Abstract
Introduction
In rural America cigarette smoking is prevalent, few cessation services are available, and healthcare providers lack the time and resources to help smokers quit. This paper describes the design and participant characteristics of Connect2Quit (C2Q), a randomized control trial (RCT) that tests the effectiveness and cost-effectiveness of integrated telemedicine counseling delivered by 2-way webcams mounted on desktop computers in participant's physician office examining rooms (ITM) versus quitline counseling delivered by telephone in participant's homes (Phone) for helping rural smokers quit.
Methods/Design
C2Q was implemented in twenty primary care and safety net clinics. Integrated Telemedicine consisted of real-time video counseling, delivered to patients in their primary care physician's (PCP) office. Phone counseling, was delivered to patients in their homes. All participants received educational materials and guidance in selecting cessation medications.
Results
The 566 participants were predominantly Caucasian (92%); 9% were Latino. Most (65%) earned < 200% of federal poverty level. One out of three lacked home internet access, 40% were not comfortable using computers, and only 4% had been seen by a doctor via telemedicine in the past. Hypertension, chronic lung disease, and diabetes were highly prevalent. Participants smoked nearly a pack a day and were highly motivated to quit.
Discussion
C2Q is reaching a rural low-income population, with comorbid chronic diseases, that would benefit greatly from quitting smoking. ITM is a good delivery model, which integrates care by holding counseling sessions in the patient's PCP office and keeps the primary care team updated on patients’ progress.
Keywords: Telemedicine, Rural, Smoking Cessation, RCT, Primary Care
BACKGROUND
Cigarette smoking is the leading preventable cause of death in the United States.[1] Although the prevalence of smoking has declined dramatically (from 42% to 21%) over the past 40 years,[2] progress in rural America lags well behind national trends. In 2009, the prevalence of smoking in non-metropolitan areas was 26%—equivalent to the U.S. prevalence of smoking in 1990.[3]
Physicians play an important role in the smoking cessation process,[4] as they see 70% of all smokers each year.[5] However, physicians face many barriers to routinely counseling patients who smoke.[6-9] Only half of smokers, seeing their physicians are asked about their smoking,[10] fewer receive clear advice to quit, and only a small subset receive pharmacotherapy.[11]
Toll-free telephone-based tobacco quitlines are effective for smoking cessation and have the potential to reach virtually every U.S. citizen—including rural smokers. Unfortunately, only 1-2% of smokers use them.[12, 13] Telemedicine, as delivered by real-time, two-way video counseling, is another promising treatment delivery system. For multiple health behaviors and outcomes, a Cochrane review of telemedicine versus face-to-face patient care found that telemedicine was as effective as face-to-face treatment and achieved high levels of satisfaction among patients and providers.[14] The only large-scale study to date evaluating telemedicine for smoking cessation is a group-based intervention trial from Canada, which achieved equivalent quit rates between groups receiving in-person versus telemedicine-delivered interventions. This study, however had a number of limitations. Participants were not randomized into groups, quit rates were based on self report, and the intervention did not include cessation medications [15].
The primary aim of the present study, Connect2Quit, was to determine the effectiveness and cost-effectiveness of integrated telemedicine (ITM) compared to traditional telephone counseling (Phone) for smoking cessation. We sought to compare the standard of care for distance-based tobacco dependence treatment—telephone counseling—to a new model for care at a distance that integrates telemedicine counseling into the patient's PCP office.
Connect2Quit employs rigorous study design features including individual randomization, fidelity monitoring of intervention procedures, and biochemical verification of smoking status. We designed the intervention to be theoretically based, simple, translatable, and sustainable to enhance its potential for widespread adoption and ultimate impact on public health. In this paper, we describe the design, study protocol, and participant baseline characteristics. We examined the characteristics of participants to ascertain how generalizable findings will be to rural smokers.
METHODS/DESIGN
We designed Connect2Quit (C2Q) to optimize use of the two cornerstones of effective tobacco treatment; counseling and pharmacotherapy.[16] We also designed C2Q to be fully integrated into the patients’ PCP office: C2Q counselors delivered all ITM sessions in the PCP office; C2Q counselors scheduled sessions with clinic receptionists, updated the primary care team on patients’ progress, and worked with the rural providers to help patients select and obtain medication prescriptions. Because telemedicine counseling occurred in the PCP office, patients had the opportunity to immediately ask their health care providers for additional advice regarding pharmacotherapy and prescriptions for smoking cessation medication. We integrated telemedicine counseling into physician offices in order to enhance autonomy support from the health care team and to facilitate support for pharmacotherapy utilization. We designed the quitline condition to be as similar as possible to the majority of quitlines available in the U.S., while at the same time delivering the same content and frequency of counseling as our ITM condition. The counseling manual, number of sessions, length of sessions and study logistics were the same for both ITM and Phone conditions. This comparison permits clear-cut evaluation of a practice innovation—telemedicine-delivered counseling, integrated into medical practice (ITM)—versus telephone quitline counseling. We examined whether our ITM innovation can do a better job of delivering effective tobacco treatment (autonomy-supportive counseling and pharmacotherapy), be more effective, and be more cost-effective, than telephone quitlines. The design did not permit us to evaluate the relative impact of a) improving communication between remote counselors and primary care providers or b) enhancing the counseling experience of the video interface. However, our design allowed us to measure if participants felt greater support from their health care providers, which may translate into greater internal motivation to quit. Nevertheless, there is some risk that patients felt external pressure to quit from providers. Measuring both internal and external motivation allowed us to detect whether provider support translates to internal, “autonomous” motivation or external motivation to quit.
Design overview
Tables 1 and 2 provide overviews of study components and assessments. Connect2Quit employed an individual-randomized design. Patients assigned to Phone received 4 sessions of in-home telephone counseling and educational materials. Patients in ITM received 4 sessions of telemedicine counseling integrated into the patient's PCP office and educational materials. In addition, all participants received individually-tailored pharmacotherapy guidance to help them select and obtain cessation medications. The counseling approach and content was the same across both conditions. ITM counseling sessions occurred in the healthcare office, in examining rooms equipped with 2-way webcams mounted on desktop computers. Study assessments were conducted at baseline and months 3, 6, and 12. The University of Kansas Medical Center Ethics Committee approved all study procedures.
Table 1.
ITM and Phone Components
| Study Components | Session 1 | Session 2 | Session 3 | Session 4 |
|---|---|---|---|---|
| ITM | ||||
| Telemedicine counseling | x | x | x | x |
| Quit Tips | x | |||
| 5-Day Plan to Quit | Completed, updated when patient desires | |||
| Pharmacotherapy guidance | x | Updated when patient desires | ||
| Phone | ||||
| Telephone counseling | x | x | x | x |
| Quit Tips | x | |||
| 5-Day Plan to Quit | Completed, updated when patient desires | |||
| Pharmacotherapy guidance | x | Updated when patient desires | ||
Table 2.
Assessments and Reimbursement
| Baseline | Month 3 | Month 6 | Month 12 | |
|---|---|---|---|---|
| Measures | ||||
| Demographics, smoking history, medication access | x | |||
| Self-reported smoking endpoints* | x | x | x | x |
| Potential mediators** | x | x | x | x |
| Potential moderators*** | x | |||
| Salivary cotinine and proxy verification**** | x | |||
| Provider/Participant costs | x | x | x | |
| Satisfaction, feasibility among patients | x | |||
| Satisfaction, feasibility among practices | Collected at end of study | |||
| Reimbursement | ||||
| For patients | $20 | $20 | $20 | $50 |
Includes self-reported 7-day abstinence, 30-day abstinence, number of cigarettes per day, exhaled carbon monoxide
Includes measures of autonomy supportiveness, autonomous motivation, perceived competence, therapeutic alliance, medication use, frequency/quality of physician counseling, counseling adherence.
Includes measures of depression (PHQ-2), alcohol (AUDIT-C), anxiety (GAD-2), health comorbidities, use/access to computers.
Salivary cotinine will be collected at baseline and 12 mos, but not analyzed at baseline.
Site recruitment
The research team assembled an initial list of 29 candidate clinics in Kansas for recruitment into the study. These clinics had to be sited in a rural location as defined by the Health Resources and Services Administration (HRSA) guidelines; in Kansas this included 88 non-metropolitan counties and other regions.[17] Clinics had to have or be willing to obtain high-speed internet; sufficient patient volume (at least 75 patients/week) to support recruitment; an exam room or other private space available for blocks of time throughout the week that was equipped with a phone and high speed internet access. Researchers sent a letter, and then telephoned all 29 practices. Most (22) clinics elected to participate and had computer equipment installed on site. However, 2 withdrew afterwards due to failure to recruit any patients into the study or inability to obtain sufficient internet capability to transmit sessions, which resulted in a final study site total of 20 clinics. The clinics were located in a wide range of counties that touched three of the Kansas four boarders, fifty percent of the clinics were located in cities with a population of less than 1,800, and three were federally-qualified health clinics. Because C2Q staff faxed prescription requests to physicians, physicians had to agree to participate in order for their patients to be included in the trial. A total of 68 physicians opted to participate in the trial. Because our study employed individual random assignment, the internal validity of the trial was not affected. Although we did not have access to patient data among non-participating physician panels, the large number of physicians opting to participate, gave us confidence that our participants were representative.
Study equipment and site orientation
At each site, a telemedicine technician met with the staff member responsible for managing internet and computer resources, cataloged their current system configurations, and installed the study computer and telemedicine counseling equipment, which facilities will keep as partial reimbursement for participating in the trial. He tested the telemedicine counseling connection, trained site staff on its use, and placed connection checklists, troubleshooting tips, and phone numbers to call in telemedicine counseling examining rooms. The technician met with internet service managers at each site and become familiar with site firewall configurations to facilitate installation and troubleshooting during counseling sessions. This was necessary as a pilot study revealed that some sites would require the creation of a port to allow high-bandwidth signals to get through clinic and internet service provider firewalls. These high quality video signals are transmitted interactively between sites using the Polycom PVX personal video conferencing system, in order to provide high quality videoconferencing from the study desktop personal computers. Polycom PVX integrates a standard web camera with the study desktop computers and clinics existing high-speed internet service. Port issues exist for only a subset of facilities as many internet providers are designing firewalls to accommodate video in response to clinic demand.
Participants
Participants were recruited through 20 primary care practices and safety net clinics in Kansas. Practice-based recruitment included direct referral of smokers via participating physicians, recruitment via medical students who were in the clinics for rural clinical preceptorships, and chart review. The chart reviews were conducted by clinic or study staff depending on the preference of the clinic. Reviews sought to identify all smokers, 18 years or older, who had been seen in clinics in the past 3 years. Study or clinic staff mailed invitations to all clinic smokers, printed on clinic letterhead, that endorsed the study, described the procedures, and notified smokers that study staff would contact patients by phone to screen for interest and eligibility. The letter included instructions for how patients could opt out from contact with study staff by calling a toll-free number. Research staff telephoned patients who had not opted out one week after the mailing to screen, verify eligibility, collect consent, and baseline data. The Project Manager randomized patients to either arm by opening concealed envelopes which contained the randomization. Counselors called participants one week after enrollment and informed participants of their randomization.
In addition, community-based recruitment efforts were conducted primarily in western Kansas to recruit Latino smokers. Latino recruitment included on-site recruitment by study staff, word of mouth, participant referrals, radio advertising, health fairs, newsletters, press releases, and recruitment tables at Latino worksites, religious organizations, and businesses.
Eligible smokers were required to have a primary care physician who was participating in the study, be 18 years of age or older, smoke 5 or more cigarettes per day for at least one year, smoke 25 out of the past 30 days, speak English or Spanish, and have a telephone. Individuals were excluded if they used other tobacco products, were currently taking quit smoking medications or participating in another quit smoking program, were breast feeding, pregnant or planning to become pregnant, planning on moving in the next year, did not have a health care provider they regularly saw at the clinic, or lived with a smoker already enrolled in the study.
Intervention and control components
Table 1 depicts the study components. Within the first week after enrollment, all study participants received a study welcome packet via mail. The welcome packets included printed educational materials on smoking cessation, a time line of the intervention, and a pharmacotherapy guidance form. Approximately one week after the packet was mailed, counselors called participants to notify them of their group assignment and to schedule their first counseling session.
Counseling format
Patients in ITM received 4 sessions of clinic-based video telemedicine counseling for smoking cessation. The counseling approach was based on Combined Behavioral Intervention (CBI), a combination of Motivational Interviewing and Cognitive Behavior Therapy.[18-20] Because most computers were located in rooms set aside for telemedicine smoking cessation during designated hours, participants could sign in at the reception and be taken directly to the room for their session. Clinic staff, either a receptionist or a nurse, called the counselor at the medical center to initiate the session. At the end of each session, counselors directed participants to go to the clinic receptionist to schedule the next session. Counselors then telephoned the receptionist while the participant was at the front desk to coordinate scheduling the next appointment. The telephone condition (Phone) was designed to mimic tobacco quitline counseling and consisted of 4 sessions delivered via telephone. At the end of each session, counselors scheduled the next counseling session with the patient.
Pharmacotherapy guidance
At baseline, study staff collected information on insurance coverage, income, prescription medication use, and pre-existing health conditions that are contraindications or cautions for cessation medications. Counselors used this information to complete a “pharmacotherapy guidance form” to help all patients identify what medications were safe for them, understand what resources they had to pay for medications, and facilitate a planning process for the patient to select and obtain medications. This form was completed based on information collected from study participants at baseline. The form was provided to participants in a personalized, pre-filled-out manner in their mailed ‘welcome packet.’ Income-eligible patients with no insurance coverage are eligible for pharmacy assistance programs (PAPs) offered through pharmaceutical drug companies. Pharmacotherapy guidance materials and quit plans were transmitted via fax to the clinic office in the ITM condition and via mail to the patients’ homes in the Phone condition.
Counseling content
The goals of session 1 (week 0) were to elicit, acknowledge, and reinforce the client's motivation to change, learn lessons from past quit attempts, review the pharmacotherapy guidance form, explore patients’ thoughts and feelings about pharmacotherapy, and invite the patient to develop their personal quit plan. Patient's not ready to quit were encouraged to set goals such as cutting down, establishing home smoking restrictions, and were encouraged to explore their ambivalence for quitting smoking. For all patients, after each session counselors either mailed (Phone) or faxed (ITM) two copies of the most recent version of the patient's quit plan which for some patient's included information to the PCP that the patient was not ready to quit and two copies of the pharmacotherapy guidance form. Counselors encouraged patients to provide materials to medical staff for inclusion in the medical record and to consult with their physician on questions and scripts for prescription medications.
In subsequent sessions (sessions 2-4 at weeks 1, 4, and 8), counselors invited patients to choose the topic of each session. Participants had 12 topics to choose from. Among participants who had quit, sessions addressed thoughts and behaviors that created challenges to remaining abstinent. Among patients who had slipped or relapsed, sessions focused on rebuilding motivation and confidence, learning from slips, and making new quit plans.
Fidelity assessment
To ensure fidelity to the counseling protocol all counselors participated in weekly supervision led by PhD-level experts in smoking cessation. During supervisions session digital audio files were reviewed and rated using an adapted version of the fidelity monitoring forms we have used successfully in prior studies.[21-23] To assess whether counseling was the same across ITM and Phone sessions we obtained independent ratings of counselor adherence on a randomly selected subset of sessions. These audio files were encrypted, blinded regarding group assignment, and emailed to an independent expert rater for coding on a monthly basis. At the end of the trial, these scores will be collapsed into a summative assessment of counseling fidelity for each study arm.
Assessment schedule and reimbursement (Table 2)
Clinics that participated in the study received a $1,000 reimbursement for incidental costs associated with the trial; $500 at enrollment and $500 at the end of the study. In addition, intervention sites received a computer and individually licensed Polycom PVX software for implementing the intervention. Clinics dedicated the equipment to the telemedicine trial for the duration of the study but kept the equipment at the end of the trial. Participants received twenty dollar shopping vouchers for completing study assessments at 0, 3, and 6 months. At twelve months participants received a fifty dollar voucher for completing the final study assessment. In addition, participants who self reported that they were quit at 12 months and provided a salivary sample received an additional fifty dollar shopping voucher. Participants are not informed of the $50 additional incentive for mailing in their saliva sample until after they self-reported their abstinence and completed the entire 12 month questionnaire in order to reduce participant incentive to misreport smoking status.
Measures
Baseline Measures
General demographic variables such as age, gender, marital status, education, employment status, income, race and ethnicity were collected (Table 3) . Smoking history included number of cigarettes per day, quitting history, previous quit smoking medication use, and age of initiation. Nicotine dependence was assessed with the six-item Fagerström Test for Nicotine Dependence scale (FTND).[24] Readiness to quit was measured using the Stages of Change 4-item questionnaire.[25] Motivation and confidence to quit smoking were measured using 10 point Likert scales with higher scores indicating greater motivation/confidence.
Table 3.
Baseline Characteristics
| Sociodemographics | Total= 566 |
|---|---|
| Age3, mean (SD), yrs | 47.5 (12.9) |
| Female1, n (%) | 368 (65.0) |
| Race3, n (%) | |
| Caucasian | 518 (91.7) |
| Black/African American | 22 (3.9) |
| American Indian/Native American/Alaskan Native | 49 (8.7) |
| Asian | 2 (0.4) |
| Native Hawaiin/Other Pacific Islander | 3 (0.5) |
| Other | 15 (2.7) |
| Refused | 1 (0.2) |
| Hispanic/Latino2, n (%) | 50 (8.9) |
| Marital Status3, n (%) | |
| Married | 238 (42.1) |
| Divorced | 134 (23.7) |
| Widowed | 29 (5.1) |
| Separated | 30 (5.3) |
| Never Married | 61 (10.8) |
| Partner | 73 (12.9) |
| Education3, n (%) | |
| Less than HS | 110 (19.5) |
| HS | 211 (37.4) |
| Some College | 204 (36.1) |
| ≥ College | 40 (7.1) |
| Employment Status3, n (%) | |
| Full Time | 235 (41.6) |
| Part Time | 63 (11.2) |
| < 200% Federal Poverty Level | 361 (64.8) |
| Health Insurance1, n (%) | 357 (63.1) |
| Prescription Cessation Medication Coverage3, 4, n (%) | 325 (57.5) |
| Medical History 3 | |
| Hypertension, n (%) | 246 (43.5) |
| High cholesterol, n (%) | 224 (39.7) |
| Chronic lung disease, n (%) | 192 (34.0) |
| Diabetes, n (%) | 104 (18.4) |
| Heart disease, n (%) | 59 (10.4) |
| Cancer, n (%) | 48 (8.5) |
| Stroke, n (%) | 24 (4.3) |
| Body Mass Index3, mean (SD), kg/m2 | |
| < 25, n (%) | 178 (32.0) |
| 25-29, n (%) | 164 (29.5) |
| ≥ 30, n (%) | 214 (38.5) |
| Mental Health Comorbidities | |
| PHQ-2, Depression3, 5, n (%) | 282 (49.9) |
| AUDIT-C6, high risk drinking n (%) | 129 (23.0) |
| GAD-27, n (%) | 226 (40.5) |
N=566
N=563
N=565
Prescription Cessation Medication Coverage, all FDA approved first line medications.
PHQ-2, with a depression cut point of > 3.
AUDIT-C, with a binge drinking cutoff of >4 Males, >3 Females.
GAD-2, with an anxiety cutoff of ≥ 3.
Study endpoints—cessation and verification
The primary outcome measure was 7-day point prevalence smoking abstinence at 12 months after enrollment (i.e. ‘having smoked no cigarettes, not even a puff in the past 7 days’).[26] Month 12 abstinence was verified via mailed salivary cotinine analysis, with a cut point of 15 ng/ml.[27] We also conducted validation via proxy report from a significant other among quitters who did not return a salivary sample.[28] Secondary outcomes at 3, 6, and 12 months included self reported point prevalence abstinence; frequency and duration of quit attempts; average number of cigarettes smoked per day; and prolonged abstinence. Prolonged abstinence as defined in this study included a “grace period” of 1-month at the beginning of treatment to allow the treatment to take effect (i.e. ‘one month after the first counseling session, having smoked no cigarettes in the past 2 consecutive weeks, including weekends’).[26]
Mediators
Potential mediators were assessed at 3, 6 and 12 months. Participants responded to the 6-item Health Care Climate Questionnaire (HCCQ)[29] to assess their perceptions of the degree to which their health care providers are autonomy-supportive in discussing smoking cessation. Participants’ autonomous motivation was assessed with the 12-item Treatment Self-Regulation Questionnaire (TSRQ).[29] Patients’ perceptions of competence for smoking cessation was measured using the 4-item Perceived Competence Scale for Cessation (PCSC).[30] In addition data were collected on adherence to counseling sessions. Adherence to pharmacotherapy was collected via self-report, which included the type, frequency, and duration of medications used.
Moderators
The two-item Patient Health Questionnaire (PHQ-2)[31] was used to measure depression with scores of 3 or higher indicating clinical depression. The two-item General Anxiety Disorder questionnaire (GAD-2) assessed anxiety with scores of 3 or higher indicating anxiety.[32] In addition, the study screening form included 3 alcohol consumption questions from the Alcohol Use Disorders Identification Test (AUDIT-C) as a brief screening test for heavy drinking and/or active alcohol abuse or dependence with a cutoff score of greater than four for males and greater than three for females.[33] Patients reported how many times they had seen their regular doctor in the past 12 months, and whether any health care provider ever told them that they had diabetes, hypertension, hyperlipidemia, stroke, chronic lung disease, heart disease, cancer, or depression.
Computer use and availability
Participants were also asked four questions related to their perceptions of using computer technology such as telemedicine for the delivery of health care. In addition, computer and internet availability within the home were assessed.
Cost Effectiveness
The purpose of our cost effectiveness analysis was to examine the differential impact and the relative cost per quit of ITM versus Phone. Program variable costs included counselor time, phone charges, and written materials. Fixed costs included personnel costs incurred in administration or oversight of the program (program director and support staff), facility costs (rent, maintenance, utilities, internet access charges), office supplies, and equipment (computers, web cams, printers, telephones).
Analyses for Connect2Quit Baseline Characteristics (Present Study)
In the present study, we report demographic characteristics, computer/internet access, attitudes related to telehealthcare, and other moderator variables that were collected during the baseline assessment. We also summarize participants’ smoking history and patterns of smoking and dependence. Categorical variables are summarized by frequencies and percentages, and quantitative variables are summarized by means and standard deviations.
Statistical and Power Analyses for Connect2Quit Outcomes (Future Outcome Studies)
Below, we briefly outline our sample size analyses and other analyses that we will conduct when the study is complete.
Sample size and power
The primary endpoint is cotinine verified 7-day point prevalence abstinence at 12 months. We expect an 8% cessation rate in the Phone arm. Studies employing in-home telephone counseling among persons who were recruited from primary care practices have reported unverified quit rates ranging from 11-12%. [REF from Grant]. With a primary endpoint of verified abstinence we anticipate a slightly lower (8%) abstinent rate in the Phone arm compared to these unverified studies. A 16% cessation rate is expected in the ITM arm based upon a meta-analysis of 45 studies found that 4-8 person-to-person treatment sessions yields a long-term abstinence rate of 18%-24%. Telemedicine has been shown repeatedly to perform as well as face-to-face office visits across a number of health outcomes, we anticipate lower quit rate as ITM will be conducted at a distance without direct counselor and clinical staff interactions post session. We implement a process recommended by Muthén and Muthén[34] to examine sufficient sample size for our structural equation modeling (SEM) analyses. Using Mplus 5 software,[35] this method took into account the expected nonindependence of observations due to cluster sampling, using Monte Carlo simulations to examine model fit and prediction of cessation by major variables of interest. Our sample size of 283 per group is more than adequate for the SEM analyses.
Hypothesis Testing
The primary analysis for Hypothesis 1 will be a comparison of ITM versus Phone 12-month, 7-day point prevalence verified abstinence rates using a chi-square test. Using the intent to treat principle, subjects lost to follow-up and those who claim to be abstinent but who fail to submit a salivary cotinine sample, will be coded as smokers. A multilevel multivariable logistic regression with being smoking status as the dependent variable will be used to adjust for covariates that might affect cessation, such as age, gender, income, and education level. The logistic regression model will include a main effect term for group.
A main focus of our analyses will be to assess how our intervention had an impact on outcome. To do so, we will use structural equation modeling (SEM), with robust maximum likelihood estimation and standard errors adjusted for clustering by clinic site, to determine how well ITM operationalizes key features of the intervention and which components of the intervention are important for smoking cessation.
RESULTS
Recruitment
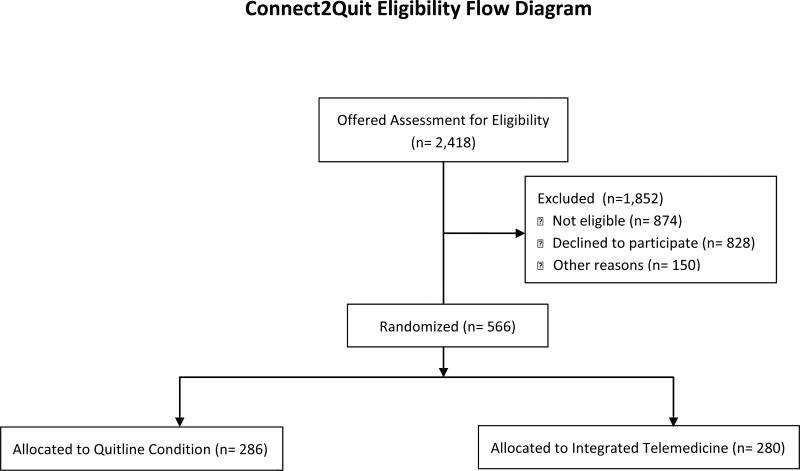
Of 2,418 individuals who were offered assessment for eligibility, 828 declined to be screened, 874 did not meet eligibility criteria, 716 were eligible and 566 were respectively randomly assigned to either ITM or Phone (see Figure 1). Reasons for ineligibility (not shown) included no longer being a smoker (481/874, 55%), did not have a regular health care provider at the clinic (85/874, 9.7%), and smoked fewer than 5 cigarettes per day (72, 8.2%), moved or is planning to move (44, 5%), pregnant or breastfeeding (44, 5%), other (used other forms of tobacco, had another household member in the trial, etc.) (104, 12%). Participants enrolled per clinic varied widely from 2 participants in one clinic to 150 participants in another with an average enrollment of 28.3 participants per clinic.
Figure 1.
Study Enrollment Flow Diagram
Participant demographics
Mean age of study participants was 47.5, and 65% of participants were female. Nearly 9% were Hispanic/Latino. Nearly 1 in 5 had less than a high school education. Well over half of our sample were living at less than 200% of the Federal Poverty Level, 63.1% had health insurance; of these, only 57.1% had insurance that covered any form of cessation medication. Most of the sample had some form of medical comorbidity, including overweight and/or obesity. Many of our participants experienced some form of mental health comorbidity, with nearly half experiencing depressive symptomology.
Telemedicine variables
Although most (nearly 70%) had a working computer at home, one out of three lacked home internet access, 40% were not comfortable using computers, and only 4% had been seen by a doctor via telemedicine in the past (Table 4). Many were not confident that personal information was kept private via technology, were not comfortable using newer communication technologies, and were not interested in receiving telecare in the home.
Table 4.
Computers, Internet, and Communication Technology
| Computer, internet, and telemedicine use | |
| Currently have a functional computer in home1, n (%) | 395 (69.9) |
| Currently have internet access in home1, n (%) | 379 (67.1) |
| Ever been seen by a doctor via telemedicine, ITV or webcam1, n (%) | 25 (4.4) |
| Attitudes toward computers, communication technology, and health technology | |
| I am comfortable using computers1, n (% agree-strongly agree) | 339 (60) |
| I am comfortable using other communication technologies, such as mobile phones, mp3 players, or web cameras1, n (% agree-strongly agree) | 355 (62.8) |
| I am interested in receiving health care in my home using computers or communication technologies1, n (% agree-strongly agree) | 326 (57.7) |
| I am confident my personal information is kept private when using communication technologies1, n (% agree-strongly agree) | 381 (67.4) |
Tobacco use
Table 5 displays tobacco use history and interest in quitting. Participants smoked on average a pack of cigarettes per day, and at a group mean of 4.9 on the FTND, had moderate nicotine dependence. They smoked on average 17 years, and most had tried some form of quit smoking medication in the past to quit. They were highly motivated to quit, with most in “preparation” to quit and on average felt it was highly important (9.4) for them to quit.
Table 5.
Smoking Patterns and History
| Current Smoking | |
| Current Cigarettes Per Day1, mean (SD) | 19.7 (10.2) |
| Fagerström Test for Nicotine Dependence (FTND)1, mean (SD) | 4.9 (2.3) |
| Smoking History | |
| Age started smoking regularly2, mean (SD), yr | 17.1 (5.0) |
| Number of quit attempts in past 12 months3, mean (SD) | 2.0 (3.1) |
| Prior use of pharmacotherapy3, n (%) | |
| Nicotine replacement patch | 287 (50.8) |
| Nicotine gum | 187 (33.1) |
| Nicotine lozenge | 61 (10.8) |
| Bupropion (Zyban) | 163 (28.9) |
| Varenicline (Chantix) | 166 (29.4) |
| Longest period of past abstinence in days, mean (SD) | 393.9 (923.4) |
| Interest in Quitting | |
| Readiness to Stop Smoking3, n (%) | |
| Precontemplation | 15 (2.7) |
| Contemplation | 221 (39.1) |
| Preparation | 329 (58.2) |
| Importance of quitting [0 (low) – 10 (high)]3, mean (SD) | 9.4 (1.5) |
N=566
N=564
N=565
DISCUSSION
Connect2Quit is the first RCT examining telemedicine for the treatment of tobacco dependence, which is the top preventable cause of death in the United States. We were able to recruit a full complement of clinics and participants into the trial, which suggests that both practitioners and patients are able and willing to participate in video counseling-delivered tobacco treatment in rural areas.
Our participant sample was diverse and included low-income smokers with comorbidities known to be prevalent in rural areas. Many clinical trials exclude high-risk participants and test interventions on highly select populations. Our study pool has representation from a wide variety of smokers, recruited from 20 different primary care practices and safety net clinics. Because we recruited through safety net clinics as well as private primary care practices, many of our participants lacked health insurance and coverage for cessation medications. Hence, our study will be representative of the majority of smokers in rural areas, who have health and mental health comorbidities and who lack access to insurance and cessation medications. Moreover, data on computer and technology use suggest ITM may be a good fit for participants, who could have difficulty accessing or navigating a computer-based intervention in their homes.
Similar to past studies, our participants have high rates of depression; we also found they experience significant anxiety. The GAD-2 is a very brief screening tool that indicates the likely presence of an anxiety disorder. There is strong evidence of a link between smoking and mental health, including anxiety disorders,[36] and the prevalence observed in this study of 40% is around double that reported in primary care and population-based surveys.[32, 37]
We designed Connect2Quit to be simple, translatable, and sustainable to enhance its potential for widespread adoption and ultimate impact on public health. The accounting cost analysis will provide useful information about the actual costs of implementing ITM in real world clinical practices. The potential health impact is large because the prevalence of smoking is high in rural areas, access to smoking cessation services is low, Medicare reimbursement creates a strong potential for widespread adoption, and the economic benefits (cost-benefits) of smoking cessation are considerable. Should ITM prove effective, it will have strong potential for improving access to high-quality tobacco treatment in rural areas.
ACKNOWLEDGEMENTS
The project described was supported by Award Number R01HL087643 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarilyvrepresent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: Clinical Trials Registration: NCT00843505
AUTHORS CONTRIBUTIONS
LM completed the first draft of this manuscript and served as project director for the first year of the project. EF contributed to the study design and assisted in recruitment of clinics. APC helped design the SEM model and oversaw all aspects of Spanish translation and recruiting Hispanic and Latino smokers. KJP designed study analyses. RS supervised telemedicine technicians and oversaw all aspects of telemedicine implementation. DC and LC designed the counseling protocol, fidelity procedures, and provided counseling supervision. LL served as project director for 3 years and oversaw all aspects of study implementation. JH directed data collection and entry and worked with NN and KJP to ensure data accuracy. NN managed the study database and generated all descriptive data. TS designed the cost-effectiveness analyses. KR oversaw all aspects of the study and worked with LM to draft the manuscript, collect feedback and approval from all authors, and submit the final version of the manuscript. All authors have seen and approved of the final manuscript.
COMPETING INTERESTS
The authors have no competing interests to declare.
REFERENCES
- 1.Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 1997-2001. MMWR Morb Mortal Wkly Rep. 2005;54(25):625–628. [PubMed] [Google Scholar]
- 2.(CDC) CfDCaP Tobacco use among adults--United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(42):1145–1148. [PubMed] [Google Scholar]
- 3.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital and health statistics Series 10, Data from the National Health Survey. 2009;(242):1–157. [PubMed] [Google Scholar]
- 4.Fiore MC, Bailey WC, Cohen SJ. Treating Tobacco Use and Dependence. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2000. al. e. [Google Scholar]
- 5.Ockene JK. Physician-delivered interventions for smoking cessation: strategies for increasing effectiveness. Prev Med. 1987;16(5):723–737. doi: 10.1016/0091-7435(87)90054-5. [DOI] [PubMed] [Google Scholar]
- 6.Wechsler H, Levine S, Idelson RK, Rohman M, Taylor JO. The physician's role in health promotion--a survey of primary-care practitioners. N Engl J Med. 1983;308(2):97–100. doi: 10.1056/NEJM198301133080211. [DOI] [PubMed] [Google Scholar]
- 7.Ockene JK. Smoking intervention: the expanding role of the physician. Am J Public Health. 1987;77(7):782–783. doi: 10.2105/ajph.77.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orleans CT, George LK, Houpt JL, Brodie KH. Health promotion in primary care: a survey of U.S. family practitioners. Prev Med. 1985;14(5):636–647. doi: 10.1016/0091-7435(85)90083-0. [DOI] [PubMed] [Google Scholar]
- 9.Wells KB, Lewis CE, Leake B, Schleiter MK, Brook RH. The practices of general and subspecialty internists in counseling about smoking and exercise. Am J Public Health. 1986;76(8):1009–1013. doi: 10.2105/ajph.76.8.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson MD, Laurent SL, Little JM., Jr. Including smoking status as a new vital sign: it works! J Fam Pract. 1995;40(6):556–561. [PubMed] [Google Scholar]
- 11.Control CfD. Physician and other health-care professional counseling of smokers to quit--United States, 1991. MMWR. 1993;42(44):854–857. [PubMed] [Google Scholar]
- 12.Ossip-Klein DJ, McIntosh S. Quitlines in North America: evidence base and applications. Am J Med Sci. 2003;326(4):201–205. doi: 10.1097/00000441-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. The American psychologist. 2010;65(4):252–261. doi: 10.1037/a0018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;(2):CD002098. doi: 10.1002/14651858.CD002098. [DOI] [PubMed] [Google Scholar]
- 15.A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiore M, United States. Tobacco Use and Dependence Guideline Panel . Treating tobacco use and dependence : 2008 update, 2008 update edn. U.S. Dept. of Health and Human Services, Public Health Service; Rockville, Md.: 2008. [Google Scholar]
- 17.Gunderson A, Menachemi N, Brummel-Smith K, Brooks R. Geographic eligibility for rural health grant programs. J Rural Health. 2006;22(3):224–228. doi: 10.1111/j.1748-0361.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller RWe. DHHS Publication No (NIH) 04-5288. Volume Volume 1, edn. DHHS Publication No. (NIH) 04-5288; Bethesda, MD: 2004. Combined Behavioral Intervention Manual: A Clinical Research Guide for Therapists Treating People with Alcohol Abuse and Dependence. [Google Scholar]
- 19.Stephens RS, Babor TF, Kadden R, Miller M. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction. 2002;97(Suppl 1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- 20.Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72(3):455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 21.Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- 22.Richter KP, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. J Addict Dis. 2005;24(4):79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- 23.Boardman T, Catley D, Grobe JE, Little TD, Ahluwalia JS. Using motivational interviewing with smokers: do therapist behaviors relate to engagement and therapeutic alliance? J Subst Abuse Treat. 2006;31(4):329–339. doi: 10.1016/j.jsat.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 25.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 27.Benowitz NL. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Rennie DC, Dosman JA. The reliability of cigarette consumption reports by spousal proxies. American journal of public health. 1995;85(12):1711–1712. doi: 10.2105/ajph.85.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams GC, Gagne M, Ryan RM, Deci EL. Facilitating autonomous motivation for smoking cessation. Health Psychol. 2002;21:40–50. [PubMed] [Google Scholar]
- 30.Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, Deci EL. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol. 2006;25(1):91–101. doi: 10.1037/0278-6133.25.1.91. [DOI] [PubMed] [Google Scholar]
- 31.de Man-van Ginkel JM, Hafsteinsdottir T, Lindeman E, Burger H, Grobbee D, Schuurmans M. An efficient way to detect poststroke depression by subsequent administration of a 9-item and a 2-item Patient Health Questionnaire. Stroke; a journal of cerebral circulation. 2012;43(3):854–856. doi: 10.1161/STROKEAHA.111.640276. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 34.LK Mn, BO Mn. How To Use A Monte Carlo Study To Decide On Sample Size and Determine Power. 2002.
- 35.LK M, BO M. Mplus user's guide: Statistical analysis with latent variables. 5 edn. Muthén & Muthén; Los Angeles, CA: 2007. [Google Scholar]
- 36.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Jama. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 37.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]