
Amino Acid Deprivation
Cells were cultured for 3 h in DMEM or the same medium lacking one or all 12 essential amino acids. Both control and amino acid-deficient media were supplemented with 10% FBS dialyzed against 0.9% saline, unless stated otherwise. In amino acid return experiments, cells received full medium for 1 h.
Purine Synthesis, and PRPP Concentration and Production
Rates of de novo purine synthesis were measured over 1 h by following [14C]formate incorporation into all cellular purines (Boss and Erbe, 1982). The PRPP concentration was measured by extracting cells in hypotonic buffer containing 10 mM EDTA and phosphatase inhibitors. After heating the extracts at 100°C for 4 min, PRPP was measured by conversion to IMP using [8-14C]hypoxanthine and purified hypoxanthine phosphoribosyltransferase (HPRT) (Boss, 1984). In control experiments, we recovered 85% of exogenous PRPP added to cell extracts. PRPP production in intact cells was measured over 1 h by following [8-14C]adenine incorporation into adenine nucleotides (Pilz et al., 1984).
Carbon Flow through the Pentose Phosphate Pathway
Flow through the oxidative and non-oxidative PPP was measured by incubating cells for 1 h with [1-14C]glucose (Boss and Pilz, 1985). For the oxidative PPP, released 14CO2 was trapped in base and measured by liquid scintillation counting. For the non-oxidative PPP, cells were extracted in 10% trichloroacetic acid, DNA and RNA were solubilized by heating at 80°C for 30 min, and radioactivity in the DNA and RNA was measured by liquid scintillation counting. Flow through both pathways was measured by following [6-14C]glucose incorporation into DNA and RNA for 1 h.
Oxygen Consumption
Cells were cultured for 16 h in XF-24 culture dishes, and then oxygen consumption was measured over 90 min in an XF-24 instrument (Seahorse Bioscience).
TKT Activity
Cells were harvested in ice-cold PBS, and extracted by sonication in 100 mM Tris, pH 7.6, 5 mM 2-mercaptoethanol, 2 mM EDTA, and protease inhibitors. Phosphatase inhibitor cocktail was included in the extraction buffer and enzyme assay except as noted. For alkaline phosphatase treatment, 0.01 U of alkaline phosphatase bound to agarose beads was incubated with samples at room temperature for 30 min, with the beads removed by centrifugation (protein-G coupled agarose beads were used as controls). TKT activity was measured using ribose 5-phosphate and xylulose 5-phosphate as substrates in a coupled enzymatic reaction following NADH oxidation (Boss and Pilz, 1985).
Animal Experiments
Eight week-old C57BL6/J male mice from Jackson Laboratories were fed normal mouse chow (Harlan Laboratory) for 5 d. Half of them were then switched to the same chow lacking lysine for 2 d. Purine synthesis in liver, spleen, and kidneys was measured in some mice by injecting 3 μCi [14C]formate into the peritoneal cavity. After 2 h, the mice were euthanized, organs were removed, homogenized, and extracted, and formate incorporation into all cellular purines was measured (Boss and Erbe, 1982). In preliminary experiments we showed the assay was linear for at least 2½ h and that >80% of cellular purines were recovered. In some mice, organs were extracted for measuring TKT activity and immunoblotting for TKT and Akt. All experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Data Presentation and Analysis
Data in bar graphs are the mean ± standard error of at least three independent experiments performed in duplicate. Immunoblots and autoradiographs are representative of at least three independent experiments. Graph Pad Prism 5 software was used for two-tailed Student t-test (to compare two groups), one-way ANOVA (to compare more than two groups), and two-way ANOVA (to compare multiple conditions in more than two groups). Bonferroni post-test analysis was used for all ANOVAs.
Supplementary Material
HIGHLIGHTS.
Amino acids regulate purine synthesis via a new Akt/TKT module
Akt phosphorylates TKT Thr382—phosphorylation is critical to TKT activity
Akt/TKT coordinate amino acid, carbohydrate, and purine metabolism
ACKNOWLEDGMENTS
We thank J.R. Woodgett for the CA- and DN-Akt plasmids, and A. Chan and T. Yuan for technical assistance. The work was supported, in part, by NIH Grant R01AR051300.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baracos VE, Mackenzie ML. Investigations of branched-chain amino acids and their metabolites in animal models of cancer. J. Nutr. 2006;136:237S–242S. doi: 10.1093/jn/136.1.237S. [DOI] [PubMed] [Google Scholar]
- Becker MA, Losman MJ, Kim M. Mechanisms of accelerated purine nucleotide synthesis in human fibroblasts with superactive phosphoribosylpyrophosphate synthetases. J. Biol. Chem. 1987;262:5596–5602. [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss GR. Decreased phosphoribosylpyrophosphate as the basis for decreased purine synthesis during amino acid starvation of human lymphoblasts. J. Biol. Chem. 1984;259:2936–2941. [PubMed] [Google Scholar]
- Boss GR, Erbe RW. Decreased purine synthesis during amino acid starvation of human lymphoblasts. J. Biol. Chem. 1982;257:4242–4247. [PubMed] [Google Scholar]
- Boss GR, Pilz RB. Phosphoribysylpyrophosphate synthesis from glucose decreases during amino acid starvation of human lymphoblasts. J. Biol. Chem. 1985;260:6054–6059. [PubMed] [Google Scholar]
- Brand K, Deckner K. Quantitative relationship between the pentose phosphate pathway and the nucleotide synthesis in ascites tumor cells. Hoppe Seylers. Z. Physiol Chem. 1970;351:711–717. doi: 10.1515/bchm2.1970.351.1.711. [DOI] [PubMed] [Google Scholar]
- Cascante M, Centelles JJ, Veech RL, Lee WN, Boros LG. Role of thiamin (vitamin B-1) and transketolase in tumor cell proliferation. Nutr. Cancer. 2000;36:150–154. doi: 10.1207/S15327914NC3602_2. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb WC, Hutti JE, Cogswell P, Cantley LC, Baldwin AS. p85a SH2 domain phosphorylation by IKK promotes feedback inhibition of PI3K and Akt in response to cellular starvation. Mol. Cell. 2012;45:719–730. doi: 10.1016/j.molcel.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comin-Anduix B, Boren J, Martinez S, Moro C, Centelles JJ, Trebukhina R, Petushok N, Lee WN, Boros LG, Cascante M. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur. J. Biochem. 2001;268:4177–4182. doi: 10.1046/j.1432-1327.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman A, Saha A, Chan A, Casteel DE, Pilz RB, Boss GR. Cell Cycle Regulation of Purine Synthesis by Phosphoribosyl- Pyrophosphate and Inorganic Phosphate. Biochem. J. 2013 doi: 10.1042/BJ20130153. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lishko VK, Herrera H, Groce A, Kubota T, Hoffman RM. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo . Cancer Res. 1993;53:5676–5679. [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Barnett SF, Layton ME, Bilodeau MT. The PI3K/Akt pathway: recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr. Cancer Drug Targets. 2008;8:7–18. doi: 10.2174/156800908783497096. [DOI] [PubMed] [Google Scholar]
- Lu X, Bennet B, Mu E, Rabinowitz J, Kang Y. Metabolomic changes accompanying transformation and acquisition of metastatic potential in a syngeneic mouse mammary tumor model. J. Biol. Chem. 2010;285:9317–9321. doi: 10.1074/jbc.C110.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke S, Neumann P, Erixon KM, Leeper F, Kluger R, Ficner R, Tittmann K. Sub-angstrom-resolution crystallography reveals physical distortions that enhance reactivity of a covalent enzymatic intermediate. Nat. Chem. 2013;5:762–767. doi: 10.1038/nchem.1728. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus EJ, Alessi DR. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat. Cell Biol. 2002;4:E214–E216. doi: 10.1038/ncb0902-e214. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, Jebailey L, Krzysik-Walker S, Villareal D, Capri M, Franceschi C, Zhang Y, Becker K, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013 doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschke L, Parthier C, Schroder-Tittmann K, Coy J, Ludtke S, Tittmann K. The crystal structure of human transketolase and new insights into its mode of action. J. Biol. Chem. 2010;285:31559–31570. doi: 10.1074/jbc.M110.149955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellasdemunt L, Tato I, Navarro-Sabate A, Ruiz-Meana M, Mendez-Lucas A, Perales JC, Garcia-Dorado D, Ventura F, Bartrons R, Rosa JL. Akt-dependent activation of the heart 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) isoenzyme by amino acids. J. Biol. Chem. 2013;288:10640–10651. doi: 10.1074/jbc.M113.455998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Pilz RB, Willis RC, Boss GR. The influence of ribose 5-phosphate availability on purine synthesis of cultured human lymphoblasts and mitogen-stimulated lymphocytes. J.Biol.Chem. 1985;259:2927–2935. [PubMed] [Google Scholar]
- Rais B, Comin B, Puigjaner J, Brandes JL, Creppy E, Saboureau D, Ennamany R, Lee WN, Boros LG, Cascante M. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich's tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456:113–118. doi: 10.1016/s0014-5793(99)00924-2. [DOI] [PubMed] [Google Scholar]
- Raivio KO, Lazar CS, Krumholz HR, Becker MA. The phosphogluconate pathway and synthesis of 5-phosphoribosyl-1-pyrophosphate in human fibroblasts. Biochim. Biophys. Acta. 1981;678:51–57. doi: 10.1016/0304-4165(81)90046-5. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schenk G, Duggleby RG, Nixon PF. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int. J. Biochem. Cell Biol. 1998 doi: 10.1016/s1357-2725(98)00095-8. [DOI] [PubMed] [Google Scholar]
- Schneider S, Ludtke S, Schroder-Tittmann K, Wechsler C, Meyer D, Tittmann K. A delta38 deletion variant of human transketolase as a model of transketolase-like protein 1 exhibits no enzymatic activity. PLoS. ONE. 2012;7:e48321. doi: 10.1371/journal.pone.0048321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J. Biol. Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- Tato I, Bartrons R, Ventura F, Rosa JL. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. J. Biol. Chem. 2011;286:6128–6142. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fridman A, Blackledge W, Connelly S, Wilson IA, Pilz RB, Boss GR. The phosphatidylinositol 3-kinase/akt cassette regulates purine nucleotide synthesis. J. Biol. Chem. 2009;284:3521–3528. doi: 10.1074/jbc.M806707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZP, Wawrousek EF, Piatigorsky J. Transketolase haploinsufficiency reduces adipose tissue and female fertility in mice. Mol. Cell Biol. 2002;22:6142–6147. doi: 10.1128/MCB.22.17.6142-6147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K–Akt signaling through downregulation of PDGFR. J. Clin. Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Mancuso A, Bui TV, Tong X, Gruber JJ, Swider CR, Sanchez PV, Lum JJ, Sayed N, Melo JV, et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene. 2010;29:2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.