Abstract
Objective
Cardiac surgery is a major cause of acute kidney injury. In this setting receipt of blood transfusions appears to associate with a higher risk of AKI, as measured using serum creatinine values. We examined this association further, using urinary biomarkers of kidney injury.
Methods
1210 adults underwent cardiac surgery and were divided into three groups based on the receipt of intraoperative packed red blood cell units (PRBC): no blood (n=894), ≤ 2 PRBC (n=206) and > 2 PRBC (n=110). AKI was defined as: i) Doubling of serum creatinine from the pre-operative value; ii) first post-operative urinary interleukin-18 in the 5th quintile; iii) first postoperative urinary neutrophil gelatinase-associated lipocalin in the 5th quintile. We determined the relative risk for AKI outcome according to PRBC group after adjusting for 12 pre-operative and surgical variables. Using the Sobel test for mediation analysis, we also evaluated the role of biomarkers in causing AKI through alternative pathways.
Results
AKI was more common in those who received >2 PRBC. In patients receiving > 2 PRBC, the adjusted RRs were 2.3 (95% CI 1.2-4.4, p 0.01), 1.36 (95% CI 1.0-1.9, p 0.05), and 1.34 (95% CI 1.0-1.8, p 0.06) for doubling of serum creatinine, urinary IL-18 in the 5th quintile (>60 pg/ml), and urinary NGAL in the 5th quintile (>102 ng/ml), respectively. Furthermore, the effect of PRBC transfusion on AKI was partially mediated by IL-18.
Conclusions
Receipt of two or more PRBC during cardiac surgery is associated with a greater risk of AKI defined by serum creatinine and kidney injury biomarkers.
Keywords: acute kidney injury, PRBC transfusion, cardiac surgery, kidney injury biomarkers
INTRODUCTION
Cardiac surgery is one of the leading contributors to acute kidney injury (AKI) in patients admitted to intensive care.1-3 It is estimated that more than 1.25 million cardiac surgeries are performed annually worldwide.4 Given the large number of cardiothoracic surgeries and associated AKI (CS-AKI), it is necessary to understand potentially modifiable risk factors for CSAKI.
Acute blood loss or drop in hematocrit due to hemodilution is common in cardiac surgery, thus blood transfusion is frequently performed during surgery in order to improve oxygen delivery to kidneys as well as other vital organs with the assumption that it would prevent ischemic injury. However, blood transfusion is not a benign intervention and has been shown to be associated with multi-organ failure including AKI in patients undergoing cardiopulmonary bypass (CPB).5,6 Since blood transfusion is a potentially modifiable factor in CS-AKI, the association between blood transfusion and outcomes in cardiac surgery has been widely investigated. More recently, data from a randomized controlled trial suggested that a restrictive protocol of blood transfusion was not inferior to a liberal protocol in patients undergoing cardiac surgery.7 To date, however, most studies investigating the relationship between blood transfusion and AKI have used creatinine or dialysis as measures of AKI. Serum creatinine is not considered a robust marker for timely diagnosis in AKI due to the effect of several non-renal factors such as fluids, drugs and muscle metabolism on serum creatinine levels. In addition, creatinine is a functional marker of renal clearance rather than direct injury. There are, however, some novel urine proteins, neutrophil gelatinase-associated lipocalin (NGAL) and Interleukin 18 (IL-18) that are associated with ischemia reperfusion injury in the kidney and have demonstrated improved sensitivity for AKI diagnosis when compared with serum creatinine.8 In addition, animal studies have demonstrated that NGAL and IL-18 are not only early biomarkers but are mediators of AKI and contribute to apoptosis and necrosis of renal tubular cells.9 As the association between packed red blood cell units (PRBC) transfusion and AKI has been examined by using serum creatinine, the objective of the present study was to determine the degree of functional and structural injury associated with transfusion of PRBCs and to determine whether urinary biomarkers of kidney injury mediate clinical AKI in this setting.
METHODS
Study Population
We performed a prospective analysis of the adult patient population who underwent coronary artery bypass grafting (CABG) or valve surgery at six academic medical centers in North America between July 2007 and December 2009. All the patients eligible for the study consented to the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) protocol.10 The main inclusion criteria required cardiac surgery with high risk for AKI defined by the presence of one or more of the following: emergency surgery, preoperative serum creatinine > 2 mg/dl (>177 _mol/L), ejection fraction < 35% or grade 3 or 4 left ventricular dysfunction, age > 70 years, diabetes mellitus, concomitant CABG and valve surgery, or repeat revascularization surgery.10,11 The cohort included patients undergoing off pump surgery (OPCAB) as well as those performed with cardiopulmonary bypass (CPB). Patients with evidence of AKI before surgery, prior kidney transplantation, preoperative serum creatinine level > 4.5 mg/dl (>398 μmol/L), or end-stage renal disease were excluded.
Sample Collection
Urine and plasma specimens were collected preoperatively and the first postoperative samples were collected soon after admission to the ICU. The remaining daily blood and urine samples were obtained at the time of routine morning blood collection performed for clinical care. Serum creatinine values were recorded for every patient throughout the hospital stay. For further details on the sample collection and processing please refer to our previous publication.10,11
AKI Biomarker Measurements
Urinary NGAL (ng/mL) and IL-18 (pg/mL) were measured with the ARCHITECT assay (Abbott Diagnostics, Abbott Park, IL). Urine creatinine was measured by the modified Jaffe reaction. The intra-assay coefficient of variation (CV) for the urine creatinine assay was 5%, whereas the CVs for the NGAL and IL-18 assays were approximately 5% and 8%, respectively.
Study Variables
Published studies suggest significant increased risk of AKI with transfusion of >2 PRBC.12-15 We therefore analyzed the cohort by dividing into three groups as receiving no blood, ≤ 2 PRBC and > 2 PRBC intraoperatively. The primary outcome was the development of AKI, defined in three ways: 1) at least a doubling in serum creatinine from the baseline preoperative value; 2) urinary IL-18 concentration in the highest quintile; or 3) urinary NGAL in the highest quintile. We limited our analysis to first postoperative biomarker values as we have previously shown the association of these values with AKI in cardiac surgery patients.10 Preoperative characteristics, operative details, and postoperative complications were collected. For postoperative complications definitions of the Society of Thoracic Surgeons database were used. The number of PRBC transfusion received intra-operatively was recorded for every patient. Cardiac catheterization if received within 48 hours of index surgery was recorded. Preoperative estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equations.16 All preoperative creatinine values were measured within 2 months before surgery.
Statistical Analysis
Patients’ baseline characteristics were compared across the three groups. We analyzed categorical and continuous variables using chi square and Kruskal Wallis statistics, respectively. We assessed the outcome of severe AKI based on serum creatinine and biomarkers as described above. We also examined the distribution of the urinary biomarkers across the three groups. We used the first postoperative values of urine biomarkers as these have been demonstrated to be associated with AKI in our prior observations.10,17 Relative risk (RR) of severe AKI was calculated using Poisson regression with robust error variance in the two groups who received either ≤ 2 or > 2 PRBC units, using the group with no blood transfusion as reference. We adjusted for various important covariates that are associated with AKI including patient demographics (white race, age, sex), clinical risk factors (preoperative eGFR, diabetes, hypertension, congestive heart failure, cardiac catheterization in the last 48 hours), and operative characteristics (elective vs. non elective surgery, CPB time and type of surgery (CABG, CABG and valve, valve)). To minimize the indication bias that is associated with PRBC use, we also used propensity scores as a covariate as well as weighted propensity scores to calculate the RR. The RR was adjusted for propensity score which provides a way to summarize covariate information about group bias (treatment selection) into a scalar value. The propensity score probability was calculated from Logistic regression after accounting for covariates listed above including PRBC groups.
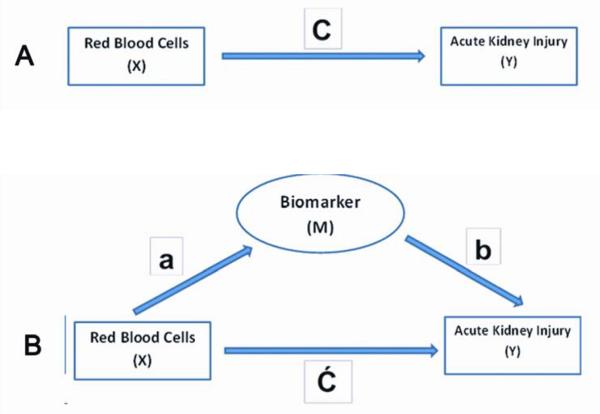
To explore the association between blood transfusion and AKI further, we sought to analyze the alternative pathways that can mediate this effect. We conducted the mediation analysis to determine if urine biomarkers are an intermediate between red blood cell usage and the development of AKI. To explore the mediation pathway, first the direct effect of independent variable X (PRBC) on dependent variable Y (AKI) was calculated as “C”. The association between X and M (biomarker) and M and Y were calculated as ‘a” and “b” respectively. Assuming there is mediation between X and Y through M, the residual direct effect of X on Y was then calculated as “Ć” (Figure 1). The equation to calculate the effect of X on Y i.e. “C” then can be written as:
Provided “a”, “b” and “C” remain significant, if Ć is non-zero but the direct effect of X to Y (i.e. “C”) is decreased then there is partial mediation through a mediator pathway. If Ć is 0 (i.e. the effect of X to Y disappears) then the effect is mediated fully through alternative pathway M which is a biomarker in this case. We used Sobel test18 to evaluate the mediation analysis. The results were considered significant with 2-sided α error <0.05. All analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC).
Figure 1.
Panel A describes the direct effect of independent variable (X) on dependent variable (Y) denoted as C. Panel B describes the effect of X on Y through a mediator (M) where the association between X and M is denoted as ‘a’, association between M and Y is denoted as ‘b’ and association of X and M to Y is denoted as Ć, such that C = Ć + ab
RESULTS
Characteristics of the Study Cohort
A total of 1210 patients were included in this analysis that underwent cardiac surgery in six university affiliated hospitals in North America. A comparison of baseline characteristics between the patients who received no blood and those who received either ≤ 2 or >2 PRBC is presented in Table 1. Mean age of all patients was 71 years. Overall, 68% of the patients were male, 94% were white and 42% had diabetes. A higher proportion of patients who either underwent non-elective surgery or a combined procedure (CABG and valve) required >2 PRBC. Also, more patients who underwent CABG with CPB required PRBC transfusion (28%) as opposed to those who had off pump surgery (OPCAB; 15%). The mean eGFR was lower (57.2 ml/min/1.73m2) in the patients requiring > 2 PRBC compared to those who did not require blood (70.3 ml/min/1.73m2).
Table 1.
Baseline Characteristics of Patients by number of units transfused
| Characteristics | All Patients (n=1210) |
No Blood (n=894) |
1-2 PRBC (n=206) |
> 2 PRBC (n=110) |
P Value* |
|---|---|---|---|---|---|
|
| |||||
| Preoperative Factors | |||||
|
| |||||
| Age, mean (SD) | 71 (10) | 71 (10) | 73 (11) | 73 (11) | <.0001 |
|
| |||||
| Male, n (%) | 820 (86) | 682 (76) | 93 (45) | 45 (41) | <.0001 |
|
| |||||
| White, n (%) | 1132 (94) | 848 (95) | 188 (91) | 96 (87) | 0.0032 |
|
| |||||
| Diabetes, n (%) | 508 (42) | 393 (44) | 74 (36) | 41 (37) | 0.0626 |
|
| |||||
| Hypertension, n (%) | 953 (79) | 697 (78%) | 165 (80%) | 91 (83%) | 0.45 |
|
| |||||
| Congestive Heart Failure, n (%) | 312 (26) | 195 (22%) | 67 (33%) | 50 (45%) | <.0001 |
|
| |||||
|
Cardiac catheterization in last 48
hours, n (%) |
72 (6) | 41 (5%) | 22 (11%) | 9 (8%) | <.01 |
|
| |||||
| Myocardial Infarction, n (%) | 310 (26) | 241 (28%) | 47 (24%) | 22 (20%) | 0.15 |
|
| |||||
| Preoperative eGFR, mean (SD) | 68 (21) | 70.33 (20.04) |
66.05 (21.98) |
57.18 (21.65) |
<.0001 |
|
| |||||
| Preoperative CKD Stage | <.0001 | ||||
| Stage 1, n (%) | 164 (14) | 131 (15%) | 24 (12) | 9 (8) | |
| Stage 2, n (%) | 612 (51) | 478 (53) | 99 (48) | 35 (32) | |
| Stage 3, n (%) | 406 (34) | 274 (31) | 75 (36) | 57 (52) | |
| Stage 4, n (%) | 28 (2) | 11 (1) | 8 (4) | 9 (8) | |
|
| |||||
| Preoperative sCr, mean (SD) | 1.09 (0.34) | 1.08 (0.32) | 1.07 (0.36) | 1.23 (0.45) | 0.0018 |
|
| |||||
| Preoperative medications | |||||
| Aspirin | 894 (74%) | 679 (76%) | 134 (65%) | 81 (74%) | 0.0 |
| Beta blockers | 890 (74%) | 665 (75%) | 139 (68%) | 86 (79%) | 0.06 |
| ACE inhibitors or ARB | 552 (46%) | 415 (47%) | 92 (45%) | 45 (41%) | 0.54 |
| Statins | 894 (74%) | 676 (76%) | 143 (69%) | 75 (69%) | 0.07 |
|
| |||||
| Status of surgery | <.0001 | ||||
| Elective, n (%) | 956 (79) | 738 (83) | 150 (73) | 68 (62) | |
| Non Elective, n (%) | 254 (21) | 156 (17) | 56 (27) | 42 (38) | |
|
| |||||
| Intraoperative Factors | |||||
|
| |||||
| Off Pump CABG (OPCAB), n (%) | 117 (10) | 100 (11) | 14 (7) | 3 (3) | 0.01 |
|
| |||||
|
Cardiopulmonary Bypass Time (m)
mean (SD) |
114 (60) | 106 (55) | 125 (60) | 160 (73) | <.0001 |
|
| |||||
| Cross clamp time (min), mean (SD) | 78 (44) | 72 (41) | 85 (47) | 108 (52) | <.0001 |
|
| |||||
| Cardioplegia, n (%) | 1068 (88) | 779 (87%) | 185 (90%) | 104 (95%) | 0.06 |
|
| |||||
|
Number of PRBC units transfused,
mean (SD) |
0.69 (1.57) | 0 (0) | 1.6 (0.49) | 4.59 (2.45) | <.0001 |
|
| |||||
| Type of surgery | <.0001 | ||||
| CABG, n (%) | 580 (48) | 482 (54) | 74 (36) | 24 (22) | |
| CABG & Valve, n (%) | 277 (23) | 176 (20) | 54 (26) | 47 (43) | |
| Valve, n (%) | 353 (29) | 236 (26) | 78 (38) | 39 (35) | |
|
| |||||
| Intra-aortic balloon pump, n (%) | 56 (3) | 31 (3%) | 11 (5%) | 14 (13%) | <.0001 |
PRBC, packed red blood cells; CABG, Coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; sCr, serum creatinine; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; Peak sCr is defined as the peak post-operative creatinine minus the pre-operative serum creatinine value.
P values were calculated using Kruskal-Wallis test for continuous variables and Chi-square test for categorical variables.
Association of PRBC Transfusion with AKI
The outcome of severe AKI defined either by serum creatinine or novel biomarkers was higher in patients who received > 2 PRBC units compared to those who did not receive any blood or received ≤ 2 PRBC units (Table 2). The first postoperative values of urinary IL-18 and urinary NGAL were significantly higher in patients who received > 2 PRBC units compared to those who did not receive any blood or received ≤ 2 PRBC units (Figure 2).
Table 2.
Association of Blood Transfusion with AKI Assessed with Serum creatinine and urine biomarkers of kidney injury
| Doubling of Serum Creatinine | Urine IL-18 >60 pg/ml‡ | Urine NGAL >102 ng/ml‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Unadjusted RR (95% CI) |
Adjusted RR† (95% CI) |
n (%) | Unadjusted RR (95% CI) |
Adjusted RR† (95% CI) |
n (%) | Unadjusted RR (95% CI) |
Adjusted RR† (95% CI) |
|
|
No
Blood (n=894) |
30 (3) | 1.0 (referent) | 1.0 (referent) | 150 (17) |
1.0 (referent) | 1.0 (referent) |
146 (16) |
1.0 (referent) | 1.0 (referent) |
|
1 - 2
PRBC (n=206) |
9 (4) | 1.30 (0.63, 2.7) |
1.06 (0.52, 2.2) |
44 (22) | 1.30 (0.96, 1.75) |
1.02 (0.75, 1.39) |
44 (22) | 1.33 (0.99, 1.8) |
0.98 (0.72, 1.34) |
|
> 2
PRBC (n=110) |
19 (17) |
5.15 (3, 8.83) |
2.29 (1.21, 4.4) |
42 (40) | 2.34 (1.77, 3.08) |
1.36 (1, 1.85) |
46 (43) | 2.63 (2.02, 3.42) |
1.34 (0.98, 1.84) |
| P Value * | <.0001 | … | 0.01 | 0.02 | … | 0.05 | 0.02 | … | 0.06 |
P values were calculated using Chi-square test.
Adjusted for race, sex, age, preoperative eGFR, diabetes, hypertension, congestive heart failure, myocardial infarction, cardiac catheterization within 48 hours, status of surgery (elective vs. non elective), type of surgery (CABG, valve or both) and perfusion time.
AKI, Acute kidney injury; PRBC, packed red blood cells.
IL-18 >60 pg/ml and NGAL >102 ng/ml represent the 5th quintile of the first post-operative biomarker values in the cardiac surgery cohort.
Figure 2.
The distribution of urinary biomarkers in different groups by blood transfusion is shown in box plot. The bars represent the inter-quartile range and the solid lines in the bars represent the median values of first post-operative urinary IL-18 and urinary NGAL. The median values of both urinary IL-18 and urinary NGAL were significantly elevated in the group that received > 2 PRBC units compared to those who received < 2 (P values for Wilcoxon test <.0001 and <.0001 respectively) or no blood (P values for Wilcoxon test <.0001 and <.0001 respectively).
* No Blood vs. More than 2 PRBC p value <.0001,
^ Less than 2 PRBC vs. More than 2 PRBC p value <.0001.
In patients receiving > 2 PRBC units, the adjusted RRs were 2.3 (95% CI 1.2-4.4, p value 0.01), 1.36 (95% CI 1.0-1.9, p value 0.05), and 1.34 (95% CI 0.98-1.8, p value 0.06) for doubling of serum creatinine, urinary IL-18 in the 5th quintile (>60 pg/mL), and urinary NGAL in the 5th quintile (>102 ng/mL), respectively (Table 2). The results for doubling of serum creatinine remained virtually unchanged when we adjusted for propensity score to receive PRBC (RR 2.3, 95% CI 1.1-4.7).
Mediation Analysis
Mediation analysis revealed that 17% of the total effect of transfusing > 2 PRBC on doubling of serum creatinine was mediated by IL-18. Mediation analysis for NGAL was not significant (Table 3).
Table 3.
Mediation Analysis to evaluate indirect effect of PRBC on AKI via Biomarkers
| Direct Pathway Estimate C |
Alternate Pathway Estimate |
Mediation Estimate Ć |
Sobel Test |
Mediation Percentage |
P Value for Mediation |
||
|---|---|---|---|---|---|---|---|
| a | b | ||||||
|
Urine IL-18
(pg/ml) |
0.973 | 0.518 | −0.32 | 0.822 | −2.19 | 17.07% | 0.02 |
|
Urine NGAL
(ng/ml) |
0.973 | 0.496 | 0.122 | 0.901 | −1.3 | 6.22% | 0.19 |
Mediation percentage is the percentage of total effect of PRBC transfusion on AKI that is mediated via biomarkers.
DISCUSSION
The association between intraoperative blood transfusion during cardiac surgery and AKI using serum creatinine has been reported by prior studies.6,13-15,19 In our analyses, we demonstrated that compared to patients who received none or ≤ 2 units, receipt of >2 PRBC was significantly associated with the risk for AKI, as defined not only by changes in serum creatinine but also by the absolute concentrations of urinary biomarkers of kidney injury (IL-18 and NGAL). This risk associated with > 2 units of blood was not attenuated in the propensity analysis models which accounted for potential treatment selection bias. In concordance with the animal studies, we also demonstrated that some of the effect of clinical AKI is mediated through IL-18 pathway.
The association between blood transfusion and AKI may be due to either a direct transfusion-related kidney injury, or an indirect effect in which the transfusion is acting as a surrogate marker for hypotension or decreased oxygen delivery. The pathophysiology of organ injury after blood transfusion is not clearly understood but there are many possible mechanisms that likely play a role. It has been reported that red blood cells preserved under hypothermic conditions undergo a series of changes that affect their viability and functionality post transfusion. Stored RBCs become ATP and 2,3-Diphosphoglycerate (2,3-DPG) depleted20 which causes cell membrane oxidative stress, resulting in cytoskeleton changes and decreased deformability.21-25 Current guidelines for blood preservation promote newer preservatives which can attenuate these metabolic prolonging the stored time period to 42 days. However, these cell membrane processes are not completely halted. In fact, Berezina et al. concluded from their study that stored RBCs can undergo significant irreversible changes and develop hemolysis during the second week of preservation long before the expiration currently set by blood banks.22 This finding has important clinical implications. In a study where the complications after cardiac surgery were correlated with the age of stored blood, authors found that patients who received blood older than 14 days had a higher incidence of AKI (2.7 vs. 1.6%).19 Depletion of RBC 2,3-DPG also shifts the oxygen dissociation curve to the left, perpetuating ischemic injury. Increased platelet activation and thrombogenesis by altered cell membranes of stored RBCs has also been shown to be another possible mechanism of organ injury after blood transfusion.23 Hemolysis of stored blood also results in release of free hemoglobin and iron in the supernatant.24-26 Transfusion of 2 units of PRBC may result in elevation of free plasma hemoglobin by approximately 10 folds above the normal limits.27 The free hemoglobin and iron can cause toxic injury to the tubules and lead to AKI.
It is possible that blood transfusion is simply a marker for higher risk patients who are more susceptible to AKI because of low baseline hematocrit or a lower intraoperative hematocrit. Huybregts et al. analyzed the association of low hematocrit and PRBC transfusion to AKI utilizing N-Acetyl-ß-D-glucosaminidase (NAG) as a marker of kidney injury.13 The authors found that the levels of NAG were relatively much higher among patients who had received ≥ 2 PRBC when compared to the group with HCT <24% suggesting that transfusion of blood may have had some role in causing AKI in addition to low HCT. Other studies have also shown a synergistic effect of blood transfusion to perioperative anemia in increasing the risk of AKI while both factors were independently associated with AKI.24,28
It is noteworthy that we also did not observe an increase in risk of severe AKI with blood transfusion of up to 2 PRBC. This observation has potentially important clinical implications. Tolerance of permissive anemia is important since perioperative blood conservation strategies are evolving and shown to be effective and associated with better outcomes.29,30 However, it is also well known that excessively low hematocrit can be associated with postoperative AKI.31,32 Thus, our observations suggest that judicious transfusion of PRBC up to 2 units may be safe and prevent postoperative AKI. It is important to note that while poor organ perfusion is a cause of organ dysfunction in critically ill patients, HCT is a poor surrogate of oxygen delivery. The evidence is currently lacking that increasing HCT would necessarily lead to improved perfusion.33
Our examination of the expression of kidney injury biomarkers in response to blood transfusion is unique compared to previous data. IL-18 is a pro-inflammatory biomarker and causes increased apoptosis in the proximal tubular cells. IL-18 is activated from its pro-from by caspase-1 pathway in response to several stressors such as ischemia, nephrotoxins or reperfusion.9 Moreover, IL-18 itself may be injurious to tissues and thus not only be a biomarker but a mediator of AKI through a caspase-1 and NLRP3 inflammasome mediated apoptosis.9 Several studies in experimental animals have demonstrated that IL-18 neutralizing antibodies can reduce the amount of tissue injury. The mediation analysis we performed supports the notion that IL-18 expression may be exacerbating clinical AKI. Thus, this approach may be used as a model to identify other potential biomarker-modifiers, which may offer therapeutic targets to reduce AKI.
A major limitation in our study was that pre- and perioperative HCT values were not collected in the original TRIBE-AKI protocol. However, prior studies have demonstrated that the lowest hematocrit during CPB is highly associated with peak fractional change in serum creatinine as well as the highest postoperative creatinine value.32 We also did not control transfusion triggers; we have simply reported the current intraoperative practices as they pertain to the institutions participating in this study. The dialysis events were low and thus we could not study this group separately. This study is also observational in nature and thus limits our ability to confirm any potential causal association between PRBC and AKI outcomes. Although we attempted to account for differences in exposures, patients who required more PRBC transfusions may have had a greater severity of illness or intraoperative complications. We also did not collect the age of PRBC transfused to our patients which may affect the outcomes of AKI as discussed above.
In conclusion, blood transfusion > 2 units is independently associated with AKI defined by both serum creatinine and urinary biomarkers of kidney injury in the setting of cardiac surgery. These findings provide additional evidence that may be used to design interventions that can alleviate kidney injury associated with blood transfusion. A major clinical implication of our study is that blood transfusion should be used judiciously in selected patients with a careful assessment of risk-benefit ratio.
Acknowledgments
The study was supported by the NIH grant RO1HL085757 (CRP) to study novel biomarkers of acute kidney injury in cardiac surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Parikh and Dr. Edelstein are named co-inventors on the IL-18 patent (no financial value). Dr. Devarajan is the co-inventor on the NGAL patents.
Members of TRIBE-AKI consortium: U of Chicago: Dr. Jai Raman, Dr. Valluvan Jeevanandam, Dr. Shahab Akhter
U of Cincinnati Children’s: Dr. Prasad Devarajan, Michael Bennett, PhD, Qing Ma, MD, Ms. Rachel Griffiths
U of Colorado: Dr. Charles Edelstein
London, Ontario: Dr. Michael Chu, Dr. Martin Goldbach, Dr. Lin Ruo Guo, Dr. Bob Kiaii, Dr. Neil McKenzie, Dr. Mary Lee Myers, Dr. Richard Novick, Dr. Mac Quantz, Ms. Virginia Schumann, Ms. Laura Webster,
Montreal Children’s: Dr. Michael Zappitelli, Dr. Ana Palijan
Yale-New Haven: Dr. Michael Dewar, Dr. Umer Darr, Dr. John Elefteriades, Dr. Arnar Geirsson
REFERENCES
- 1.Falvo A, Horst HM, Rubinfeld I, et al. Acute renal failure in cardiothoracic surgery patients: what is the best definition of this common and potent predictor of increased morbidity and mortality. Am J Surg. 2008 Sep;196(3):379–383. doi: 10.1016/j.amjsurg.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 2.Kallel S, Triki Z, Abdenadher M, Frikha I, Jemel A, Karoui A. [Acute renal failure after cardiac surgery: Evaluation of the RIFLE criteria.] Nephrol Ther. 2012 Aug 21; doi: 10.1016/j.nephro.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005 Aug 17;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Garg AX, Devereaux PJ, Yusuf S, et al. Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularisation Study (CORONARY): kidney substudy analytic protocol of an international randomised controlled trial. BMJ Open. 2012;2(2):e001080. doi: 10.1136/bmjopen-2012-001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Santo L, Romano G, Della Corte A, et al. Preoperative anemia in patients undergoing coronary artery bypass grafting predicts acute kidney injury. J Thorac Cardiovasc Surg. 2009 Oct;138(4):965–970. doi: 10.1016/j.jtcvs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Habib RH, Zacharias A, Schwann TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005 Aug;33(8):1749–1756. doi: 10.1097/01.ccm.0000171531.06133.b0. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010 Oct 13;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 8.Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med. 2009 Feb;37(2):553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 9.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001 May;107(9):1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011 Sep;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Coca SG, Wang Z, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011 Sep;58(3):366–373. doi: 10.1053/j.ajkd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant. 2012 Jan;27(1):153–160. doi: 10.1093/ndt/gfr275. [DOI] [PubMed] [Google Scholar]
- 13.Huybregts RA, de Vroege R, Jansen EK, van Schijndel AW, Christiaans HM, van Oeveren W. The association of hemodilution and transfusion of red blood cells with biochemical markers of splanchnic and renal injury during cardiopulmonary bypass. Anesth Analg. 2009 Aug;109(2):331–339. doi: 10.1213/ane.0b013e3181ac52b2. [DOI] [PubMed] [Google Scholar]
- 14.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009 Feb 3;119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 15.Karkouti K, Wijeysundera DN, Yau TM, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology. 2011 Sep;115(3):523–530. doi: 10.1097/ALN.0b013e318229a7e8. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012 May;23(5):905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. The New England journal of medicine. 2008 Mar 20;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 20.Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfus Med Rev. 2005 Apr;19(2):127–142. doi: 10.1016/j.tmrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe LC. Oxidative injuries to the red cell membrane during conventional blood preservation. Semin Hematol. 1989 Oct;26(4):307–312. [PubMed] [Google Scholar]
- 22.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002 Jan;102(1):6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 23.Stadler I, Toumbis CA, Ambrus JL. Influence of cold storage altered red cell surface on the function of platelets. J Med. 1994;25(6):353–361. [PubMed] [Google Scholar]
- 24.Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. British journal of anaesthesia. 2012 Dec;109(Suppl 1):i29–i38. doi: 10.1093/bja/aes422. [DOI] [PubMed] [Google Scholar]
- 25.Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free radical biology & medicine. 2002 Apr 1;32(7):568–576. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- 26.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011 Jul 26;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011 Jul;142(1):1–11. doi: 10.1016/j.jtcvs.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Loor G, Li L, Sabik JF, 3rd, Rajeswaran J, Blackstone EH, Koch CG. Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. J Thorac Cardiovasc Surg. 2012 Sep;144(3):654–662. e654. doi: 10.1016/j.jtcvs.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 29.Moskowitz DM, McCullough JN, Shander A, et al. The impact of blood conservation on outcomes in cardiac surgery: is it safe and effective? Ann Thorac Surg. 2010 Aug;90(2):451–458. doi: 10.1016/j.athoracsur.2010.04.089. [DOI] [PubMed] [Google Scholar]
- 30.Passik CS, Fernandes E, D’Amico A, Maldarelli W, Parikh C. Spare the blood, but save the kidneys. Ann Thorac Surg. 2011 Jun;91(6):2022–2023. doi: 10.1016/j.athoracsur.2010.11.051. author reply 2023-2024. [DOI] [PubMed] [Google Scholar]
- 31.Karkouti K, Beattie WS, Wijeysundera DN, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005 Feb;129(2):391–400. doi: 10.1016/j.jtcvs.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. 2003 Sep;76(3):784–791. doi: 10.1016/s0003-4975(03)00558-7. discussion 792. [DOI] [PubMed] [Google Scholar]
- 33.Ranucci M, Aronson S, Dietrich W, et al. Patient blood management during cardiac surgery: do we have enough evidence for clinical practice? J Thorac Cardiovasc Surg. 2011 Aug;142(2):249, e241–232. doi: 10.1016/j.jtcvs.2011.04.007. [DOI] [PubMed] [Google Scholar]