Abstract
In a primary care population of 367 older adults (age 60+) with osteoarthritis (OA) pain and insomnia, we examined the relationship between short-term improvement in sleep and long-term sleep, pain and fatigue outcomes through secondary analyses of randomized controlled trial data. Study participants, regardless of experimental treatment received, were classified as either Improvers (≥30% baseline to 2-month reduction on the Insomnia Severity Index [ISI]) or Non-Improvers. After controlling for treatment arm and potential confounders, Improvers showed significant, sustained improvements across 18 months compared to Non-Improvers in Pain Severity (p<.001, Adjusted Mean Difference = −0.51 [95% Confidence Interval: −0.80, −0.21]), Arthritis Symptoms (p<.001, 0.63 [0.26, 1.00]), and Fear Avoidance (p=.009, −2.27 [−3.95, −0.58]) but not in Catastrophizing or Depression. Improvers also showed significant, sustained improvements in ISI (p<.001, −3.03 [−3.74, −2.32]), Pittsburgh Sleep Quality Index Total (p<.001, −1.45 [−1.97, −0.93]) and General Sleep Quality (p<.001, −.28 [−.39, −.16]) scores, Flinders Fatigue Scale (p<.001, −1.99 [−3.01, −0.98]), and Dysfunctional Beliefs about Sleep (p=.037, −2.44 [−4.74, −0.15]), but no improvements on the Functional Outcomes of Sleep Questionnaire or the Epworth Sleepiness Scale. We conclude that short-term (2-month) improvements in sleep predicted long-term (9- and 18-month) improvements for multiple measures of sleep, chronic pain, and fatigue. These improvements were not attributable to non-specific benefits for psychological well-being such as reduced depression. These findings are consistent with benefits of improved sleep for chronic pain and fatigue among older persons with osteoarthritis pain and co-morbid insomnia if robust improvements in sleep are achieved and sustained.
Keywords: Aging, Cognitive-Behavioral Therapy, Fatigue, Insomnia, Pain, Osteoarthritis, Sleep
1. Introduction
A growing literature suggests that poor nighttime sleep is associated with reduced pain and increased next-day pain reports [15,19,34,36]. If so, improving sleep in pain populations might improve chronic pain outcomes [31]. Several trials evaluating cognitive behavioral therapy for insomnia (CBT-I) in pain populations have found improved sleep outcomes, but benefits of CBT-I for pain outcomes have been inconsistent [6,10,18,38]. However, these studies had significant limitations including small sample sizes, inadequate controls, recruitment of convenience samples, and short follow-ups, making it difficult to draw clear conclusions on the impact of improved sleep on pain.
Recently, we reported results from “Lifestyles”, a randomized controlled trial of CBT for pain and insomnia among older adults with co-morbid osteoarthritis pain and insomnia [23,39]. The Lifestyles trial had notable strengths: a large population-based sample (N=367); broad eligibility criteria; primary care treatment delivery; high participant retention; and a highly credible, well-accepted attention control. Over a 9-month assessment period, a combination CBT for pain and insomnia (CBT-PI) was associated with more favorable outcomes for insomnia severity than either CBT for pain alone (CBT-P) or control [39]. However, at 18 months benefits were non-significant for all treatment arms [23]. Post-hoc analyses of participants with greater baseline insomnia and pain severity showed significant reductions in pain for CBT-PI compared to CBT-P, and moderate, albeit non-significant treatment effects for insomnia severity and sleep efficiency in the CBT-PI group [23]. Further, although unadjusted effect sizes for sleep and pain were attenuated over time, they were greater at 18 months for both outcomes for CBT-PI compared to the other two treatment arms [23]. Thus Lifestyles trial results showed a pattern of treatment estimates consistent with the a priori hypothesis that improving sleep could improve pain.
The failure to find statistically significant and sustained improvements may have resulted from trial limitations [23,39] including: (1) many participants had relatively mild pain and insomnia at study entry, attributable to screening to baseline regression to the mean; and (2) greater than planned intraclass correlations of pain and sleep because of group-based interventions, which reduced the effective sample size of the trial [1,28]. Given these unanticipated limitations, it is possible that Lifestyles was unable to detect clinically meaningful benefits of CBT-PI for sleep and pain outcomes, particularly among the patients with less severe insomnia at baseline. Fortunately, the Lifestyles trial provides an opportunity to assess the relationship of short-term improvement of sleep, regardless of experimental treatment received in the trial, with long-term sleep, pain, and fatigue outcomes, by comparing persons from all treatment groups whose sleep improved short-term to those whose sleep did not.
Here we report secondary analyses of Lifestyles data, testing the hypotheses that short-term (2-month) improvements in sleep predict long-term benefits in sleep, pain, and fatigue outcomes over 9–18 months.
2. Methods
The “Lifestyles” trial was a double-blind, cluster-randomized, controlled trial of a six-week cognitive-behavioral pain coping skills intervention (CBT-P), cognitive-behavioral therapy for pain and insomnia (CBT-PI), and an education only attention control (EOC), all delivered as group interventions to improve sleep and pain outcomes. The study was approved by Group Health, an integrated practice healthcare management organization in Western Washington State, and University of Washington institutional review boards. Study recruitment began January, 2009 and the last 18 month assessment was made May, 2012. Details describing Lifestyles’ study design rationale, recruitment, screening, randomization procedures, and intervention protocols have been published elsewhere [22,42], as have the primary outcome results from initial (post-treatment and 9-month) and long-term (18-month) assessments [23,39].
2.1 Participants
Three hundred sixty-seven Western Washington members of Group Health, an integrated practice healthcare management organization, age 60 or older, were enrolled in the Lifestyles trial (see Figure 1). When screened for trial eligibility, all participants had clinically significant pain and insomnia. Significant arthritis pain was defined by Grade II, III or IV pain on the Graded Chronic Pain Scale (GCPS [41]). Significant insomnia was defined by self-reported sleep difficulties (trouble falling asleep, difficulty staying asleep, waking up too early, or waking up unrefreshed) three or more nights per week during the past month with at least one daytime sleep-related problem, consistent with established research diagnostic criteria [9].
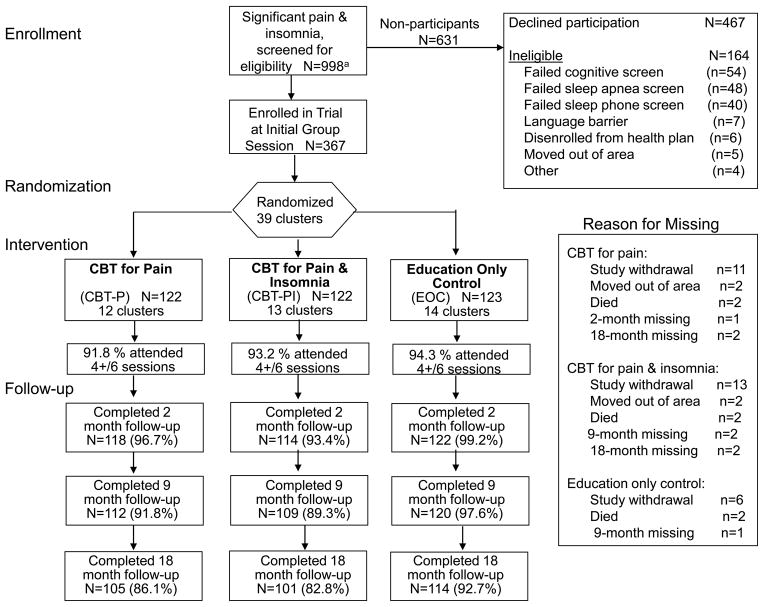
Figure 1.
Consort Flow Diagram for enrollment of potentially eligible participants.
Exclusion criteria were initially determined through electronic health records and included diagnosis of rheumatoid arthritis, obstructive sleep apnea, periodic leg movement disorder, restless leg syndrome, sleep-wake cycle disturbance, rapid eye movement behavior disorder, dementia or receiving cholinesterase inhibitors, Parkinson’s disease, cancer in the past year, receiving chemotherapy or radiation therapy in the past year, and inpatient treatment for congestive heart failure within the prior 6 months [42]. Additional screening occurred during telephone contact by study staff; potentially eligible subjects with a score of 7 or greater on the Blessed Short Orientation Memory and Concentration Test, or with a score greater than 32 on the sleep apnea sub-scale of the Sleep Disorders Questionnaire were also excluded as well as those who self-reported any of the following limitations or chronic conditions: unable to read a newspaper; difficulty hearing in a group situation; unable to walk across a room without help; and persons reporting the following chronic conditions: periodic leg movement disorder; rapid eye movement behavior disorder; sleep apnea; Parkinson’s disease; rheumatoid arthritis [42].
2.2 Comparison groups
For the current analyses, the 367 Lifestyles participants, regardless of randomly assigned treatment arm, were defined as either Improvers (a 30% or greater baseline to 2-month [post-treatment] reduction on the Insomnia Severity Index [ISI]) or Non-Improvers. We employed a 30% or greater reduction in the ISI to identify improvers based on a convention established for defining clinically significant improvement in pain severity [7], since a standard for identifying clinically significant improvement has not been defined for sleep outcomes.
2.3 Data collection
Baseline, 2-month (post-treatment), and 9 and 18-month follow-up assessments were each carried out at two visits to participants’ homes one week apart. Actigraphy and sleep diary data were collected during the intervening week.
3. Measures
3.1 Pain outcomes
Pain Severity – Six Graded Chronic Pain Scale (GCPS [41]) items assessing arthritis pain intensity (average pain, worst pain, pain right now), and interference with usual work, recreational, social, and family activities (possible range 0–10; higher is worse).
Arthritis Symptoms - A 3-item arthritis symptom subscale from the Arthritis Impact Measurement Scales Version 2, Short Form, Revised (AIMS [14,24,29]), which queried three pain-related questions: How often did you have severe pain from your arthritis? How often did your morning stiffness last more than one hour from the time you woke up? How often did your pain make it difficult for you to sleep? (possible range 1–10, higher is better).
Catastrophizing – The Pain Catastrophizing Scale (PCS [33]) consists of 13 items describing different thoughts and feelings that individuals may experience when they are in pain (possible range 0–52, higher is worse).
Fear Avoidance – Participants completed a 10 item version of the Tampa Scale for Kinesiophobia [5,25,40]. This ten-item scale measures fear of movement, pain and injury on a four-point scale (possible range 17–68, higher is worse).
3.2 Sleep and Fatigue outcomes
Insomnia Severity – Score on the Insomnia Severity Index (ISI [26]), a 7-item questionnaire assessing global insomnia severity (possible range 0–28; higher is worse).
Sleep Efficiency - Average time asleep as a percent of time in bed (possible range 0–100; higher is better), measured using wrist actigraphy (Actiwatch-2; Respironics, Inc., Bend, Oregon) for one week at each assessment. The night (in-bed) period was defined as “lights out” at bedtime until the final morning rising. Bed and rising times were derived from a daily sleep log kept by participants.
Sleep Quality – Total score on the 19-item Pittsburgh Sleep Quality Index (PSQI [2,3]) (possible range 0–21; higher is worse). Component #1 of the PSQI (PSQI-1) was also analyzed separately (possible range 0–3; higher is worse).
Sleep Beliefs and Attitudes – Total score on the 10-item version of the Dysfunctional Beliefs and Attitudes About Sleep scale (DBAS-10 [8,11] (possible range 0–100; higher is worse).
Fatigue – Total fatigue measured by the 7-item Flinders Fatigue Scale (FFS [13]) (possible range 0–31; higher is worse).
Daytime Sleepiness -Total score on the 8-item Epworth Sleepiness Scale (ESS [17]) (possible range 0–24; higher is worse).
Daytime Function – Total score on the 10-item version of the Functional Outcomes of Sleep Questionnaire (FOSQ-10 [4] assessing the daytime functional impact of disturbed sleep (possible range 5–20; higher is better).
3.3 Other outcomes
Depression – The Geriatric Depression Scale (GDS [44]), a 30-item questionnaire assessing depressive symptoms in older persons (possible range 0–30, higher is worse).
3.4 Baseline covariate measures
Patient characteristics were collected from Group Health electronic medical records and baseline Lifestyle’s participant surveys including age, gender, and chronic illness comorbidity. Information about education, retirement status, and race were obtained by self-report in the baseline interview.
Mental Status – The Modified Mini-Mental State Examination (3MS [35]), a 100-point cognitive screen based on an expanded version of Folstein’s Mini-Mental State Examination (possible range 0–100, higher is better).
Antidepressant, analgesic, and/or sedating medication use – Participant self-report of current medication use to relieve pain and/or improve sleep.
3.5 Statistical Analysis
Multiple imputation was used with 10 imputations to accommodate missing information in statistical analyses. Demographics and observed pain, insomnia, and functional outcomes collected at all assessments were used in the imputation models. Fully conditional specification was used to estimate imputation models and to multiply impute information from missed visits, missed items during study visits (including missing actigraphy data), and missing information from participant drop out [37,43].
Sleep, pain, fatigue and other outcomes for Improvers and Non-improvers at baseline, post-treatment, 9-month, and 18-month assessments were compared. At baseline, Improvers and Non-improvers were compared on all covariates and outcome variables using linear regression for continuous variables and logistic regression for categorical variables combing over all 10 imputations. Baseline variables that differed between Improvers and Non-improvers (p-value < 0.10) were included as covariates in regression models assessing differences in long-term outcomes (9 and 18 months) between Improvers and Non-improvers. At two months, Improvers and Non-improvers were compared on all outcome variables using linear regression for continuous variables and logistic regression for categorical variables combing over all 10 imputations and using the robust sandwich estimator for account for clustering due to intervention class [45]. Unadjusted percentages for categorical data and means and standard deviations were calculated for Improvers and Non-improvers at baseline and 2 months, averaged over all 10 imputations with standard deviations adjusted using Rubin’s formula [20].
To compare long-term sleep, pain, fatigue, and other outcomes for Improvers versus Non-improvers, repeated measures linear regression with outcomes measured at 9 and 18 months were used. Regression models used generalized estimating equations with an independence working correlation matrix [45]. The robust sandwich estimator was used to account for within-person correlation over time. In order to account for the cluster randomization implemented in the Lifestyles trial, a small-sample adjustment [21] was employed because when fewer than 40 groups are included, standard error estimates using the sandwich estimator are known to be biased downward [12,27]. All regression models comparing Improvers and Non-improvers were adjusted for treatment assignment; age; baseline values of the outcome of interest; ISI, GDS, and 3MS score; antidepressant, analgesic, and sedating medication use recorded at baseline; an indicator for whether the outcome was measured at 18 months; and the clinic at which the intervention was delivered.
Sensitivity analyses to assess if the comparison between Improvers and Non-improvers differed between treatment groups or if the comparison changed over time were performed by including interactions of improver status and treatment assignment or improver status and time in the linear regression models. Summary information was calculated and comparison tests at baseline and 2 months were performed using SAS 9.2 (SAS Institute, Inc, Cary, NC). Imputation and regression models were estimated using Stata© 11.1 (Stata Corp., College Station, TX) [30].
4. RESULTS
The Lifestyles trial included 367 participants assigned to three experimental arms. Among these 367 trial participants, there were 131 individuals whose ISI scores were improved (a 30% or greater baseline to 2-month [post-treatment] reduction), 223 participants whose ISI scores were not improved at 2-month assessment, and 13 persons with a missing ISI score at 2 months whose improvement status varied across the 10 imputed analyses.
Table 1 reports descriptive statistics and comparisons between Improvers versus Non-Improvers on baseline covariates and baseline values of sleep, pain, fatigue, and other outcomes. Improvers as a group were two years younger (p=0.03) and used more anti-depressant medications (p=0.02) than Non-Improvers. None of the other 23 baseline variables examined differed significantly at the 0.05 level between the two groups.
Table 1.
Baseline mean (standard deviation) of demographic, health and sleep and pain measures by Improver versus Non-Improver status (Improver: ≥ 30% reduction of ISI score between baseline and 2 months). Imputed data used.
| Improvers | Non-Improvers | P** | |
|---|---|---|---|
| N* | 131 | 223 | - |
| Age*** | 71.9 (7.9) | 73.8 (8.2) | 0.03 |
| Women (%)*** | 79.1 | 78.1 | 0.82 |
| Retired (%) | 79.3 | 77.9 | 0.75 |
| Caucasian (%) | 89.3 | 92.4 | 0.32 |
| Some College (%) | 89.9 | 84.3 | 0.14 |
| 3MSE | 93.0 (5.1) | 93.7 (4.7) | 0.20 |
| 3MSE < 90 (%) | 19.3 | 15.5 | 0.35 |
| Chronic Illness (%)*** | 53.4 | 52.5 | 0.87 |
| Anti-depressants (%) | 29.1 | 18.5 | 0.02 |
| Anti-psychotics (%) | 0.7 | 0.4 | 0.71 |
| Anxiolytics (%) | 11.0 | 9.1 | 0.55 |
| Hypnotics (%) | 22.1 | 15.2 | 0.10 |
| Analgesics (%) | 88.3 | 91.7 | 0.29 |
| ISIa | 11.7 (5.1) | 11.4 (4.9) | 0.64 |
| Sleep Efficiencyb | 81.8 (8.8) | 83.0 (8.9) | 0.21 |
| PSQIa | 9.2 (3.3) | 9.6 (3.7) | 0.32 |
| PSQI-1a | 1.4 (0.6) | 1.5 (0.7) | 0.28 |
| DBASa | 47.9 (16.9) | 49.1 (16.4) | 0.50 |
| FOSQa | 17.4 (1.9) | 17.4 (1.9) | 0.88 |
| FFSa | 11.5 (6.4) | 11.2 (6.1) | 0.66 |
| ESSa | 6.9 (4.1) | 7.3 (4.1) | 0.38 |
| Pain Severity | 4.5 (1.6) | 4.3 (1.5) | 0.16 |
| Arthritis Symptoms | 5.9 (2.2) | 6.1 (2.2) | 0.42 |
| Catastrophizing | 11.9 (9.9) | 10.5 (8.9) | 0.15 |
| Fear avoidance | 37.0 (9.3) | 36.3 (8.9) | 0.53 |
| GDSa | 7.3 (5.5) | 6.7 (4.6) | 0.30 |
Thirteen participants had a missing ISI score at 2 month follow-up, thus their improvement status was missing. Their status was imputed in all analyses and varied between the 10 imputations.
P-values correspond to estimated treatment effect of Improvement status in linear or logistic regression models for the baseline values. Standard errors were adjusted to account for multiple imputations.
Derived from electronic medical record; all other variables assessed during the baseline data collection.
ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index; PSQI-1 = Component #1 (subjective sleep quality) of the PSQI; DBAS = Dysfunctional Beliefs About Sleep scale; FOSQ = Functional Outcomes of Sleep Questionnaire; FFS = Flinders Fatigue Scale; ESS = Epworth Sleepiness Scale
Sleep Efficiency based on actigraphic recording.
Table 2 reports the 2-month (post-treatment) unadjusted means and standard deviations for Improvers versus Non-Improvers for sleep, pain, fatigue and other outcomes. In addition to significant improvement on the ISI (p<.0001, used to define improver status), Improvers showed significant 2-month improvements compared to Non-Improvers on self-reported sleep quality (PSQI and PSQI-1, p<.0001), sleepiness (ESS, p=0.04), fatigue (FFS, p<.0001), and Arthritis Symptoms (p=0.0003). Improvers did not show 2-month improvements on Sleep Efficiency, sleep beliefs and attitudes (DBAS), function (FOSQ), Pain Severity, Catastrophizing, Fear Avoidance, or depression (GDS).
Table 2.
Post-treatment (2-month) means (standard deviations) of sleep, pain and related measures by Improver versus Non-Improver status (Improver: ≥ 30% reduction of ISI* score between baseline and 2 months). Imputed data used.
| Improvers | Non-Improvers | P** | |
|---|---|---|---|
| N* | 131 | 223 | - |
| ISI*** | 5.3 (3.5) | 11.3 (4.5) | <.0001 |
| Sleep Efficiencya | 82.8 (9.2) | 82.6 (8.6) | 0.79 |
| PSQI*** | 6.5 (2.9) | 8.7 (3.5) | <.0001 |
| PSQI-1*** | 0.9 (0.6) | 1.3 (0.6) | <.0001 |
| DBAS*** | 43.5 (16.4) | 46.7(16.2) | 0.08 |
| FOSQ*** | 18.1 (1.7) | 17.8 (1.7) | 0.11 |
| FFS*** | 7.0 (5.5) | 10.2 (6.1) | <.0001 |
| ESS*** | 6.2 (3.5) | 6.8 (4.1) | 0.04 |
| Pain Severity | 3.9 (1.6) | 4.1 (1.6) | 0.42 |
| Arthritis Symptoms | 7.3 (1.9) | 6.5 (2.2) | 0.0003 |
| Catatrophizing | 8.4 (9.1) | 8.7 (8.4) | 0.71 |
| Fear avoidance | 35.6 (9.0) | 35.6 (9.5) | 0.98 |
| GDS*** | 6.0 (4.7) | 6.1 (4.4) | 0.81 |
Thirteen participants had a missing ISI score at 2 month follow-up, thus their improvement status was missing. Their status was imputed in all analyses and varied between the 10 imputations.
P-values correspond to estimated treatment effect of Improvement status in linear or logistic regression models for the post-treatment values. Standard errors were adjusted to account for multiple imputations.
ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index; PSQI-1 = Component #1 (subjective sleep quality) of the PSQI; DBAS = Dysfunctional Beliefs About Sleep scale; FOSQ = Functional Outcomes of Sleep Questionnaire; FFS = Flinders Fatigue Scale; ESS = Epworth Sleepiness Scale; GDS = Geriatric Depression Scale
Sleep Efficiency based on actigraphic recording.
Results of analyses comparing Improvers and Non-Improvers at long-term (9 and 18 month) follow-up for sleep and fatigue outcomes are reported in Table 3. Improvers showed significant sustained improvement in Insomnia Severity at 9 and 18 months compared to Non-Improvers (Adjusted Mean Difference = −3.03 [95% Confidence Interval −3.74, −2.32]). Significant sustained improvements in the Improver versus Non-Improver groups were also observed in overall sleep quality (total PSQI, p<.001, −1.45 [−1.97, −0.93]; PSQI-1; p<.001, −.28 [−.39, −.16]), sleep beliefs and attitudes (DBAS, (p=.037, −2.44 [−4.74, −0.15]), and fatigue (FFS, p<.001, −1.99 [−3.01, −098]), but not in daytime function or sleepiness.
Table 3.
Adjusted group means and 95% confidence intervals in Improvers and Non Improvers for sleep and fatigue outcome measures for Improvers and Non-Improvers. Adjusted mean differences and corresponding p-values are for between group comparisons across 9 and 18 months. Imputed data used.
| Variable | Group | 9 Months** | 18 Months** | Adjusted mean difference** | P value |
|---|---|---|---|---|---|
| ISI* | Improvers | 7.17 [5.24, 9.09] | 6.72 [4.72, 8.72] | −3.03 [−3.74, −2.32] | <0.001 |
| Non-Improvers | 10.2 [8.45, 11.94] | 9.75 [7.92, 11.58] | |||
| SE* | Improvers | 82.94 [77.95, 87.93] | 83.17 [77.82, 88.51] | 1.29 [−0.18, 2.76] | =0.084 |
| Non-Improvers | 81.65 [77.12, 86.19] | 81.88 [76.96, 86.8] | |||
| PSQI* | Improvers | 6.76 [5.33, 8.20] | 6.59 [5.13, 8.06] | −1.45 [−1.97, −0.93] | <0.001 |
| Non-Improvers | 8.21 [6.95, 9.48] | 8.04 [6.72, 9.36] | |||
| PSQI-1* | Improvers | 0.98 [0.67, 1.28] | 0.95 [0.63, 1.27] | −.28 [−.39, −.16] | <0.001 |
| Non-Improvers | 1.25 [0.97, 1.53] | 1.23 [0.93, 1.52] | |||
| DBAS* | Improvers | 41.88 [33.69, 50.07] | 41.88 [33.69, 50.07] | −2.44 [−4.74, −0.15] | 0.037 |
| Non-Improvers | 44.32 [36.76, 51.89] | 44.32 [36.76, 51.89] | |||
| FOSQ* | Improvers | 18.06 [17.35, 18.77] | 18.00 [17.26, 18.74] | 0.20 [−0.03, 0.43] | 0.085 |
| Non-Improvers | 17.86 [17.22, 18.5] | 17.8 [17.12, 18.47] | |||
| FSS* | Improvers | 6.89 [3.87, 9.92] | 7.04 [3.82, 10.26] | −1.99 [−3.01, −0.98] | <0.001 |
| Non-Improvers | 8.89 [6.08, 11.69] | 9.04 [5.99, 12.08] | |||
| ESS* | Improvers | 6.46 [4.68, 8.23] | 6.19 [4.31, 8.08] | −0.35 [−1.00, 0.29] | 0.276 |
| Non-Improvers | 6.81 [5.28, 8.34] | 6.55 [4.89, 8.21] |
ISI = Insomnia Severity Index; GDS = Geriatric Depression Scale; 3MS = Modified Mini-Mental State Examination; SE = Actigraphically measured sleep efficiency; PSQI = Pittsburgh Sleep Quality Index; PSQI-1 = Component #1 (subjective sleep quality) of the PSQI; DBAS = Dysfunctional Beliefs About Sleep scale; FOSQ = Functional Outcomes of Sleep Questionnaire; FFS = Flinders Fatigue Scale; ESS = Epworth Sleepiness Scale
Means and mean differences adjusted for intervention arm, baseline values of relevant outcome, age, ISI score, GDS score, modified Mini-Mental State Examination score, analgesic use, hypnotic use, antidepressant use, an indicator of whether the outcome was measured at 9 months, and the clinic at which the intervention was delivered.
Results of analyses comparing Improvers and Non-Improvers at long-term (9 and 18 month) follow-up for pain and depression outcomes are reported in Table 4. Improvers showed significant sustained improvement compared to Non-Improvers in Pain Severity (p<.001, −0.51 [−0.80, −0.21]), Arthritis Symptoms (p<.001, 0.63 [0.26, 1.00]), and Fear Avoidance (p<.01, −2.27 [−3.95, −0.58]) but not on Catastrophizing or GDS.
Table 4.
Adjusted group means and 95% confidence intervals for pain and depression outcome measures for Improvers and Non-Improvers. Adjusted mean differences and corresponding p-values are for between group comparisons across 9 and 18 months. Imputed data used.
| Variable | Group | 9 Months** | 18 Months** | Adjusted mean difference** | P value |
|---|---|---|---|---|---|
| Pain Severity | Improvers | 3.55 [2.73, 4.36] | 3.88 [3.12, 4.64] | −0.51 [−0.80, −0.21] | <0.001 |
| Non-Improvers | 4.05 [3.3, 4.8] | 3.38 [2.54, 4.21] | |||
| Arthritis Symptoms | Improvers | 7.5 [6.57, 8.44] | 7.46 [6.4, 8.52] | 0.63 [0.26, 1.00] | <0.001 |
| Non-Improvers | 6.87 [6.04, 7.7] | 6.83 [5.89, 7.77] | |||
| Catastro-phizing | Improvers | 7.41 [3.74, 11.09] | 7.07 [3.21, 10.94] | 1.33 [−2.94, 0.29] | 0.108 |
| Non-Improvers | 8.74 [5.85, 11.63] | 8.4 [5.34, 11.46] | |||
| Fear Avoidance | Improvers | 33.08 [28.95, 37.22] | 32.92 [28.61, 37.24] | −2.27 [−3.95, −0.58] | 0.009 |
| Non-Improvers | 35.35 [32.04, 38.65] | 35.19 [31.7, 38.68] | |||
| GDS* | Improvers | 5.60 [3.32, 7.88] | 6.09 [3.7, 8.48] | −0.52 [−1.36, 0.32] | 0.223 |
| Non-Improvers | 6.12 [4.18, 8.06] | 6.61 [4.51, 8.70] |
ISI = Insomnia Severity Index; GDS = Geriatric Depression Scale; 3MS = Modified Mini-Mental State Examination
Means and mean differences adjusted for intervention arm, baseline values of relevant outcome, age, ISI score, GDS score, modified Mini-Mental State Examination score, analgesic use, hypnotic use, antidepressant use, an indicator of whether the outcome was measured at 9 months, and the clinic at which the intervention was delivered.
Figure 2 provides an overview of the pattern of study findings reporting raw (unadjusted) mean values for the ISI, PSQI, Pain Severity, Arthritis Symptoms, FFS and GDS for the Improver and Non-Improver groups at baseline, 2-month (post-treatment), and 9 and 18-month (follow-up) assessments. The figure shows that the criterion improvement in ISI (the criterion variable for group assignment) seen at 2 months in the Improver versus Non-Improver groups was sustained across 9 and 18 months in the Improvers. It also shows both short-term (2-month) and sustained sleep improvement (9 and 18 months) in the PSQI and fatigue (FFS) in the Improvers versus Non-Improvers. Improvements in Arthritis Symptoms over time paralleled sleep quality and fatigue changes. Pain Severity declined in the Improvers versus the Non-Improvers; however, it is noteworthy that this improvement lagged improvements in sleep. Finally, depression (GDS) remained unchanged in both groups across all time points.
Figure 2.
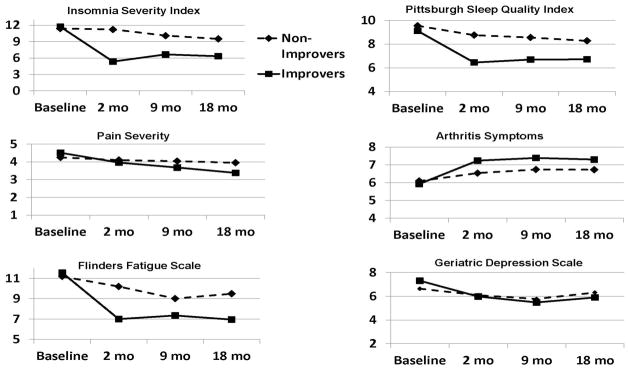
Raw (unadjusted) data for the Improver and Non-Improver groups at 0 (baseline), 2 (post-treatment), 9 and 18 (follow-up) month assessments for the Insomnia Severity Index, Pittsburgh Sleep Quality Index, Pain Severity, Arthritis Symptoms, Flinders Fatigue Scale, and Geriatric Depression Scale.
Finally, an analysis for pain improvers (a baseline to post treatment reduction of 30% or more in Pain Severity, N=65) analogous to that for Insomnia Severity was conducted in order to determine the ability of short-term improvements in Pain Severity to predict long-term improvements in the various OA symptom measures. Short-term pain improvement predicted long-term improvements in Pain Severity (p<.001, −0.86 [−1.27, −0.45]) and Arthritis Symptoms (p = 0.05, 0.55 [0.00, 1.10]), but did not predict long-term improvement in Insomnia Severity nor fatigue and only marginally predicted long-term improvements in sleep (PSQI) (p = 0.046, −0.62 [−1.23, −0.01]) and depression (GDS) (p = 0.04, −0.81 [−1.58, −0.04]).
5. DISCUSSION
Regardless of the experimental treatment received in the Lifestyles trial, participants with clinically significant improvement in insomnia symptom severity from baseline to 2 months showed sustained beneficial sleep outcomes on multiple measures of self-reported sleep quality over the 18-month follow-up period, while Non-Improvers showed substantially less favorable long-term sleep outcomes. Crucially, these initial and sustained improvements in sleep quality were also associated with clinically significant long-term improvements in measures of Pain Severity, Arthritis Symptoms and fatigue (FFS). The fact that these pain outcomes which had only marginally improved (Arthritis Symptoms) or not improved at all (Pain Severity) at 2 months subsequently showed sustained improvements at 9 and 18 months suggests that benefits of improved sleep for pain outcomes may have accumulated over time in the context of sustained, clinically significant improvements in sleep.
It is also notable that improved short-term sleep did not have a statistically significant association with improvements in depressive symptoms or pain catastrophizing, although modest improvements in pain fear-avoidance and dysfunctional beliefs about sleep were observed. This suggests that any benefits for pain outcomes associated with improved sleep were not likely to have been achieved primarily through general improvements in psychological well-being that might influence pain perceptions or reporting. However, it is possible that failure to observe improvements in depression and catastrophizing may have been due to the sub-clinical level of impairment on these measures at baseline for most study participants.
The observed longitudinal reductions in daytime fatigue observed in the Improver group are also noteworthy because of the importance of daytime activation for successful management of chronic pain. Although we did not observe longitudinal improvements in daytime function, this may reflect the FOSQ being designed for use with sleep apnea patients. Also, generally low baseline scores may have made this measure less sensitive to clinically significant change in sleep in this general primary care population.
These findings demonstrate that OA patients with co-morbid pain and insomnia whose sleep quality improves in the short-term (2 months) are likely to show sustained (up to 18 months) improvements in sleep on multiple measures of sleep quality. Our findings further demonstrate that sustained improvements of sleep of sufficient magnitude may influence cumulative benefits for arthritis pain outcomes and for fatigue, relative to persons whose sleep does not initially improve.
While short-term improvements in insomnia severity predicted long-term reductions in pain severity, arthritis symptoms, fear avoidance, insomnia severity, sleep quality, sleep beliefs/attitudes, and fatigue; a parallel analysis found that short-term improvements in pain severity predicted long-term reductions in pain severity, arthritis symptoms, and depression, but modest and inconsistent improvements in sleep outcomes, possibly because of the smaller number of short-term Pain Improvers (N=65) compared to short-term Sleep Improvers (N = 131) in the study sample.
Differences between these results and those of the Lifestyles randomized trial have several potential explanations. The randomized trial did not find robust benefits for pain outcomes subsequent to an intervention (CBT-PI) that did yield improved sleep outcomes. The ability of the randomized trial to detect cumulative benefits of improved sleep for pain outcomes may have been reduced by reductions in sleep and pain severity (regression to the mean) between the screening and baseline assessments, and by the larger than anticipated intraclass correlation within treatment groups. We estimate that the larger than expected intraclass correlations for both pain and insomnia severity (ICC=0.10) reduced the effective sample size of the LIFESTYLES trial by 62 percent [16,39]. The observable benefits of the CBT-PI intervention for sleep and pain outcomes, relative to CBT-P and the education-only control group, may also have been attenuated somewhat by non-specific benefits of participation in the well-received CBT-P and EOC group interventions. Further, dividing the focus of treatment between insomnia and pain in the CBT-PI intervention may have diluted insomnia treatment efficacy and potentially pain treatment efficacy of that integrated intervention arm; particularly in the context of a lack of meaningful CBT-P treatment effects. This lack of compelling CBT-P treatment effects supports the notion that future research on this question might best advance the field by concentrating on the impact of insomnia-focused treatment alone on pain, without the additional effort, expense and subject burden of complementary pain-focused treatment. In any case, current results suggest that detection of benefits of improved sleep for chronic pain outcomes may depend on robust and sustained improvements in sleep status as these benefits may accumulate over time, at least among moderately impaired community-based osteoarthritis patients.
There is a growing body of evidence supporting the notion that improvements in sleep can improve pain [32,38]. Smith and colleagues [32] recently noted that disturbed sleep has become increasingly recognized as a direct contributor to both hyperalgesia and impaired endogenous pain modulation. They have described putative pathways by which sleep disturbance interacts directly with central pain and inflammatory processes, and indirectly with mood and physical functioning to exacerbate clinical OA pain [32]. If so, improved sleep, particularly when sustained long term, would be expected to result in sustained improvements in pain, such as were demonstrated here.
The current study has weaknesses. It is a secondary analysis of data gathered in a randomized controlled trial and so is effectively descriptive and can only describe associations and not ascribe causal relationships among the variables reported. It is possible that there is unmeasured confounding in this study independent of treatment that led to both improved short and long term sleep outcomes and to improved long-term pain and fatigue outcomes. While baseline use of analgesics and hypnotics and depression (GDS scores) were co-variates in the analyses of long-term outcomes, changes in subjects’ medication scheduling or dosing over time including use of anti-depressant medications were not analyzed.
Nevertheless, the study also has considerable strengths. It employed a large, well characterized study sample that is representative of a primary care population of older adults with OA pain and insomnia with multiple long-term follow-up assessments [22,23,39,42]. The trial had excellent subject retention (89% at 18 months) which required minimal lost data to be imputed. Finally, the analytic design involved assigning participants’ improver status based on ISI change from baseline to 2-month assessment, while sustained changes in sleep, pain and related outcomes of Improvers and Non-Improvers were analyzed using 9 and 18 month data with baseline data as covariates, providing a time-order to the observed sleep/pain relationships consistent with a sleep to pain causality.
In conclusion, this secondary analysis of the Lifestyles trial findings demonstrated that OA patients with co-morbid pain and insomnia whose sleep improved in the short-term (2 months), were likely to show continued long-term (up to 18 months) improvement in sleep. These sustained and robust improvements in sleep were accompanied by significant and sustained benefits in chronic pain and fatigue outcomes relative to OA patients whose sleep did not improve initially. It is noteworthy that benefits for pain and fatigue outcomes did not occur in the context of general benefits for psychological well-being, since depression and pain catastrophizing outcomes did not show differences by insomnia improver status. These findings may be particularly salient given that commonly prescribed drug therapies for controlling chronic pain (e.g. NSAIDs and opioids) have clinically significant and potentially life-threatening adverse effects, particularly among older adult, and often less than desired analgesic efficacy.
These findings support the hypothesis that successful treatment of sleep disturbance in pain populations with co-morbid insomnia may yield benefits for reduced pain over the long-term, contingent on achieving robust and sustained improvements in sleep between intervention and control patients.
Summary.
Sleep, pain and fatigue were longitudinally examined in 367 osteoarthritic older adults. Two-month sleep improvement predicted eighteen-month in sleep, pain, and fatigue improvements.
Acknowledgments
Funding: This study was supported by NIH grant R01-AG031126 (MVV, SMMc and MVK - Multiple Principal Investigators).
The authors wish to thank Dr. Jack Edinger for suggesting concept of the improver analyses reported in this paper. The authors also wish to thank; Martha Cagley, Amy Cunningham, Fredda Jaffe, Shirley Meyer, Katie Saunders, Janyce Vick, Kendra Wight, and Patty Yarbro for their invaluable assistance in conducting this study.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT01142349
Disclosure Statement: Dr. Von Korff receives funding from grants awarded to Group Health Research Institute by Johnson and Johnson and by Pfizer. Dr. Shortreed has received funding from grants awarded to Group Health Research Institute by Bristol Meyers Squibb. Drs. Von Korff’s and Shortreed’s grant support does not represent a financial conflict of interest with the work reported here. The other authors have indicated no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baskerville NB, Hogg W, Lemelin J. The effect of cluster randomization on sample size in prevention research. J Fam Pract. 2001;50:W241–246. [PubMed] [Google Scholar]
- 2.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Reyholds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 4.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: A short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crombez G, Vlaeyen JWS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 6.Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68:407–416. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA, Rauschkolb C, Sampaio C. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Wohlgemuth WK. Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire. Sleep Med. 2001;2:493–500. doi: 10.1016/s1389-9457(01)00078-8. [DOI] [PubMed] [Google Scholar]
- 9.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM, Stepanski EJ. Derivation of Research Diagnostic Criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 10.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: A randomized clinical trial. Arch Intern Med. 2005;165:2527–2535. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 11.Espie CA, Inglis SJ, Harvey L, Tessier S. Insomniacs’ attributions: Psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. J Psychosom Res. 2000;48:141–148. doi: 10.1016/s0022-3999(99)00090-2. [DOI] [PubMed] [Google Scholar]
- 12.Feng Z, McLerran D, Grizzle J. A comparison of statistical methods for clustered data analysis with Gaussian error. Stat Med. 1996;15:1793–1806. doi: 10.1002/(SICI)1097-0258(19960830)15:16<1793::AID-SIM332>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Gradisar M, Lack LC, Richards H, Harris JH, Gallasch J, Boundy M, Johnston A. The Flinders Fatigue Scale: Preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3:722–728. [PMC free article] [PubMed] [Google Scholar]
- 14.Guillemin F, Coste J, Pouchot J, Ghézail M, Bregeon C, Sany J. The AIMS2-SF: A short form of the Arthritis Impact Measurement Scales 2. French Quality of Life in Rheumatology Group. Arthritis Rheum. 1997;40:1267–1274. doi: 10.1002/1529-0131(199707)40:7<1267::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145:136–141. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 18.Jungquist CR, O’Brien C, Matteson-Rusby S, Smith MT, Pigeon W, Xia Y, Lu N, Perlis ML. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11:302–309. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: John Wiley & Sons; 2002. [Google Scholar]
- 21.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57:126–134. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 22.McCurry SM, Von Korff M, Vitiello MV, Saunders K, Balderson BH, Moore AL, Rybarczyk BD. Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: Predictors of enrollment in a randomized treatment trial. J Psychosom Res. 2011;71:296–299. doi: 10.1016/j.jpsychores.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurry SM, Shortreed SM, Von Korff M, Balderson BH, Baker LD, Rybarczyk BD, Vitiello MV. Who benefits from CBT for insomnia in primary care: Longitudinal results form the Lifestyles trial. Sleep. 2014;37(2):299–308. doi: 10.5665/sleep.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 25.Moore JE, Von Korff M, Cherkin D, Saunders K, Lorig K. A randomized trial of a cognitive-behavioral program for enhancing back pain self care in a primary care setting. Pain. 2000;88:145–153. doi: 10.1016/S0304-3959(00)00314-6. [DOI] [PubMed] [Google Scholar]
- 26.Morin CM. Insomnia: Psychological assessment and management. New York: NY: Guilford Press; 1993. [Google Scholar]
- 27.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: A review of recent methodological developments. Am J Public Health. 2004;94:423–432. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preisser JS, Reboussin BA, Song EY, Wolfson M. The importance and role of intracluster correlations in planning cluster trials. Epidemiology. 2007;18:552–560. doi: 10.1097/ede.0b013e3181200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren XS, Kazis L, Meenan RF. Short-form Arthritis Impact Measurement Scales 2: Tests of reliability and validity among patients with osteoarthritis. Arthritis Care Res. 1999;12:163–171. doi: 10.1002/1529-0131(199906)12:3<163::aid-art3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Royston P, White IR. Multiple imputation by chained equations (Mice): Implementation in Stata. J Stat Software. 2011;45:1–20. [Google Scholar]
- 31.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 32.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: A conceptual model. Curr Pain Headache Rep. 2009;13:447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan MJ, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 34.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: A multilevel daily process study. Sleep. 2012;35:675–687A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 36.Tiede W, Magerl W, Baumgärtner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049–1064. [Google Scholar]
- 38.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–362. [PMC free article] [PubMed] [Google Scholar]
- 39.Vitiello MV, McCurry SM, Shortreed SM, Balderson BH, Baker LD, Keefe FJ, Rybarczyk BD, Von Korff M. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: The Lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61:947–956. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 41.Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M, Vitiello MV, McCurry SM, Balderson BH, Moore AL, Baker LD, Shortreed S, Yarbro P, Saunders K, Keefe FJ, Rybarczyk BD. Group interventions for co-morbid insomnia and osteoarthritis pain in primary care: The Lifestyles cluster randomized trial design. Contemp Clin Trials. 2012;33:759–768. doi: 10.1016/j.cct.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 44.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, Leirer VO. Development and validation of a Geriatric Depression Screening Scale: A preliminary report. J Psychiatric Res. 1982–83;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 45.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]