Abstract
Background & Aims
Perilipin-5 (PLIN5) is a member of the perilipin family of lipid droplet (LD)-associated proteins. PLIN5 is expressed in oxidative tissues including the liver, and is critical during LD biogenesis. Studies showed that statins reduce hepatic triglyceride contents in some patients with non-alcoholic fatty liver disease and in rodent models of diet-induced hepatosteatosis. Whether statins alter triglyceride synthesis, storage, and/or utilization within the hepatocyte is unknown, though. Here we tested the hypothesis that statins alter the metabolism of LD in the hepatocyte during physiological conditions, such as fasting-induced steatosis.
Methods
Mice were gavaged with saline or atorvastatin, and the expression of LD-associated genes was determined in fed and fasted animals. The accumulation of triglycerides and LD was studied in mouse or human primary hepatocytes in response to statins, and following knock-down of SREBP2 or PLIN5.
Results
We show that statins decrease the levels of PLIN5, but not other LD-associated genes, in both mouse liver and mouse/human primary hepatocytes, which is paralleled by a significant reduction in both intracellular triglycerides and the number of LD. We identify an atypical negative sterol regulatory sequence in the proximal promoter of mouse/human PLIN5 that recruits the transcription factor SREBP2 and confers response to statins. Finally, we show that the statin-dependent reduction of hepatocyte triglyceride contents is mimicked by partial knock-down of PLIN5; conversely, ectopic overexpression of PLIN5 reverts the statin effect.
Conclusions
PLIN5 is a physiological regulator of triglyceride metabolism in the liver, and likely contributes to the pleiotropic effects of statins.
Keywords: PLIN5, liver, triglycerides, fatty acid oxidation, steatosis
Introduction
Lipid droplets (LDs) are energy-storage organelles that play a remarkably complex role in triglyceride homeostasis. Thus, LDs both prevent the lipotoxic effects of non-esterified fatty acids (FFAs), and support cellular needs by releasing FFAs for β-oxidation and membrane synthesis. These organelles contain a core of neutral lipids (mostly triglycerides (TG) but also cholesteryl esters) surrounded by a phospholipid monolayer, and are lined with specific proteins that include members of the perilipin (PLIN) family [1]. Perilipins are grouped into two categories depending on their stability when not associated to LD: non-exchangeable and exchangeable. The former include PLIN1 and -2, which are only found in an LD-bound state and are rapidly degraded when not associated to lipids [1, 2]. The latter include PLIN3, -4, and -5, which exist either in an LD-bound state or soluble in the cytosol [1, 2]. PLIN1 and -4 are abundant in white adipose tissue [1], PLIN2 and -3 are expressed in many cell types [1, 3], and PLIN5 is mainly expressed in oxidative tissues such as liver, heart, muscle and brown adipose tissue [4–6].
PLIN5 was identified by three independent laboratories, which showed that both mRNA and protein are induced in the heart, liver, and skeletal muscle during fasting via the peroxisome proliferator-activated receptor α (PPARα) [4–6]. PPARγ and PPARδ were also reported to induce PLIN5 expression in adipose tissue [5] and skeletal muscle [7], respectively. These early studies established a role for PLIN5 on LD metabolism, likely inhibiting triglyceride lipolysis and/or decreasing fatty acid oxidation, and contributing to an overall intracellular lipid accumulation [4–6]. Mice deficient in Plin5 showed a striking cardiac phenotype: they lacked detectable LDs, had decreased TG levels, increased β-oxidation, increased reactive oxygen species, and developed heart failure with age [8]. Conversely, cardiac-specific overexpression of Plin5 resulted in severe TG accumulation and a robust increase in LDs [9, 10]. These latter authors showed that PLIN5-coated LDs are resistant to TG hydrolysis and that mitochondrial function is decreased, suggesting that PLIN5 acts as a lipolytic barrier to prevent uncontrolled TG mobilization [9, 10].
Patients with hypercholesterolemia are normally prescribed statins, competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR, the rate-limiting enzyme of the de novo cholesterol synthesis pathway). Interestingly, several data suggest that statins can decrease hepatic TG contents in patients [11–15] and rodents [16–18]. The mechanisms by which statins exert beneficial effects on pathological hepatosteatosis are not well understood, however. Likewise, whether statins control physiological hepatic TG homeostasis is unknown. Here we tested the hypothesis that statins alter the metabolism of LD in the hepatocyte by stimulating fatty acid β-oxidation, and show that the transcription of PLIN5, but not other perilipins, is controlled by statins via SREBP2.
Materials and methods
Mice
Male, 8–10 week-old C57BL/6 mice were maintained in a 12h/12h light/dark cycle with unlimited access to food and water. All studies were approved by the IACUC at SLU.
Primary hepatocytes
Normal human primary hepatocytes were obtained from Lonza (CC-2591) and cultured in Hepatocyte Basal Medium (Lonza). Mouse primary hepatocytes were isolated using Perfusion and Digest buffers (Invitrogen) as described [19]. Cells were seeded in 12- or 6-well BioCoat Collagen I plates (BD), and incubated at 37°C and 5% CO2 in William’s E media + Hepatocyte Supplements (Invitrogen). For siRNA, cells were transfected with anti-SREBP2 (M-050073-01-0005), anti-PLIN5 (M-0557756-01-05), or control (D-001210-01-05) oligonucleotides (siGENOME SMART pool, ThermoScientific), using Dharmafect 1 reagent (ThermoScientific). For adenovirus-mediated overexpression, cells were transduced with Adeno-SREBP2, Adeno-Plin5, or Adeno-empty vectors at moi=3. Where indicated, cells were cultured in media supplemented with 5 μmol/L statins (Sigma) for 48 h. For oleate challenge, cells were pre-treated with simvastatin for 24 h, before addition of 600 μmol/L oleate:BSA (1:3) for an additional 24 h.
Additional Materials and Methods are provided as Supplemental Data.
Results
Statins decrease hepatic PLIN5 levels and fasting-induced steatosis
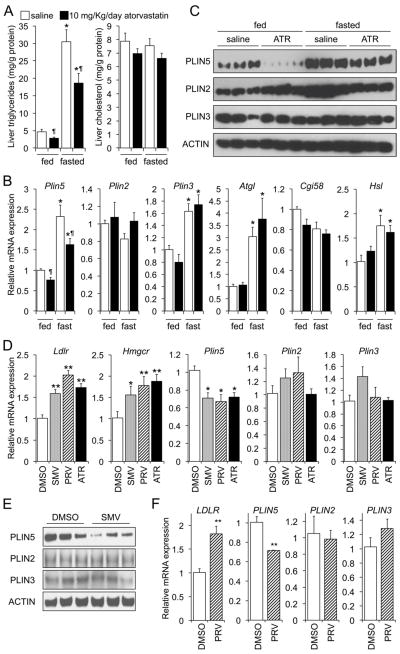
To test the hypothesis that statins influence the metabolism of TG in the hepatocyte, we measured the effects of atorvastatin on hepatic TG contents and on the expression of selected genes encoding lipogenic and LD-associated proteins. We gavaged chow-fed mice with saline or 20 mg/Kg/day atorvastatin for 10 days; then some mice were allowed access to food, while others were fasted overnight, before sacrifice. We found no significant changes in body weight, plasma lipids, and transaminases between groups (data not shown). As expected, fasting induced significant hepatic TG accumulation, compared to feeding, with no change in hepatic cholesterol levels (Fig. 1A, open bars); and atorvastatin induced the hepatic expression of Srebp-2 and SREBP-2 targets (Hmgcr, Pcsk9) (Fig. S1A). Our data show that both groups of mice (fasted and non-fasted) had a significant decrease (~35%) in liver triglycerides when treated with atorvastatin, compared to those gavaged with saline (Fig. 1A, closed vs. open bars), which was independent on changes in Pparα and PPARα target genes (Fig. S1B), and Srebp1c and SREBP1C target genes (Fig. S1C). Analysis of selected LD-associated genes in presented in Fig. 1B and C. Data show that the levels of Plin2, Plin5, Atgl, and Hsl were induced in fasted, compared to fed animals, as expected [3, 5, 20, 21]. However, only the expression of Plin5 was differentially regulated by the statin: a 25% and 30% decline in fed and fasted mice, respectively, compared to saline (Fig. 1B). These latter changes were more pronounced at the protein level (Fig. 1C). Our data are also consistent with those of other investigators who showed that fasting induces both PLIN2 and PLIN5, but not PLIN3, in the livers of mice [3, 5, 22]. The reduction in PLIN2 in the livers of fasted, atorvastatin-treated mice (Fig. 1C) is also consistent with the degradation of this protein following the decrease in intracellular TG, since this perilipin is unstable in the cytosol when not bound to LDs [8].
Fig. 1. Statins reduce hepatic TG contents and PLIN5 expression.
(A–C) Chow-fed mice were gavaged saline or atorvastatin for 10 days, and then fasted for 24 h or allowed access to food (n=4/group). Hepatic TG and cholesterol contents (A), mRNA (B) and protein (C) levels of specific LD-related genes were measured in the same livers. Data were analysed by 2-way ANOVA followed by Mann-Whitney U-test. *P≤0.05, fasted vs. fed; ¶P≤0.05, atorvastatin vs. saline. Mouse (D, E), and human (F) primary hepatocytes were incubated 48 h in media supplemented with vehicle, or 5 μmol/L simvastatin (SMV), pravastatin (PRV) or atorvastatin (ATR). The relative levels of hepatic perilipins were determined by RT-qPCR or immunoblot. Data are shown as mean ± SEM of 2 independent experiments in quadruplicate, and were analysed by one-way ANOVA and post hoc Tukey’s test. *P≤0.05, statin vs. DMSO; **P≤0.01, statin vs. DMSO.
To confirm that the repressing effect on PLIN5 was shared by different statins, we tested simvastatin, pravastatin, and atorvastatin in mouse primary hepatocytes. Data in Fig. 1D show that all three statins reduced the mRNA levels of Plin5, but not Plin2 or Plin3, compared to vehicle. The transcript levels of other LD-related genes were unaffected by either statin (Fig. S2A). As expected, all statins induced the expression of classic SREBP2 targets (Fig. 1D and S2A). In agreement with these results, immunoblots performed in a different set of primary hepatocytes revealed that the abundance of PLIN5, but not PLIN2 or PLIN3, was significantly decreased following incubation with simvastatin (Fig. 1E). Finally, experiments in human primary hepatocytes showed that the regulation of PLIN5, but not other LD-related genes, by statins is conserved in humans (Figs. 1F and S2B). Collectively, the data suggest that statins alter LD metabolism via down-regulation of 2B). PLIN5, likely limiting hepatic TG accumulation in both mice and humans.
Statins decrease the expression of PLIN5 through SREBP2
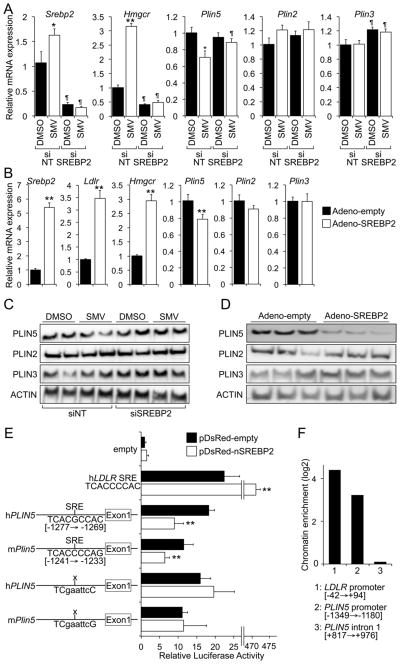
To test whether the decrease in PLIN5 following treatment with statins is SREBP2-dependent, we assessed the impact of either SREBP2 knock-down or overexpression in mouse primary hepatocytes (Fig. 2A–D). Hence, we transfected cells with either non-targeting (siNT) or anti-Srebp2 (siSREBP2) oligonucleotides, and later cultured them in the presence or absence of simvastatin. Data in Fig. 2A show that, as expected, knock-down of Srebp2 prevented the induction of both Ldlr and Hmgcr by simvastatin. Importantly, the statin-dependent repression of Plin5 was also abolished in siSREBP2-tranfected cells at both the mRNA and protein levels (Fig. 2A, C), with no change in the expression of Plin2 or Plin3 (Fig. 2A, C). Gain-of-function studies by ectopically expressing a constitutively active SREBP2 show that the levels of Plin5 mRNA and protein were significantly decreased following SREBP2 overexpression, while known SREBP2 targets (Ldlr and Hmgcr) were induced, and the expression of Plin2 and Plin3 did not change under the same conditions (Fig. 2B, D). Taken together, these data demonstrate that statins control the expression of PLIN5 through an SREBP2-dependent mechanism.
Fig. 2. Statin-mediated decrease in Plin5 is SREBP2–dependent.
(A) Mouse primary hepatocytes were transfected with non-targeting (siNT) or anti-SREBP-2 (siSREBP2) oligonucleotides, and then incubated with or without 5 μmol/L simvastatin. The relative expression of genes of interest was analysed by RT-qPCR. (B) Mouse primary hepatocytes were transduced with an empty or a constitutively active SREBP2 (nSREBP2) adenovirus. The mRNA levels of genes of interest was analysed by RT-qPCR. Data are shown as mean ± SEM of 2 independent experiments in quadruplicate, and were analysed by Student’s t-test. **P≤0.01. (C, D) Immunoblots of selected proteins in parallel sets of cells treated as in A or B, respectively. (E) Luciferase reporters containing the human or mouse PLIN5 promoter were transfected into HEK293 cells, with or without an expression vector for nSREBP2. Data are shown as mean ± SEM of 3 independent experiments in triplicate, and were analysed by Student’s t-test. **P≤0.01. (F) Recruitment of SREBP2 to the SRE was assayed by ChIP in HuH7 hepatoma cells (see Methods). Data are shown as chromatin amplification enrichment vs. non-specific IgG immunoprecipitates for regions surrounding the human PLIN5 SRE, human LDLR SRE (positive control), and intron 1 of PLIN5 (negative control).
Analysis in silico of both mouse and human PLIN5 proximal promoters with the TF-Search algorithm [23], identified a conserved putative sterol regulatory element (SRE), suggesting that SREBP2 binds to the PLIN5 promoter. Fig. 2E shows that this SRE differs only 1 nucleotide from the SRE in the human LDLR promoter. Luciferase reporter constructs containing a 3.5 Kb fragment (which includes the potential SRE) of the mouse or human PLIN5 promoter where tested in transfection experiments. Data in Fig. 2E show that the activity of both mouse and human PLIN5 reporters was reduced following co-transfection with a plasmid encoding a constitutively active SREBP2, while an empty reporter did not respond to SREBP2 co-transfection. Importantly, site-directed mutagenesis of the putative SRE abolished the effect of SREBP2 (Fig. 2E), thus identifying those sequences as functional SREBP2 responsive elements. The recruitment of SREBP2 to this region of the human PLIN5 promoter was verified by chromatin immunoprecipitation (ChIP), using extracts from HuH7 hepatocytes transfected with a Flag-nSREBP2 expression plasmid and anti-Flag or unrelated IgG antibodies (Fig. 2F). Taken together, data in Fig. 2 demonstrate that the statin-dependent downregulation of mouse and human PLIN5 is mediated by the recruitment of SREBP2 to the proximal PLIN5 promoter.
Reduction of PLIN5 with siRNA mimics the effects of statins on TG contents and LD numbers/distribution in mouse primary hepatocytes
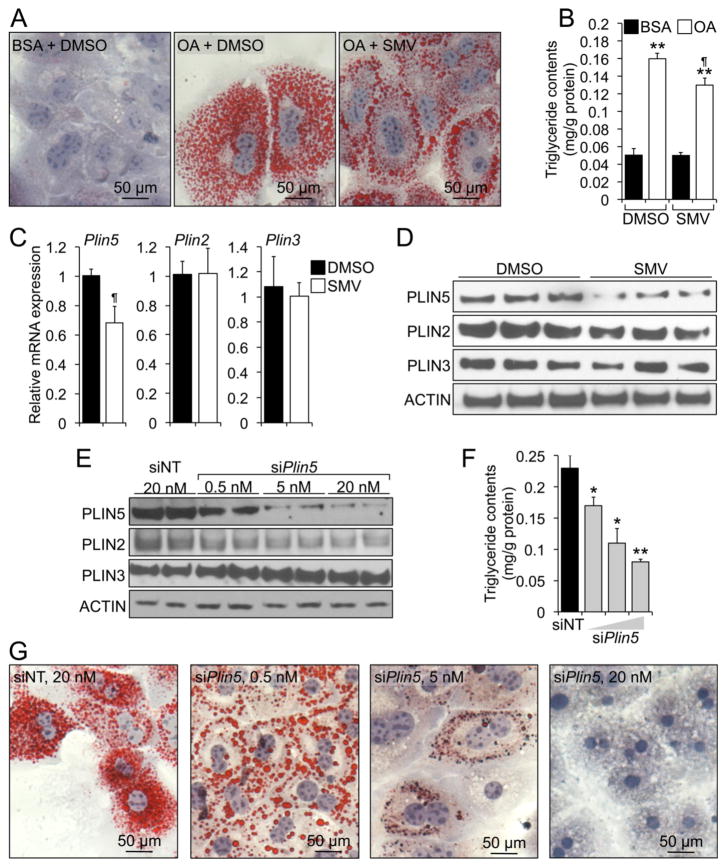
We next examined the functional consequences of statins and decreased PLIN5 on LD organization in mouse primary hepatocytes. In a first set of experiments, cells were cultured in the presence or absence of 5 μmol/L simvastatin for 24 h, and then with BSA (control) or 0.6 mmol/L oleate (conjugated to BSA; to induce the formation of LDs), for an additional 24 h. The oleate challenge resulted in numerous intracellular LD and increased TG contents, compared to BSA-treated cells (Fig. 3A, centre vs. left panel; and Fig. 3B, lane 2 vs. 1). Interestingly, LDs in simvastatin-treated cells were less numerous and appeared to distribute mostly in the periphery of the cell, as opposed to LDs in DMSO-treated cells that seemed more uniformly distributed (Fig. 3A, right vs. centre panel). In agreement with data in Fig. 1, the presence of simvastatin in oleate-loaded cells resulted in a ~20% decrease in intracellular TG contents, compared to DMSO (Fig. 3B, lane 4 vs. 2). No changes were noted on TG content in BSA-treated cells (Fig. 3B, lane 3 vs. 1). Notably, the effects of the statin on TG contents in oleate-challenged hepatocytes were paralleled by a significant decrease in PLIN5, but not PLIN2 or PLIN3, mRNA and protein (Fig. 3C, D). A possible explanation for the changes in LD/TG following treatment with the statin is that they are due to toxic effects of the drug. Data in Fig. S3 show that neither the oleate challenge nor the statin resulted in significant changes in cell viability, as measured by both lactate dehydrogenase (LDH) activity released into the media and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cleavage activity. Therefore, we conclude that the changes in intracellular TG observed in Fig. 3A, B are due to physiological, non-toxic effects of the statin.
Fig. 3. Statins and PLIN5 control LD contents in hepatocytes.
(A–D) Mouse primary hepatocytes were incubated in media supplemented with BSA or BSA:oleate, in the presence or absence of 5 μmol/L simvastatin. The statin decreased TG accumulation, as measured by oil red O staining (A) and enzymatically (B). The relative mRNA (C) and protein (D) expression of hepatic perilipins in oleate-challenged hepatocytes was analysed in a parallel set of cells. Data are shown as mean ± SEM of 2 independent experiments in triplicate or quadruplicate, and were analysed by Student’s t-test. **P≤0.01, oleate vs. BSA. ¶P≤0.01, SMV vs. DMSO. (E–G) siRNA transfection of mouse primary hepatocytes showed that dose-dependent decrease in PLIN5 expression (E) was paralleled by a concomitant decrease in TG contents as measured enzymatically (F) and by oil red O staining (G). Data in F are mean ± SEM of 2 independent experiments in triplicate, and were analysed by one-way ANOVA and post hoc Tukey’s test. *P<0.05; **P<0.01
Next, we investigated the consequences of decreased PLIN5 activity on LD formation/metabolism and TG accumulation. We aimed at studying conditions with only a modest-to-moderate knock-down of PLIN5, so as to mimic the changes noted in statin-treated livers (Fig. 1C) or hepatocytes (Figs. 1E and 3D). Hence, mouse primary hepatocytes were transfected with non-targeting (siNT) or anti-Plin5 (siPlin5) oligonucleotides, followed by oleate challenge. Data show a dose-dependent specific reduction in PLIN5 protein (Fig. 3E) and mRNA (Fig. S4A) in cells transfected with siPlin5 oligonucleotides, compared to cells receiving siNT oligonucleotides. Such decline in PLIN5 was paralleled by a reduction in both intracellular TG contents (Fig. 3F) and LDs (Fig. 3G), that occurred in the absence of changes in the amounts of Pparα and PPARα targets (Fig. S4A). It is remarkable that the modest knock-down of PLIN5 by the lower siRNA dose [which was similar to the decline in PLIN5 levels noted with statins (compare Fig. 3E vs. 3D)] phenocopied the effects of the statins on both LD number and distribution within the cell, and TG contents (Fig. 3G, first and second panel vs. 3A, centre and right panel). Importantly, only the highest siPlin5 treatment induced significant cell toxicity, as measured by either LDH activity in the cultured media, or intracellular MTT activity (Fig. S4B). Interestingly, we found that almost-complete abolition of Plin5 expression in oleate-loaded hepatocytes was also paralleled by the induction of caspase-3 activity, increased production of reactive oxygen species, and elevated Tnfα expression (Fig. S4B). Collectively, these data suggest that PLIN5 activity is crucial to maintain TG homeostasis within the hepatocyte during episodes of intense fatty acid influx, such as during in vitro oleate challenge or in vivo fasting. Nevertheless, data in Fig. 3 strongly suggest that either statins or loss of PLIN5 attenuate LD formation and TG accumulation following oleate challenge.
Plin5 overexpression prevents the effects of statins on LD and TG metabolism in mouse primary hepatocytes
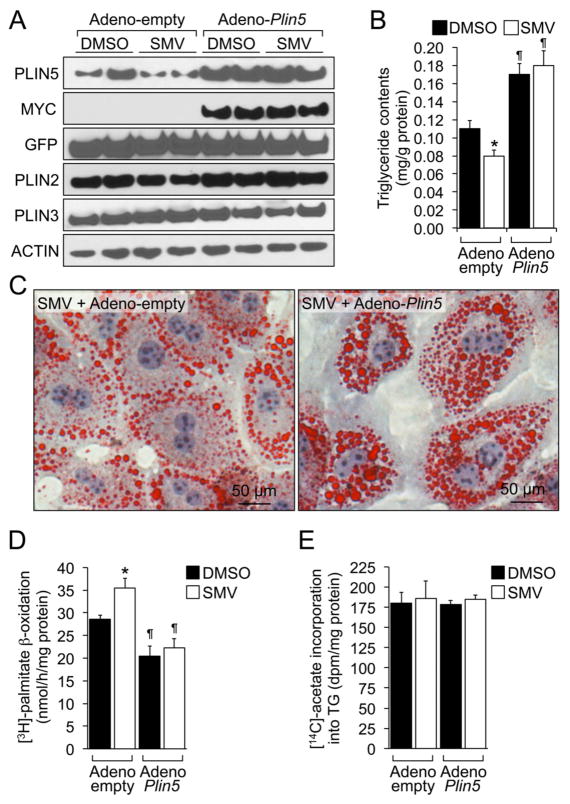
To further demonstrate that PLIN5 mediates the effects of statins on LDs, we tested whether PLIN5 overexpression could reverse the statin-dependent decrease in intracellular TG. Hence, mouse primary hepatocytes were transduced with an empty- or a Myc-Plin5-encoding adenovirus, and incubated for 48 h in media supplemented with 0.3 mmol/L oleate:BSA in the presence or absence of simvastatin. The amount of PLIN5 adenovirus was titrated to avoid excessive, supra-physiological amounts of PLIN5 protein (Fig. 4A). Importantly, introduction of PLIN5 did not alter PLIN2 or PLIN3 mRNA/protein levels (Figs. 4A and S5). Again, treatment with the statin in control cells led to a significant decrease in intracellular TG contents (Fig. 4B, lane 2 vs. 1). However, overexpression of PLIN5 resulted in a ~40% increase in TG levels and, importantly, abrogated the effect of the statin (Figs. 4B, lanes 3, 4 vs 1, 2; and 4C).
Fig. 4. Plin5 reverses the effects of statins on TG contents and fatty acid oxidation.
Mouse primary hepatocytes were transduced with an empty or a Myc-Plin5 adenovirus, and then incubated in media supplemented with DMSO or 5 μmol/L simvastatin (SMV). (A) Immunoblots of selected hepatic proteins. (B) Intracellular TG contents, as determined enzymatically. Data are shown as mean ± SEM of 2 independent experiments in triplicate, analysed by two-way ANOVA followed by Mann-Whitney U-test. *P≤0.05 SMV vs. DMSO; ¶P≤0.05, Adeno-Plin5 vs. Adeno-empty. (C) Representative oil Red O staining. (D) Fatty acid oxidation capacity was assessed in cells incubated with [3H]-palmitate, by measuring the release of [3H]-water. (E) De novo TG synthesis was assessed in cells incubated with [14C]-acetate (see Methods). Data in D and E are shown as mean ± SEM of 2 independent experiments in triplicate, and were analysed by two-way ANOVA followed by Mann-Whitney U-test. *P≤0.05 SMV vs. DMSO; ¶P≤0.05, Adeno-Plin5 vs. Adeno-empty.
Since PLIN5 has been shown to prevent fatty acid utilization in various cell types [2], we hypothesized that the statin-dependent decrease in cellular TG might be due to accelerated fatty acid oxidation. Hence, we measured the catabolism of [3H]-palmitate in primary hepatocytes incubated in the presence or absence of simvastatin (see Methods). Data show that simvastatin does indeed increase the ability of cells to oxidize fatty acids (Fig. 4, lane 2 vs. 1). As expected, overexpression of PLIN5 decreased fatty acid oxidation, compared to control cells (Fig. 4D, lane 3 vs 1). Importantly, PLIN5 also abrogated statin induced fatty acid oxidation (Fig. 4D, lane 4 vs. 3), suggesting that the effects of statins on fatty acid utilization are mediated by PLIN5. Reduced intracellular TG may also result from impaired de novo lipogenesis. Data in Fig. 4E, however, suggest that neither statins nor PLIN5 overexpression affect the incorporation of [14C]-acetate into TG. Taken together, data in Fig. 4 suggest that PLIN5 mediates the effects of statins of LD metabolism.
Discussion
Statins are the most commonly prescribed drugs to manage the cardiovascular risk associated to hypercholesterolemia [24]. However, studies on whether these drugs exert any effects on hepatic TG metabolism are notoriously scarce in the literature. Recent clinical studies revealed an unexpected reduction in liver TG contents in NAFLD patients taking statins [11–15]. The mechanisms behind such effect are poorly understood, however. Studies in rodents suggested that the induction of hepatic steatosis in rats fed either a high-fructose [16] or a high-fat [17] diet, or in aromatase-deficient mice [18], is linked to reduced PPARα signalling which can be rescued by treatment with statins. On the other hand, Horton et al reported that one out of two lines of nSREBP2 transgenic mice developed mild TG fatty liver [25], although this is an extreme model with constitutive, massive SREBP2 overexpression that likely resulted in non-physiological induction of lipogenic genes (which are SREBP1 targets).
Contrary to these diet-dependent or genetic animal models, fasting elicits physiological hepatic steatosis due to the accelerated influx of FFA released from adipose tissue that are subsequently re-esterified in the liver. Fasting also results in stimulation of PPARα signalling in several tissues, including the liver [4–6, 22]. Here we show that mice gavaged with atorvastatin exhibit reduced hepatic TG contents, compared to mice gavaged saline, both in fed and fasted conditions and independent on the levels of PPARα and classic PPARα targets such as Plin2, Cpt1α, and Pdk4. Our data suggest that statins modulate physiological TG accumulation through down-regulation of PLIN5. To our knowledge, this is the first report describing the effect of statins/SREBP2 on hepatocyte LD remodelling and liver TG metabolism in chow-fed wild-type mice. Importantly, our results were consistent among 3 different statins (atorvastatin, simvastatin, and pravastatin) and, importantly, conserved in human primary hepatocytes. Future studies will determine whether statins can also ameliorate hepatosteatosis in diet-induced or genetic NAFLD animal models.
We identified a conserved SRE in the proximal promoter of PLIN5 that confers response to statins/SREBP2. Yet, PLIN5 is an atypical target of SREBP2 since it is repressed, rather than induced, by this transcription factor. The only other instances of SREBP2-mediated transrepression described in the literature are the genes encoding microsomal triglyceride transfer protein (MTTP) [26], hepatocyte nuclear factor 4α (HNF4α) [27], and caveolin [28]. It is interesting that statins exert modest effects on the expression of PLIN5, suggesting that the SREBP2 likely binds to the PLIN5 SRE with relative low affinity, compared to other SREBP2 targets. This would ensure that PLIN5 levels are reduced only when significant amounts of mature SREBP2 are present, such as following cholesterol depletion (see below) or treatment with statins. Nonetheless, we show that even a modest reduction in PLIN5 levels using siRNA leads to changes in intracellular TG levels in the absence of changes in other LD-related genes. Collectively, these data suggest that PLIN5 activity is particularly important for the metabolism of LDs in the liver, a fact that had been overlooked in previous studies.
Two different cardiomyocyte-specific transgenic rodent models revealed that overexpression of PLIN5 resulted in cardiac steatosis and hypertrophy [9, 10]. These studies suggested that PLIN5 activity restricts ATGL/CGI58-mediated lipolysis of TG in LDs, thus preventing efficient mobilization of FFA for mitochondrial β-oxidation [2, 8–10]. However, a paradoxical observation was made in Plin5−/− mice, where hepatic TG contents were decreased in fed animals (consistent with PLIN5 promoting LD formation and preventing FFA utilization) but increased in fasted animals, compared to controls [8]. The reasons behind these latter contradictory results remain obscure, and no data was provided regarding the expression of other LD-associated genes in those livers, further complicating the interpretation of the data. Nevertheless, Li et al. showed that >95% ablation of PLIN5 in mouse primary hepatocytes using antisense oligonucleotides resulted in virtual absence of LDs, decreased TG contents, accelerated lipase activity, and enhanced β-oxidation, compared to control oligonucleotides [29]; conversely, adenoviral-mediated overexpression of PLIN5 resulted in more abundant LDs and increased TG contents [29]. The relevance of these phenotypes to human physiology might be limited due to the complete absence [8] or drastic decrease [29] of PLIN5 in those studies. The data presented herein show for the first time that modest changes in PLIN5, such as those following treatment with statins, can regulate hepatic TG metabolism.
SREBP2 signalling is reduced during fasting, which might result in increased levels of PLIN5 at a time when FFA are stored into LD in the hepatocyte. Conversely, SREBP2-mediated transrepression of PLIN5 may also be physiologically relevant during sterol depletion episodes, where accelerated FFA β-oxidation could provide acetyl-CoA for de novo cholesterol synthesis. In this latter context, SREBP2 would control cholesterogenesis by both upregulating the expression of enzymes (i.e. HMGCR) and promoting substrate availability. Nevertheless, we propose that PLIN5 links TG and cholesterol metabolism via SREBP2. Physiologically, the role of PLIN5 seems particularly important during physiological fasting-induced hepatosteatosis. Whether this mechanism might provide additional therapeutic benefit to patients taking statins remains to be determined.
Supplementary Material
Acknowledgments
Financial support: This work was supported in part by NIH Grant HL107794 (to Á.B.).
We thank members of the Center for Cardiovascular Research at SLU for helpful discussions.
Abbreviations
- LD
lipid droplet
- LDLR
low-density lipoprotein receptor
- NAFLD
non-alcoholic fatty liver disease
- FFA
free fatty acids
- PLIN
perilipin
- PPAR
peroxisome proliferator-activated receptor
- SRE
sterol regulatory element
- SREBP
sterol regulatory element binding protein
- TG
triglycerides
Footnotes
COI: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Sztalryd C. Oxidative tissue: perilipin 5 links storage with the furnace. Trends Endocrinol Metab. 2011;22:197–203. doi: 10.1016/j.tem.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, et al. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006;47:329–340. doi: 10.1194/jlr.M500203-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 5.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 6.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Bindesboll C, Berg O, Arntsen B, Nebb HI, Dalen KT. Fatty acids regulate perilipin5 in muscle by activating PPARdelta. J Lipid Res. 2013;54:1949–1963. doi: 10.1194/jlr.M038992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak NM, Schweiger M, Jaeger D, Kolb D, Kumari M, Schreiber R, et al. Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J Lipid Res. 2013;54:1092–1102. doi: 10.1194/jlr.M034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Sreenivasan U, Gong DW, O’Connell KA, Dabkowski ER, Hecker PA, et al. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J Lipid Res. 2013;54:953–965. doi: 10.1194/jlr.M032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nseir W, Mograbi J, Ghali M. Lipid-lowering agents in nonalcoholic fatty liver disease and steatohepatitis: human studies. Dig Dis Sci. 2012;57:1773–1781. doi: 10.1007/s10620-012-2118-3. [DOI] [PubMed] [Google Scholar]
- 12.Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 13.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Are statins ‘IDEAL’ for non-alcoholic fatty liver disease? Curr Med Res Opin. 2013;30:229–231. doi: 10.1185/03007995.2013.855192. [DOI] [PubMed] [Google Scholar]
- 14.Nseir W, Mahamid M. Statins in nonalcoholic fatty liver disease and steatohepatitis: updated review. Curr Atheroscler Rep. 2013;15:305. doi: 10.1007/s11883-012-0305-5. [DOI] [PubMed] [Google Scholar]
- 15.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Roglans N, Sanguino E, Peris C, Alegret M, Vazquez M, Adzet T, et al. Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. J Pharmacol Exp Ther. 2002;302:232–239. doi: 10.1124/jpet.302.1.232. [DOI] [PubMed] [Google Scholar]
- 17.Ji G, Zhao X, Leng L, Liu P, Jiang Z. Comparison of dietary control and atorvastatin on high fat diet induced hepatic steatosis and hyperlipidemia in rats. Lipids Health Dis. 2011;10:23. doi: 10.1186/1476-511X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egawa T, Toda K, Nemoto Y, Ono M, Akisaw N, Saibara T, et al. Pitavastatin ameliorates severe hepatic steatosis in aromatase-deficient (Ar−/−) mice. Lipids. 2003;38:519–523. doi: 10.1007/s11745-003-1093-x. [DOI] [PubMed] [Google Scholar]
- 19.Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, et al. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen TS, Vendelbo MH, Jessen N, Pedersen SB, Jorgensen JO, Lund S, et al. Fasting, but not exercise, increases adipose triglyceride lipase (ATGL) protein and reduces G(0)/G(1) switch gene 2 (G0S2) protein and mRNA content in human adipose tissue. J Clin Endocrinol Metab. 2011;96:E1293–1297. doi: 10.1210/jc.2011-0149. [DOI] [PubMed] [Google Scholar]
- 21.Bertile F, Raclot T. ATGL and HSL are not coordinately regulated in response to fuel partitioning in fasted rats. J Nutr Biochem. 2011;22:372–379. doi: 10.1016/j.jnutbio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26:362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 25.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato R, Miyamoto W, Inoue J, Terada T, Imanaka T, Maeda M. Sterol regulatory element-binding protein negatively regulates microsomal triglyceride transfer protein gene transcription. J Biol Chem. 1999;274:24714–24720. doi: 10.1074/jbc.274.35.24714. [DOI] [PubMed] [Google Scholar]
- 27.Xie X, Liao H, Dang H, Pang W, Guan Y, Wang X, et al. Down-regulation of hepatic HNF4alpha gene expression during hyperinsulinemia via SREBPs. Mol Endocrinol. 2009;23:434–443. doi: 10.1210/me.2007-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bist A, Fielding PE, Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci U S A. 1997;94:10693–10698. doi: 10.1073/pnas.94.20.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Song Y, Zhang LJ, Gu Y, Li FF, Pan SY, et al. LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid beta-oxidation of lipid droplets. PLoS One. 2012;7:e36712. doi: 10.1371/journal.pone.0036712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.