Abstract
Primary cilia were the largely neglected non-motile counterparts of their better-known cousin, the motile cilia. For years these non-motile cilia were considered evolutionary remnants of little consequence to cellular function. Fast-forward 10 years and we now recognize primary cilia as key integrators of extracellular ligand-based signaling and cellular polarity, which regulate neuronal cell fate, migration differentiation, as well as a host of adult behaviors. Important future questions will focus on structure-function relationships, their roles in signaling and disease, and as areas of target for treatments.
Keywords: primary cilium, ciliopathies, nervous system, development, treatment
Introduction
The role of primary cilia are well known in special sensory cells involving hearing, vision, taste and olfaction, as the location of stimulus transduction, but their identification in other neural types came as somewhat of a surprise. Evidence for primary cilia in the vertebrate nervous system was first documented during electron microscopic examination of brain sections. The observation was initially assumed to be simply evidence of an evolutionary remnant. The finding harkened back to our evolutionary roots as flagellates, when cells needed a motile flagella for movement. For decades the observation that most neural cells display a primary cilia was ignored.
All of that began to change with several key observations: 1] The protein products of genes mutated in murine hydrocephalus are localized to the primary cilium. 2] Sonic hedgehog signaling in vertebrates is altered with mutations in flagellar genes. 3] Human cerebellar disorders are due to mutations in genes encoding ciliary proteins, thus defining the ciliopathies. Recent studies have demonstrated diverse roles for primary cilia, which include regulation of signal transduction that goes well beyond their sensory roles (Goetz and Anderson, 2010).
The primary cilium is increasingly viewed as critical for the development of the vertebrate nervous system, playing either essential or modulatory roles in specific neurodevelopmental signaling pathways such as Sonic hedgehog (Shh) and Wnt (Han et al., 2008). Cilia also play important roles in regulation of stem cells and regeneration in the adult nervous system, where cell cycle and specific morphogen pathways recapitulate developmental functions to facilitate cell fate decisions in ciliated tissues. Moreover, primary cilia are implicated in neuronal signaling and central control of appetite (Berbari et al., 2013; Spassky et al., 2008), but the specific mechanisms are still the subject of debate.
Ciliopathies: Human Disease Class Related to Ciliary Dysfunction
The ciliopathies are a group of genetic disorders caused by defects in primary ciliary structure and/or function. Ciliopathies are characterized by pleiotropic clinical features, with mutations disrupting ciliary function and leading to distinctive developmental and/or degenerative phenotypes in several tissues, including the retina, kidney and nervous system (Lee and Gleeson, 2011). However, cilia still form in cells with mutations in these cilia genes, although they may be altered morphologically. Therefore, it is important to differentiate between the role of a specific gene and the role of the entire cilium in a cell. By studying ciliopathies we can begin to understand the key roles of cilia in development and homeostasis, but must recognize that cilia may still maintain some function even in the setting of specific ciliopathy gene mutations.
More than a dozen disorders are now considered to be within the ciliopathy spectrum, with a plethora of names to distinguish the subtypes including named syndromes Joubert (JBTS), Nephronophthisis (NPHP), Senior-Løken (SLS), Leber congenital amaurosis (LCA), Meckel-Gruber (MKS), Alström (AS) and Bardet-Biedl (BBS). Ciliopathies demonstrate a range of clinical features including early fetal lethality, polydactyly (extra fingers or toes), cardiac defects, cystic kidneys, hepatic fibrosis, obesity and nervous system defects. Although most any of the specific subtypes can display involvement in any of these organs, each has a classical presentation, and most patients can be diagnosed specifically. Altogether, these conditions involve nearly every major body organ and emphasize the important role of the primary cilium in development and homeostasis (Goetz and Anderson, 2010).
Although it is now clear that mutations in over 50 different genes lead to ciliopathies, much less is known about specific ciliary signaling pathways and the pathogenic principles that ultimately result in disease phenotypes at the tissue and organ levels. The phenotypic characterization of the disorders, the identification of causative genes and research focusing on gene function are providing new insights that link ciliary function with key developmental signaling pathways. Nervous system related defects include neural tube, migration and cerebellar developmental, retinal degeneration, anosmia and obesity. The plethora of nervous system effects suggests many potential roles of cilia in development and function.
JBTS is a genetically and phenotypically heterogeneous disorder with several subtypes united by the defining feature of hindbrain defects observed on axial brain MRI sections, known as the ‘molar tooth’ sign. JBTS is sometimes associated with hydrocephalus (i.e. enlarged cerebral ventricles), anatomical abnormalities of the cerebral cortex, retinal dystrophy and cognitive deficits including intellectual disability and autism spectrum disorders (Romani et al., 2013). BBS is also genetically heterogeneous and is characterized by intellectual disability, anosmia, obesity, polydactytly, hypogonadism, and retinal degeneration (Zaghloul and Katsanis, 2009). MKS is an early lethal disorder, genetically overlapping with JBTS and BBS, with occipital encephalocele, cystic kidney, and polydactyly (Barker et al., 2013). Alström syndrome, caused by mutations in the ALMS1 gene, is associated with obesity and retinal degeneration, but unlike BBS or JBTS, mental defect, polydactytly and hypogonadism are not featured (Collin et al., 2002). One might expect that tissue expression of the responsible genes correlate with disease organ specificity or time of disease onset, but no such correlations have emerged to date. Thus it is fascinating to consider how these tiny subcellular organelles can mediate so many diverse cellular functions, and how they may be potentially disrupted in so many different ways to produce such specific syndromes.
Structure-Function Relationships of the Cilium
The primary cilium is a slender extension of the cell membrane protruding from the surface of most cells, most notable in epithelial cells. The cilium is assembled within a ciliary membrane extended over the axoneme and is anchored to the cell by the basal body. Primary (i.e. non-motile) ciliary axonemes classically contain nine doublet microtubules (9+0 axoneme), whereas secondary (i.e. motile) cilia axonemes contain nine doublet microtubules and an extra central pair of microtubules that are attached to a dynein motor to generate movement (9+2 axoneme). Therefore, the ultrastructure of the axoneme can predict whether a given cilium is likely to be motile or non-motile. In the brain, motile cilia are restricted to ependymal cells lining the ventricle and some choroid plexus cells (Lee, 2013), whereas primary cilia are evident on virtually all brain cells including progenitors, neurons and astrocytes.
A key feature of cilia is that they contain no vesicles and thus utilize methods different from the rest of the cell to transport lipids and transmembrane proteins. While the ciliary membrane is contiguous with the plasma membrane, it has a unique set of sensory and transduction proteins to respond to extracellular signals. The cytoplasm of cilia is mostly isolated from the rest of the cell by a transition zone (TZ) at the base, which acts as a selective pore, and by the GTPase Septin in the membrane, thereby establishing a barrier to protein diffusion as well as a loading-unloading zone for transport into and out of the cilium (Reiter et al., 2012).
Proteins selected for entry are carried along the axoneme by intraflagellar transport (IFT) (Kozminski et al., 1993), mediated by two protein complexes, IFT-B and IFT-A. IFT-B complex moves cargo from the cilia base towards the tip under the control of the anterograde kinesin-2 (Kif3 motor complex), whereas IFT-A moves cargo in the opposite direction utilizing the retrograde axonemal dynein motors. Mutations in IFT-B components such as Ift172, Ift88, Ift52 and Ift57 lead to complete absence of cilia and, surprisingly, severely blunted Shh signaling (Huangfu et al., 2003). In contrast, some mutations in IFT-A proteins lead to the formation of abnormally bulbous or elongated ends, consistent with a cargo backup, and, even more surprisingly, result in in activation rather than repression of Shh signaling (Qin et al., 2011). The consensus is that disruption of IFT-A may disturb trafficking of Shh pathway components differentially, causing phenotypes distinct from those observed in mutants in which cilia are absent.
The encoded ciliopathy proteins localize mostly to the ciliary base or axoneme, now a standard assessment for confirmation of newly proposed ciliopathy factors. While initial observations focused on simple reduction in the percent of ciliated cells or length of cilia in mutant cells, the field has come to appreciate that this is too crude a measure of disrupted function. Recent observations point to important defects in specific signaling pathways in ciliary ultrastructure such as impaired axonemal tubulin modifications or structure of the 9+0 arrangement (Lee and Gleeson, 2011). Making things more complicated is the finding that these same pathways can themselves regulate ciliogenesis, spacing and orientation of cilia, and whether a cell builds a motile or non-motile cilium (Boskovski et al., 2013).
For instance, most JBTS genes identified to date encode proteins localized predominantly to the transition zone or the ciliary axoneme. One of these genes mutated in JBTS, ARL13B, encodes a small GTPase localized to the ciliary axoneme, which regulates the graded response to Shh signaling (Caspary et al., 2007). In the absence of Arl13b in mice, Smo is constitutively enriched in cilia (Larkins et al., 2011). Mutant mammalian cells show reduced cilia length and defective axonemal tubulin structure, whereas overexpression results in artificially lengthened cilia. In arl13 mutant worms, disrupted IFT integrity is linked to dissociation between IFT-A and IFT-B and compromised ciliogenesis. Still many questions remain, including how Arl13b controls tubulin structure, how the defect in IFT contributes to defective Shh signaling, and the identity of Arl13b guanine exchange factors (GEFs) and GTP activating proteins (GAPs).
Cilia in Sonic Signaling
The importance of primary cilia in vertebrate development was first identified by genetic experiments demonstrating that ciliary proteins are required for murine embryonic patterning, linking Shh signaling to cilia function in mammals (Huangfu et al., 2003). Shh signaling is essential for embryonic development of numerous tissues, controlling the pattern of cellular differentiation in a concentration-dependent fashion, and disruptions lead to neural tube and dorso-ventral patterning defects. Although the Shh pathway can exhibit low-level activity in the absence of cilia, in vertebrates, cilia are nonetheless required for signal amplification when Shh ligand is present. The result is defects in spinal cord patterning, and alterations in cerebellar granule neuron pools expansion, both under Shh control. There are also midline defects including optic colobomas, corpus callosal defects, hydrocephalus, failed closure of the rostral neural tube (i.e. exencephaly) and open posterior neural tube (i.e. occipital encephalocele).
Shh regulates the balance of Gli transcriptional activators and repressors, dependent upon the ability of Smo and Ptch signaling components to travel through cilia (Humke et al., 2010). The ciliary-associated kinesin Kif7 is the top candidate for ciliary transport of these factors, acting downstream of Smo and upstream of Gli2, and having both negative and positive roles in Shh signal transduction (Goetz and Anderson, 2010). The role of cilia in hedgehog signaling extends to vertebrates other than mammals, and to hedgehogs other than sonic, suggesting ancient evolutionary connections. Several other signaling proteins including protein kinase A, Gpr161, pituitary adenylate cyclase activating polypeptide and forkhead transcription factor Foxj1 mediate cilia- and Gli-dependent Shh signaling (Cruz et al., 2010; Mukhopadhyay et al., 2013; Niewiadomski et al., 2013; Tuson et al., 2011) and a complete understanding of mechanisms is one of the holy grails of Dev Biol (Figure 1).
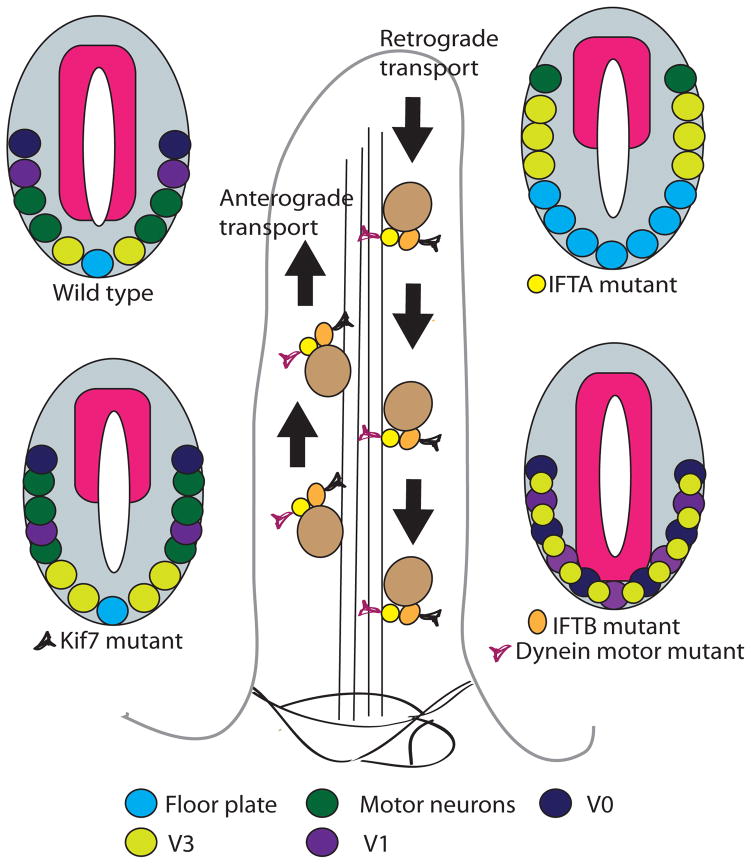
Figure 1. Neural patterning phenotypes in intraflagellar transport mutants.
Axonemal and membrane components are transported by intraflagellar transport (IFT) particles (complexes B and A) along the axoneme toward the tip complex (anterograde transport) by kinesins and in the opposite direction (retrograde transport) by the motor dyneins, respectively. In wt embryos, ventral neural cell fates are specified by Shh gradient. Anterograde IFT-B mutants Ift88 or Ift172 lack cilia, Shh signaling is reduced and the neural tube is dorsalized. Retrograde IFT-A mutants like Ift139 exhibit excess Shh signaling and expanded ventral cell pools. Mutations that disrupt the kinesin Kif7 cause a partial activation of the Shh pathway, with an expansion of motor neurons that require intermediate levels of Shh. V0, V1, motor neurons, V3 and floor plate represent progressive more ventral cell types in the developing spinal cord. (Adapted from (Goetz and Anderson, 2010))
Cilia interplay with PCP and Wnt signaling
Disruptions of PCP protein function are also linked to neural tube defects (NTDs), but in more caudal areas, probably reflecting a requirement for cell polarity cues during complex convergent extension cell-cell interactions at the dorsal midline (Wallingford, 2012). But unlike cilia in regulation of Shh signaling, which is well established, it is less clear if cilia regulate the PCP pathway, vice versa, or both. The best evidence is from the Vangl1/2 double knockout mouse, the two homologues of the Drosophila core PCP component, which shows intact ciliogenesis, cilium motility, Shh signaling and basal body docking, but completely disrupted cellular polarity and as a result, failed left-right asymmetry at the node (Song et al., 2010). The results suggest that PCP controls cellular and ciliary positioning, but not signaling.
Are cilia involved in PCP or Wnt signal transduction? Inversin, a key cilia protein mutated in cystic kidney disease, acts in primary cilia to inactivate canonical Wnt signaling during kidney morphogenesis and promote convergence extension, thus functioning as a switch between these two Wnt pathways (Simons et al., 2005). Ahi1, mutated in JBTS, is a Wnt potentiator, facilitating beta-catenin nuclear accumulation (Lancaster et al., 2009). On the other hand, some evidence points to a role for cilia in negative regulation of Wnt signaling, as embryos without cilia (i.e. kif3a nulls) show dramatically enhanced Wnt reporter activation (Corbit et al., 2008). The data suggest a role for cilia in repressing Wnt signaling, not by silencing the pathway, but by maintaining a discrete range of Wnt responsiveness (Lancaster et al., 2011). Yet not all studies are in agreement, most notably a comprehensive study of canonical Wnt signaling in mutant embryos that lack primary cilia due to loss of IFT components found no Wnt phenotype or signaling defects (Ocbina et al., 2009). Thus results vary, and we can at least conclude that if cilia regulate Wnt signaling, it may be in subtle, perhaps indirect ways or in specific settings.
Primary cilia, autophagy, and cell cycle entry
The assembly of primary cilia is a dynamic process initiated once cells exit the cell cycle to enter quiescence. In cultured cells, primary cilium assembly and autophagy are both triggered by serum withdrawal and thus it is not surprising that part of the molecular machinery involved in ciliogenesis also participates in the early steps of the autophagic process, and recent evidence link these processes together (Pampliega et al., 2013; Tang et al., 2013). In Parmpliega’s paper, a previous unknown reciprocal relationship between ciliogenesis and autophagy was identified finding that autophagy inhibits ciliogenesis by limiting ciliary trafficking of components required for ciliary growth. In contrast, Tang’s paper demonstrates that autophagic degradation of the ciliopathy protein OFD1 at centriolar satellites promotes primary ciliary biogenesis. These apparently contradictory findings remain unresolved, but suggest cross-talk between the pathways.
Cilia role in Neurogenesis, Migration and Axon Guidance
Shh signaling is the major proliferative driver for cerebellar granule neuron precursors (CGPs), an effect directly mediated by cilia and a likely explanation for the cerebellar defects observed in ciliopathies. CGPs display cilia, and are essential for Shh-dependent proliferation of CGPs during cerebellar development (Spassky et al., 2008). Accordingly, the conditional removal of other Shh targeted ciliary genes (e.g. Ift88 or Stumpy) in CGPs gives rise to striking dysgenesis and abnormal foliation of the cerebellum due to decreased proliferation (Breunig et al., 2008; Chizhikov et al., 2007). In addition, mutations of ciliary genes including Fantom, Ofd1 and Smo, also show cerebellar defects (Ferrante et al., 2006; Spassky et al., 2008; Vierkotten et al., 2007). Moreover, the link between cilia and CGP proliferation extends to medulloblastoma, as CGPs are the likely cell source of the tumor, and cilia regulate tumorogenesis (Han et al., 2009).
Freely motile vertebrate cells like sperm use their modified motile cilium (i.e. flagella) for motility, but can primary cilia also regulate cellular motility? Cilia are critical for some forms of Platelet-Derived Growth Factor (PDGF) signaling, a stimulant for fibroblast migration (Schneider et al., 2010). Do neurons behave similarly? Shh has potent chemotactic effects, but surprisingly ciliary mutants show enhanced rather than diminished Shh chemotactic responsiveness (Bijlsma et al., 2012), and thus any effects on migration are likely to be Shh-independent. Although migration defects are not widely recognized in ciliopathies, evidence suggests that BBS patients have defective neural crest cell migration as cause for facial dysmorphisms and gut motility defects, thus implicating primary cilia albeit indirectly (Tobin et al., 2008).
The best evidence for cilia in neuronal migration comes from study of the ciliary protein Arl13b in the cerebral cortex, where interneuron mutants show a specific defect in directional navigation but not general motility. Ciliary receptors such as GPCRs essential for guidance cue responsivity fail to localize in Arl13b mutant cells (Higginbotham et al., 2012), potentially as a result of defective PKA-mediated phosphorylation of cilia-localized proteins (Niewiadomski et al., 2013). These defects in cilia-dependent interneuron migration may in part underlie the neurological defects in JBTS patients (Figure 2).
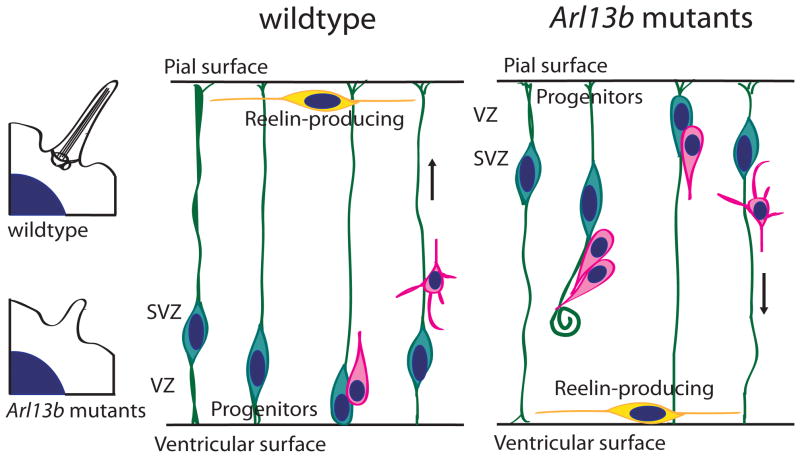
Figure 2. Primary cilia participate neural migration during cortical development via Arl13b.
Arl13b is critical for the formation of the polarized radial progenitor scaffold (wildtype). Deletion of Arl13b led to a reversal in radial glial apical-basal polarity, inversion of position of reelin-producing cells, and consequently to aberrant neuronal migration, i.e. from the pial surface to the ventricular surface (arrow indicates direction).
In the JBTS patients studied at postmortem, a striking finding was failed decussation of the corticospinal tract (CST) and superior cerebellar peduncles (SCP) (Yachnis and Rorke, 1999). The CST is the main motor output of the cerebral cortex, decussating at the cervicomedullary junction, and the SCP is the main outflow tract of the cerebellum, decussating at the red nucleus, so this finding suggests an axon guidance defect in JBTS patients. Moreover, functional imaging and diffusion tensor imaging support defects in axon guidance in JBTS patients (Poretti et al., 2007), but mouse models have failed to show correlated defects (Lancaster et al., 2011). The obvious question is how cilia, located at the soma, influence direction or guidance of the growth cone. Thus ciliary roles and mechanisms in axon guidance remain to be elucidated.
Primary Cilia and Cortical Development
Convergent data point to a role for cilia-associated factors in mammalian brain patterning (i.e. the progressive subdivision of the neural tube along the dorsal-ventral and rostral-caudal axis). Genetic screens have uncovered mutations in Ift88 (an anterograde IFT component essential for ciliogenesis), Ttc21b (encoding retrograde IFT139) and Slb (encoding anterograde IFT172) in mice with profound Shh-related cortical pattern defects (Gorivodsky et al., 2009; Stottmann et al., 2009; Willaredt et al., 2008).
It is important to differentiate between roles for primary cilia and roles for the centrioles in cortical development, even though the mother centriole forms the base of the cilium. Asymmetric divisions of radial glia progenitors produce both self-renewing radial glia and differentiating neuroblasts simultaneously in the ventricular zone (VZ). Whereas differentiating neuroblasts leave the VZ to constitute the future neocortex, renewing radial glia progenitors stay in the VZ for subsequent divisions. In both fly and mouse, the cell that inherits the older centriole preferentially remains proliferative whereas the younger centriole marks the differentiating neuroblast (Wang et al., 2009; Yamashita et al., 2007). In agreement, the vast majority of genes mutated in human primary microcephaly encode centrosome-associated proteins, suggesting non-ciliary roles for the centrosome in proliferation and stem cell maintenance, probably functioning at the mother centriole.
It is also obviously important to differentiate between the requirement for an organelle and the requirement for proteins localized to the organelle. While cilia are identified on nearly all cortical projection and interneurons, many murine mutants with aberrant ciliogenesis show remarkably normal neuronal migration, laminar organization and polarity (for example the Stumpy mutant) (Arellano et al., 2012), whereas for other mutants there are notable developmental defects. One such example is Arl13b, deletion of which leads to a reversal in radial glial apical-basal polarity and consequently aberrant neuronal migration (Higginbotham et al., 2013). The fascinating aspect of the phenotype, novel in mammalian cortical mutants to date, was a complete inversion of the entire cortical organization, i.e. the apical aspect was located basally and vice versa. Whether this phenotype is the result of exencephaly or hinting a role for cilia in radial glial polarity is still controversial, but as a result, proliferation occurred adjacent to the pia and the reelin-positive layer 1 was adjacent to the ventricle. The phenotype was recapitulated with conditional removal using Foxg1 but not Nestin or Gfap-driven Cre, suggesting a requirement during polarization but not maintenance of radial glia.
Interestingly, the targeted ablation of Arl13b in projection neurons did not alter their migration but did induce axonal outgrowth and connectivity defects (Arellano et al., 2012). Moreover, disruption of ciliogenesis in layer 2/3 cortical neurons, as well as in neurons destined for the deeper layers of the cortex reduces dendritogenesis, supporting a link between cilia and dendrite outgrowth (Guadiana et al., 2013). Together, these results, pending validation, suggest that the impact of ciliogenesis on neuronal development and maturation is dependent on both developmental age and neuronal subtype.
Rotatin is a centrosome-associated protein that colocalizes with the basal bodies at the primary cilium. (Kheradmand Kia et al., 2012). Humans with mutations show an abnormal cortical organization called polymicrogyria, and structural abnormalities of the cilium. Some BBS patients also exhibit enlarged cerebral ventricles, and a ciliopathy mouse model for BBS shows increased apoptosis and reduced neurogenesis progressing to neonatal hydrocephalus as a result of defective development (Carter et al., 2012). These results hint at requirements for ciliary proteins in human cortical development, but specific mechanisms will require more work.
Defective neuronal migration and resulting aberrant connectivity may represent a key to understanding the various neurological symptoms exhibited by patients diagnosed with ciliopathies, including cognitive deficits, autism spectrum disorders, seizures, schizophrenia, and developmental dyslexia. For instance, the frequent cognitive impairments in JBST are likely to be accompanied by less obvious defects in the cerebral cortex as nearly 40% of patients are diagnosed with autism spectrum disorders (Alvarez Retuerto et al., 2008). The specific changes induced in the neural network or in signaling pathways by ciliary defects may depend on the nature of the defect and the type of neuron affected.
Role of Cilia in Special Sensory Neurons
Sensory neurons in the olfactory, visual, and acousticovestibular systems display a highly modified and specialized primary cilium. Specialized sensory cilia are located between the outer and inner segments of the retinal rods and cones and on the dendrites of olfactory receptor neurons (Louvi and Grove, 2011). Primary cilia in olfactory neurons respond to odorants via olfactory G-protein-coupled receptors (GPCRs), producing a cAMP-mediated depolarization, whereas photoreceptor outer segments mediate phototransduction via the rhodopsin family of GPCR, producing a cGMP-mediated hyperpolarization (Hengl et al., 2010). The modified cilium of photoreceptors is especially sensitive to disruptions, and without an intact connecting cilium photoreceptors apoptosis, a condition known as Leber Congenital Amaurosis (LCA) (if early onset) and retinitis pigmentosa (RP) (if later onset) (Adams et al., 2007). LCA/RP as well anosmia are well described in ciliopathies including JS, NPHP, and BBS, among others (Arts et al., 2007; Delous et al., 2007). Acousticovestibular cells show a primary cilium-like kinocilium, and thus it is not surprising that deafness is a frequent accompaniment in ciliopathies such as in Usher syndrome. Although GPCRs mediate gustatory responses, there is a paucity of literature on gustatory cilia.
Primary Cilia in the Adult Nervous System: Neurogenesis and Signaling?
New neurons are continuously produced to control circuit plasticity, learning and memory in the hippocampal dentate gyrus (DG) and anterior subventricular zone (aSVZ). Shh signaling is not only crucial for neurodevelopment, but also participates in the expansion of postnatal progenitors (Breunig et al., 2008). Is adult neurogenesis under the control of cilia? Treatment with Smo inhibitor decreases proliferation in vivo, and mice lacking Smo in GFAP-positive neural precursors exhibit hippocampal neurogenesis defects (Machold et al., 2003), and loss of ciliary genes (Kif3a, Ift88, or Ftm) leads to defective hippocampal neurogenesis due to premature cell cycle exit (Amador-Arjona et al., 2011; Han et al., 2008). Interestingly, loss of either primary cilia or Shh signaling has no effect on the generation of other cell types such as astrocytes, suggesting that this mechanism is cell-type specific.
Although hippocampal defects have not been extensively described in human ciliopathy patients, learning disabilities are common, and reports suggest that up to half of BBS patients have neocortical and hippocampal volume loss. Moreover, there are connections between hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with BBS, emphasizing the impact of primary cilia in cognition (Baker et al., 2011; Bennouna-Greene et al., 2011). Murine models also indicate a delay in spatial learning and alteration of spatial novelty recognition with decreased adult neurogenesis by conditional ablation of cilia-required genes (Amador-Arjona et al., 2011). Whether the cognitive deficits observed in ciliopathies are related to the alteration in Shh signaling or other signaling pathways remains to be determined. One interesting possibility is altered cilia-localized GPCR signaling. For instance, somatostatin receptor 3 (SSTR3) is localized to the neuronal cilium, and Sstr3 mouse mutants show impaired object recognition and hippocampal LTP (Einstein et al., 2010; Stanic et al., 2009).
Might GPCR or other receptors use cilia as signaling centers or hubs to integrate signals and regulate synapses? TULP1, mutated in humans with RP, is required for photoreceptor synaptic ribbon development in mice prior to the onset of retinal degeneration, showing disrupted spatial relationship between the ribbon-associated proteins, Bassoon and Piccolo (Grossman et al., 2010). Overexpression of dominant negative KIF3A blocks ciliogenesis in adult hippocampal-derived neural progenitors and as a result there is enhanced Wnt signaling and defects in dendritic refinement and glutamatergic synapse formation (Kumamoto et al., 2012). Although these results point to potential roles for neuronal cilia in synapse development, further confirmation in other systems is missing.
In the case of special sensory photoreceptor and olfactory neurons, signals must pass through the cilium to the synapse to relay information, but how much capacity do primary cilia have for conveying signals to the cell body? Chlamydomonas uses compartmentalized calcium signaling in motile cilia to drive intraflagellar transport (Collingridge et al., 2013), but visualizing calcium fluctuations in cilia is only now possible. Cilium-localized calcium indicators now provide evidence of calcium signaling in cilia upon chemical or mechanical stimulation (Su et al., 2013). By patch clamping onto ciliary membrane (the difficulty of which should not be underappreciated), the Polycystin proteins were identified as ciliary TRP-like calcium channels, modulating Shh signaling. However, the Pkd2l1 mutant mice present a mild phenotype when compared to other Shh-deficient mutant mice, suggesting that calcium is probably also adjusted by other PKD members or even other as-yet-unidentified ion pumps or transporters in the cilium during development. The ciliary membrane is richly populated with calcium-permeant, relatively non-selective cation channels, but the ciliary compartment is mostly isolated electrophysiologically and chemically from cellular compartments (DeCaen et al., 2013; Delling et al., 2013). The degree to which these tools will enable new discoveries in ciliary signaling is unknown.
Primary Cilia and Obesity
Obesity is a common feature in syndromes such as BBS and Alström, suggesting that cilia may modulate neural circuitry that monitors food intake and appetite. Recent data indicate that obesity in BBS mutant mice is due to defects in leptin receptor trafficking and downstream Stat3 signaling leading to leptin resistance (Rahmouni et al., 2008), because obesity occurs despite elevations in leptin, a satiety signal, and because loss of cilia specifically in leptin-responsive proopiomelanocortin (POMC) neurons results in obesity (Davenport et al., 2007). Bbs1 interacts with the leptin receptor and mediates its trafficking, and mice lacking Bbs2, Bbs4, and Bbs6 also present leptin resistance, failing to reduce food intake in response to leptin (Seo et al., 2009) (Figure 3).
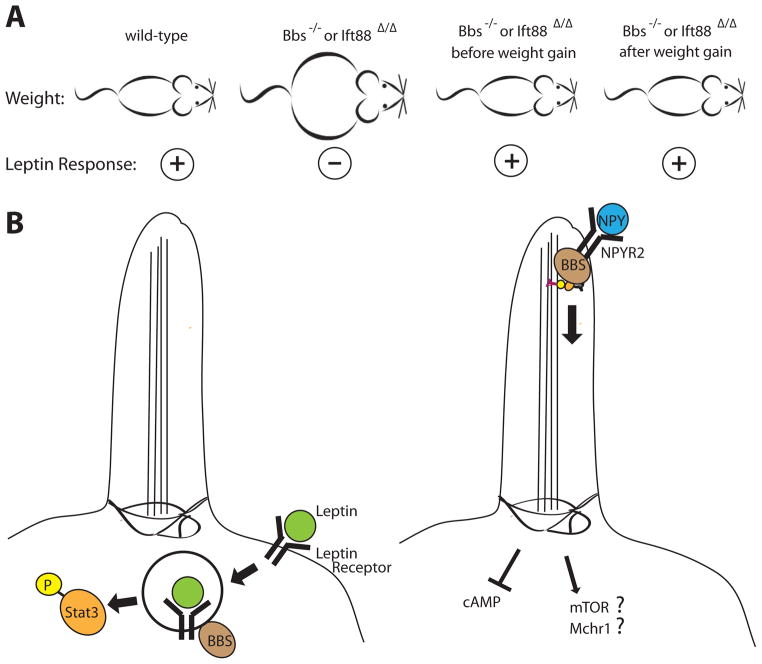
Figure 3. Proposed roles for cilia in regulation of weight and predisposition to obesity.
A. Bbs knockout or IFT88 conditional knockout mice are obese and show reduced leptin responsiveness. However, both before weight gain and after weight loss the knockouts show intact leptin responsiveness, suggesting the leptin insensitivity is secondary to obesity, and is independent of ciliary function. B. Models for the roles of ciliopathy proteins in obesity. Left: proposes leptin receptors at the cell membrane require BBS for trafficking and downstream Stat3 signaling. Right: proposes other satiety signals like NPY, signaling through its cilia-localized NPY2R GPCR receptor, requires BBS components for trafficking and modulation of cAMP levels. Alternatively, cilia may modulate satiety through pathways such as mTOR or Mchr1.
But more recent work suggests that Bbs4 and Ift88 mutant mice present hyperphagia-associated obesity independent from defective leptin signaling and ciliary function, as both before weight gain and after weight loss the knockouts show intact leptin responsiveness. Furthermore, other phenotypes associated with leptin signaling defects are not consistent between cilia mutant mice and leptin deficient ob/ob mice, suggesting that leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice (Berbari et al., 2013). There are several posited mechanisms for the obesity observed in ciliopathies other than leptin-signaling defects. The melanin concentrating hormone receptor 1 (Mchr1), present on hypothalamic neurons, is mis-targeted in Bbs mutant mice (Berbari et al., 2008). Altered Mchr1 signaling in the absence of cilia could result in hyperphagia, inducing obesity. Another possibility is alteration of mTOR activity due to cilia loss (Berbari et al., 2011; Boehlke et al., 2010). Although treatment of cilia mutant mice with rapamycin can partially correct some of the ciliary phenotypes (Shillingford et al., 2006), the connection between cilia, mTOR signaling and obesity requires further evaluation. Obesity in BBS mice may alternatively result from defects of hypothalamus patterning, due to misregulated Shh signaling. Accordingly, Bbs mutant mice have a decrease number of POMC neurons (Seo et al., 2009). Thus, it will be interesting to evaluate whether modulating cilia-dependent signaling activities can alter feeding behavior and energy homeostasis. A recent paper proposes other satiety signals like NPY, signaling through its cilia-localized NPY2R receptor, requires BBS components for trafficking and modulation of cAMP levels to control energy balance in mammals (Loktev and Jackson, 2013).
Recently, homozygous mutations in CEP19, a highly conserved protein that localizes to the centrosome and primary cilia, were found in a newly described morbid-obesity syndrome. Moreover, Cep19-knockout mice are morbidly obese, hyperphagic, glucose intolerant, and insulin resistant (Shalata et al., 2013). The identification of CEP19 as the cause of morbid obesity in both humans and mice provides an additional link between cilia dysfunction and obesity.
Treatment of Ciliopathies
Although the spectrum of mutations contributing to the ciliopathies has advanced in recent years, current therapies are limited primarily to symptom management, and there are no curative therapies currently available to patients. Challenges to the development of treatments for the ciliopathies include the pleiotropy of genes involved, including 89 described gene mutations (and counting) and 23 known syndromes, variable penetrance resulting in symptomatic heterogeneity within a single mutation, and effects on multiple different organs and cell types such that clinical manifestations can be found in a multitude of different organ systems. Phenotypes may be due to neurodevelopmental-associated defects, sensory impairments in vision, hearing and olfaction, or in progressive tissue degeneration as a result of liver or kidney disease.
A spectrum of clinical trials is underway aimed at improving symptomatic treatment, specifically targeting polycystic kidney disease and RD. Several treatments targeting the development of renal cysts in polycystic kidney disease have shown promise in rodent models and are currently in clinical development (Bukanov et al., 2006; Shillingford et al., 2006). However, human clinical trials have shown only limited success to date (Chang and Ong, 2012; Torres et al., 2012). These trials target pathways implicated in renal cyst development, specifically inhibiting mammalian target of rapamycin (mTOR), cyclic adenosine monophosphate (cAMP), or cyclin dependent kinases, and therefore may not be efficacious in other organ systems affected in the ciliopathies.
Retinal degeneration (RD) could be an ideal target for therapy, as the retina represents a convenient, immunologically privileged site for injection of therapeutics. Ciliary neurotrophic factor (CNTF) slows RD in an animal model and has shown some promising results for a variety of RD syndromes by direct slow release injection of encapsulated cell technology in clinical trials (Birch et al., 2013). Tauroursodeoxycholic acid, the active ingredient in bear bile, has anti-apoptotic effects in many RD models, and in Bbs1 mutants rescues loss of photoreceptors (Drack et al., 2012). Likewise, the Bbs12 mutant demonstrated slowed RD upon polytherapy to block the unfolded protein response (Mockel et al., 2012). Overall pharmacologic management of symptoms associated with ciliopathies offers the prospect of delayed disease progression while lacking curative potential.
Gene therapy
Gene therapy offers a promising avenue for long-term targeted rather than symptomatic treatment. Numerous gene delivery approaches have shown promise in early studies and have restored cilia function in ex vivo and in vivo models of varying ciliopathies. Retina and olfactory epithelium is appealing for gene delivery in ciliopathies, as these cells are accessible and show time-dependent degeneration. Adeno-associated virus (AAV) has successfully been utilized in several animal ciliopathy models of retinal degeneration. AAV-mediated photoreceptor transduction of RPE65 led to overtly improved vision in humans (Maguire et al., 2008), and since then a plethora of RD animal model and human trials have appeared. In Bbs4 mutants, AAV5-mediated restoration rescued rhodopsin mislocalization and downstream RD (Simons et al., 2011). Taken together these studies show the promise of photoreceptor-directed gene therapy. Treatment of anosmia due to ciliary defects in olfactory sensory neurons is a potential target for gene delivery, via intranasal injection. AV-mediated IFT88 transduction in fully differentiated olfactory sensory neurons in Ift88 mutant mice saw restoration of ciliary structures and rescue of olfactory function (McIntyre et al., 2012).
Lentivirus has recently been proven beneficial for gene therapy against childhood lysosomal disorders (Aiuti et al., 2013; Biffi et al., 2013), however lentivirus has not yet been widely utilized for gene delivery in ciliopathies except for in vitro studies. One proof of concept study utilized cultured respiratory epithelial cells from a patient with primary ciliary dyskinesia with a mutation in DnaI1, showing restoration of normal ciliary beating frequency in transduced cells (Chhin et al., 2009). Persistent difficulties of viral methods include limiting expression in unintentional cell types, potential detrimental effects of gene overexpression/misregulation, and most importantly the risk of malignancy due to viral integration into deleterious gene loci.
An alternative approach is manipulation of DNA splicing. This approach circumvents difficulties associated with full-length gene delivery. To this end, modified U1 small nuclear RNAs and antisense oligonucleotides have been engineered to correct splicing defects due to donor site mutations, successfully utilized in two different ciliopathy models in vitro. The single most common cause of LCA is a founder intronic CEP290 splice site mutation, which can be corrected by antisense oligonucleotide therapy (Collin et al., 2012). The potential challenge is off-target splicing effects on other transcripts, yet to be addressed. Thus RNA-based therapies, while potentially immunogenic due to activation of Toll-like receptors, represent an exciting avenue for treatment.
Perspectives
The link between the structure of primary cilia and signaling pathways has become a framework for understanding the pathogenesis of ciliopathies. Until recently, studies utilized germline constitutive knockout animal models, which provided useful loss-of-function phenotypes, but were limited in mechanistic interpretation. We are beginning to see more intricate analysis with cell-type-specific or temporal-specific gene loss. These will be especially important as we relate gene function to specific ciliary defects such as the requirement of one gene on ciliary localization of a different gene’s encoded protein, or the particular role of a gene on intricate 3D ciliary ultrastructure (Garcia-Gonzalo et al., 2011; Williams et al., 2011).
Although the role of primary cilia during nervous system development is now well established, many questions about its involvement in adult nervous system functions remain. Adult-specific ciliopathy gene mutations to date, with the exception of neurogenesis defects, have failed to show major neurological abnormalities, yet the link with GPCR, Wnt, calcium and other signaling pathways suggests discoveries remain, perhaps around complex behavior and brain plasticity. In this regard, neuropsychiatric risk genes were linked to ciliation in a provocative study. Using RNA interference to broadly test expressed candidate genes associated with schizophrenia, bipolar affective disorder, autism spectrum disorder and intellectual disability, approximately half of the candidates were found to produce a ciliary phenotype (Marley and von Zastrow, 2012). In this light, DISC1, one of the best genetic candidates for schizophrenia, has been implicated in ciliogenesis (Marley and von Zastrow, 2010). In addition, variable neuropsychiatric phenotypes in patients with BBS and JS suggest primary cilia may regulate complex CNS functions (Bennouna-Greene et al., 2011).
Little attention has been focused on how primary cilia could influence other neurological diseases until recently. Mice lacking tau tubulin kinase 2 (TTBK2), a microtubule-associated kinase, fail ciliogenesis, and exhibit neural tube defects (Goetz et al., 2012). This same gene with truncating mutations around residues 122–137 causes dominant spinocerebellar ataxia type 11 (SCA11) (Houlden et al., 2007). Furthermore, when these truncated cDNAs are transfected into cells there is inhibition of ciliogenesis, suggesting SCA may relate to ciliary defects. Ataxin10, displaying a trinucleotide expansion as the cause for spinocerebellar ataxia-10 (SCA10), showed a homozygous splice mutation in a patient with JBTS (Sang et al., 2011), These examples suggest a potential connection between cerebellar development and degeneration linked by cilia, but will require specific human alleles introduced into in vivo models to clarify these links.
Acknowledgments
We wish to thank members of the Gleeson lab for discussions, and Eva Anton, Bradley Yoder, John Wallingford, Val Sheffield, and Peter Jackson for comments. A.G.-G. is supported by a UC MEXUS-CONACYT postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, Dionisio F, Calabria A, Giannelli S, Castiello MC, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, Geschwind DH. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet. 2008;17:3887–3896. doi: 10.1093/hmg/ddn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011;31:9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR. Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. 2012;520:848–873. doi: 10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nature Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Baker K, Northam GB, Chong WK, Banks T, Beales P, Baldeweg T. Neocortical and hippocampal volume loss in a human ciliopathy: A quantitative MRI study in Bardet-Biedl syndrome. Am J Med Genet A. 2011;155A:1–8. doi: 10.1002/ajmg.a.33773. [DOI] [PubMed] [Google Scholar]
- Barker AR, Thomas R, Dawe HR. Meckel-Gruber syndrome and the role of primary cilia in kidney, skeleton and central nervous system development. Organogenesis. 2013:10. doi: 10.4161/org.27375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennouna-Greene V, Kremer S, Stoetzel C, Christmann D, Schuster C, Durand M, Verloes A, Sigaudy S, Holder-Espinasse M, Godet J, et al. Hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with Bardet-Biedl syndrome underline complex CNS impact of primary cilia. Clin Genet. 2011;80:523–531. doi: 10.1111/j.1399-0004.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Kin NW, Sharma N, Michaud EJ, Kesterson RA, Yoder BK. Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Dev Biol. 2011;360:66–76. doi: 10.1016/j.ydbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Nat Acad Sci. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, Yoder BK. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc Nat Acad Sci. 2013;110:7796–7801. doi: 10.1073/pnas.1210192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Damhofer H, Roelink H. Hedgehog-stimulated chemotaxis is mediated by smoothened located outside the primary cilium. Science signaling. 2012;5:ra60. doi: 10.1126/scisignal.2002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 2013;156:283–292. e281. doi: 10.1016/j.ajo.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nature Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovski MT, Yuan S, Pedersen NB, Goth CK, Makova S, Clausen H, Brueckner M, Khokha MK. The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature. 2013;504:456–459. doi: 10.1038/nature12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Nat Acad Sci. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- Carter CS, Vogel TW, Zhang Q, Seo S, Swiderski RE, Moninger TO, Cassell MD, Thedens DR, Keppler-Noreuil KM, Nopoulos P, et al. Abnormal development of NG2+PDGFR-alpha+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nature Med. 2012;18:1797–1804. doi: 10.1038/nm.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Developmental cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chang MY, Ong AC. Mechanism-based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clin Pract. 2012;120:c25–34. doi: 10.1159/000334166. discussion c35. [DOI] [PubMed] [Google Scholar]
- Chhin B, Negre D, Merrot O, Pham J, Tourneur Y, Ressnikoff D, Jaspers M, Jorissen M, Cosset FL, Bouvagnet P. Ciliary beating recovery in deficient human airway epithelial cells after lentivirus ex vivo gene therapy. PLoS Genet. 2009;5:e1000422. doi: 10.1371/journal.pgen.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nature Genet. 2002;31:74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- Collin RW, den Hollander AI, van der Velde-Visser SD, Bennicelli J, Bennett J, Cremers FP. Antisense Oligonucleotide (AON)-based therapy for Leber congenital amaurosis caused by a frequent mutation in CEP290. Mol Ther Nucleic Acids. 2012;1:e14. doi: 10.1038/mtna.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge P, Brownlee C, Wheeler GL. Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Current biology : CB. 2013;23:2311–2318. doi: 10.1016/j.cub.2013.09.059. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development. 2010;137:4271–4282. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Current biology : CB. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nature Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Drack AV, Dumitrescu AV, Bhattarai S, Gratie D, Stone EM, Mullins R, Sheffield VC. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci. 2012;53:100–106. doi: 10.1167/iovs.11-8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein EB, Patterson CA, Hon BJ, Regan KA, Reddi J, Melnikoff DE, Mateer MJ, Schulz S, Johnson BN, Tallent MK. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J Neurosci. 2010;30:4306–4314. doi: 10.1523/JNEUROSCI.5295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nature Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Liem KF, Jr, Anderson KV. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151:847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, Malik N, Huttner W, Westphal H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol. 2009;325:24–32. doi: 10.1016/j.ydbio.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GH, Pauer GJ, Narendra U, Hagstrom SA. Tubby-like protein 1 (Tulp1) is required for normal photoreceptor synaptic development. Advances in experimental medicine and biology. 2010;664:89–96. doi: 10.1007/978-1-4419-1399-9_11. [DOI] [PubMed] [Google Scholar]
- Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, Sarkisian MR. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci. 2013;33:2626–2638. doi: 10.1523/JNEUROSCI.2906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nature Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nature neuroscience. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hengl T, Kaneko H, Dauner K, Vocke K, Frings S, Mohrlen F. Molecular components of signal amplification in olfactory sensory cilia. Proc Nat Acad Sci. 2010;107:6052–6057. doi: 10.1073/pnas.0909032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Developmental cell. 2012;23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nature neuroscience. 2013;16:1000–1007. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Johnson J, Gardner-Thorpe C, Lashley T, Hernandez D, Worth P, Singleton AB, Hilton DA, Holton J, Revesz T, et al. Mutations in TTBK2, encoding a kinase implicated in tau phosphorylation, segregate with spinocerebellar ataxia type 11. Nature Genet. 2007;39:1434–1436. doi: 10.1038/ng.2007.43. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand Kia S, Verbeek E, Engelen E, Schot R, Poot RA, de Coo IF, Lequin MH, Poulton CJ, Pourfarzad F, Grosveld FG, et al. RTTN mutations link primary cilia function to organization of the human cerebral cortex. Am J Hum Genet. 2012;91:533–540. doi: 10.1016/j.ajhg.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Nat Acad Sci. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature neuroscience. 2012;15:399–405. S391. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nature Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nature Cell Biol. 2011;13:700–707. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22:4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genomic Med. 2011;3:59. doi: 10.1186/gm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res. 2013;91:1117–1132. doi: 10.1002/jnr.23238. [DOI] [PubMed] [Google Scholar]
- Loktev AV, Jackson PK. Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep. 2013;5:1316–1329. doi: 10.1016/j.celrep.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. New Eng J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. DISC1 regulates primary cilia that display specific dopamine receptors. PloS one. 2010;5:e10902. doi: 10.1371/journal.pone.0010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PloS one. 2012;7:e46647. doi: 10.1371/journal.pone.0046647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JC, Davis EE, Joiner A, Williams CL, Tsai IC, Jenkins PM, McEwen DP, Zhang L, Escobado J, Thomas S, et al. Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nature Med. 2012;18:1423–1428. doi: 10.1038/nm.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockel A, Obringer C, Hakvoort TB, Seeliger M, Lamers WH, Stoetzel C, Dollfus H, Marion V. Pharmacological modulation of the retinal unfolded protein response in Bardet-Biedl syndrome reduces apoptosis and preserves light detection ability. J Biol Chem. 2012;287:37483–37494. doi: 10.1074/jbc.M112.386821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the Hedgehog pathway by PKA. Cell Signal. 2013;25:2222–2230. doi: 10.1016/j.cellsig.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PloS one. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A, Boltshauser E, Loenneker T, Valente EM, Brancati F, Il’yasov K, Huisman TA. Diffusion tensor imaging in Joubert syndrome. Am J Neurorad. 2007;28:1929–1933. doi: 10.3174/ajnr.A0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Nat Acad Sci. 2011;108:1456–1461. doi: 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Micalizzi A, Valente EM. Joubert syndrome: congenital cerebellar ataxia with the molar tooth. Lancet neurology. 2013;12:894–905. doi: 10.1016/S1474-4422(13)70136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genetic. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalata A, Ramirez MC, Desnick RJ, Priedigkeit N, Buettner C, Lindtner C, Mahroum M, Abdul-Ghani M, Dong F, Arar N, et al. Morbid obesity resulting from inactivation of the ciliary protein CEP19 in humans and mice. Am J Hum Genet. 2013;93:1061–1071. doi: 10.1016/j.ajhg.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Nat Acad Sci. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DL, Boye SL, Hauswirth WW, Wu SM. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Nat Acad Sci. 2011;108:6276–6281. doi: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Malmgren H, He H, Scott L, Aperia A, Hokfelt T. Developmental changes in frequency of the ciliary somatostatin receptor 3 protein. Brain Res. 2009;1249:101–112. doi: 10.1016/j.brainres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Tran PV, Turbe-Doan A, Beier DR. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009;335:166–178. doi: 10.1016/j.ydbio.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nature Meth. 2013;10:1105–1107. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, Zhong Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502:254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Nat Acad Sci. 2008;105:6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. New Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, Gojak CP, Gorgas K, Bradford CL, Spatz J, Wolfl S, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis AT, Rorke LB. Neuropathology of Joubert syndrome. J Child Neurol. 1999;14:655–659. doi: 10.1177/088307389901401006. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]