Abstract
Perivascular adipose tissue (PVAT), long assumed to be nothing more than vessel-supporting connective tissue, is now understood to be an important, active component of the vasculature, with integral roles in vascular health and disease. PVAT is an adipose tissue with similarities to both brown and white adipose tissue, although recent evidence suggests that PVAT develops from its own precursors. Like other adipose tissue depots, PVAT secretes numerous biologically active substances that can act in both autocrine and paracrine fashion. PVAT has also proven to be involved in vascular inflammation. While PVAT can support inflammation during atherosclerosis via macrophage accumulation, emerging evidence suggests that PVAT also has anti-atherosclerotic properties related to its abilities to induce non-shivering thermogenesis and metabolize fatty acids. We here discuss the accumulated knowledge of PVAT biology, and related research on models of hypertension and atherosclerosis.
Keywords: Perivascular adipose tissue, atherosclerosis, hypertension
Introduction
Adipose tissue is a complex set of cell types, including adipocytes, macrophages, T cells, collagen fibers, nerves and capillaries, spread throughout the body. Traditionally, adipose tissue was classified into two types: white adipose tissue (WAT), which comprises the visceral and subcutaneous fat tissues, and brown adipose tissue (BAT), which is found in the interscapular region in both rodents and human infants, with recent reports of BAT in adults.1 While WAT is composed of adipocytes with a large, single fat droplet and is presumed to be the main depot for lipid storage, BAT contains several smaller fat droplets and numerous mitochondria, and is involved in heat production. BAT is defined by the expression of uncoupling protein-1 (UCP-1), a long-chain fatty acid/H+ symporter that produces heat by “uncoupling” fuel oxidation from ATP synthesis.2 More recently, “beige” adipocytes have been characterized. These cells were first reported in rodents, and express UCP-1, like BAT cells, but also express unique cell surface markers, including CD137 and Tmem26.3 Beige adipocytes appear to be programmed to be flexible, with the ability to store lipids and produce heat under different circumstances such as cold stimuli.4 The presence of brown and beige fat in humans is still under debate, with reports of human adipose tissues that display similarity to both brown and beige fat of rodents.4–8 Interestingly, it is being revealed that both white and beige cells have the ability to upregulate thermoregulation in response to reduced temperature,9 a process known as “browning.” In addition to cold, several other signals have been reported to induce browning of white and beige adipocytes, including cardiac hormones10 and exercise-induced irisin.11 Irisin has gained significant attention recently, since it browns adipocytes via the p38 MAPK and ERK pathways12 and is responsible for the cold-induced browning signal in rodents and humans.13 WAT displays significant variability as well, with visceral adipose tissue now understood to be more harmful, as it is associated with insulin resistance and cardiovascular events, due to its greater inflammatory characteristics. Conversely, subcutaneous WAT has been shown to have a higher expression of UCP-1, indicating its greater ability to be “browned.”14 These results underscore the plasticity and adaptability of adipocytes.
Historically, adipose tissue was thought to be simply lipid-rich connective tissue.15 Similarly, the sheath of adipose tissue surrounding most blood vessels, known as PVAT, was long assumed to provide mechanical protection to the vessels during contraction of neighboring tissues.16 However, with an increased understanding of the differentiation and function of adipose tissue in health and disease, PVAT research is undergoing its own renaissance. In addition to the structural role of PVAT, it is increasingly being appreciated that this tissue plays many other roles in vascular function. These include the secretion of metabolically active adipokines, chemokines and hormone-like factors, such as leptin, adiponectin and resistin, free fatty acids, and vasoactive substances.17 With complex endocrine and paracrine functions, PVAT regulate vascular tone in both rodents and humans. In addition, PVAT appears to be altered in obesity and diabetes, expanding and accumulating inflammatory cells and altering the production of various adipokines and inflammatory cytokines. This dysfunctional PVAT has been suggested as a mechanistic link between metabolic syndrome and atherosclerosis,18 and may contribute to or modulate hypertension, though a causal role has not yet been established.
Clinical association of PVAT with vascular diseases
The role of PVAT in human vascular disease is becoming increasingly apparent. For example, a recent study measured higher levels of adipokines secreted by PVAT biopsies taken from stenotic coronary artery segments, versus non-stenotic segments.19 Similarly, the Framingham Heart Study is providing insights to the role PVAT plays in cardiovascular disease (CVD) risk. In a recent report from this study, thoracic PVAT was measured via multidetector computed tomography.20 High thoracic PVAT was found to be significantly associated with a higher prevalence of CVD, even in individuals without high visceral adipose tissue. In addition, other CVD risk factors have been demonstrated to have links with PVAT. For example, smoking has been reported to increase the inflammation of PVAT by enhancing the expression and activity of the P2X7R-inflammasome complex,21 and systemic lupus erythematosus, a known CVD risk factor for women, is associated with greater aortic PVAT and calcification of vascular beds.22 Clearly, the emerging data from the clinic compels us to develop models to better understand the effects of PVAT in vascular (patho)physiology.
PVAT: White, Beige, Brown, or something else?
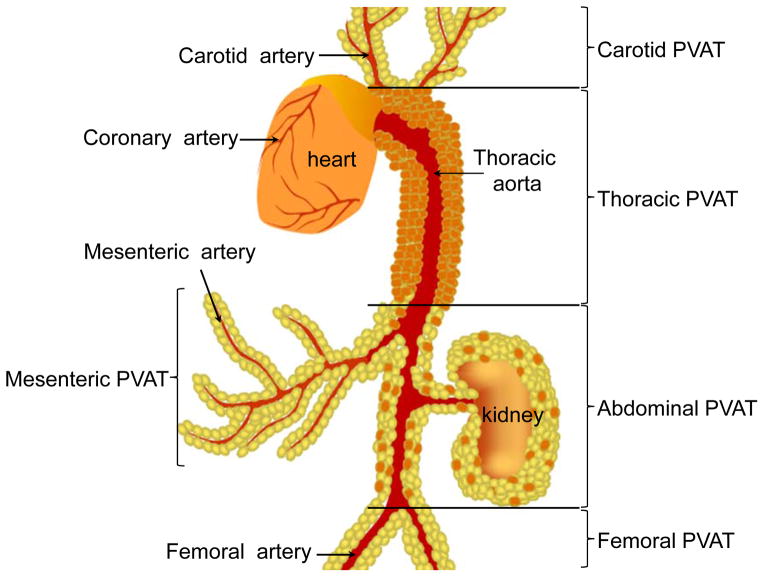
PVAT differs between species and anatomic location. The mesenteric artery, the coronary artery and the aorta are three distinct vessels particularly associated with CVD complications. In rodents, the mesenteric artery is surrounded by WAT (traditionally categorized as visceral WAT), while the thoracic aorta is surrounded by BAT-like tissue, and the abdominal aorta is surrounded by adipose tissue with a mixture of white and brown adipocytes (Fig. 1). While there is no fat tissue surrounding the murine coronary artery, adipose tissue surrounds all these vessels in humans and other large experimental animals, including rabbits and pigs, although the morphological status of PVAT in these other species is not as well defined as murine PVAT. However, indirect evidence suggests that human PVAT shares characteristics of both WAT and BAT.4 WAT acts as an endocrine organ, secreting circulating adipokines that mediate cross-talk between visceral or subcutaneous WAT and cardiovascular tissues. Many of these adipokines, including adiponectin, leptin and inflammatory cytokines such as IL-6 and tumor necrosis factor-α (TNF-α), are also produced by PVAT.23 Furthermore, since PVAT is an integral part of the vasculature, it may have more immediate and direct effects on the vessels it envelops, as compared to visceral or subcutaneous WAT, which would require long-distance transport of messengers. The close proximity of PVAT and the underlying fibroblasts, VSMCs or endothelial cells also suggests the possibility of paracrine signaling between these tissues. However, while PVAT is involved in adipokine secretion, several studies have uncovered that PVAT shares several important features with BAT. These include morphological characteristics, including several small, multilocular lipid droplets and abundant mitochondria. The similarities extend to the transcriptional profile as well, with nearly overlapping gene expression profiles between BAT and PVAT in a rodent model, including high expression of UCP-1, Cidea, and other genes known to be expressed by BAT.24 Our own study also found a similar proteomic profile between thoracic PVAT and BAT.25 Moreover, in accordance with published reports of BAT’s role in clearing lipids under extreme low temperature stimulation26, we also found that PVAT-free mice were impaired in their ability to regulate triglyceride levels and intravascular temperature.25 It is now accepted that white (and beige) adipocytes do not share a common lineage with brown adipocytes. White and beige adipocytes derive from a Pdgfr-α+ precursor.27 Furthermore, there is a possibility that mature white adipocytes may be capable of directly differentiating into beige adipocytes under appropriate conditions. A recent study demonstrated that beige adipocytes may derive from smooth muscle-like precursors28. On the other hand, brown adipocytes share a lineage with skeletal muscle cells (15, 27 and Fig. 2). Unexpectedly, our study suggested that the origin of PVAT adipocytes may yet be distinct from either white or brown adipocytes. Using PPARγ-floxed mice crossed to SM22α-Cre knock-in mice we were able to generate mice completely devoid of PVAT in the aortic and mesenteric regions. Surprisingly, however, both interscapular BAT and gonadal/inguinal/subcutaneous WAT were intact in these mice, implying that BAT, WAT and PVAT have different origins in mice. While SM22α is a marker of SMCs early in development,29 our results indicate that SM22α must either be transiently expressed in PVAT-precursor cells, or that PVAT and VSMCs share a common precursor. It is of note that this latter situation would be similar to the prevailing view of BAT development, which shares precursors with skeletal muscle cells, as discussed above. Nevertheless, our findings indicate that PVAT may indeed be a fourth kind of adipose tissue, distinct from white, beige and brown fat as they are now understood. However, as the majority of PVAT characterization studies have been performed in mouse models, it remains to be seen how much of these results can be translated to humans. As it stands, the main area of PVAT studies focus on its effects related to vascular function.
Figure 1. Types of PVAT.
PVAT can be classified as white, brown and beige according to different anatomic locations. In rodents, brown PVAT surrounds the thoracic aorta, and white PVAT surrounds small arteries such as mesenteric, carotid and femoral arteries, while the abdominal aorta is surrounded by beige PVAT. PVAT is absent from the murine coronary artery.
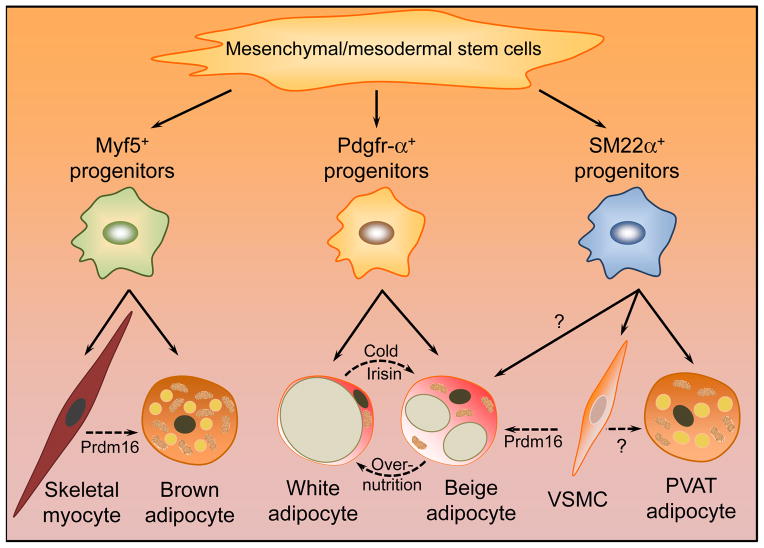
Figure 2. Origin of different types of adipocytes.
Even though all types of adipocytes differentiate form mesenchymal/mesodermal stem cells,98 brown, white and beige adipocytes arise from different progenitors. Interscapular brown adipocytes differentiate from Myf5+ precursors common to skeletal muscle cells.15, 27 Subcutaneous and visceral white and beige adipocytes derive from Pdgfr-α+ progenitors.27 Aortic and mesenteric PVAT adipocytes are derived from SM22α-positive cells,25 although it is unclear whether pre-adipocytes of PVAT share common precursors with VSMCs and whether VSMCs can trans-differentiate into PVAT adipocytes. Additionally, with ectopic expression of PRDM16, skeletal myocytes trans-differentiate into brown adipocytes,99 and VSMCs into beige adipocytes.28 White adipocytes can be “browned” by stimulation with cold temperature or certain factors such as irisin.9, 11 (Solid arrows indicate direct differentiation, and dashed arrows indicate trans-differentiation.)
Functions of PVAT
1. Mechanical protection
The classical understanding of blood vessel anatomy includes the intima, media, and adventitia. These layers are formed by strong networks of collagen and elastic fibers, whereas the perivascular area is filled by thin lamellae of PVAT.30 The amount of PVAT surrounding the vessels varies based on anatomical location and caliber of the vessel; PVAT is very abundant on the aorta, and absent from cerebral- and micro-vasculature.31 It has long been accepted that PVAT offers mechanical protection of the vessels against neighboring tissue during contraction.32 Indeed, methods for preparing blood vessels for experimental manipulation ex vivo routinely begin by “cleaning” the vessel, essentially removing the PVAT. While these mechanical protective functions are undoubtedly important to large vessels, such as the aorta, it is becoming increasingly clear that there is considerably more to PVAT biology.
2. Vasodilator effects
As PVAT was thought to only have a mechanical role as a connective tissue, its removal was deemed to have little effect on the contractile function of blood vessels. The first hint of an expanded function for PVAT came in 1991 with a report of PVAT-mediated contractile regulation in rat aorta.33 Still, more than a decade passed before PVAT function was studied in earnest. Like other adipose tissues, PVAT acts as an endocrine organ, secreting a wide range of bioactive molecules that influence VSMC contraction, proliferation and migration. PVAT-derived factors may also directly influence endothelial function to relax vessels. In addition, the entire perivascular tissue is involved in the inflammatory response to vascular injury.34 This suggests that communication flows bi-directionally between PVAT and cells of the vessel wall. In support of this, there is accumulating evidence that PVAT has vasodilator effects (also termed anti-contractile effects) in various vascular beds, and this function has been shown to be impaired in hypertension35–38 and metabolic syndrome.35, 39–43 Substantial evidence exists that adipose-derived factors, such as leptin, resistin, and TNF-α, secreted under conditions of inflammation, can attenuate vasodilatation,44–50 and such factors may be produced by PVAT. Indeed, a recent study demonstrated the importance of inflammation in PVAT-mediated regulation of vascular tone.51 Mice were generated to lack rictor, an essential mammalian target of rapamycin complex 2 (mTORC2) component, which acts to limit inflammation, specifically in adipose tissue, including PVAT. The resultant mice had increased markers of inflammation in PVAT, including IL-6, MIP-1α and TNF-α, and decreased ability of PVAT to regulate vascular tone.51 While it is clear that PVAT exerts a dynamic effect on vascular tone, no single factor responsible for this vasodilator effect has been identified. In the meantime, the term PVAT-derived relaxing factor (PVRF, originally adventitium-derived relaxing factor [ADRF]) has been coined.52 Several compounds have been proposed to constitute PVRF, including adiponectin,53, 54 H2S,55 nitric oxide (NO),56 angiotensin (Ang) 1–7,57 and palmitic acid methyl ester.58 We have also reported that PVAT-derived prostacyclin may be a PVRF.25 While prostacyclin is a potent vasodilator secreted by endothelial cells,59 it is also readily detectable in PVAT.25 It is well established that aging and hypertensive subjects have vascular dysfunction characterized by acetylcholine-induced vessel constriction.60 We demonstrated that incubation with PVAT completely blocked the acetylcholine-induced constriction of vessel rings from aged mice, while this effect was blocked with a prostacyclin receptor antagonist, reinforcing that PVAT-derived prostacyclin acts on other vascular cells to reduce contractility,25 and defining it as a putative PVRF. In support of our findings using a murine model, a recent study has found both prostacyclin and prostaglandin E2 from PVAT to induce relaxing effects in human saphenous vein graft preparations.61 However, the same study found prostanoids to be dispensable for the relaxing effects of PVAT on internal mammary arties, suggesting that PVAT of different locations may employ different PVRFs.
As for the downstream effects of PVRF, release of NO and subsequent K+ channel activation may be involved. Experimental evidence for this includes the relaxation of PVAT-stripped aortic rings ex vivo after transfer into an incubation solution containing PVAT. This PVAT-dependent effect was further blocked by endothelial cell removal, NO synthase inhibition, scavenging of NO, high extracellular K+, or blockade of calcium-dependent K+ channels.56 Additionally, PVRF may act through endothelium-independent mechanisms involving H2O2 production and subsequent activation of guanylyl cyclase (sGC).56 However, these experiments have been carried out on vessel rings isolated from rodents, in the presence or absence of the PVAT layer. Therefore, the applicability in vivo, especially in regards to human physiology, remains to be determined.
3. Contractile effects
In addition to the vasodilator effects of PVAT, there is also considerable evidence of contractile functions of PVAT on the underlying vascular bed. Save for renin, all of the components of the renin-angiotensin system have been detected in PVAT,59 as well as AT(1a) and AT(1b) receptors.62 Electrical stimulation-induced contraction of vessel rings was dependent on intact PVAT, and this effect was shown to involve AngII.33 Furthermore, in vivo studies have also demonstrated that PVAT-derived AngII is involved in electrical-induced vessel contraction.63 Norepinephrine (NE) is found in PVAT,64 and we observed that alpha-adrenergic receptor antagonists block PVAT-induced constriction of vessel rings (unpublished data). Furthermore, PVAT was shown to enhance the mesenteric arterial contractile response to perivascular nerve stimulation via superoxide production.65 During the last year there has been a surge of reports on the contractile effects of PVAT, especially in the context of obesity. Meyer et al. described the vasocontractile effects of PVAT from obese mice, and named the putative molecule(s) responsible for this effect “adipose-derived contracting factor” (ADCF). This report found cyclooxygenase (COX) to be responsible for the contractile effects of PVAT in obesity,66 while an article from a different group reported chemerin to be responsible for vasoconstriction in obesity.67 A study using a porcine model uncovered that the pro-contractile effects of PVAT were enhanced in obese swine.68 Interestingly, while one report excluded superoxide anions, NO synthase, or endothelin receptors as vasoconstrictive agents in obesity,66 a separate study reported that superoxide production by PVAT was responsible for arterial stiffening in aged mice,69 indicating that PVAT may produce multiple ADCFs. However, the contractile effects of PVAT on vessels depend on the overall physiology of the organism and the anatomic location of the PVAT. Indeed, we have unpublished data suggesting that the hierarchies of PVAT contractile ability are as follows: thoracic PVAT>abdominal PVAT>mesenteric PVAT, and PVAT of lean mice > PVAT of obese mice.
4. Thermoregulation
While white adipocytes are involved in energy storage, brown and beige adipocytes are associated with dissipating energy during non-shivering thermogenesis. Both rodent and human thoracic PVAT are comprised of UCP-1-positive brown or beige adipocytes, indicating that PVAT is also capable of thermogenesis. This capability is physiologically and phathophysiologically significant. Our recent study using a mouse model lacking PVAT demonstrated that intravascular temperature was indeed regulated by PVAT. Similar to the ability of BAT to enhance clearance of plasma cholesterol, PVAT reduces plasma cholesterol in response to stimuli by moderate cold temperature (16°C). This function of PVAT is important for the biology of the vasculature since the development of atherosclerosis was reduced when the mice were housed in 16°C25. Additionally, it is known that a blood temperature gradient exists in humans, with the vasculature closest to the heart having the highest temperatures,70 and it is very likely that PVAT plays an essential role in maintaining this gradient. With a possible role for the metabolism of lipids and atherogenesis, PVAT-dependent thermoregulation is an area that requires further study, both in humans and animal models.
5. Autocrine/paracrine effects
PVAT produces many putative vasoactivators, ADCFs and ADRFs. In addition, PVAT has been reported to produce several other molecules with possible autocrine or paracrine effects, which has recently been extensively reviewed.71 These include adipokines, such as leptin, adiponectin and resistin, visfatin, hepatic growth factor, and others. Adipose tissue is intimately associated with inflammation, and PVAT releases several cytokines including TNF-α, IL-1, IL-6, IL-8, and MCP-1, reactive oxygen species (superoxide, NO, H2O2) and H2S. Hormones including prostaglandins and angiotensin 1–7 are also produced. Many of these molecules have effects on the development of atherosclerosis, and will be discussed below. It is clear that PVAT is a complex, active organ with several functions beyond mechanical protection for the underlying vascular bed.
In summary, vascular beds are surrounded by PVAT that varies with anatomical location and developmental origin, and which can be characterized either as WAT or BAT. While all PVAT shares functions common with adipose tissue, including autocrine/paracrine effects, some specific differences are apparent. For example, thoracic PVAT is distinct from mesenteric PVAT, as thoracic PVAT most closely resembles thermoactive BAT. These differences are illustrated in Fig. 1 & 3. These distinct PVAT depots constitute an area ripe for study. Thus, it is currently unclear whether there are any differences regarding pro- or anti-contractile effects between thoracic PVAT and mesenteric PVAT. Additionally, the functional analysis of PVAT bioenergetics will help determine the impact of PVAT thermogenesis on systemic metabolism, highlighting possible avenues for future research.
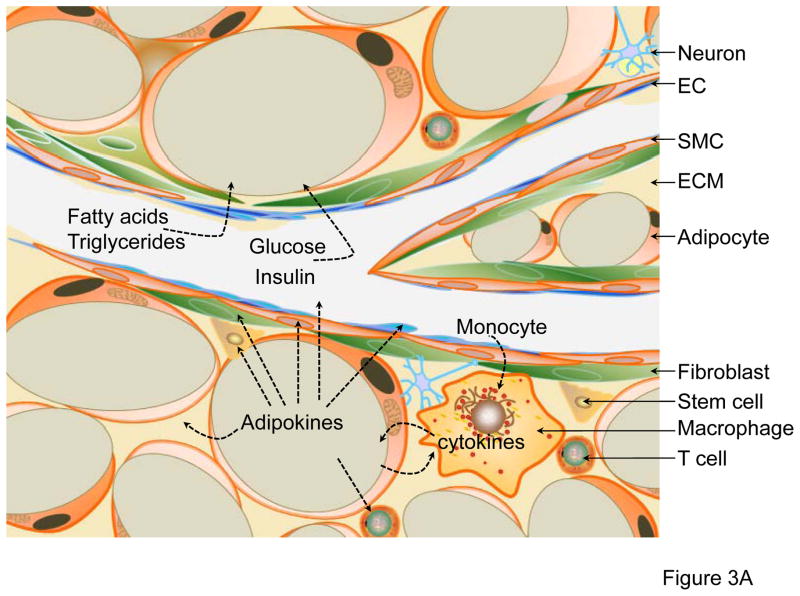
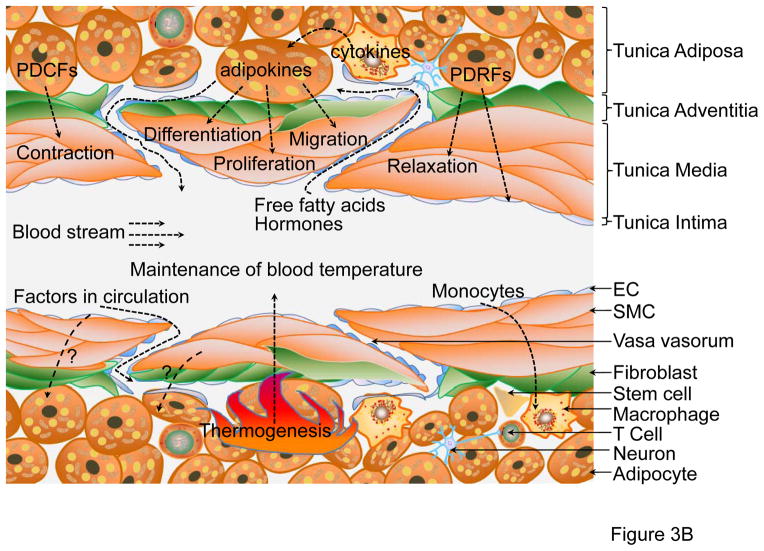
Figure 3. Functions of PVAT.
(A) PVAT surrounding the mesenteric artery consists of WAT, also known as visceral WAT. The major functions of this depot are: 1) lipid storage by utilization of circulating free fatty acids, triglycerides and glucose; 2) inflammation by macrophage infiltration; and 3) autocrine/paracrine secretion of adipokines. The adipokines secreted from adipocytes may act on themselves, T-cells, macrophages, stem cells, fibroblasts in adventitia, SMCs in media, endothelium or other organs/tissues through transport via blood circulation. (B) PVAT surrounding the thoracic aorta is similar to BAT. Adipokines released from brown adipocytes may act on SMCs and induce SMC differentiation, migration and proliferation. Additionally, PVAT-derived contracting factors (PDCFs) and relaxing factors (PDRFs) constrict or relax SMC, respectively. A distinctive function of thoracic PVAT is thermogenesis in response to cold stimuli or hormones such as irisin in circulation, which is critical to maintaining blood temperature. Crosstalk between PVAT and other tissues happens either through factors released from adipocytes or factors in circulation.
Pathologies in animal models with reduced or absent PVAT
1. Regulation of BP and metabolism
There are now several published rodent models with reduced or absent PVAT. The A-ZIP/F mouse expresses the dominant-negative protein A-ZIP/F under the control of the adipose-specific aP2 promoter.72 These mice are free of WAT, and have dramatically reduced BAT and PVAT throughout their lives. The loss of WAT induces complex physiological phenotypes in these mice, including diabetes72 and hypertension.35, 73 They also display altered vascular contractile functions, but ex vivo incubation of A-ZIP/F aortas with WT PVAT does not rescue these defects,35 indicating that the transgenic mice may have dysfunctional aortas unrelated to the absence of PVAT. This conclusion is supported by the finding that, compared to WT aortas, A-ZIP/F aortas have higher expression of AT1, but not AT2, receptors.35
Similar to the A-ZIP/F mouse, an innovative model of inducible adipose deletion has been generated.74 This transgenic mouse, dubbed FAT-ATTAC (fat apoptosis through targeted activation of caspase 8) makes use of a caspase 8-FKBPv fusion protein under control of the adipocyte-specific Fabp4 promoter. Mice grow normally, including normal development of all adipose tissues, until fusion protein dimerization is induced by the FK1012 analog AP20187. Two weeks post-induction, adipose tissues are reduced to near-knockout levels. Induced FAT-ATTAC mice develop phenotypes similar to A-ZIP/F mice, with glucose intolerance and reduced systemic inflammation. Notably, the fusion protein induces apoptosis and depletion of both WAT and BAT, although the effects on PVAT and blood pressure are unknown at this time.
The MORE-PGKO mouse is a transgenic strain that lacks interscapular BAT, as well as mesenteric, perirenal, subcutaneous, epidiymal and periovarian adipose tissue.73 This strain was generated to rescue the embryonic lethality of global PPARγ knockout by breeding Mox2-Cre (MORE) mice with floxed PPARγ mice to inactivate PPARγ in the embryo but not the trophoblast. These transgenic mice are hypotensive, and have other phenotypes relevant to cardiovascular disease, including insulin resistance and lipodystrophy. These mice have impaired contraction of the VSMCs in response to α-adrenergic agents, and the angiotensin-aldosterone system is mildly activated. However, there are currently no reports on the PVAT status of these animals.
We generated a fourth murine model, deficient in peroxisome proliferator-activated receptor-γ in smooth muscle cells (SMPG KO). These mice have VSMC-specific deletion of PPARγ.25 Differing from the models described above, SMPG KO mice have normal glucose metabolism, WAT and BAT depots, but are completely devoid of PVAT. Similar to the MORE-PGKO mice, our SMPG KO mice display hypotension in the resting period of the circadian cycle. However, these mice also have increased β2-adrenergic receptor as a result of the PPARγ deletion in the SMCs, complicating the interpretation of whether loss of PVAT is responsible for the observed hypotension.25 However, there are other lines of evidence suggesting that hypotension in SMPG KO mice is not caused by PPARγ deletion in SMCs, as two published mouse models display a hypertensive phenotype with altered VSMC-PPARγ level or function.75, 76 Notably, PVAT is present in both of these models.
Taken together, these mouse models demonstrate that BP is lower in mice that lack PVAT, while mice with intact PVAT are hypertensive. Of course, each of these models has its limitations when used to evaluate the effects of PVAT on the regulation of BP. A-ZIP/F, FAT-ATTAC and MOPG KO mice have insulin resistance and lipodystrophy, which could affect BP. Even our SMPG KO mice, which have normal metabolism and adipose depots (aside from PVAT), have the major limitation that PPARγ is also deleted in VSMCs. The obvious solution would be to develop a new animal model with specific PVAT removal. As mentioned, PVAT may share a common lineage with VSMC, thus making the targeting of only PVAT via the Cre strategy rather difficult.
2. Vascular remodeling effects of PVAT
In addition to the effects on vascular tone, PVAT is involved in atherosclerosis, a vascular disease with a strong inflammatory component.77 While the endothelium and media are the major players of the development of atherosclerotic lesion, there is increasing evidence of important roles played by other layers of the vessel. For example, the adventitia, comprised of fibroblasts, has been implicated in vascular remodeling and constriction of the external lamina by the accumulation of alpha smooth muscle-containing myofibroblasts in the area surrounding the injury site.78 Indeed, inhibition of myofibroblast proliferation and/or recruitment affects vascular remodeling and reduces vessel constriction.79 Similarly, the inflammatory response to arterial angioplasty includes the PVAT.34, 79 These results suggest that PVAT is closely involved with vascular remodeling, and underscores the idea that PVAT constitutes an integral layer of the vasculature. Regarding the roles of PVAT on development of atherosclerosis, current research indicates dual effects: pro-atherosclerotic and anti-atherosclerotic.
3. Pro-atherosclerotic effects of PVAT
The inflammatory cells resident in and recruited by PVAT have been hypothesized to be responsible for myofibroblast recruitment or proliferation, contributing to vascular remodeling.34 Consistent with this, a recent study using a murine model of chronic inflammation via TNF-α injection found that PVAT inflammation led to MMP-mediated TGF-β production, resulting in neointima formation.80 In addition, vascular injury has been reported to upregulate proinflammatory adipokines and downregulate anti-inflammatory adiponectin in PVAT in both mice and rats.81 Furthermore, a high-fat diet in mice was found to induce a proinflammatory phenotype in the PVAT.82 This same study also analyzed depots of human adipose tissue. In comparison to subcutaneous and visceral adipose tissue, PVAT was found to have less-differentiated adipocytes, and a more inflammatory signature, with lower expression of adiponectin and higher IL-6, IL-8 and MCP-1. More recently, a study highlighted the effect of leptin on neointima formation after vascular injury.83 Diet-induced obesity increased leptin levels in WT mice, leading to increased vascular remodeling after injury, though this effect was not observed in leptin-deficient ob/ob mice. Adenoviral vector-induced overexpression of leptin also led to increased neointima formation in this model. Interestingly, the authors also found leptin-independent effects of inflamed PVAT on vascular remodeling.83 These results suggest that PVAT is primed for inflammatory responses. Indeed, the accumulation of macrophages and T cells at the PVAT-adventitia interface in human atherosclerotic aortas indicate that PVAT recruits proinflammatory cells in atherogenesis.84 The idea that perivascular adipose tissue can play such a significant role in the inflammatory response to atherosclerosis was experimentally tested by transplanting adipose tissue to the mid-perivascular area of the common carotid arteries, which do not normally develop atherosclerosis, in apolipoprotein-E-deficient mice.85 Transplant of proinflammatory visceral WAT resulted in atherosclerotic lesions and increased inflammatory markers, compared to transplantation of noninflammatory subcutaneous WAT. A postmortem study of atherosclerotic patients likewise found that the PVAT mass was positively correlated with atherosclerotic plaque size.86 Additionally, PVAT adipocytes release more angiogenic factors including acidic fibroblast growth factor, thrombospondin-1, serpin-E1, MCP-1, insulin-like growth factor-binding protein-3, and hepatocyte growth factor (HGF), compared to other adipocyte cell types.87 PVAT was found to be the only adipose tissue that independently correlated with serum HGF levels in patients. This implies that PVAT-derived HGF, which stimulates endothelial cell growth and cytokine release from SMC, is a mediator of PVAT effects in vascular remodeling. In addition, chronic kidney disease is a risk factor for atherosclerosis, and a recent study demonstrated that PVAT plays a role in this effect. Uninephrectomized mice were found to have activation of the renin-angiotensin system in PVAT, which led to increased atherosclerosis.88
4. Anti-Atherosclerotic Properties of PVAT
Aside from the role inflammation plays in atherosclerosis development, impaired energy metabolism in the blood vessels is associated with atherogenesis.89 Temperature has long been recognized to influence energy metabolism,90 and one of the main roles of BAT is to provide adaptive thermogenesis.91 As PVAT has a phenotype similar to BAT, including expression of UCP-1 which is necessary for non-shivering thermogenesis,24, 25 it is possible that heat generation is involved in vascular physiology. Indeed, we recently reported that PVAT is thermogenic, and critical to the maintenance of intravascular temperature.25 In mammals, the vasculature reacts to changes in temperature,92 which involve both endothelial and SMC function. In humans, an intravascular temperature gradient exists, with temperature increasing in large veins as blood approaches the heart.70 Human BP is also increased following exposure to either hot or cold stimulation,93 although it is not yet known if this function is associated with PVAT. At the same time, it is not known if intravascular temperature regulates vessel energy metabolism, thereby influencing atherogenesis. However, as local energy metabolism affects atherosclerosis development, as discussed above, it can be proposed that increased energy production in PVAT affects vessel biology under pathological conditions. Indeed, we were able to activate PVAT thermogenesis by housing mice at a reduced temperature (16°C), which was associated with reduced development of atherosclerosis.25 Importantly, plasma triglyceride levels were reduced under these conditions, suggesting that the increased metabolic activity of PVAT may result in lipid clearance from the vasculature, thereby reducing atherogenesis. PVAT-free mice housed in similar cold conditions did not have comparable reductions in atherosclerosis, underscoring the necessity of PVAT for this phenotype. Human studies have reported that individuals living in cold climates have active BAT in the peri-aortic region of adults,94 and that activation of BAT26 and PVAT25 in rodents results in reduced plasma lipid levels. However, it is unclear if cold exposure in humans activates PVAT thermogenesis leading to protection from atherosclerosis. Exposure to both heat and cold are associated with increased incidences of mortality from heart attacks in humans,95, 96 though we need carefully-controlled epidemiological studies to determine if cold exposure is beneficial in preventing the development of atherosclerosis.
As discussed above, vascular inflammation is pro-atherogenic, although we did not observe a decrease in PVAT inflammation in high-fat diet-fed mice housed in a cold environment,25 indicating that that the anti-atherogenic effects of cold stimulation on PVAT likely act through a different pathway. However, a study demonstrated that mice fed a high-fat diet had relatively less induction of inflammation in PVAT and BAT, compared to WAT,24 suggesting that PVAT may have a nominally anti-inflammatory effect on the vasculature. From these observations, it is clear that PVAT has a profound effect on the development of atherosclerosis. As extensively reviewed previously,97 PVAT inflammation occurs during high-fat diet challenge and is intimately linked to atherosclerosis development. On the other hand, the thermogenic properties of PVAT may reduce plasma triglyceride levels, leading to reduced atherosclerosis. These paradoxical effects nevertheless suggest that PVAT may be an attractive target for atherosclerosis interventions, and warrants further study of the role of this tissue on vascular disease.
Perspective
PVAT is increasingly being accepted as an integral part of the vasculature, and it is clear that functional PVAT is necessary to maintain vascular physiology. Regarding the effects of PVAT on vascular diseases, it is still unclear if dysfunctional PVAT leads to vascular disease or if vascular lesions lead to dysfunctional PVAT. Current evidence from experimental animals and the clinic do not adequately answer this question. There is an urgent need for animal models that modify genes or proteins solely in PVAT. Additionally, the anatomy of PVAT is complex: 1) while most vessels are surrounded by PVAT, some, including cerebral vasculature, are not; 2) PVAT of vessels in different locations exhibit different phenotypes, with characteristics resembling white, brown, beige or perhaps a new type of adipose tissue; and 3) the type of PVAT differs between species.
Along with the investigation of the effects of PVAT on vascular diseases such as hypertension and atherosclerosis, it is essential to study the effects of PVAT on cardiovascular complications of other diseases such as diabetes, systemic immune disease, etc. Conversely, it is also important to study the effects of these diseases on PVAT biology. So far there has been considerable data on factors released by PVAT, including the PVRFs and PVCFs, although there is a dearth of information on the molecular targets of these factors, and which cells they may target. It is important to delineate the receptors on fibroblasts, VSMCs and ECs that receive the signals produced by PVAT to investigate the crosstalk between all of the cell types of the vasculature. Finally, the possibility that PVAT-mediated thermogenesis and PVAT energy metabolism at large could play a protective role in vascular disease should be systematically addressed as a new potential target for intervention.
Acknowledgments
The authors thank Dr. Minerva Garcia-Barrio at Morehouse School of Medicine for critical reading of the manuscript.
Sources of Funding
This work was supported by the National Institutes of Health Grants HL068878, HL105114, and HL088391 (to Y.E.C.), and by the American Heart Association National Scientist Development Grant (09SDG2230270 to L.C.).
Abbreviations
- PVAT
Perivascular adipose tissue
- WAT
white adipose tissue
- BAT
brown adipose tissue
- UCP-1
uncoupling protein-1
- CVD
cardiovascular disease
- PVRF
PVAT-derived relaxing factor
- ADCF
adipose-derived contracting factor
- BP
blood pressure
Footnotes
Disclosure: None
References
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American journal of physiology. Endocrinology and metabolism. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 2.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent ucp1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. Journal of lipid research. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerback S. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, Tseng YH. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Moller K, Scheele C. A classical brown adipose tissue mrna signature partly overlaps with brite in the supraclavicular region of adult humans. Cell metabolism. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human bat possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: The new brown fat activators. Cell metabolism. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 map kinase and erk map kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 13.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and fgf21 are cold-induced endocrine activators of brown fat function in humans. Cell metabolism. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szasz T, Webb RC. Perivascular adipose tissue: More than just structural support. Clinical science. 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of cellular and molecular medicine. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabro P, Yeh ET. Obesity, inflammation, and vascular disease: The role of the adipose tissue as an endocrine organ. Sub-cellular biochemistry. 2007;42:63–91. [PubMed] [Google Scholar]
- 19.Verhagen SN, Buijsrogge MP, Vink A, van Herwerden LA, van der Graaf Y, Visseren FL. Secretion of adipocytokines by perivascular adipose tissue near stenotic and non-stenotic coronary artery segments in patients undergoing cabg. Atherosclerosis. 2014;233:242–247. doi: 10.1016/j.atherosclerosis.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Britton KA, Pedley A, Massaro JM, Corsini EM, Murabito JM, Hoffmann U, Fox CS. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the framingham heart study. Journal of the American Heart Association. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi C, Santini E, Chiarugi M, Salvati A, Comassi M, Vitolo E, Madec S, Solini A. The complex p2x7 receptor/inflammasome in perivascular fat tissue of heavy smokers. European journal of clinical investigation. 2014;44:295–302. doi: 10.1111/eci.12232. [DOI] [PubMed] [Google Scholar]
- 22.Shields KJ, Barinas-Mitchell E, Gingo MR, Tepper P, Goodpaster BH, Kao AH, Manzi S, Sutton-Tyrrell K. Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis. 2013;231:129–135. doi: 10.1016/j.atherosclerosis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: Epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Annals of medicine. 2012;44 (Suppl 1):S74–84. doi: 10.3109/07853890.2012.663928. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American journal of physiology. Heart and circulatory physiology. 2011;301:H1425–1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 27.Harms M, Seale P. Brown and beige fat: Development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 28.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ, Jr, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Miano JM, Cserjesi P, Olson EN. Sm22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circulation research. 1996;78:188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 30.de Souza RR, Ferraz de Carvalho CA, Merluzzi Filho TJ, Andrade Vieira JA. Functional anatomy of the perivascular tissue in the adductor canal. Gegenbaurs morphologisches Jahrbuch. 1984;130:733–738. [PubMed] [Google Scholar]
- 31.Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Current pharmaceutical design. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 32.Szasz T, Webb RC. Perivascular adipose tissue: More than just structural support. Clinical science. 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clinical and experimental hypertension. Part A, Theory and practice. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 35.Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, Vinson C, Lee RM. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–372. doi: 10.1161/01.HYP.0000255576.16089.b9. [DOI] [PubMed] [Google Scholar]
- 36.Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M, Fernandez-Alfonso MS. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Frontiers in pharmacology. 2012;3:103. doi: 10.3389/fphar.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. European journal of pharmacology. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, Su CJ. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clinical and experimental hypertension. 2009;31:355–363. doi: 10.1080/10641960902977916. [DOI] [PubMed] [Google Scholar]
- 39.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 40.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, Ruiz-Gayo M, Fernandez-Alfonso MS, Somoza B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–3306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the ampk/mtor pathway in high-fat diet-induced obese rats. Hypertension research: official journal of the Japanese Society of Hypertension. 2010;33:446–453. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- 42.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase c-beta pathway. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunker AK, Laughlin MH. Influence of exercise and perivascular adipose tissue on coronary artery vasomotor function in a familial hypercholesterolemic porcine atherosclerosis model. Journal of applied physiology. 2010;108:490–497. doi: 10.1152/japplphysiol.00999.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dick GM, Katz PS, Farias M, 3rd, Morris M, James J, Knudson JD, Tune JD. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. American journal of physiology. Heart and circulatory physiology. 2006;291:H2997–3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- 45.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. American journal of physiology. Heart and circulatory physiology. 2005;289:H48–56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 46.Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, Tune JD. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via pkc-beta-dependent phosphorylation of nitric oxide synthase. American journal of physiology. Heart and circulatory physiology. 2009;297:H460–465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beltowski J, Wojcicka G, Marciniak A, Jamroz A. Oxidative stress, nitric oxide production, and renal sodium handling in leptin-induced hypertension. Life sciences. 2004;74:2987–3000. doi: 10.1016/j.lfs.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circulation research. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 50.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. American journal of physiology. Heart and circulatory physiology. 2007;292:H904–911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya I, Dragert K, Albert V, Contassot E, Damjanovic M, Hagiwara A, Zimmerli L, Humar R, Hall MN, Battegay EJ, Haas E. Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2105–2111. doi: 10.1161/ATVBAHA.112.301001. [DOI] [PubMed] [Google Scholar]
- 52.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 53.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovascular research. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Xu A, Wang Y, Lam KS, Vanhoutte PM. Vascular actions of adipokines molecular mechanisms and therapeutic implications. Advances in pharmacology. 2010;60:229–255. doi: 10.1016/B978-0-12-385061-4.00008-8. [DOI] [PubMed] [Google Scholar]
- 55.Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous h2s formation in perivascular adipose tissue. Pharmacological research: the official journal of the Italian Pharmacological Society. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: The role of endothelium and hydrogen peroxide. British journal of pharmacology. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. Journal of hypertension. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 58.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 59.Hermenegildo C, Oviedo PJ, Garcia-Perez MA, Tarin JJ, Cano A. Effects of phytoestrogens genistein and daidzein on prostacyclin production by human endothelial cells. The Journal of pharmacology and experimental therapeutics. 2005;315:722–728. doi: 10.1124/jpet.105.090456. [DOI] [PubMed] [Google Scholar]
- 60.Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and cox2−/− knockout but not cox1−/− knockout mice. Journal of cardiovascular pharmacology. 2005;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- 61.Ozen G, Topal G, Gomez I, Ghorreshi A, Boukais K, Benyahia C, Kanyinda L, Longrois D, Teskin O, Uydes-Dogan BS, Norel X. Control of human vascular tone by prostanoids derived from perivascular adipose tissue. Prostaglandins & other lipid mediators. 2013;107:13–17. doi: 10.1016/j.prostaglandins.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. The Journal of endocrinology. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 63.Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: The role of adipocyte-derived angiotensin ii. European journal of pharmacology. 2010;634:107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Mamiya Y, Suzuki M, Namima M, Ouno H. the influence of the sympathetic nervous system on brown adipose tissue: An experimental and histopathologic study on aging. Nihon Naibunpi Gakkai zasshi. 1991;67:1252–1262. doi: 10.1507/endocrine1927.67.11_1252. [DOI] [PubMed] [Google Scholar]
- 65.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: The role of superoxide anion. Cardiovascular research. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PloS one. 2013;8:e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R, Fink GD. Chemerin connects fat to arterial contraction. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1320–1328. doi: 10.1161/ATVBAHA.113.301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging cell. 2013 doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson S. Physiological effects of heat and cold. Annual review of physiology. 1952;14:73–96. doi: 10.1146/annurev.ph.14.030152.000445. [DOI] [PubMed] [Google Scholar]
- 71.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vascular health and risk management. 2013;9:105–116. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: A transgenic mouse. Genes & development. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan SZ, Ivashchenko CY, Whitesall SE, D’Alecy LG, Duquaine DC, Brosius FC, 3rd, Gonzalez FJ, Vinson C, Pierre MA, Milstone DS, Mortensen RM. Hypotension, lipodystrophy, and insulin resistance in generalized ppargamma-deficient mice rescued from embryonic lethality. The Journal of clinical investigation. 2007;117:812–822. doi: 10.1172/JCI28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: A new mouse model of inducible and reversible lipoatrophy. Nature medicine. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 75.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular ppargamma controls circadian variation in blood pressure and heart rate through bmal1. Cell metabolism. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with ppar gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 78.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 79.Wilcox JN, Okamoto EI, Nakahara KI, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: A role for circulating myofibroblast precursors? Annals of the New York Academy of Sciences. 2001;947:68–90. doi: 10.1111/j.1749-6632.2001.tb03931.x. dicussion 90–62. [DOI] [PubMed] [Google Scholar]
- 80.Moe KT, Naylynn TM, Yin NO, Khairunnisa K, Allen JC, Wong MC, Chin-Dusting J, Wong P. Tumor necrosis factor-alpha induces aortic intima-media thickening via perivascular adipose tissue inflammation. Journal of vascular research. 2013;50:228–237. doi: 10.1159/000350542. [DOI] [PubMed] [Google Scholar]
- 81.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 82.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circulation research. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch FS, Chalikias G, Konstantinides S, Schafer K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:980–987. doi: 10.1161/ATVBAHA.113.301393. [DOI] [PubMed] [Google Scholar]
- 84.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 85.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein e deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A postmortem study. Atherosclerosis. 2012;225:99–104. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9. [DOI] [PubMed] [Google Scholar]
- 88.Kawahito H, Yamada H, Irie D, Kato T, Akakabe Y, Kishida S, Takata H, Wakana N, Ogata T, Ikeda K, Ueyama T, Matoba S, Mori Y, Matsubara H. Periaortic adipose tissue-specific activation of the renin-angiotensin system contributes to atherosclerosis development in uninephrectomized apoe−/− mice. American journal of physiology. Heart and circulatory physiology. 2013;305:H667–675. doi: 10.1152/ajpheart.00053.2013. [DOI] [PubMed] [Google Scholar]
- 89.Mayr M, Chung YL, Mayr U, Yin X, Ly L, Troy H, Fredericks S, Hu Y, Griffiths JR, Xu Q. Proteomic and metabolomic analyses of atherosclerotic vessels from apolipoprotein e-deficient mice reveal alterations in inflammation, oxidative stress, and energy metabolism. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2135–2142. doi: 10.1161/01.ATV.0000183928.25844.f6. [DOI] [PubMed] [Google Scholar]
- 90.Balogh L, Donhoffer S, Mestyan G, Pap T, Toth I. The effect of environmental temperature on the o2-consumption and body temperature of rats under the acute action of some drugs affecting energy exchange and body temperature. Acta physiologica Hungarica. 1952;3:367–375. [PubMed] [Google Scholar]
- 91.Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201–202. doi: 10.1038/206201b0. [DOI] [PubMed] [Google Scholar]
- 92.Drettner B. Vascular reactions on the intake of food and drink of various temperatures. Acta oto-laryngologica. Supplementum. 1964;188(SUPPL 188):249. doi: 10.3109/00016486409134570. [DOI] [PubMed] [Google Scholar]
- 93.Zeman V, Novak J, Holecek V. blood pressure response to swimming in icy and warm water in individuals adapted to cold. Casopis lekaru ceskych. 1982;121:1035–1038. [PubMed] [Google Scholar]
- 94.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 95.Taggart P, Parkinson P, Carruthers M. Cardiac responses to thermal, physical, and emotional stress. British medical journal. 1972;3:71–76. doi: 10.1136/bmj.3.5818.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheldahl LM, Wilke NA, Dougherty S, Tristani FE. Cardiac response to combined moderate heat and exercise in men with coronary artery disease. The American journal of cardiology. 1992;70:186–191. doi: 10.1016/0002-9149(92)91273-7. [DOI] [PubMed] [Google Scholar]
- 97.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 98.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. Prdm16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]