Abstract
Purpose
EC145 (vintafolide), a conjugate of folic acid and the vinca alkaloid desacetylvinblastine hydrazide (DAVLBH), is a ligand for the folate receptor (FR), with activity against FR-positive tumor xenografts in vivo. This phase I study determined the maximum-tolerated dose (MTD) of EC145 administered as a bolus intravenous injection or 1-hour infusion in patients with refractory solid tumors.
Patients and Methods
EC145 was administered as a bolus injection or 1-hour infusion on days 1, 3, and 5 and days 15, 17, and 19 of each 28-day cycle with dose escalation in cohorts of three to six patients until the MTD was identified. Plasma pharmacokinetics were determined on days 1 and 3 of the first cycle.
Results
The MTD of EC145 was 2.5 mg when administered as either a bolus injection or 1-hour infusion. Constipation was the dose-limiting toxicity with both routes. Constipation, nausea, fatigue, and vomiting were the most commonly reported adverse events. One partial response to therapy was observed in a patient with metastatic ovarian cancer.
Conclusion
EC145 administered by bolus injection or as a 1-hour infusion at a dose of 2.5 mg on days 1, 3, and 5 and days 15, 17, and 19 of a 28-day cycle has an acceptable safety profile in patients with advanced cancer. On the basis of these findings, phase II studies of EC145 have been initiated in patients with advanced epithelial ovarian cancer and non–small-cell lung cancer.
INTRODUCTION
Toxicity to normal tissue restricts the use of cytotoxic agents. Limitations include a narrowed therapeutic index and recovery from toxicity before repeat dosing, with lowered dose or modified administration schedule from what might provide maximum antitumor effect.1 Limiting drug exposure may lead to development of resistance, with decrease in clinical efficacy for cytotoxic drugs.
One solution involves drug conjugates with targeting moieties to guide cytotoxic payloads to cancer cells. Antibody-drug conjugates are one example.2,3 However, the utility of antibody conjugates is limited by their large molecular size. Alternative approaches use small-molecule targeting ligands. EC145 (vintafolide) is such a construct, using folic acid as a high-affinity folate receptor (FR)–targeting ligand conjugated to the microtubule-destabilizing agent, desacetylvinblastine monohydrazide (DAVLBH), via a self-immolative disulfide-based linker system. FR is a membrane protein expressed on a variety of cancers including those of the ovary, lung, breast, and endometrium.4–8 Different from DAVLBH, which displays minor antitumor activity with a narrow therapeutic index in preclinical models, EC145 has been characterized as having a potent (curative) effect specifically against FR-expressing tumor xenografts without significant toxicity, thereby prompting the phase I clinical study reported here.7,8 The objectives of this phase I study were to assess the maximum-tolerated dose (MTD), safety and tolerability, antitumor activity, and pharmacokinetics of EC145 when administered as a bolus intravenous injection or 1-hour infusion.
PATIENTS AND METHODS
Eligibility
Eligible patients were men and nonpregnant women with refractory solid tumors, age ≥ 18 years, with Eastern Cooperative Oncology Group performance status ≤ 2 and adequate bone marrow, liver, and renal function. The protocol was approved by the institutional review boards of the Marlene and Stewart Greenebaum Cancer Center, University of Maryland (Baltimore, MD), and the Barbara Ann Karmanos Cancer Institute, Wayne State University (Detroit, MI), in accordance with the Helsinki Declaration. All patients provided written informed consent.
Dosage and Administration
EC145 was supplied (by Endocyte, West Lafayette, IN) in single-use vials (concentration, 5 mg/mL). Vials were kept frozen until use. For bolus injection cohorts (planned doses, 1.2, 2.5, and 4.0 mg), the appropriate volume of EC145 solution was withdrawn into a syringe and injected over 10 seconds. The intravenous hub was flushed with 10 mL of normal saline before and after bolus administration of EC145.
For the 1-hour infusion cohorts (planned doses, 2.5, 3.0 and 4.0 mg), the appropriate volume of EC145 solution was withdrawn, added to 100 mL of normal saline, and infused through a venous catheter over 1 hour. The EC145 infusion was followed by 25 mL to allow for clearance of the EC145 from the line.
Study Design
EC145 was administered as either a bolus or 1-hour infusion on days 1, 3, and 5 and on days 15, 17, and 19 of each 28-day cycle. The bolus route was explored first, followed by the 1-hour infusion. The bolus starting dose of EC145 was 1.2 mg, representing one sixth of the human-dose equivalent of the MTD in dogs (the most sensitive species tested in toxicology studies), based on a human body surface area of 1.6 m2. The starting dose of EC145 for the 1-hour infusion was 2.5 mg, representing the MTD of EC145 when administered by bolus.
Dose-limiting toxicity (DLT) was defined as any of the following first-cycle drug-related toxicities: grade 2 nonhematologic toxicity that failed to recover to grade 1 in time for the second cycle, grade 3 nonhematologic toxicity (except for nausea/vomiting without maximal symptomatic/prophylactic treatment), any grade 4 hematologic toxicity, or any other toxicity that in the judgment of the investigator would prevent use of the drug dose or regimen by the general oncology community. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.9
EC145 was dose escalated in subsequent cohorts until the MTD was reached. At least three patients were treated in each cohort. If one of the first three patients treated at a given dose level experienced DLT, an additional three patients were treated at that dose level. If ≤ one of six patients experienced DLT, enrollment proceeded to the next dose level. If ≥ two of six patients experienced DLT, the MTD was considered exceeded, and escalation of the EC145 dose ceased. The MTD was defined as the dose at which no more than one of six patients experienced DLT.
Patients completing the first cycle of therapy were allowed to receive additional cycles of EC145 until disease progression (PD) or unacceptable toxicity. Once three patients had completed the first cycle at a higher dose level without DLT, escalation of EC145 dose was allowed in those who did not experience significant toxicity at a lower dose. Reductions and re-escalations in EC145 dose were allowed for individual patients based on tolerability. Treatment could be delayed for up to 2 weeks to allow for recovery from toxicity.
Assessment of Safety
Patients were assessed during each clinic visit or by telephone contact during off weeks. A physical examination was performed during the first week of each cycle. Laboratory studies were obtained on days 1 and 15 of each cycle. Vital signs were measured before and after EC145 administration on days 1, 3, 5, 15, 17, and 19 of each cycle.
Assessment of Response
Imaging studies occurred every 8 weeks. Response was assessed by RECIST, version 1.0.10
Pharmacokinetic Analysis
Blood samples were collected on days 1 and 3 of the first cycle of therapy. In bolus cohorts, samples were collected within 15 minutes before EC145 injection and at 5, 15, 30, 45, 60, and 90 minutes postinjection. For the 1-hour infusion cohorts, blood samples were collected within 15 minutes of the start of the infusion and at 30, 60, 75, 90, 105, and 120 minutes after the start of the infusion. Plasma concentrations of EC145 were determined as described elsewhere.11
Statistical Analysis
Patient demographics, adverse events, clinical laboratory evaluations, and vital signs were summarized descriptively. Quantitative laboratory measurements were categorized according to the normal reference range (low, normal, high), and shift tables of the pretreatment versus post-treatment category were prepared.
RESULTS
Patient Demographics
Thirty-two patients were enrolled and treated with EC145 (Table 1). Patients entering this trial had received a median of eight prior chemotherapies (range, two to 22), with 84% having prior exposure to a platin, and 65% undergoing prior irradiation or local treatment. Sixteen patients each received bolus injections of EC145 or 1-hour infusions of EC145 on days 1, 3, and 5 and days 15, 17, and 19 of each 28-day cycle.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Route of Administration |
Total (N = 32) |
||||
|---|---|---|---|---|---|---|
| Bolus IV Injection (n = 16) |
1-Hour IV Infusion (n = 16) |
|||||
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 10 | 63 | 8 | 50 | 18 | 56 |
| Female | 6 | 38 | 8 | 50 | 14 | 44 |
| Race | ||||||
| White | 12 | 75 | 9 | 63 | 21 | 66 |
| Black/African American | 4 | 25 | 7 | 25 | 11 | 34 |
| Age, years | ||||||
| Mean ± SD | 57.7 ± 9.8 | 56.4 ± 13.2 | 57.1 ± 11.4 | |||
| ECOG performance status | ||||||
| 0 | 1 | 6 | 3 | 19 | 4 | 13 |
| 1 | 13 | 81 | 9 | 56 | 22 | 69 |
| 2 | 2 | 13 | 4 | 25 | 6 | 19 |
| Tumor type | ||||||
| Colorectal | 4 | 25 | 5 | 31 | 9 | 28 |
| Head and neck | 3 | 19 | 3 | 19 | 6 | 19 |
| Lung | 1 | 6 | 4 | 25 | 5 | 16 |
| Ovarian | 2 | 13 | 0 | 0 | 2 | 6 |
| Pancreatic | 1 | 6 | 1 | 6 | 2 | 6 |
| Bladder | 1 | 6 | 0 | 0 | 1 | 3 |
| Breast | 1 | 6 | 0 | 0 | 1 | 3 |
| Renal | 1 | 6 | 0 | 0 | 1 | 3 |
| Thyroid | 1 | 6 | 0 | 0 | 1 | 3 |
| Esophageal | 0 | 0 | 1 | 6 | 1 | 3 |
| Other | 1 | 6 | 2 | 13 | 3 | 9 |
| Prior anticancer therapy | ||||||
| Surgery | 16 | 100 | 16 | 100 | 32 | 100 |
| Chemotherapy | 15 | 94 | 16 | 100 | 32 | 96 |
| Radiotherapy | 10 | 63 | 10 | 63 | 20 | 63 |
| Investigational therapy | 10 | 63 | 8 | 50 | 18 | 56 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SD, standard deviation.
The study included 18 men and 14 women (mean age, 57.1 years; Table 1). Twenty-six patients had baseline Eastern Cooperative Oncology Group performance status of 0 or 1. All patients (100%) had undergone prior surgery; 31 (96.9%) had received prior systemic chemotherapy; 20 (62.5%) had received radiation therapy; and 18 (56.3%) had received other investigational agents.
Patient Disposition
At analysis, 31 of the 32 patients were off study. The primary reason for discontinuation was PD (69%; 22 of 32). Three patients—one (6%) in the bolus arm and two (13%) in the infusion arm—discontinued treatment because of adverse events. Other reasons for discontinuation were physician decision (9%; three of 32) and patient withdrawal of consent (9%; three of 32).
Dose Escalation and Toxicity
The starting bolus dose of EC145 was 1.2 mg; both this dose and the next higher dose (2.5 mg) were well tolerated during the first cycle, with no DLT among the three patients treated at either dose level (Table 2). With administration of the third bolus dose level (4 mg), one patient encountered grade 2 constipation after 1 week of therapy (three doses of EC145), and another experienced reversible grade 3 ileus after one dose. Dose escalation was halted, because these toxicities (acute onset constipation ± ileus) were of sufficient severity to prevent use of this dose by the general oncology community. Seven more patients were enrolled at the 2.5-mg EC145 dose (total of 10 patients) to further characterize toxicity at 2.5 mg. No first-cycle DLT occurred in any of the 10 patients treated with bolus 2.5-mg EC145. The MTD of EC145 when administered as bolus was therefore determined to be 2.5 mg.
Table 2.
Dose Escalation and Toxicity (first cycle)
| Dose Level | Dose (mg) | No. of Patients | No. of Cycles |
No. of Patients With Unacceptable Toxicity in First Cycle | |
|---|---|---|---|---|---|
| Total | Median | ||||
| Bolus IV injection | |||||
| 1 | 1.2 | 3 | 5 | 1 | 0 |
| 2 | 2.5* | 10 | 32 | 2 | 0 |
| 3 | 4.0 | 3 | 4 | 1 | 2 |
| 1-hour IV infusion | |||||
| 1 | 2.5* | 10 | 30 | 1.5 | 1 |
| 2 | 3.0 | 6 | 8 | 1 | 2 |
Abbreviation: IV, intravenous.
Maximum-tolerated dose.
After the bolus MTD was established, rather than explore an intermediate-dose level between 2.5 and 4 mg, and having demonstrated tolerability of the 2.5-mg bolus, we next amended the protocol to determine the 1-hour infusion MTD, because toxicity could possibly relate to a peak effect of the bolus. The starting dose for the infusion was 2.5 mg (ie, the MTD for bolus), and this infusion dose was well tolerated by the first three patients (Table 2). With administration of the next dose (3 mg), two of six patients encountered acute-onset grade 2 constipation. These findings would also prevent use of this dose by the general oncology community. No further dose escalation was undertaken. An additional seven patients were treated with a 1-hour infusion of 2.5 mg of EC145 (for a total of 10 patients treated at this dose level). First-cycle DLT (grade 3 ileus) was observed in one of these patients. On the basis of these findings, the 1-hour infusion MTD was also determined to be 2.5 mg. The fact that in the 1-hour infusion schedule, 3 mg was not tolerated, did not suggest the likely value of exploring intermediate doses (between 2.5 and 4 mg) by the bolus route.
Constipation, nausea, fatigue, and vomiting were the most commonly reported adverse events by either route of administration; however, these events were primarily grade 1 to 2. RECIST version 3.0 grade 2 constipation as DLT requires persistence despite regular use of laxatives or enemas. The usual regimen suggested to patients, but not quantitatively assessed with respect to usage, included a softener such as docusate, to which the patient could then add senna and then Miralax or lactulose. Symptomatic constipation or overt ileus despite these measures constituted the basis for definition of DLT. No important toxicity differences were observed between the bolus and 1-hour infusion administration schedules (Table 3).
Table 3.
No. of Occurrences of Most Common Drug-Related Toxicities During EC145 Therapy*
| Toxicity | Bolus IV Injection (grade) |
1-Hour IV Infusion (grade) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.2 mg (n = 3) |
2.5 mg (n = 10) |
4.0 mg (n = 3) |
2.5 mg (n = 10) |
3.0 mg (n = 6) |
|||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Constipation | 1 | — | — | 1 | 3 | 1 | — | 1 | 1 | 1 | 3 | — | 1 | 1 | 2 |
| Nausea | — | — | — | 2 | 4 | — | 1 | 1 | — | 3 | — | — | 1 | — | — |
| Vomiting | 1 | — | — | 2 | 3 | — | — | 1 | — | 2 | — | — | 2 | — | — |
| Fatigue | 1 | 1 | — | 1 | 2 | 1 | — | 2 | — | 1 | 2 | 1 | — | 3 | — |
| Peripheral sensory neuropathy | 1 | 1 | — | 3 | 1 | — | — | — | — | 2 | — | 1 | 2 | — | — |
Abbreviation: IV, intravenous.
Reported in ≥ 10% of patients.
Other Clinical Toxicities
Adverse events were primarily grade 1 to 2 in severity, with few grade 3 toxicities reported for either route of administration (Appendix Table A1, online only). On review of neutrophil counts, only three of 29 patients had grade 1 decrease. No grade 4 events were reported in patients treated with bolus EC145. Grade 4 events were reported in four of 10 patients treated with 2.5 mg of EC145 as a 1-hour infusion. Events included hyponatremia (patient 019); pulmonary embolism (patient 030); pulmonary thrombosis (patient 031); and cardiac arrest, hypotension, somnolence, and acute renal failure (patient 033). None of these events were attributed to the study medication.
Among the 32 patients treated with bolus injection or 1-hour infusion of EC145, constipation occurred in 16 (50%): grade 1 to 2 in 12 (38%), and grade 3 in 4 (13%); no cases of grade 4 constipation occurred. Constipation was exacerbated by concomitant use of narcotic analgesics.
Peripheral sensory neuropathy (PSN) was reported in nine patients (28%) and peripheral neuropathy in two (6%). The incidence of neuropathy was comparable for bolus injection and 1-hour infusion. Most cases of neuropathy were grade 1 or 2. PSN was commonly observed in patients who had previously received radiation therapy for head and neck cancers. Specifically, of six patients with head and neck cancer, four experienced PSN (66%); five (19%) of 26 patients with non–head and neck cancer had PSN. The single patient who experienced grade 3 PSN (Appendix Table A1, online only) entered the trial with pre-existing grade 1 neuropathy.
Three deaths (9.4%) were reported among 32 patients treated with EC145. Deaths in these patients were attributed to complications of underlying disease or PD; no deaths were attributed to EC145. No significant hematologic, liver, or renal toxicity or effects on blood pressure, heart rate, or respiratory rate were observed after administration of EC145.
Pharmacokinetics
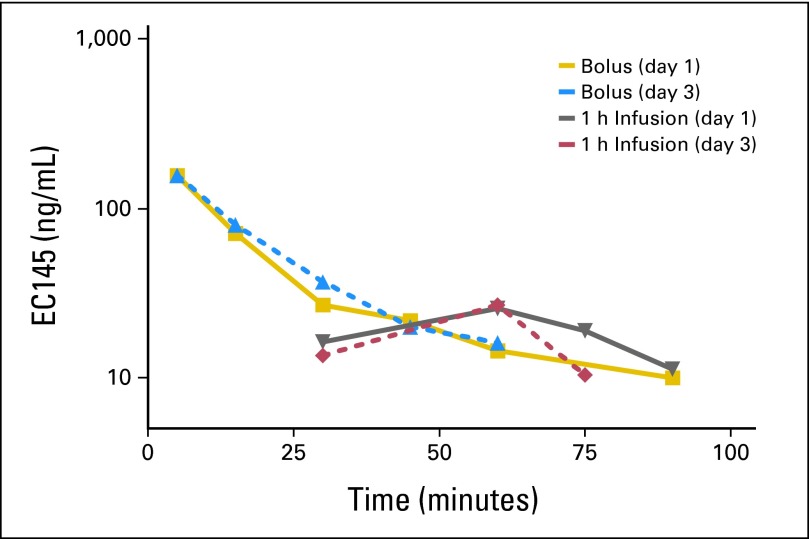
After bolus injection or 1-hour infusion of 2.5 mg of EC145, maximum plasma concentrations of EC145 were observed at 5 minutes after administration both on day 1 and on day 3. Plasma concentrations of EC145 declined to predose levels by 1.5 hours after injection or infusion (Fig 1).
Fig 1.
Representative EC145 concentration-time profiles after 2.5-mg bolus intravenous dose and 2.5-mg 1-hour infusion of EC145.
The EC145 mean maximum plasma concentration, adjusted for protein binding (estimated at 54%), exceeded the 50% inhibition concentration in vitro for EC145 (ie, 9 nmol/L in KB cells) by both routes of administration.7 No significant changes in exposure to drug or total clearance of drug were observed between days 1 and 3 for either route of administration (Table 4). Full pharmacokinetic data obtained from this study can be found in a separate report.11
Table 4.
Pharmacokinetic Parameters for EC145
| Parameter | Bolus IV Injection (2.5 mg) |
1-Hour IV Infusion (2.5 mg) |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 (n = 10) |
Day 3 (n = 7) |
Day 1 (n = 10) |
Day 3 (n = 10) |
|||||
| Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | |
| Cmax, ng/mL | 134.3 | 43.8 | 123.2 | 6.2 | 38.6 | 64.7 | 44.8 | 43.1 |
| Tmax, minutes | 5.5 | 15.5 | 5.1 | 6.2 | 66.0 | 19.6 | 66.0 | 18.6 |
| AUClast, ng × h/mL | 43.0 | 48.3 | 40.2 | 38.0 | 36.0 | 79.0 | 43.2 | 47.2 |
| AUC∞, ng × h/mL | 50.1 | 38.1 | 49.9 | 25.3 | 57.6 | 71.5 | 70.9 | 25.3 |
| t1/2, minutes | 19.9 | 52.9 | 21.5 | 38.6 | 25.3 | 30.6 | 33.6 | 34.3 |
| CL, L/h | 46.9 | 50.2 | 56.8 | 46.0 | 59.7 | 54.6 | 41.6 | 39.7 |
| Vd, L | 21.9 | 38.1 | 26.6 | 32.6 | 32.7 | 41.4 | 32.9 | 60.6 |
| λz, h−1 | 3.0 | 36.5 | 2.3 | 38.6 | 1.8 | 39.6 | 1.5 | 50.8 |
Abbreviations: λz, apparent terminal-phase disposition rate constant (first order); AUC∞, area under the concentration-time curve from zero to infinity; AUClast, AUC from zero to the last observable concentration; Cmax, peak (maximum) plasma concentration; CL, clearance; IV, intravenous; t1/2, half-life; Tmax, time to peak (maximum) plasma concentration; Vd, volume of distribution.
Response to Therapy
Seven patients maintained stable disease (SD) for periods ranging from 42 to 211 days during EC145 therapy. One RECIST-defined partial response (PR) of 111 days duration was observed (Table 5). This patient had metastatic ovarian cancer, and tumor shrinkage was accompanied by a decrease in cancer antigen 125 levels over the response period (Appendix Table A2, online only). This patient had received two prior courses of carboplatin plus paclitaxel, in addition to phospholipid-formulated doxorubicin, gemcitabine, topotecan, and altretamine. A second patient with metastatic ovarian cancer maintained SD for 172 days during EC145 therapy; decreases in cancer antigen 125 levels were also observed during the time SD was maintained. This patient had received two prior courses of carboplatin plus paclitaxel, as well as docetaxel, phospholipid-formulated doxorubicin, gemcitabine, topotecan, INNO-105, and benzoylphenylurea.
Table 5.
Patients With Response or SD During EC145 Therapy
| Patient No. | Dose Cohort (mg) | Mode of Administration | Tumor Type | Sex | Age (years) | Race | Best Response | Duration of PR or SD (days) |
|---|---|---|---|---|---|---|---|---|
| 003 | 1.2 | Bolus | Head and neck | M | 61 | White | SD | 95 |
| 004 | 2.5 | Bolus | Ovarian | F | 64 | White | SD | 172 |
| 005 | 2.5 | Bolus | Lung | M | 67 | Black | SD | 42 |
| 010 | 2.5 | Bolus | Ovarian | F | 65 | White | PR | 111 |
| 011 | 2.5 | Bolus | Nerve sheath | M | 33 | White | SD | 82 |
| 015 | 2.5 | Bolus | Head and neck | M | 57 | Black | SD | 117 |
| 021 | 2.5 | Infusion | Head and neck | F | 43 | White | SD | 211 |
Abbreviations: PR, partial response; SD, stable disease.
DISCUSSION
Progress in cancer therapeutics will include novel strategies to deliver cytotoxic agents selectively to tumors expressing specific molecular targets. FR-based targeting capitalizes on increased expression of FR in a variety of tumors, and the intrinsically efficient endocytotic movement of FR into the cell after engagement of ligand.12 EC145 is the first agent in the clinic where the folate moiety is linked through a hydrolysable linker to desacetylvinblastine hydrazide. Although other FR-targeted constructs have been proposed (eg, folate-targeted liposomes, folate-targeted nanoparticles, or polyethylene glycol nanoemulsions), the complex nature of these approaches renders them potentially difficult to standardize for production in comparison with the straightforward chemical coupling approach used in producing EC145.13–15 Thus, EC145 and related molecules allow clinical examination of whether FR-directed targeting can achieve clinical activity without characteristic toxicities normally related to vinca alkaloids.
This phase I study of EC145 allows for the following conclusions: First, the MTD and recommended phase II dose on a three times per week, every other week, schedule is 2.5 mg administered either as a bolus injection or 1-hour infusion; second, EC145 has an acceptable safety profile, without evidence of myelosuppression at the doses explored; third, pharmacologic studies confirmed levels of EC145 consistent with those necessary for cytotoxicity mediated by targeting the FR; and finally, evidence of clinical benefit was observed, with a PR in one patient with ovarian cancer and disease control (complete response + PR + SD) in 37.5% of patients (six of 16) receiving intravenous bolus EC145. The indication of some clinical benefit in patients with ovarian carcinoma is of interest, owing to the overexpression of the FR in ovarian carcinoma.16 At the recommended phase II dose on the bolus schedule, there was no significant grade 3 or 4 thrombocytopenia or granulocytopenia, even in this heavily pretreated population. This experience differs from the observations of Rossi et al,17 who observed a 25% incidence of granulocytopenia and 17% incidence of constipation when using vinorelbine.
Published preclinical results using human tumor xenograft-bearing mice8 confirmed that response to intravenous EC145 was schedule dependent, with more favorable outcomes occurring in those cohorts receiving more frequent drug exposure. This effect is supported by the ability of the FR to unload EC145 inside the cell and recycle back to the cell surface in less than 24 hours,18 suggesting that a dose-dense regimen might prove to be more effective than a more intermittent schedule of administration.
The prominence of GI toxicity manifested by vinca alkaloids in comparison with taxanes has raised the possibility that hepatobiliary metabolism may not fully eliminate metabolites that once excreted into the bile, potentiate GI toxicity.19 Indeed, recent preclinical studies have demonstrated unconjugated desacetylvinblastine hydrazide in the bile of bile duct–canulated rats after dosing with EC145, thus providing a basis for the GI toxicity of EC145 in humans observed here.20 Although nearly all patients involved in the study were receiving concomitant analgesic narcotics, a majority of those treated at the proposed phase II dose tolerated the agent well. What constipation did occur at the 2.5-mg dose level was easily managed by stool softeners and bowel-stimulating agents.
The favorable pharmacologic features of EC145, with rapid clearance (half-life: bolus, approximately 20 minutes; 1-hour infusion, approximately 25 minutes), afforded peak concentrations (bolus, approximately 130 ng/mL; 1-hour infusion, approximately 42 ng/mL) consistent with allowing FR-mediated internalization and subsequent cytotoxicity.7 The rapid clearance is consistent with the goal of maximally loading FR in the tumor while avoiding prolonged marrow exposure, thus decreasing myelotoxicity.
Patients entering this initial phase I trial of EC145 were not preselected by expression of the FR. At the initiation of this trial, antibody staining of tissue for FR had, and continues to have, severe limitations including tumor heterogeneity, antibody nonspecificity, and reproducibility of staining. Thus, FR immunohistochemistry was not made a condition for entry into this phase I trial. The need to define the presence of FR for future development of EC145 encouraged the development of EC20, a technetium-folate conjugate. Fisher et al21 recently described EC20 as a useful FR-directed imaging probe that may allow real-time evaluation of FR expression status in patients before therapy with EC145. Tissues collected retrospectively under informed consent from this trial, as well as from subsequent phase II studies of EC145 (NCT00507741, NCT00511485, and NCT00722592) in patients with advanced ovarian and non–small-cell lung cancers, will be studied with respect to FR tissue expression as part of ongoing evaluation of EC20. EC20 may be ultimately more useful than immunohistochemistry to identify patients whose tumors express FR.
The results of this trial are encouraging and indicate a need for further exploration of EC145 in FR-expressing malignancies. On the basis of our findings, phase II studies were started exploring the utility of EC145 in patients with advanced epithelial ovarian and non–small-cell lung cancers. Initial results from a phase II randomized study of EC145 in combination with pegylated liposomal doxorubicin indicate a significant improvement in progression-free survival in patients with platinum-resistant ovarian cancer.22
Appendix
Table A1.
Related Adverse Events During Bolus IV Injection or 1-Hour IV Infusion of EC145 (all cycles)*
| System Organ Class/Preferred Term† | Bolus IV Injection (n = 16) |
1-Hour IV Infusion (n = 16) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Grades |
Grades 1-2 |
Grade 3 |
All Grades |
Grades 1-2 |
Grade 3 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 13 | 1 | 6 | 1 | 6 |
| GI disorders | ||||||||||||
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 19 | 2 | 13 | 1 | 6 |
| Constipation | 5 | 31 | 5 | 31 | 0 | 0 | 7 | 44 | 5 | 31 | 2 | 13 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 13 | 2 | 13 | 0 | 0 |
| Nausea | 2 | 13 | 2 | 13 | 0 | 0 | 5 | 31 | 5 | 31 | 0 | 0 |
| Vomiting | 2 | 13 | 2 | 13 | 0 | 0 | 2 | 13 | 2 | 13 | 0 | 0 |
| General disorders and administration site conditions | ||||||||||||
| Fatigue | 2 | 13 | 1 | 6 | 1 | 6 | 5 | 31 | 5 | 31 | 0 | 0 |
| Metabolism and nutrition disorders | ||||||||||||
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 13 | 2 | 13 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||||||||
| Pain in jaw | 1 | 6 | 1 | 6 | 0 | 0 | 2 | 13 | 2 | 13 | 0 | 0 |
| Nervous system disorders | ||||||||||||
| Peripheral neuropathy | 2 | 13 | 2 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 4 | 25 | 4 | 25 | 0 | 0 | 5 | 31 | 4 | 25 | 1 | 6 |
Abbreviation: IV, intravenous.
Reported in ≥ 10% of patients.
Medical Dictionary for Regulatory Activities system organ classes and preferred terms were used and are listed in alphabetic order. Adverse events are listed with presumed relationship to the study medication of possibly, probably, or definitely related. All adverse events that were reported in ≥ 10% of patients who were either treated by bolus IV injection or continuous 1-hour IV infusion are included in the tabulation.
Table A2.
Cancer Antigen 125 Levels (U/mL) in Patients With Ovarian Cancer
| Patient No. | Best Response | Baseline Value | In-Study Values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | |||
| 010 | PR | 493 | 669 | 619 | 694 | 401 | 409 | 371 | 408 | 342 | 293 |
| 004 | SD | 271 | 290 | 257 | 253 | 225 | — | — | — | — | — |
Abbreviations: PR, partial response; SD, stable disease.
Footnotes
Supported by Endocyte, West Lafayette, IN.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2007.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00308269.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Susan L. Bever, Endocyte (C); Patrick J. Klein, Endocyte (C); Christopher P. Leamon, Endocyte (C); Richard A. Messmann, Endocyte (C) Consultant or Advisory Role: Patricia M. LoRusso, Endocyte (U); Martin J. Edelman, Endocyte (U); Edward A. Sausville, Endocyte (U) Stock Ownership: Susan L. Bever, Endocyte; Patrick J. Klein, Endocyte Honoraria: None Research Funding: Patricia M. LoRusso, Endocyte; Martin J. Edelman, Endocyte; Edward A. Sausville, Endocyte Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Patricia M. LoRusso, Martin J. Edelman, Christopher P. Leamon, Richard A. Messmann, Edward A. Sausville
Provision of study materials or patients: Patricia M. LoRusso, Martin J. Edelman, Karen M. Forman, MaryJo Pilat, Mary F. Quinn, Elisabeth I. Heath, Lisa M. Malburg, Edward A. Sausville
Collection and assembly of data: Susan L. Bever, Karen M. Forman, MaryJo Pilat, Mary F. Quinn, Elisabeth I. Heath, Lisa M. Malburg, Edward A. Sausville
Data analysis and interpretation: Patricia M. LoRusso, Martin J. Edelman, Jing Li, Lisa M. Malburg, Patrick J. Klein, Richard A. Messmann, Edward A. Sausville
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Chatelut E, Delord JP, Canal P. Toxicity patterns of cytotoxic drugs. Invest New Drugs. 2003;21:141–148. doi: 10.1023/a:1023565227808. [DOI] [PubMed] [Google Scholar]
- 2.Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A phase 1 weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematological malignancies. Clin Cancer Res. 2012;18:248–255. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 3.LoRusso PM, Weiss D, Guardino E, et al. Trastuzumab emtansine: A unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–6447. doi: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- 4.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Reddy JA, Westrick E, Vlahov I, et al. Folate receptor specific anti-tumor activity of folate-mitomycin conjugates. Cancer Chemother Pharmacol. 2006;58:229–236. doi: 10.1007/s00280-005-0151-z. [DOI] [PubMed] [Google Scholar]
- 6.Vlahov IR, Santhapuram HK, Kleindl PJ, et al. Design and regioselective synthesis of a new generation of targeted chemotherapeutics: Part 1—EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett. 2006;16:5093–5096. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Leamon CP, Reddy JA, Vlahov I, et al. Comparative preclinical activity of the folate-targeted vinca alkaloid conjugates EC140 and EC145. Int J Cancer. 2007;121:1585–1592. doi: 10.1002/ijc.22853. [DOI] [PubMed] [Google Scholar]
- 8.Reddy JA, Dorton R, Westrick E, et al. Preclinical evaluation of EC145, a folate-vinca alkaloid conjugate. Cancer Res. 2007;67:4434–4442. doi: 10.1158/0008-5472.CAN-07-0033. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events v3.0 (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Sausville EA, Klein PJ, et al. Clinical pharmacokinetics and exposure-toxicity relationship of a folate-vinca alkaloid conjugate EC145 in cancer patients. J Clin Pharmacol. 2009;49:1467–1476. doi: 10.1177/0091270009339740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bathori G, Cervenak L, Karadi I. Caveolae: An alternative endocytotic pathway for targeted drug delivery. Crit Rev Ther Drug Carrier Syst. 2004;21:67–95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. [DOI] [PubMed] [Google Scholar]
- 13.Yamada A, Le Berre M, Yoshikawa K, et al. Spontaneous generation of giant liposomes from an oil/water interface. Chembiochem. 2007;8:2215–2218. doi: 10.1002/cbic.200700473. [DOI] [PubMed] [Google Scholar]
- 14.Rosenholm JM, Meinander A, Peuhu E, et al. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano. 2009;3:97–206. doi: 10.1021/nn800781r. [DOI] [PubMed] [Google Scholar]
- 15.Ohguchi Y, Kawano K, Hattori Y, et al. Selective delivery of folate-PEG-linked, nanoemulsion-loaded aclacinomycin A to KB nasopharyngeal cells and xenograft: Effect of chain length and amount of folate-PEG linker. J Drug Target. 2008;16:660–667. doi: 10.1080/10611860802201464. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Nymoen DA, Dong HP, et al. Expression of the folate receptor genes FOLR1 and FOLR3 differentiates ovarian carcinoma from breast carcinoma and malignant mesothelioma in serous effusions. Hum Pathol. 2009;40:1453–1460. doi: 10.1016/j.humpath.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Rossi A, Gridelli C, Gebbia V, et al. Single agent vinorelbine as first-line chemotherapy in elderly patients with advanced breast cancer. Anticancer Res. 2003;23:1657–1664. [PubMed] [Google Scholar]
- 18.Paulos CM, Reddy JA, Leamon CP, et al. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 19.Lemke T. Baltimore, MD: Lippincott, Williams & Wilkins; 2008. Cancer and chemotherapy, in Foye's Principles of Medicinal Chemistry (ed 6) pp. 1184–1186. [Google Scholar]
- 20.Leamon CP, Reddy JA, Klein PJ, et al. Reducing undesirable hepatic clearance of a tumor-targeted vinca alkaloid via novel saccharopeptidic modifications. J Pharmacol Exp Ther. 2011;336:336–343. doi: 10.1124/jpet.110.175109. [DOI] [PubMed] [Google Scholar]
- 21.Fisher RE, Siegel BA, Edell SL, et al. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J Nucl Med. 2008;49:899–906. doi: 10.2967/jnumed.107.049478. [DOI] [PubMed] [Google Scholar]
- 22.Naumann RW, Symanowski JT, Ghamande SA. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum-resistant ovarian cancer. J Clin Oncol. 2010;28(suppl):393s. doi: 10.1200/JCO.2013.49.7685. abstr LBA5012b. [DOI] [PubMed] [Google Scholar]