Abstract
Objective
There is evidence that pregnancy-specific stress is associated with preterm birth. The purpose of this study is to examine the association between change in pregnancy-specific stress over the course of pregnancy and birth outcomes (i.e., preterm birth and gestational age) in an understudied, but vulnerable group using a theoretically-derived model.
Methods
Multivariate linear and logistic regression techniques were used to examine the association between pregnancy-specific stress (measured in second and third trimester) and length of gestation (i.e. preterm birth and gestational age) among a sample of 920 Black and/or Latina adolescent and young women.
Results
Second trimester pregnancy-specific stress was not associated with preterm birth or gestational age. Third trimester pregnancy-specific stress was associated with preterm birth, but not with gestational age. Change in pregnancy-specific stress between second and third trimester was significantly associated with increased likelihood of preterm delivery and shortened gestational age, even after controlling for important biological, behavioral, psychological, interpersonal, and sociocultural risk factors.
Conclusions
Findings emphasize the importance of measuring pregnancy-specific stress across pregnancy, as the longitudinal change from second to third trimester was significantly associated with length of gestation measured both as a dichotomous variable (preterm birth) and a continuous variable (gestational age). Furthermore, this is the first study to observe the association of pregnancy-specific stress with length of gestation in this understudied population–unique in age, race, and ethnicity.
Keywords: pregnancy-specific stress, pregnancy anxiety preterm birth, gestational age, birth outcomes
Preterm birth is the leading cause of perinatal morbidity and mortality in developed countries (Goldenberg, Culhane, Iams, & Romero, 2008). Preterm birth has been associated with 75% of perinatal mortality and more than 50% of long-term morbidity, including developmental issues in childhood and adverse health effects through adulthood (Glynn, Schetter, Hobel, & Sandman, 2008; Goldenberg et al., 2008). The current rate of preterm birth in the United States is 12.0%, which is higher than other developed countries (Goldenberg et al., 2008; Martin et al., 2012). Rates are even higher among certain populations, as disparities exist by race and ethnicity (Goldenberg et al., 2008; Martin et al., 2012; Martin, Osterman, & Sutton, 2010). Among women under 25, the 2010 rate of preterm birth for non-Hispanic Blacks was 16.6% compared to 11.6% and 10.9% for Hispanic and non-Hispanic White infants (Martin et al., 2012).
Variations in the range of gestational age have been associated with cognitive, behavioral, and health outcomes from infancy through adulthood (Davis, Glynn, Waffarn, & Sandman, 2011; Davis, Buss, et al., 2011; Dong & Yu, 2011; Loftin et al., 2010; Yang, Bergvall, Cnattingius, & Kramer, 2010; Yang, Platt, & Kramer, 2010). Even a one-week increase in gestational age can improve health outcomes and reduce healthcare costs (Loftin et al., 2010). Therefore, it is imperative for birth outcomes research to consider not only preterm birth as a risk category, but also gestational age as a continuous outcome.
Given the health implications of preterm birth, it is critical to identify risk factors for preterm birth and gestational age to develop methods for intervention and prevention (Behrman & Butler, 2007).Dunkel Schetter and Lobel (2012) highlight the need for multilevel models of prenatal health that encompass biological, psychological, and social aspects of health from the individual level through community level. Well-established risk factors of preterm birth include very young or advanced maternal age, lower parity, Black and Hispanic race and ethnicity, low pre-pregnancy body mass index, smoking, alcohol, substance use, increased stress, single marital status or marital strain, depression, maternal sexually transmitted infections, lower self-esteem, and inadequate social support (Berkowitz & Papiernik, 1993; Cokkinides, Coker, Sanderson, Addy, & Bethea, 1999; Neggers, Goldenberg, Cliver, & Hauth, 2004; Nkansah-Amankra, Dhawain, Hussey, & Luchok, 2010; Shumway et al., 1999). A history of low birth weight, preterm birth, stillborn birth or neonatal death, and other complications are also associated with increased risk of preterm birth and shortened length of gestation (Behrman & Butler, 2007; Berkowitz & Papiernik, 1993).
In addition to these risk factors, pregnancy-specific stress has gained attention as an important factor associated with length of gestation (Dunkel Schetter & Tanner, 2012; Dunkel Schetter, 2011). Pregnancy-specific stress refers to “fears about the health and well-being of one’s baby, the impending childbirth, of hospital and health-care experiences (including one’s own health and survival in pregnancy), birth and postpartum, and of parenting or the maternal role” (Dunkel Schetter, 2011, p. 535). It has been operationalized using various measures and referred to using various terms (e.g., pregnancy anxiety, pregnancy-specific distress, and pregnancy-related stress; Alderdice, Lynn, & Lobel, 2012). Nonetheless, evidence consistently suggests pregnancy-specific stress is an independent and often better predictor of preterm birth than other measures of generalized psychological distress (Alderdice et al., 2012; Dunkel Schetter, 2011).
Several studies among diverse populations provide evidence that pregnancy-specific stress has a negative association on length of gestation, meaning that gestational age decreases as pregnancy-specific stress increases (Coussons-Read et al., 2012; Dole et al., 2003; Kramer et al., 2009; Lobel, Cannella, et al., 2008; Mancuso, Schetter, Rini, Roesch, & Hobel, 2004; Misra, Guyer, & Allston, 2003; Orr et al., 1996; Rini, Dunkel-Schetter, Wadhwa, & Sandman, 1999; Roesch, Schetter, Woo, & Hobel, 2004; Wadhwa, Sandman, Porto, Dunkel-Schetter, & Garite, 1993). Additionally, while there are medical risk conditions that may confound the association between pregnancy-specific stress and preterm birth, there is evidence that this association remains even after controlling for risk factors such as pre-pregnancy body mass index, maternal age, and parity (Dole et al., 2003; Dunkel Schetter, 2011; Rini et al., 1999). In fact, observed effect sizes range from small to moderate, which is equal to or larger than reported effect sizes of risk factors such as medical risk and smoking (Dunkel Schetter, 2009, 2011; Goldenberg et al., 2008).
There is evidence of disparate rates of adverse birth outcomes according to race, ethnicity, and socioeconomic status (Foster et al., 2000; Lieberman, Ryan, Monson, & Schoenbaum, 1987; Martin et al., 2012; J. Parker, Schoendorf, & Kiely, 1994; Rosenthal & Lobel, 2011; Zhang & Bracken, 1995). However, our review found no studies on the relationship between pregnancy-specific stress and length of gestation using a sample of young, majority Black and/or Latina women. In fact, we found only two studies to date that have included women under the age of 18, and one study that included a predominately Latina sample (Coussons-Read et al., 2012; Dole et al., 2003, 2004; Lynn, Alderdice, Crealey, & McElnay, 2011). It may be particularly important to study correlates of adverse birth outcomes among young women of color living in urban settings given their relatively high risk for adverse birth outcomes and elevated stress (Chandra, Schiavello, Ravi, Weinstein, & Hook, 2002; Corcoran, Franklin, & Bennett, 2000).
More specifically, stress may affect birth outcomes through a variety of biological mechanisms including neuroendocrine, inflammatory, and behavioral (Dunkel Schetter, 2011; Hobel, Goldstein, & Barrett, 2008; Sandman, Davis, & Glynn, 2012). Neuroendocrine mechanisms have the greatest evidence related to pregnancy-specific stress (Dunkel Schetter, 2011; Hobel et al., 2008). Maternal stressors activate the hypothalamic-pituitary-adrenal axis, which is modulated by the corticotropin-releasing hormone.
Some hypothesize that placental production of cortisol and corticotropin-releasing hormone constitute a “placental clock” that may be activated by stress, although the pathway by which this occurs is not clear (McLean et al., 1995; Sandman et al., 2012). The “placental clock” hypothesis has been shown in animal models with supportive human studies (Hobel, Dunkel-Schetter, Roesch, Castro, & Arora, 1999; Sandman et al., 2012; Smith, 1999; Wadhwa et al., 2004). In this model corticotropin-releasing hormone serves as a mediator between maternal stress and length of gestation, by providing a way for the fetus to adjust the timing of birth according to maternal state (Hobel et al., 2008; Pike, 2005). Corticotropin-releasing hormone likely serves as a signal for normal labor, thus corticotropin-releasing hormone induced by stress could contribute to premature labor (Kramer et al., 2009; McLean et al., 1995).
Additionally, there is evidence that stress-induced corticotropin-releasing hormone can result in a release of proinflammatory ctyokines, and the elevated inflammatory markers can lead to adverse birth outcomes (Coussons-Read et al., 2012; Coussons-Read, Okun, Schmitt, & Giese, 2005; Kalantaridou et al., 2010; Mancuso et al., 2004; V. Parker & Douglas, 2010; Pearce et al., 2010). A recent study by Coussons-Read (2012) is the first to provide evidence that elevated pregnancy-specific stress and increased inflammatory cytokines (i.e. interleukin-6 and tumor necrosis factor alpha) are associated with preterm birth and shortened gestation. The study also provides initial support for inflammatory cytokines as partial mediators of the association between pregnancy-specific stress and gestational age.
While much is known about the relationship between pregnancy-specific stress and length of gestation, there is debate about the point in pregnancy at which pregnancy-specific stress has the greatest effect on length of gestation. Although some research suggests that the critical time is during the second trimester, other evidence points to third trimester as more predictive of preterm birth and length of gestation (Hedegaard, Henriksen, Sabroe, & Secher, 1993; Rini et al., 1999; Wadhwa et al., 1993). Mancuso et al. (2004) found that pregnancy-specific stress measured at 28–30 weeks was predictive of gestational age at delivery, but pregnancy-specific stress measured at 18–20 weeks was not predictive of gestational age at delivery. The majority of studies have assessed the effect of pregnancy-specific stress measured at one time point on birth outcomes; however, Roesch et al. (2004) found an association with shortened gestation when pregnancy-specific stress was measured over the course of pregnancy (from 18 weeks gestation through third trimester). They found change in pregnancy-specific stress to have stronger association with shortened gestation than pregnancy-specific stress measured at one time point or a composite average of several time points (Roesch et al., 2004). Two other longitudinal studies were identified, however, one study used the mean average of pregnancy-related stress over the course of pregnancy and the other study was not sufficiently powered to observe a small to moderate change in pregnancy-specific stress over time (Coussons-Read et al., 2012; Gennaro, Shults, & Garry, 2008).
Cross-sectional measurements of pregnancy-specific stress that are prevalent in the literature prevent firm conclusions regarding the time when pregnancy-specific stress has the greatest effect on birth outcomes (Lobel, Hamilton, & Cannella, 2008). This gap also prevents understanding of the relationship between change in pregnancy-specific stress and length of gestation.
The purpose of this study is to investigate longitudinally the association of pregnancy-specific stress with length of gestation in an understudied, but vulnerable sample of majority Black and/or Latina young women. The hypothesis is that pregnancy-specific stress is independently associated with preterm birth and gestational age, and therefore higher levels of pregnancy-specific stress will be associated with increased risk of preterm birth and shortened gestational age. Pregnancy-specific stress is measured in both the second and third trimester of pregnancy. The association between change in pregnancy-specific stress over the course of pregnancy and length of gestation is assessed, given that this is an existing gap in the literature. Building upon the multilevel biopsychosocial approach proposed by Dunkel Schetter and Lobel (2012), models include well-established risk factors for length of gestation at the individual, interpersonal, and sociocultural levels, in order to understand the unique contribution of pregnancy-specific stress (Table 1). In addition, appropriate analyses were conducted to rule out the possibility that associations of pregnancy-specific stress with length of gestation are confounded by the relationship of pregnancy-specific stress with obstetric risk conditions.
Table 1.
Sample characteristics by preterm birth status (N=920)
| Primary Outcome |
Term (37+ weeks) (n=831) |
Preterm (<37 weeks) (n=89) |
|
| Gestational Age at Birth (weeks) (mean ± SD)* | 39.78 ± 1.22 | 34.35 ± 2.92 | |
| Primary Predictor | Pregnancy-specific Stress Score (mean ± SD) | ||
| Second Trimester | 14.76 ± 7.19 | 14.16 ± 7.21 | |
| Third Trimester | 12.56 ± 7.00 | 13.55 ± 7.27 | |
| Control Variables: Additional Biopsychosocial Factors | Individual-Level Factors: Biological/Medical | ||
| History of Adverse Pregnancy Outcome | 160 (19.25%) | 23 (25.84%) | |
| Pre-pregnancy Body Mass Index (mean ± SD) | 26.97± 7.18 | 26.75 ± 7.75 | |
| Pre-pregnancy Body Mass Index Category | |||
| Underweight | 46 (5.54%) | 5 (5.62%) | |
| Normal weight | 363 (43.68%) | 38 (42.70%) | |
| Overweight/Obese | 422 (50.78%) | 46 (51.69%) | |
| Maternal Age (mean ± SD) | 20.43 ± 2.60 | 20.44 ± 2.87 | |
| Nulliparous | 533 (64.14%) | 54 (60.67%) | |
| Maternal Antenatal Complications During Pregnancy* | 40 (4.81%) | 14 (15.73%) | |
| Positive STI Test During Pregnancy | 190 (22.86%) | 20 (22.47%) | |
| Gestational Age at Study Interview | |||
| Second Trimester* | 18.24 ± 3.50 | 17.08 ± 3.34 | |
| Third Trimester | 34.45 ± 3.17 | 34.58 ± 4.52 | |
| Individual-Level Factors: Behavioral | |||
| Smoking During Pregnancy | 187 (22.50%) | 16 (17.98%) | |
| Substance Use During Pregnancy | 137 (16.49%) | 14 (15.73%) | |
| Individual-Level Factors: Psychological | |||
| Depression (mean ± SD) | |||
| Second Trimester | 12.43 ± 8.23 | 12.06 ± 7.61 | |
| Third Trimester | 11.71 ± 8.73 | 10.67 ± 6.94 | |
| Perceived General Stress | |||
| Second Trimester | 17.74 ± 6.99 | 17.51 ± 6.51 | |
| Third Trimester | 16.83 ± 7.07 | 15.94 ± 6.19 | |
| Self-esteem (mean ± SD) | |||
| Second Trimester | 33.46 ± 5.18 | 33.79 ± 5.12 | |
| Third Trimester | 34.11 ± 5.11 | 34.05 ± 4.76 | |
| Interpersonal-Level Factors | |||
| Social Support (mean ± SD) | |||
| Second Trimester | 29.22 ± 6.03 | 30.11 ± 5.36 | |
| Third Trimester | 29.97 ± 5.40 | 30.06 ± 5.07 | |
| In a relationship | 659 (79.30%) | 73 (82.02%) | |
| Sociocultural-Level Factors | |||
| Race and Ethnicity* | |||
| Black | 645 (77.62%) | 75 (84.27%) | |
| Latina | 113 (13.60%) | 12 (13.48%) | |
| White and Other | 73 (8.78%) | 2 (2.25%) | |
| Education > High School Diploma/GED | 142 (17.09%) | 11 (12.36%) | |
| Employed Full-time or Part-time | 264 (31.17%) | 32 (35.96%) | |
| Treatment Group | Control | 310 (37.30%) | 38 (42.70%) |
| CPP | 265 (31.89%) | 24 (26.97%) | |
| CPP+ | 256 (30.81%) | 27 (30.34%) | |
Note. SD= Standard Deviation.
p<0.05
Methods
Data were drawn from a randomized controlled trial to test the effects of an innovative model of group prenatal care on birth and reproductive health outcomes (Ickovics et al., 2007). The original dataset included 1047 women between the ages of 14 and 25 recruited from two university-affiliated obstetric clinics in in Atlanta, Georgia and New Haven, Connecticut between 2001 and 2004. Inclusion criteria were (a) pregnancy less than 24 weeks gestation, (b) less than 25 years of age, (c) not considered a high-risk pregnancy, (d) ability to speak English or Spanish, and (e) willingness to participate in a randomized clinical trial. Women were not included if they were HIV-positive or had a clinical diagnosis for a psychological disorder at intake. All participants received a detailed explanation of the study and provided informed consent. Parental consent was not required for women under the age of 18, as state laws allow minors to consent to reproductive healthcare and research (Boonstra & Nash, 2000). All procedures were approved by the Yale and Emory University Institutional Review Boards. Women were randomized into two intervention groups and one control group. One intervention group received prenatal care in a group setting, while the other intervention group received the same group prenatal care sessions with enhancements for HIV and sexually transmitted disease prevention. The control group received standard individual prenatal care.
Women completed structured interviews via audio computer-assisted self-interviews at four time points during pregnancy and one year postpartum. Data from Time 1 and 2 interviews were utilized for this study. Time 1 interviews occurred in second trimester [6 – 24 weeks gestation (M=18.23 weeks, SD=3.50)]. Time 2 interviews occurred at the end of third trimester [32 –42 weeks gestation (M= 34.46 weeks, SD = 3.32)]. Medical record review was conducted by trained medical abstractors who were independent of care and blinded to study assignment. Additional details of the original study have been published elsewhere (Ickovics et al., 2007; Kershaw, Magriples, Westdahl, Rising, & Ickovics, 2009).
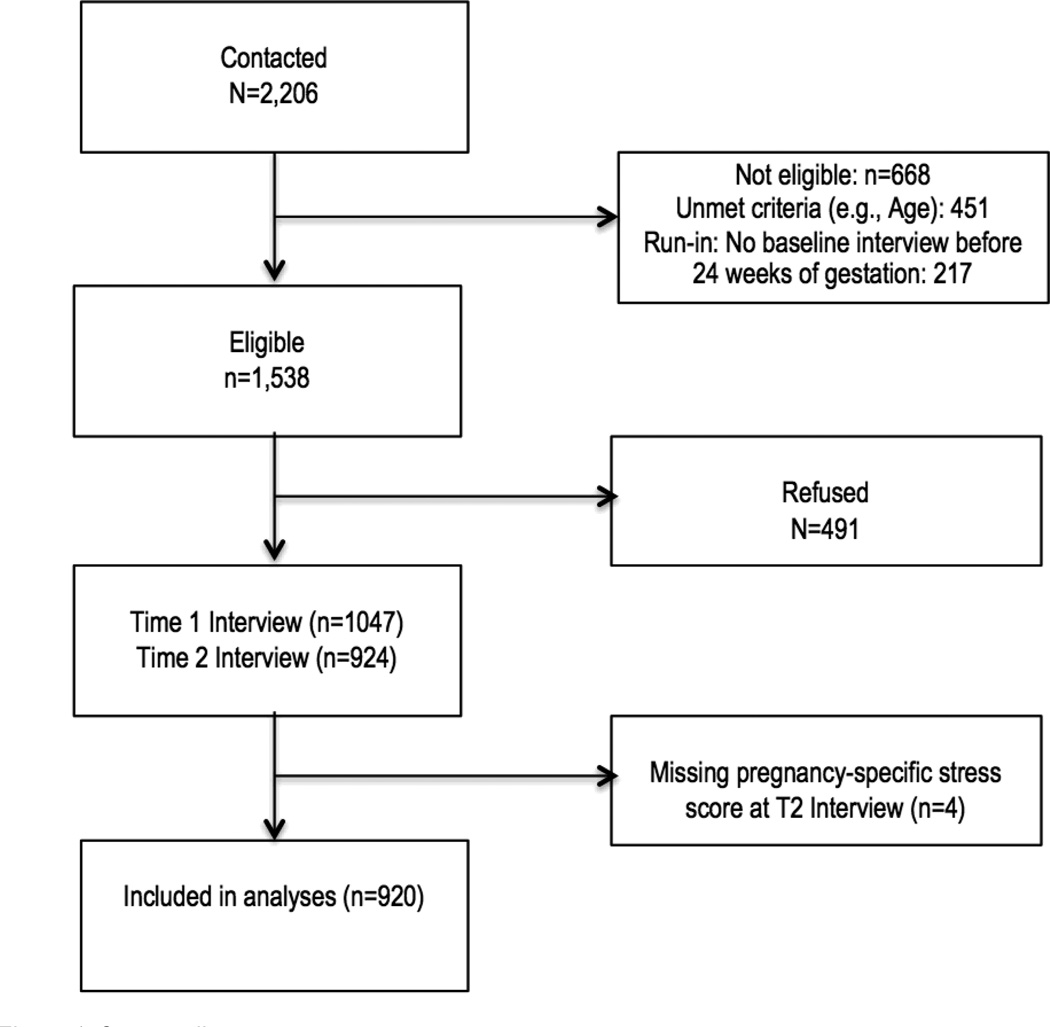
The cohort was limited to only those women with complete data for second and third trimester interviews and recorded gestational age, resulting in a sample size of 920 (see Figure 1). Compared to those included in this analytic sample, those excluded were more likely to be Black, have lower pre-pregnancy body mass index, and have history of adverse pregnancy. They also had lower self-esteem and higher pregnancy-specific stress, perceived general stress, and depression at the baseline interview and were more likely to have a sexually transmitted disease and report substance use during pregnancy.
Figure 1. Consort diagram.
Note. Because this was a longitudinal study, an initial run-in period was part of the eligibility criteria and consented participants were deemed ineligible if they could not be re-contacted before they were out of the eligibility window for their first interview (24 weeks gestation).
Measures
Measures used in this study have all been successfully used in populations with characteristics similar to the sample in this study (e.g. pregnant, adolescent, racially and ethnically diverse) (Alderdice et al., 2012; Amaro, Zuckerman, & Cabral, 1989; Arnold, Lewis, Maximovich, Ickovics, & Kershaw, 2011; Dailey, Humphreys, Rankin, & Lee, 2011; Lanz, 1995). Our study is the first to use the Revised Prenatal Distress Scale in a population that is young, majority Black and Latina, and of lower socioeconomic status; however, results for the association of preterm birth are consistent with that of other populations.
Primary outcome
Gestational age at birth was calculated by estimated date of delivery according to the participant’s reported last menstrual period. All participants underwent second-trimester ultrasound examination for confirmation of dating and anatomy. Estimated date of delivery was established by a consulting obstetrician who was independent of the study, and the date was confirmed by ultrasonography. Gestational age was treated as a continuous variable. Preterm birth (gestational age < 37 weeks) was coded with a 1, and term birth (gestational age ≥ 37 weeks) was coded with a 0.
Primary predictor
Pregnancy-specific stress was measured using the Revised Prenatal Distress Questionnaire at second and third trimester interviews (Lobel, Cannella, et al., 2008). Change in pregnancy-specific stress was represented by modeling third trimester pregnancy-specific stress, controlling for second trimester pregnancy-specific stress in the model. The scale is used to measure how much women were “bothered, worried, or upset” about various aspects of the pregnancy (e.g. physical symptoms, parenting concerns, relationship strains, bodily changes, anxiety about labor and delivery, the baby’s health) (Lobel, Cannella, et al., 2008). The scale consists of 3 versions, including items that are specific to each of the three trimesters of pregnancy (Lobel, Cannella, et al., 2008). In order to assess pregnancy-specific stress during the course of pregnancy, all unique items were included at both time points. The 17-item scale was measured on a 3-point response category, with 0 = not at all, 1 = somewhat, and 2 = very much. Reponses were summed to create a pregnancy-specific stress score, ranging from 0 to 34. At both time points, the scale demonstrated good internal reliability, with Cronbach’s alphas of .86 and .87. Participants completed this measure via audio-computer assisted self-interviewing.
Several instruments have been used to measure pregnancy-specific stress. In a recent review of these measures, Alderdice et al. (2012) identified the Revised Prenatal Distress Questionnaire to be among the most appropriate measures for predictive validity for preterm birth. The scale’s strengths include the ability for prospective measurement and differentiation of sources of stress (Alderdice et al., 2012).
Control variables- additional biopsychosocial factors
To examine the relationship of pregnancy-specific stress with preterm birth independent of other factors, additional established risk factors for preterm birth were included in each model according to Dunkel Schetter and Lobel’s multilevel biopsychosocial approach (Dunkel Schetter & Lobel, 2012). Risk factors, chosen a priori according to evidence in the literature, represent individual-level, interpersonal, and sociocultural factors (see Table 1).
Individual-level factors- biological/medical
History of adverse pregnancy outcomes were assessed via medical record review and self-report. Conditions considered for this dichotomous variable were previous preterm pregnancy and history of spontaneous abortion, ectopic pregnancy, stillbirth, or fetal demise. Having at least one of these conditions was coded with a 1, and having none was coded with a 0.
Maternal antenatal complications of the current pregnancy were identified from medical record review. A dichotomous value was assigned for women who experienced gestational diabetes, pregnancy-induced hypertension, preeclampsia, or oligohydramnios during the current pregnancy. Having at least one of these conditions was coded with a 1, and having none was coded with a 0.
Positive sexually transmitted disease during pregnancy was measured by self-report in the second trimester interview, and biological ligase chain reaction testing for gonorrhea and chlamydia at the third trimester interview. Self-report or biological evidence of at least one STI over the course of pregnancy was coded with a 1, and no STI was coded with a 0.
Pre-pregnancy body mass index was assessed through self-report of height and weight at the second trimester interview. Women were asked to report their pre-pregnancy height and weight.
Age and Parity were assessed at second trimester interview through self-report. Age was treated as a continuous variable. Nulliparity was coded with a 1, while primiparity and multiparity were coded with a 0.
Individual-level factors- behavioral
Smoking was assessed by self-report during the second and third trimester interviews, using a questionnaire developed by the investigators. Items in the questionnaire asked the participant to indicate whether or not she had used cigarettes since pregnancy began. Those responding “yes” were prompted to indicate the amount and frequency of cigarette use. Any report of cigarette use was coded with a 1, and report of no cigarette use was coded with a 0.
Substance use during pregnancy was assessed by self-report during the second and third trimester interviews, using a questionnaire developed by investigators. Items asked the participant to indicate whether or not she had used alcohol and/or illicit drugs since pregnancy began. Those responding “yes” were prompted to indicate the amount and frequency of each behavior. Illicit drug use included using marijuana, crack, cocaine, and “other hard drugs;” sharing needles; and injection drug use. Any report of alcohol or illicit drug use was coded with a 1, and report of no alcohol or illicit drug use was coded with a 0.
Individual-level factors- psychological
Depression during pregnancy was measured using the Center for Epidemiologic Studies- Depression Scale (CES-D) at second and third trimester interviews (Radloff, 1977). The items focused on the affective component of depressed mood (e.g., feelings of failure, guilt, hopelessness, and sadness). As in other studies with pregnancy women, five psychophysiologic items were dropped because pregnancy may cause physical disturbances (e.g., changes in appetite, sleep). Respondents indicated how often they had experienced each of the items in the past week on the following 4-point scale: 0 = less than 1 day, 1 = 1–2 days, 2 = 3–4 days, and 3 = 5–7 days. All items were summed to form a total score, ranging from 0 to 45. At both time points, the scale demonstrated good internal reliability, with Cronbach’s alphas of .84 and .87.
Perceived General Stress during pregnancy was assessed using the Perceived Stress Scale-10 at second and third trimester interviews (Cohen & Williamson, 1988). The scale assessed the degree to which participants perceive situations in their life to be stressful in the past month. For each of the 10 items, responses were on a 4-point scale, ranging from 1= never to 4= very often. Items were summed to form a score ranging from 0 to 40. At both time points, the scale demonstrated good internal reliability, with Cronbach’s alpha of .81 in both second and third trimester.
Self-esteem during pregnancy was measured using the Rosenberg Self-Esteem Scale at second and third trimester interviews (Rosenberg, 1965). For each of the 10-items, responses were on a 4-point scale, ranging from 1 = strongly disagree to 4 = strongly agree. Items were summed to form a score ranging from 10 to 40. The scale demonstrated good internal reliability at both time points, with Cronbach’s alphas of .85.
Interpersonal-level factors
Social support during pregnancy was assessed using the Social Support Subscale of the Social Relationship Scale at second and third trimester interviews (O’Brien, Wortman, Kessler, & Joseph, 1993). The subscale consists of 7 items and responses were on a 5-point scale, ranging from 1 = definitely not, to 5 = definitely yes. The 7 items were summed to form a total score, ranging from 7 to 35. The scale demonstrated good internal reliability, with Cronbach’s alpha of .89 and .90.
Relationship status at the beginning of the pregnancy was collected at the second trimester interview through self-report. In a relationship was coded with a 1, and not in a relationship was coded with a 0.
Sociocultural-level factors
Race and Ethnicity, Education, and Employment status at beginning of pregnancy were collected at the second trimester interview through self-report. Education and employment status at beginning of pregnancy were included as proxies for socioeconomic status. Participants were asked to self-identify their race and ethnicity. Categories were collapsed to “Black”, “Latina”, and “White and Other” with Black as the referent group. More education than a high school diploma or GED was coded with a 1, and less than a high school diploma or GED was coded with a 0. Employed full-time or part-time was coded with a 1, and not employed was coded with a 0.
Statistical Analysis
Analyses were conducted using SAS software version 9.2 (SAS, 2008). Sample characteristics were described for the overall sample, stratified by timing of birth (term vs. preterm). Means and standard deviations are provided for continuous variables, and frequencies for categorical variables (see Table 1). Analyses were conducted to separately model the association of pregnancy-specific stress with preterm birth (see Table 2) and gestational age (see Table 3), while controlling for known risk factors.
Table 2.
Final models predicting preterm birth
| Characteristic | OR | 95% CI | OR | 95% CI | OR | 95% CI |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| Second Trimester Pregnancy-specific Stressa | 0.99 | 0.96, 1.02 | -- | -- | -- | -- |
| Third Trimester Pregnancy-specific Stressb | -- | -- | 1.02 | 0.99, 1.05 | ||
| Change in Pregnancy-specific Stressc | -- | -- | -- | -- | 1.05 | 1.01, 1.10* |
| Adjusted | ||||||
| Second Trimester Pregnancy-specific Stressd | 1.00 | 0.96, 1.04 | -- | -- | -- | -- |
| Third Trimester Pregnancy-Specific Stresse | -- | -- | 1.06 | 1.01, 1.10* | -- | -- |
| Change in Pregnancy-specific Stressf | -- | -- | -- | -- | 1.08 | 1.02, 1.13** |
| Control Variables | ||||||
| History of Adverse Pregnancy Outcome | 1.46 | 0.84, 2.51 | 1.56 | 0.90, 2.69 | 1.56 | 0.90, 2.73 |
| Pre-pregnancy Body Mass Index | 1.00 | 0.96, 1.03 | 0.99 | 0.96, 1.03 | 0.99 | 0.96, 1.03 |
| Maternal Age | 0.98 | 0.88, 1.09 | 0.97 | 0.88, 1.08 | 0.98 | 0.88, 1.09 |
| Nulliparous | 0.79 | 0.44, 1.40 | 0.71 | 0.40, 1.24 | 0.77 | 0.43, 1.38 |
| Maternal Antenatal Complications During Pregnancy | 4.09 | 2.05, 8.17** | 3.73 | 1.88, 7.44** | 3.75 | 1.84, 7.62* |
| Positive STI Test During Pregnancy | 0.99 | 0.57, 1.71 | 0.93 | 0.54, 1.62 | 0.99 | 0.56, 1.75 |
| Smoking During Pregnancy | 0.90 | 0.47, 1.70 | 0.79 | 0.41, 1.52 | 0.89 | 0.46, 1.71 |
| Substance Use During Pregnancy | 1.04 | 0.53, 2.05 | 1.05 | 0.53, 2.09 | 1.00 | 0.50, 2.01 |
| Depression | 1.00 | 0.96, 1.05 | 0.97 | 0.93, 1.02 | 0.96 | 0.92, 1.01 |
| Perceived General Stress | 1.00 | 0.96, 1.05 | 0.96 | 0.92, 1.01 | 0.96 | 0.91, 1.01 |
| Self-esteem | 0.99 | 0.93, 1.05 | 0.96 | 0.90, 1.02 | 0.94 | 0.88, 1.02 |
| Social Support | 1.03 | 0.98, 1.08 | 1.00 | 0.95, 1.05 | 1.03 | 0.97, 1.09 |
| In a relationship | 1.13 | 0.62, 2.05 | 1.21 | 0.67, 2.19 | 1.16 | 0.63, 2.12 |
| Race and Ethnicity | ||||||
| Black | Ref | Ref | Ref | Ref | Ref | Ref |
| Latina | 0.99 | 0.50, 1.97 | 0.78 | 0.40, 1.53 | 0.99 | 0.49, 2.00 |
| White and Other | 0.27 | 0.06, 1.13 | 0.21 | 0.05, 0.90* | 0.24 | 0.06, 1.05 |
| Education > High School Diploma/GED | 0.65 | 0.32, 1.32 | 0.67 | 0.33, 1.35 | 0.67 | 0.33, 1.38 |
| Employed Full-time or Part-time | 1.27 | 0.78, 2.07 | 1.23 | 0.76, 2.01 | 1.27 | 0.77, 2.08 |
| Treatment Group | 0.70 | 0.44, 1.12 | 0.71 | 0.44, 1.14 | 0.67 | 0.41, 1.09 |
Note. OR= odds ratio; CI= confidence interval.
p<0.05
p<.01;
All analyses controlled for experimental group membership. All adjusted models controlled for gestational age at study interview.
McFadden’s Pseudo R2= .002;
McFadden’s Pseudo R2= .003;
McFadden’s Pseudo R2= .008;
McFadden’s Pseudo R2= .039;
McFadden’s Pseudo R2= .040;
McFadden’s Pseudo R2= .055.
Table 3.
Final models predicting gestational age
| Characteristic | B | SE | β | t | p-value | B | SE | β | t | p-value | B | SE | β | t | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | |||||||||||||||
| Second Trimester Pregnancy-specific Stressa | 0.02 | 0.01 | 0.06 | 1.86 | .064 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Third Trimester Pregnancy-specific Stressb | -- | -- | -- | -- | -- | −0.01 | 0.01 | −0.02 | −0.51 | .613 | -- | -- | -- | -- | -- |
| Change in Pregnancy-specific Stressc | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.03 | 0.01 | −0.11 | −2.40 | .017* |
| Adjusted | |||||||||||||||
| Second Trimester Pregnancy-specific Stressd | 0.02 | 0.01 | 0.06 | 1.34 | .182 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Third Trimester Pregnancy-specific Stresse | -- | -- | -- | -- | -- | −0.02 | 0.01 | −0.06 | −1.39 | .166 | -- | -- | -- | -- | -- |
| Change in Pregnancy-specific Stressf | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.04 | 0.02 | −0.12 | −2.31 | .021* |
| Control Variables | |||||||||||||||
| History of Adverse Pregnancy Outcome | −0.53 | 0.19 | −0.10 | −2.84 | .005** | −0.53 | 0.19 | −0.10 | −2.86 | .004** | −0.53 | 0.19 | −0.10 | −2.86 | .004** |
| Pre-pregnancy Body Mass Index | 0.01 | 0.01 | 0.03 | 0.88 | .378 | 0.01 | 0.01 | 0.03 | 0.94 | .346 | 0.01 | 0.01 | 0.03 | 0.98 | .330 |
| Maternal Age | −0.01 | 0.03 | −0.01 | −0.30 | .763 | −0.01 | 0.03 | −0.01 | −0.29 | .771 | −0.01 | 0.03 | −0.01 | −0.32 | .751 |
| Nulliparous | 0.06 | 0.18 | 0.01 | 0.31 | .758 | 0.15 | 0.18 | 0.03 | 0.87 | .386 | 0.07 | 0.18 | 0.02 | 0.39 | .689 |
| Maternal Antenatal Complications During Pregnancy | −0.94 | 0.31 | −0.10 | −3.09 | .002** | −0.91 | 0.31 | −0.10 | −2.98 | .003** | −0.88 | 0.31 | −0.10 | −2.88 | .004** |
| Positive STI Test During Pregnancy | −0.02 | 0.17 | 0.00 | −0.11 | .903 | 0.00 | 0.17 | −0.01 | 0.04 | .994 | −0.02 | 0.18 | −0.01 | −0.15 | .883 |
| Smoking During Pregnancy | 0.00 | 0.19 | 0.00 | 0.02 | .987 | 0.09 | 0.19 | 0.02 | 0.49 | .627 | 0.01 | 0.19 | 0.00 | 0.05 | .957 |
| Substance Use During Pregnancy | 0.21 | 0.21 | 0.04 | 0.99 | .324 | 0.23 | 0.21 | 0.04 | 1.06 | .289 | 0.22 | 0.22 | 0.04 | 1.03 | .304 |
| Depression | 0.00 | 0.01 | 0.00 | −0.09 | .925 | 0.01 | 0.01 | 0.02 | 0.40 | .692 | 0.01 | 0.01 | 0.03 | 0.57 | .566 |
| Perceived General Stress | 0.00 | 0.02 | 0.00 | −0.04 | .968 | 0.02 | 0.02 | 0.06 | 1.13 | .257 | 0.02 | 0.02 | 0.06 | 1.14 | .253 |
| Self-esteem | 0.02 | 0.02 | 0.05 | 0.96 | .338 | 0.02 | 0.02 | 0.04 | 0.91 | .362 | 0.02 | 0.02 | 0.04 | 0.74 | .461 |
| Social Support | −0.01 | 0.01 | −0.02 | −0.45 | .654 | 0.01 | 0.02 | 0.02 | 0.46 | .643 | 0.01 | 0.02 | 0.03 | 0.63 | .528 |
| In a relationship | −0.30 | 0.18 | −0.06 | −1.64 | .101 | −0.33 | 0.18 | −0.06 | −1.81 | .071 | −0.30 | 0.18 | −0.06 | −1.64 | .103 |
| Race and Ethnicity | |||||||||||||||
| Black | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Latina | 0.34 | 0.22 | 0.05 | 1.54 | .123 | 0.44 | 0.22 | 0.07 | 2.04 | .042* | 0.33 | 0.22 | 0.05 | 1.51 | .130 |
| White and Other | 0.67 | 0.27 | 0.08 | 2.47 | .013* | 0.73 | 0.27 | 0.09 | 2.69 | .007** | 0.69 | 0.27 | 0.09 | 2.52 | .011* |
| Education > High School Diploma/GED | 0.31 | 0.20 | 0.05 | 1.54 | .125 | 0.32 | 0.20 | 0.06 | 1.57 | .117 | 0.29 | 0.20 | 0.05 | 1.45 | .147 |
| Employed Full-time or Part-time | −0.21 | 0.16 | −0.05 | −1.34 | .181 | −0.18 | 0.16 | −0.04 | −1.15 | .251 | −0.22 | 0.16 | −0.05 | −1.37 | .171 |
| Treatment Group | 0.03 | 0.15 | 0.01 | 0.20 | .839 | 0.02 | 0.15 | 0.01 | 0.16 | .875 | 0.03 | 0.16 | 0.01 | 0.19 | .850 |
Note. B= unstandardized regression coefficients, β= standardized regression coefficients, SE= standard error;
p<0.05;
p<.01.
All analyses controlled for experimental group membership. All adjusted models controlled for gestational age at study interview.
Model R2= .004;
Model R2= < .001;
Model R2= .010;
Model R2= .051;
Model R2= .044;
Model R2= .059.
Multivariate logistic and linear regression models were used to assess the association of pregnancy-specific stress with preterm birth and gestational age, respectively. The primary predictor was considered in the following ways: second trimester pregnancy-specific stress, third trimester pregnancy-specific stress, and change in pregnancy-specific stress between second and third trimester. Change was modeled using third trimester pregnancy-specific stress score while controlling for second trimester score. Each main effects model also controlled for possible confounders, chosen a priori according Dunkel Schetter and Lobel’s multilevel biopsychosocial conceptual approach (Dunkel Schetter & Lobel, 2012). The following factors were included in all adjusted models: history of adverse pregnancy outcome, pre-pregnancy body mass index, maternal age, parity, maternal antenatal complications during pregnancy and sexually transmitted disease; smoking and substance use during pregnancy; depression, perceived general stress, and self-esteem; social support and relationship status; race, education, and employment status; gestational age at study interview. All analyses controlled for experimental group membership by inclusion of treatment group as a dichotomous covariate (control group vs. both intervention groups). Unadjusted and adjusted associations are presented in Table 2 for preterm birth and Table 3 for gestational age.
To address the concern of confounding by indication, we tested additional interaction effects with the adjusted models (de Koning et al., 2005; Psaty et al., 1999). In the context of this study, confounding by indication refers to the possibility that women who experience complications early in the current pregnancy, or have had an adverse event related to a prior pregnancy know that they are at greater risk for adverse pregnancy outcomes, and therefore have greater anxiety throughout the pregnancy. In order to address this possibility, we tested interaction effects between maternal antenatal complications and each pregnancy-specific stress main effect. Additionally, we tested interaction effects between history of adverse pregnancy outcome and each pregnancy-specific stress main effect.
To assess the impact of missing data on the model estimates, sensitivity analysis was conducted using extreme case analysis. Analysis of the relationship between pregnancy-specific stress (second trimester, third trimester, and change between second and third trimesters) and preterm birth was reconsidered under the following assumptions for missing data: (a) women missing third trimester pregnancy-specific stress were assumed to have the lowest possible value, and those missing preterm birth status were assumed to have delivered preterm; (b) women missing third trimester pregnancy-specific stress were assumed to have the highest possible value, and those missing preterm birth status were assumed to have delivered preterm; (c) women missing third trimester pregnancy-specific stress were assumed to have the lowest possible value, and those missing preterm birth status were assumed to have delivered at term; and (d) women missing third trimester pregnancy-specific stress were assumed to have the highest possible value, and those missing preterm birth status were assumed to have delivered at term.
Results
Sample characteristics are provided in Table 1. The average age of women in the sample was 20.42 (SD= 2.63). The majority of the women were Black, unemployed, and in a relationship at the beginning of the pregnancy. Most were having their first child and had earned less than a high school diploma. Nearly 20% of the sample had a history of adverse pregnancy outcome, while 6% experienced complications during the current pregnancy. The average pre-pregnancy body mass index, 26.95 (SD=7.23), falls within clinical guidelines for overweight. Reports of sexually transmitted disease, smoking, and substance use during pregnancy ranged from 16% to 23%.
Pregnancy-specific stress decreased from second trimester to third trimester (Mean difference: −2.04, t=−10.81, p< .001). Depression (Mean difference: −0.78, t= −3.12, p= 0.002), and general stress (Mean difference: −0.98, t= −4.98, p<0.001) also decreased. Self-esteem (Mean difference: 0.61, t=4.57, p< .001) and social support (Mean difference: 0.67, t=4.30, p< .001) increased from second to third trimester.
About 90% of infants in the sample (n=831) were born full-term, with the average gestational age of full-term births being 39.78 weeks (SD=1.22). The average gestational age of preterm births (n=89) was 34.35 weeks (SD=2.92). There were no deliveries before the third trimester interview (24 weeks gestation).
The final logistic regression models predicting preterm birth are presented in Table 2. Second trimester pregnancy-specific stress was not significantly associated with preterm birth. Third trimester pregnancy-specific stress and change in pregnancy-specific stress were significantly associated with preterm birth. Each unit increase in third trimester pregnancy-specific stress was associated with a 5% increase in the odds of having preterm birth (OR= 1.05, 95% CI: 1.01, 1.09). Additionally, each unit increase in pregnancy-specific stress between second and third trimester was associated with a 7% increase in the odds of having a preterm birth (OR= 1.07, 95% CI: 1.02, 1.13). There was little difference in point estimates and confidence intervals between unadjusted and adjusted models.
The final multivariate regression main effects models predicting gestational age are presented in Table 3. Neither second nor third trimester pregnancy-specific stress models predicted gestational age. However, change in pregnancy-specific stress was inversely related to gestational age: an increase in pregnancy-specific stress from second to third trimester was associated with decreased gestational age (β= −0.12; p= .022). To assess confounding by indication, interaction effects between each pregnancy-specific stress main effect and maternal antenatal complications such as previous preterm birth, ectopic pregnancy or fetal demise were independently added to adjusted models for preterm birth and gestational age. None of these interaction effects was significant. Similar interaction effects were tested for history of adverse pregnancy outcome and none of these was significant.
None of the assumptions of missing data values had an effect on the statistical significance of the results for the relationship between pregnancy-specific stress and preterm birth, regardless of whether pregnancy-specific stress was measured in the second trimester, third trimester, or as change between second and third trimesters. Neither the point estimates nor significance levels for pregnancy-specific stress were altered in any of the alternative models. For the purposes of this analysis the control and intervention groups were retained. The influence of pregnancy-specific stress and preterm birth was not moderated by group status, and there was no relationship between group status and pregnancy-specific stress; this in addition to statistical control of group status, provides confidence that inclusion of the intervention groups does not unduly influence the results, while allowing maximum power.
Discussion
Pregnancy-specific Stress and Length of Gestation
Findings from this study suggest that change in pregnancy-specific stress from second to third trimester is a significantly associated with both length of gestation among a sample of mostly Black and/or Latina, socioeconomically disadvantaged young mothers. Increases in pregnancy-specific stress over the course of pregnancy were significantly associated with increased likelihood of preterm delivery and shortened gestational age, while taking into account important multi-level biopsychosocial risk factors. Additionally, third trimester pregnancy-specific stress was associated with preterm birth, but not gestational age. The observed associations were also independent of other indicators of maternal psychological stress and emotion (i.e. general stress, depression, self-esteem). Change in pregnancy-specific stress was found to significantly predict timing of birth, measured both as a dichotomous variable (preterm birth) and a continuous variable (gestational age). This suggests that the processes linking pregnancy-specific stress and birth outcomes influence length of gestation for term and preterm births, with cases of preterm birth representing the more extreme instances. These results are in line with previous findings in the literature and extend our understanding of the association between pregnancy-specific stress and length of gestation (Dole et al., 2003; Kramer et al., 2009; Lobel, Hamilton, et al., 2008; Mancuso et al., 2004; Orr, Reiter, Blazer, & James, 2007; Roesch et al., 2004).
Notably, the association of third trimester pregnancy-specific stress with preterm birth, and the association of change in pregnancy-specific stress with preterm birth and gestational age remained significant after controlling for well-known risk factors for preterm birth, such as history of adverse pregnancy outcome and maternal antenatal complications of the current pregnancy. The association of pregnancy-specific stress with length of gestation may occur independently of these other risk factors.
Additionally, previous adverse pregnancy outcomes and antenatal complications are biological factors that are well studied for their effect on preterm birth and gestational age, as they can impact length of gestation through both spontaneous and medically indicated preterm birth (Behrman & Butler, 2007; Shapiro-Mendoza & Lackritz, 2012). It is possible that women who have experienced prior complications of pregnancy or are aware that they are at greater risk for complications in the current pregnancy may be more likely to worry; thus causing an effect where women at most risk are also those who have the highest pregnancy-specific stress. Therefore to address confounding by indication, interaction effects were tested between maternal antenatal complications and each main effect, and none of the interactions was significant. Similar interaction effects were tested for history of adverse pregnancy outcome, and none of these interactions was significant either. This provides further evidence that the observed associations of pregnancy-specific stress with length of gestation are independent, regardless of antenatal complications of the current or past pregnancies.
Timing of Pregnancy-specific Stress Measurements
Results contribute to the debate on the best time during pregnancy to measure pregnancy-specific stress in order to explore the relationship with preterm birth and gestational age. Pregnancy-specific stress was assessed during both second and third trimesters, but not first trimester. Only change in pregnancy-specific stress was significantly associated with both preterm birth and gestational age. Third trimester pregnancy-specific stress was significantly associated with preterm birth only, and the magnitude of effect was less than that of the change over the course of pregnancy; thus indicating that change in pregnancy-specific stress has a stronger association with length of gestation than that measured in a cross-sectional manner. Similarly, Roesch et al. (2004) found that a latent variable representing change in pregnancy-specific stress across several time points during pregnancy had stronger association with shortened gestation than that measured at one time point, or a composite average of multiple time points.
Future research is needed to provide insight as to why change in pregnancy-specific stress has a stronger association with preterm birth and gestational age than mean levels assessed at one time during pregnancy. Findings are in line with Glynn et al. (2008) who found that change in perceived stress and state anxiety over the course of pregnancy had stronger associations with preterm birth than either variable at a single time point. They posit that such findings provide support for the hypothesis that changes in the prenatal stress profile mirror the dampening of the physiological stress response, and are therefore related to the prenatal physiological processes. More evidence, including longitudinal measurement and assessment of pregnancy-specific stress and related biomarkers, is necessary to explore the underlying biological mechanisms of the relationship between change in pregnancy-specific stress and length of gestation. Attenuation in the cortisol awakening response (a measure of hypothalamic-pituitary-adrenal axis responsiveness) over the course of pregnancy provides promise as a potential mechanism. Higher cortisol in late pregnancy and less pronounced dampening from early to late pregnancy is associated with shortened gestation (Buss et al., 2009).
Strengths, Limitations, and Future Directions
It is evident that there are factors other than pregnancy-specific stress that influence birth outcomes, especially in a population at such high risk for known risk factors for preterm birth (Ananth & Vintzileos, 2006; Goldenberg et al., 2008). Women in this sample were not only majority Black and/or Latina, populations known to be at risk for adverse birth outcomes, but they were also adolescent and young mothers. Therefore, it is important that we fully explore all possible risk factors for preterm birth including pregnancy-specific stress, taking into consideration clinical subtypes of preterm birth (e.g., spontaneous preterm birth, medically indicted preterm birth), in an attempt to improve clinical and behavioral knowledge in this area (Ananth & Vintzileos, 2006).
Strengths of this study include inclusion of multilevel biopsychosocial factors known to be associated with preterm birth, use of both a continuous and dichotomous length of gestation outcome, repeated assessments of pregnancy-specific stress throughout pregnancy, consideration of confounding by indication due to antenatal complications of the current or past pregnancies, and a unique sample comprised of an understudied group. The rate of preterm birth was lower in this sample compared to norms for women with similar characteristics, possibly because participants were engaged in prenatal care (Behrman & Butler, 2007).
Future research should attend to ways in which changes in pregnancy-specific stress over the course of pregnancy activate biological mechanisms associated with length of gestation, and reasons why pregnancy-specific stress is unique from other indicators of maternal psychological distress. Furthermore, no study in the literature (including this study) has included a measure of pregnancy-specific stress in the first trimester. There is evidence that first trimester is a sensitive period for the occurrence of stress (Glynn et al., 2008; Hobel et al., 1999; Lederman et al., 2004). In fact, for particular types of stress such as major life event stress, there is consensus that the associations between major life event stress and birth outcomes are stronger when the major life event occurs at the beginning of pregnancy as opposed to the end (Glynn et al., 2008; Lederman et al., 2004). Studies considering pregnancy-specific stress and other biopsychosocial factors longitudinally-- beginning in first trimester, are necessary for a more nuanced understanding of the time-varying relationship between pregnancy-specific stress and length of gestation.
This study included multi-level biopsychosocial factors associated with preterm birth at the individual, interpersonal, and sociocultural levels in order to understand the unique contribution of pregnancy-specific stress to length of gestation. Although Dunkel Schetter and Lobel (2012) highlight the importance of community-level factors such as residential segregation, neighborhood poverty, and access to healthcare in the multilevel biopsychosocial approach to birth outcomes, there are no such factors included in this study. Nonetheless, young women included in this study were all from low-resource urban neighborhoods and had similar access to care (Medicaid supported). Dunkel Schetter and Lobel (2012) acknowledge the challenge of including all potential factors in multilevel biopsychosocial models; however, when possible, studies should explicitly measure factors at each level to gain better understanding of pregnancy-specific stress and birth outcomes. In addition to primary data collection, opportunities may exist to combine existing datasets and utilize census tract data.
Future research should continue to examine these associations among women diverse in race, ethnicity, socioeconomic status, and age. Despite concerns about generalizability, it may be particularly important to identify and intervene to reduce adverse birth outcomes among young women of color given their relatively high risk for adverse birth outcomes. Given that only two studies to date included women under the age of 18, and majority of each sample was White women, the current work draws empirical attention to an understudied, yet vulnerable population (Dole et al., 2003, 2004; Lynn et al., 2011).
An additional limitation of this study is that women with missing observations were excluded from the sample and these women were more likely to have delivered preterm and more likely to have risk factors associated with preterm birth than those included in the sample. Although sensitivity analysis shows that missing data did not greatly influence the observed effect, future work should seek to replicate results with datasets with fewer missing observations. Also, behavioral data were assessed by survey and thus may be subject to bias if women underreport behaviors that have social stigma, such as smoking or substance use. Future research may benefit from the use of biomarkers to gain even more accurate assessments of risk behaviors.
Proposed mechanisms for preterm birth and low birthweight are distinct, despite the fact that both are correlated, and evidence of the association between pregnancy-specific stress and low birthweight is less definitive than that for preterm birth (Dunkel Schetter, 2011). Because this study was specifically interested in building a greater understanding of pregnancy-specific stress, length of gestation was chosen as the outcome as opposed to birthweight. Future research might examine if findings extend to birthweight.
Findings have implications for interventions and clinical practice to improve birth outcomes, particularly among young Black and Latina pregnant women. Within both intervention and clinical settings, it may be important to address pregnancy-specific stress over the course of pregnancy as opposed to only one time point. Continuous monitoring and subsequent intervention to reduce pregnancy-specific stress may benefit all women; however, future research is needed to ascertain whether it is possible to reduce this type of stress and the best methods to accomplish this, given that pregnancy-specific stress it is distinct from perceived general stress and other psychosocial measures. Additionally, because pregnancy-specific stress includes aspects directly related to tangible resources such as money, food security, and housing, it is important to consider intervention on these factors in addition to psychosocial factors.
Conclusion
Change in pregnancy-specific stress is significantly associated with gestational age and preterm birth among a sample of majority Black and/or Latina adolescents and young women. Third trimester pregnancy-specific stress is also significantly associated with preterm birth. The magnitude of effect for pregnancy-specific stress and length of gestation remained after other biopsychosocial risk factors were considered. Decreases in pregnancy-specific stress over the course of pregnancy predicted reduced odds of preterm birth and longer gestation. Findings from this study highlight the importance of studying pregnancy-specific stress longitudinally, exploring the mechanisms through which pregnancy-specific stress influences birth outcomes, and exploring solutions to address pregnancy-specific stress over the course of pregnancy. This work is imperative, as any lengthened gestation may decrease morbidity, improve health outcomes, and alleviate the social and financial burden of preterm birth in the United States (Behrman & Butler, 2007; Loftin et al., 2010; Shapiro-Mendoza & Lackritz, 2012).
Acknowledgments
This research was funded by the National Institute of Mental Health (R01 MH/HD61175 and T32 MH020031), with additional support from the Center for Interdisciplinary Research on AIDS (NIMH P30 MH062294). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
The authors would like to thank Claire Westdahl, CNM, MPH and Sharon Schindler Rising, CNM, MSN for their contribution to the study, as well as three anonymous reviewers and Dr. Annmarie Cano for their helpful comments on earlier versions of this manuscript.
Contributor Information
Heather J. Cole-Lewis, School of Public Health and Center for Interdisciplinary Research on AIDS
Trace S. Kershaw, School of Public Health and Center for Interdisciplinary Research on AIDS
Valerie A. Earnshaw, Center for Interdisciplinary Research on AIDS, Yale University
Kimberly Ann Yonkers, School of Medicine, Yale University.
Haiqun Lin, School of Public Health and Center for Interdisciplinary Research on AIDS.
Jeannette R. Ickovics, School of Public Health and Center for Interdisciplinary Research on AIDS
References
- Alderdice F, Lynn F, Lobel M. A review and psychometric evaluation of pregnancy-specific stress measures. Journal of Psychosomatic Obstetrics & Gynecology. 2012;33(2):62–77. doi: 10.3109/0167482X.2012.673040. [DOI] [PubMed] [Google Scholar]
- Amaro H, Zuckerman B, Cabral H. Drug use among adolescent mothers: profile of risk. Pediatrics. 1989;84(1):144–151. [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. Journal of Maternal-Fetal and Neonatal Medicine. 2006;19(12):773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- Arnold A, Lewis J, Maximovich A, Ickovics J, Kershaw T. Antecedents and consequences of caregiving structure on young mothers and their infants. Maternal and child health journal. 2011;15(7):1037–1045. doi: 10.1007/s10995-010-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman R, Butler A, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US); 2007. [PubMed] [Google Scholar]
- Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiologic reviews. 1993;15(2):414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- Boonstra H, Nash E. Minors and the right to consent to health care (The Guttmacher Report on Public Policy) Guttmacher Institute; 2000. Retrieved from http://www.guttmacher.org/pubs/tgr/03/4/gr030404.pdf. [PubMed] [Google Scholar]
- Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, Wadhwa PD. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. American Journal of Obstetrics and Gynecology. 2009;201(4):398.e1–398.e8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Chandra PC, Schiavello HJ, Ravi B, Weinstein AG, Hook FB. Pregnancy outcomes in urban teenagers. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2002;79(2):117–122. doi: 10.1016/s0020-7292(02)00240-0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Thousand Oaks, CA, US: Sage Publications, Inc.; 1988. pp. 31–67. [Google Scholar]
- Cokkinides VE, Coker AL, Sanderson M, Addy C, Bethea L. Physical violence during pregnancy: maternal complications and birth outcomes. Obstetrics and gynecology. 1999;93(5 Pt 1):661–666. doi: 10.1016/s0029-7844(98)00486-4. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Franklin C, Bennett P. Ecological factors associated with adolescent pregnancy and parenting. Social Work Research. 2000;24(1):29–39. [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, Behavior, and Immunity. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine. 2005;67(4):625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Dailey DE, Humphreys JC, Rankin SH, Lee KA. An Exploration of Lifetime Trauma Exposure in Pregnant Low-income African American Women. Maternal and Child Health Journal. 2011;15(3):410–418. doi: 10.1007/s10995-008-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, Sandman CA. Children’s Brain Development Benefits from Longer Gestation. Frontiers in Psychology. 2011;2 doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52(2):119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning JS, Klazinga NS, Koudstaal PJ, Prins A, Borsboom GJ, Mackenbach JP. The role of “confounding by indication” in assessing the effect of quality of care on disease outcomes in general practice: results of a case-control study. BMC Health Services Research. 2005;5(1):10. doi: 10.1186/1472-6963-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal Stress and Preterm Birth. American Journal of Epidemiology. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psychosocial factors and preterm birth among African American and White women in central North Carolina. American journal of public health. 2004;94(8):1358–1365. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Yu J-L. An overview of morbidity, mortality and long-term outcome of late preterm birth. World Journal of Pediatrics. 2011;7(3):199–204. doi: 10.1007/s12519-011-0290-8. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Stress Processes in Pregnancy and Preterm Birth. Current Directions in Psychological Science. 2009;18(4):205–209. [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual review of psychology. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Lobel M. Pregnancy and birth outcomes: A multilevel analysis of prenatal maternal stress and birth weight. In: Baum A, Revenson TAA, Singer J, editors. Handbook of Health Psychology. 2nd Edition. CRC Press; 2012. [Google Scholar]
- Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Current opinion in psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster HW, Wu L, Bracken MB, Semenya K, Thomas J, Thomas J. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in African Americans. Journal of the National Medical Association. 2000;92(5):213–221. [PMC free article] [PubMed] [Google Scholar]
- Gennaro S, Shults J, Garry DJ. Stress and preterm labor and birth in Black women. Journal of obstetric, gynecologic, and neonatal nursing: JOGNN / NAACOG. 2008;37(5):538–545. doi: 10.1111/j.1552-6909.2008.00278.x. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2008;27(1):43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. BMJ: British Medical Journal. 1993;307(6898):234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180(1, Supplement 2):S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Goldstein A, Barrett ES. Psychosocial Stress and Pregnancy Outcome. Clinical Obstetrics and Gynecology. 2008;51(2):333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, Rising SS. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstetrics and gynecology. 2007;110(2 Pt 1):330–339. doi: 10.1097/01.AOG.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. Journal of Reproductive Immunology. 2010;85(1):33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kershaw TS, Magriples U, Westdahl C, Rising SS, Ickovics J. Pregnancy as a Window of Opportunity for HIV Prevention: Effects of an HIV Intervention Delivered Within Prenatal Care. American Journal of Public Health. 2009;99(11):2079–2086. doi: 10.2105/AJPH.2008.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Platt RW. Stress Pathways to Spontaneous Preterm Birth: The Role of Stressors, Psychological Distress, and Stress Hormones. American Journal of Epidemiology. 2009;169(11):1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Lanz JB. Psychological, Behavioral, and Social Characteristics Associated with Early Forced Sexual Intercourse among Pregnant Adolescents. Journal of Interpersonal Violence. 1995;10(2):188–200. [Google Scholar]
- Lederman SA, Rauh V, Weiss L, Stein JL, Hoepner LA, Becker M, Perera FP. The Effects of the World Trade Center Event on Birth Outcomes among Term Deliveries at Three Lower Manhattan Hospitals. Environmental Health Perspectives. 2004;112(17):1772–1778. doi: 10.1289/ehp.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman E, Ryan KJ, Monson RR, Schoenbaum SC. Risk factors accounting for racial differences in the rate of premature birth. The New England journal of medicine. 1987;317(12):743–748. doi: 10.1056/NEJM198709173171206. [DOI] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2008;27(5):604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Lobel M, Hamilton JG, Cannella DT. Psychosocial Perspectives on Pregnancy: Prenatal Maternal Stress and Coping. Social and Personality Psychology Compass. 2008;2(4):1600–1623. [Google Scholar]
- Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, DeFranco EA. Late Preterm Birth. Reviews in Obstetrics and Gynecology. 2010;3(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Lynn FA, Alderdice FA, Crealey GE, McElnay JC. Associations between maternal characteristics and pregnancy-related stress among low-risk mothers: An observational cross-sectional study. International Journal of Nursing Studies. 2011;48(5):620–627. doi: 10.1016/j.ijnurstu.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal Prenatal Anxiety and Corticotropin-Releasing Hormone Associated With Timing of Delivery. Psychosomatic Medicine. 2004;66(5):762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Matthews T. Births: Final data for 2010. National vital statistics reports. 2012;61(1) [PubMed] [Google Scholar]
- Martin JA, Osterman MJK, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS data brief. 2010;(39):1–8. [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nature Medicine. 1995;1(5):460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Misra DP, Guyer B, Allston A. Integrated perinatal health framework: A multiple determinants model with a life span approach. American Journal of Preventive Medicine. 2003;25(1):65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Neggers Y, Goldenberg R, Cliver S, Hauth J. Effects of domestic violence on preterm birth and low birth weight. Acta obstetricia et gynecologica Scandinavica. 2004;83(5):455–460. doi: 10.1111/j.0001-6349.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- Nkansah-Amankra S, Dhawain A, Hussey JR, Luchok KJ. Maternal social support and neighborhood income inequality as predictors of low birth weight and preterm birth outcome disparities: analysis of South Carolina Pregnancy Risk Assessment and Monitoring System survey, 2000–2003. Maternal and child health journal. 2010;14(5):774–785. doi: 10.1007/s10995-009-0508-8. [DOI] [PubMed] [Google Scholar]
- O’Brien K, Wortman CB, Kessler RC, Joseph JG. Social relationships of men at risk for AIDS. Social science & medicine (1982) 1993;36(9):1161–1167. doi: 10.1016/0277-9536(93)90236-w. [DOI] [PubMed] [Google Scholar]
- Orr ST, James SA, Miller CA, Barakat B, Daikoku N, Pupkin M, Huggins G. Psychosocial stressors and low birthweight in an urban population. American journal of preventive medicine. 1996;12(6):459–466. [PubMed] [Google Scholar]
- Orr ST, Reiter JP, Blazer DG, James SA. Maternal Prenatal Pregnancy-Related Anxiety and Spontaneous Preterm Birth in Baltimore, Maryland. Psychosomatic Medicine. 2007;69(6):566–570. doi: 10.1097/PSY.0b013e3180cac25d. [DOI] [PubMed] [Google Scholar]
- Parker J, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, premature delivery in the United States. Annals of epidemiology. 1994;4(4):271–278. doi: 10.1016/1047-2797(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Parker V, Douglas A. Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. Journal of Reproductive Immunology. 2010;85(1):86–92. doi: 10.1016/j.jri.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Grove J, Bonney EA, Bliwise N, Dudley DJ, Schendel DE, Thorsen P. Interrelationship of Cytokines, Hypothalamic-Pituitary-Adrenal Axis Hormones, and Psychosocial Variables in the Prediction of Preterm Birth. Gynecologic and Obstetric Investigation. 2010;70(1):40–46. doi: 10.1159/000284949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike IL. Maternal stress and fetal responses: Evolutionary perspectives on preterm delivery. American Journal of Human Biology. 2005;17(1):55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Furberg CD. Assessment and control for confounding by indication in observational studies. Journal of the American Geriatrics Society. 1999;47(6):749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1999;18(4):333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Schetter CD, Woo G, Hobel CJ. Modeling the types and timing of stress in pregnancy. Anxiety, Stress & Coping. 2004;17(1):87–102. [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Social science & medicine (1982) 2011;72(6):977–983. doi: 10.1016/j.socscimed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Glynn LM. Psychobiological Stress and Preterm Birth. In: Morrison J, editor. Preterm Birth - Mother and Child In Tech. 2012. Retrieved from http://www.intechopen.com/books/preterm-birth-mother-and-child/psychobiological-stress-and-preterm-birth. [Google Scholar]
- SAS. Cary, NC, USA: SAS Institute; 2008. [Google Scholar]
- Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Seminars in fetal & neonatal medicine. 2012;17(3):120–125. doi: 10.1016/j.siny.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway J, O’Campo P, Gielen A, Witter FR, Khouzami AN, Blakemore KJ. Preterm labor, placental abruption, and premature rupture of membranes in relation to maternal violence or verbal abuse. The Journal of maternal-fetal medicine. 1999;8(3):76–80. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<76::AID-MFM2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Smith R. The timing of birth. Scientific American. 1999;280(3):68–75. doi: 10.1038/scientificamerican0399-68. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. American journal of obstetrics and gynecology. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. American journal of obstetrics and gynecology. 1993;169(4):858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Yang S, Bergvall N, Cnattingius S, Kramer MS. Gestational age differences in health and development among young Swedish men born at term. International Journal of Epidemiology. 2010;39(5):1240–1249. doi: 10.1093/ije/dyq070. [DOI] [PubMed] [Google Scholar]
- Yang S, Platt RW, Kramer MS. Variation in Child Cognitive Ability by Week of Gestation Among Healthy Term Births. American Journal of Epidemiology. 2010 doi: 10.1093/aje/kwp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bracken MB. Tree-based risk factor analysis of preterm delivery and small-for-gestational-age birth. American journal of epidemiology. 1995;141(1):70–78. doi: 10.1093/oxfordjournals.aje.a117347. [DOI] [PubMed] [Google Scholar]