Abstract
The presynaptic protein α-synuclein is central to the pathogenesis of α-synucleinopathies. We show that the presence of endogenous mouse α-synuclein leads to higher number of dopaminergic neurons in the substantia nigra of wild-type C57Bl/6J mice compared with C57Bl/6S mice with a spontaneous deletion of the α-synuclein gene or C57Bl/6J mice with a targeted deletion of the α-synuclein gene. This effect of α-synuclein on dopaminergic neuron occurs during development between E10.5 and E13.5 and persists in adult life supporting the involvement of α-synuclein in the development of a subset of dopaminergic neurons.
Keywords: α-Synuclein, Dopaminergic, Embryonic development, Parkinson’s disease, Transgenic mouse
Introduction
α-Synuclein is a presynaptic protein central to the pathogenesis of Parkinson’s disease (PD), the most common movement disorder (Lees et al., 2009). Lewy bodies (LBs) containing fibrillous α-synuclein and dopaminergic cell death in the substantia nigra (SN) are defining neuropathological features of PD, and mutations or multiplications of the α-synuclein gene (SNCA) are a cause of familial PD (Gasser, 2010; Polymeropoulos et al., 1997; Singleton et al., 2003; Spillantini et al., 1997, 1998).
The physiological role of α-synuclein is still unclear although it is known to bind to lipid membranes and to be implicated in SNARE-mediated exocytosis and synaptic vesicle transport (Chandra et al., 2005; Darios et al., 2010; Garcia-Reitbock et al., 2010; Burre et al., 2010). We have previously reported a transgenic mouse in which truncated (1-120) human α-synuclein is expressed in an endogenous α-synuclein null background under the control of the tyrosine hydroxylase (TH) promoter in dopaminergic cells (Tofaris et al., 2006). α-Syn(1-120) transgenic mice develop a motor phenotype associated with fibrillar/granular α-synuclein inclusions in the SN, coupled to a reduction in striatal dopamine levels and impaired dopamine release associated with SNARE protein redistribution in the absence of dopaminergic cell death (Garcia-Reitbock et al., 2010; Tofaris et al., 2006).
We hypothesized that the presence of the endogenous mouse α-synuclein protein might exacerbate the pathology in our model. To test this we crossed the 1-120 α-syn mice with wild type (wt) C57Bl/6J mice expressing the endogenous protein, generating the new α-syn(1-120)E (E = endogenous) line (Garcia-Reitbock et al., 2010). Surprisingly, the presence of endogenous mouse protein was associated with a dose-dependent increase in the number of TH+ neurons in the SN pars compacta of transgenic α-syn(1-120)E mice. In addition, the absence of endogenous α-synuclein is also associated with a reduced number of dopaminergic cells in the SN of wt CB57Bl/6S mouse subline with a spontaneous deletion of the α-synuclein gene and in α-synuclein knockout C57Bl/6J mice (Robertson et al., 2004) compared to wt C57Bl/6J mice. This difference in dopaminergic neuron number between wt C57Bl/6J mice and mice without endogenous α-synuclein becomes apparent after embryonic day 10.5 (E10.5), indicating that the endogenous mouse α-synuclein is involved in the development of a subset of dopaminergic neurons in the SN.
Methods
Generation of transgenic mice
The genotyping and characteristics of α-syn (1-120) transgenic mice have previously been described (Garcia-Reitbock et al., 2010; Tofaris et al., 2006). α-Syn(1-120) mice are in C57Bl/6JOlahsd background also known as C57Bl/6S (Harlan Laboratories) which lacks endogenous mouse α-synuclein due to a spontaneous deletion in the mouse genome (Specht and Schoepfer, 2001). To obtain α-syn(1-120)E mice with endogenous α-synuclein, α-syn(1-120) mice were crossed with wt C57Bl/6J mice and the homozygosity of the endogenous protein was determined by qPCR as well as western blotting and test breeding. α-Synuclein knockout mice were previously described (Abeliovich et al., 2000; Robertson et al., 2004). Littermate wild type C57Bl/6J mice were used as control for the heterozygous and homozygous α-synuclein KO mice.
Immunohistochemistry
For immunohistochemistry, mice at different ages were anesthetized and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4, and processed as previously described (Garcia-Reitbock et al., 2010; Tofaris et al., 2006). E10.5 and E13.5 C57Bl/6S and C57Bl/6J embryos were fixed in 4% PFA by immersion for 6 h, followed by soaking in 30% sucrose in PBS. Twelve micrometer coronal sections were then serially sectioned on a cryostat and collected in 3 groups of 10 slides — the first 10 slides covering the anterior part of the embryo, then the middle and posterior part of the embryo. About 6 sections were present on each slide; the mesencephalic areas including substantia nigra were present on 2-3 sections either in the end of anteri-or group, or in the beginning of middle group. E10.5 and E13.5 α-synuclein knockout embryos were cut into 14 μm sections using a cryostat and sections were serially distributed onto 6 glass slides. Free floating sections or slides were then processed for immunohistochemistry as described before (Garcia-Reitbock et al., 2010; Tofaris et al., 2006). The primary antibodies used were: monoclonal mouse anti-α-synuclein (Syn1, BD Transductions, 1:1000, recognizes both mouse and human α-synuclein), monoclonal anti-tyrosine hydroxylase MAB318 (Chemicon, 1:2000), and polyclonal anti-tyrosine hydroxylase (Pel-Freeze, 1:1000).
Western blotting
Brains were dissected and tissue lysed on ice in 10 volumes or 150 μl of 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X100, 0.5% Deoxycholic acid (Calbiochem), 0.1% SDS and protease inhibitor cocktail (Roche). Protein amounts were determined using the BCA Protein Assay Kit (Pierce), 30 μg of protein were loaded on a 10-15% SDS-PAGE and immunobloting performed as previously indicated (Garcia-Reitbock et al., 2010; Tofaris et al., 2006) using Western Lightning Chemiluminescence Reagent Plus Kit (PerkinElmer, UK).
Measurement of dopamine and metabolites by HPLC were performed as previously described (Garcia-Reitbock et al., 2010).
Stereological cell counting
Every 6th brain section (30 micrometer thick, and 6 in totals) of perfused mouse brain tissue was processed for immunohistochemistry for TH as described above. Cells were counted on an Olympus B×50 microscope using the Castgrid software from the same manufacturer. To separate the SN from the ventral tegmental area, a vertical line was drawn through the medial end of the cerebellar peduncle. All TH+ cells lateral to the line were counted. Between 5 and 6 animals/group were counted for each experiment.
The number of cells in the SN pars compacta of adult brain was determined blind to the genotype of the mice and using the following calculations (West, 1999): N = Nv × Vref, in which Nv = Σ (Number of cells counted)/(number of windows × area of window × height of window) and Vref = Σ structure areas × 1 / Frequency × section thickness.
As it is difficult to determine exactly the location of the prospective SN pars compacta in E10.5 and E13.5 embryos, all TH+ cells in mesencephalic area of the neural tube were counted in 3-4 embryos of each line of mice. TH+ neurons in embryos were quantified blind to genotype using optical fractionator method and Stereo Investigator 9 Software (MicroBright Field Bioscience Inc.), connected to Leica microscope equipped with motorized stage and digital camera. Every 6th section of the mouse brain was quantified, the area was selected under low magnification, and quantification was performed with oilimmersion objective ×100. Counting frame was 40 × 40 μm, and counting grid size was 168 × 187 μm. The total number of neurons was calculated using software in-built equations. In total, 2-4 sections were counted, depending on the age of embryos. In addition, the total number of Cresyl violet-positive cell nuclei within the same area was quantified to assess the total number of the cells per structure.
Results
Endogenous α-synuclein increases the number of dopaminergic neurons
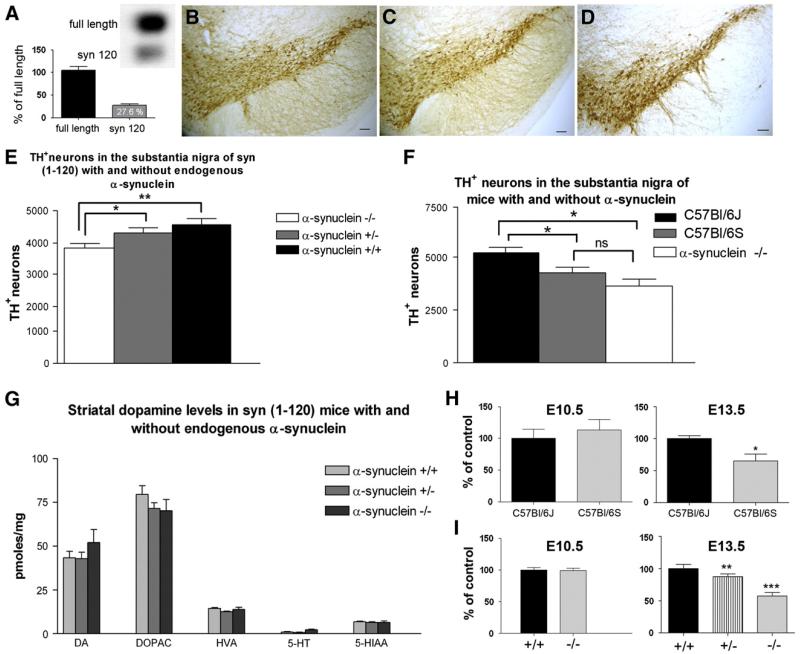
To investigate whether the presence of endogenous mouse α-synuclein could influence the toxicity of transgenic truncated 1-120 human α-synuclein we crossed α-syn (1-120) mice with wt C57Bl/6J mice that express endogenous α-synuclein to generate α-syn(1-120)E transgenic mice (Garcia-Reitbock et al., 2010). Western blot analysis of olfactory bulb extracts confirmed the expression of the endogenous mouse protein and showed that the transgenic 1-120 α-synuclein was about 30% of the endogenous mouse α-synuclein (Fig. 1A).
Figure 1. Western blot analysis of olfactory bulb (OB) extract demonstrates the expression of truncated human 1-120 α-synuclein in the presence of endogenous α-synuclein in α-syn (1-120)E mice.
Quantification was performed by analyzing the relative optic density of the western blot bands from 3 animals (A); Staining with anti-TH antibody in the substantia nigra of C57Bl/6S control (B), α-syn (1-120) mice (C), and homozygous α-syn(1-120)E mice (D) shows an increase in stereologically quantified TH+ cell numbers in Syn (1-120)E mice (E), scale bar 100 μm, *p < 0.05, **p < 0.01; (F) Stereological quantification of TH+ neurons in the substantia nigra of 6 month-old wt C57BL/6J, C57Bl/6S mice and C57Bl/6J α-synuclein knockout mice. *p < 0.05, ns not significant; (G) HPLC analysis does not show a significant difference in striatal dopamine and dopamine metabolite levels between α-syn (1-120)E and α-syn (1-120) mice with and without endogenous α-synuclein; TH+ cells in ventral mesencephalic areas (substantia nigra) of C57Bl/6J and C57Bl/6S wild type mice embryos at E10.5 and E13.5 days of gestation (H), as well as α-synuclein heterozygous (+/−) and homozygous (−/−) knockout mouse embryos at E10.5 and E13.5 days of gestation (I). Cell numbers were quantified stereologically and presented as percentage of control groups. The statistically significant difference was observed between C57Bl/6J and C57Bl/6S embryos at E13.5 (n = 3, p = 0.0143, two tailed t-test) and in the group of α-synuclein +/+, α-synuclein −/− and α-synuclein +/− (n = 4, 6 and 4 respectively, ** and *** by one-way ANOVA).
Surprisingly, TH staining showed an increase of TH+ cells in the SN of 18 month-old α-syn(1-120)E mice when compared to α-syn(1-120) transgenic (Fig. 1B-D). Stereological counting of neurons confirmed that the presence of endogenous α-synuclein increased the number of dopaminergic cells in the SN pars compacta of α-syn(1-120)E mice in a dose-dependent manner, with 13% (4346 ± 163 cells, p = 0.03, n = 6/group) and 20% (4601 ± 192 cells, p = 0.005, n = 5/group) increase respectively in mice heterozygous and homozygous for the endogenous mouse protein compared to α-synuclein deficient mice (3844 ± 143 cells, n = 5) (Fig. 1E). The effect of endogenous α-synuclein on dopaminergic cell number in the SN was independent of transgene expression, as a similar increase in TH+ neurons (5337 ± 252 cells, n = 6/group) was found in 6 month-old wt C57Bl/6J mice with the endogenous protein compared to wt C57Bl/6S mice (4251 ± 262 cells, p = 0.01, n = 6/group) that do not express endogenous α-synuclein due to a spontaneous deletion of the α-synuclein gene locus (Fig. 1F). Furthermore, quantification of TH+ cells in C57Bl/6J mice where the α-synuclein gene was specifically knocked out (3629 ± 322 cells, p = 0.001, n = 6/group) supported our findings that α-synuclein deficiency alone is sufficient to affect the number of TH+ neurons in the SN (Fig. 1F).
However, this increase in dopaminergic cell number did not affect striatal dopamine levels in the α-syn(1-120)E mice (Fig. 1G) which remained lower compared with wt C57Bl/6S mice as previously reported in α-syn(1-120) mice (Garcia-Reitbock et al., 2010; Tofaris et al., 2006).
Endogenous α-synuclein affects dopaminergic cell number during development
α-Synuclein is expressed at detectable levels at E12 (Hsu et al., 1998), while TH+ cells appear in the mesencephalic areas between E10 and E11 (Wallen et al., 1999). In order to determine when the effect of α-synuclein on TH+ cells occurs during development, TH+ neurons were counted in E13.5 and E10.5 embryos from α-synuclein KO C57Bl/6J mice as well as wt C57Bl/6S and C57Bl/6J mice. No difference in the number of TH+ neurons was found between the α-synuclein deficient and normal wt strains at E10.5 (Figs. 1H, I). On the contrary, at E13.5 we observed a clear difference in the number of TH+ neurons between the strains with and without α-synuclein, with C57Bl/6S embryos having 33% less TH+ cells compared to C57Bl/6J embryos (p = 0.0143, n = 3 per group) of the same age (Fig. 1H). Homozygous and heterozygous α-synuclein knockout E13.5 embryos had 43% (4370 ± 477, n = 4) and 13% less TH+ cells (6659 ± 657, n = 6) respectively, when compared to wt littermates (7605 ± 994, n = 4) used as control (Fig. 1I). This change in TH+ neurons number is not due to the general cell loss in homozygous α-synuclein knockout E13.5 embryos, as Nissl staining did not reveal a statistically significant difference between the groups (data not shown). These data indicate that endogenous α-synuclein exerts its effect during development, between E10.5 and E13.5.
Discussion
Although α-synuclein’s function is not yet completely clear it is known to be involved in plasticity (George et al., 1995), in regulation of neurotransmitter release and synaptic vesicle pool (Abeliovich et al., 2000; Anwar et al., 2011; Garcia-Reitbock et al., 2010; Greten-Harrison et al., 2010; Burre et al., 2010; Scott and Roy, 2012), and its involvement in neurodevelopment (Simon et al., 2001) has also been suggested.
We show here that the presence of endogenous mouse α-synuclein is associated with an increase in the number of TH+ cells in the SN of both transgenic α-syn(1-120)E and wt C57Bl/6J mice compared to the transgenic line α-syn(1-120) and wt C57Bl/6S. This effect is dose-dependent with homozygous mice for the endogenous protein exhibiting a significantly higher number of TH+ cells compared to heterozygous mice. The same effect of α-synuclein and an increase in dopaminergic cell numbers was seen in both young and old mice, indicating that during development α-synuclein either prevents cell death or promotes differentiation of dopaminergic neurons in the SN conditioning their number during life.
We cannot exclude that the absence of endogenous α-synuclein can influence behavior. C57BL/6S mice which lack the α-synuclein gene have been reported to have reduced rotarod performance compared to C57BL/6J mice (Bryant et al., 2008), although we demonstrated here that levels of striatal dopamine are similar in the presence or absence of α-synuclein in the α-syn(1-120) transgenic mice. We did not find any difference in young mice in the open field test that was affected only in 18 month-old α-syn(1-120) mice (Tofaris et al., 2006). Unfortunately, due to the poor breeding of the α-syn(1-120)E mice it was not possible to investigate whether they had a defect in dopamine release in the striatum following K+ stimulation as we have done in the α-syn(1-120) mice (Garcia-Reitbock et al., 2010).
Since α-syn(1-120) line was produced on C57Bl/6S mice background, where the endogenous α-synuclein genomic locus is spontaneously deleted, an increase in the number of TH+ cells in the SN of transgenic α-syn(1-120)E is due to the α-synuclein presence alone, as a similar degree of TH+ cell difference was observed between C57Bl/6S and C57Bl/6J. We have demonstrated here that endogenous α-synuclein increases the number of TH+ neurons in α-syn(1-120) mice excluding toxicity in young mice, however the number of TH+ neurons in older α-syn(1-120)E mice is lower than in younger C57Bl/6J mice and as we have described full-length endogenous α-synuclein in the synaptic aggregates of α-syn(1-120)E mice (Garcia-Reitbock et al., 2010), we cannot exclude its contribution to toxicity in older mice. More studies are needed to clarify this point. The difference in the number of TH+ neurons could also be due to the different age of the animals (18 months vs 6 months), which is consistent with a previous study that has demonstrated that the number of dopaminergic neurons in the substantia nigra of mice declines with age (Tatton et al., 1991).
While this is the first observation of the lower number of TH+ cell in SN in a wild-type strain of mice, carrying spontaneous deletion of α-synuclein genomic locus (C57Bl/6S vs C57Bl/6J), conflicting results were observed in α-synuclein knock-out animals. Some groups did not report changes in TH+ cell numbers in substantia nigra of α-synuclein knockout mice (Abeliovich et al., 2000), others found minor but significant decrease in mice without α-synuclein (Robertson et al., 2004; Al-Wandi et al., 2010; Thomas et al., 2011). Here we show that α-synuclein knockout mice have less TH+ neurons in SN when compared to endogenous α-synuclein containing strain C57Bl/6J, strengthening the idea that α-synuclein deficit alone is sufficient to result in dopaminergic neuronal number difference. This effect of endogenous α-synuclein occurred between E10.5 and E13.5 because while at E10.5 we found no difference in TH+ neuron number between wt mice with and without the endogenous protein and α-synuclein targeted KO mice, a difference between strains with and without α-synuclein was evident in E13.5 embryos (Michell et al., 2007; and present work). α-Synuclein has been reported to be present at very low levels in some areas in the rodent nervous system at E9.5 but in the SN it appears only at E11.5, becomes consistent at E12.5 and increases later in development reaching its highest level in adult brain (Hsu et al., 1998; Zhong et al., 2010). In this study we did not detect consistent α-synuclein staining in E10.5 mouse embryos. Our novel and unexpected result suggests that α-synuclein expression is necessary for the appearance of a subset of TH+ neurons in the SN during mouse embryonic development, unfortunately no markers are available to identify these neurons and to determine whether α-synuclein influences their differentiation or death.
Whether α-synuclein stimulates proliferation of precursor cells, prevents neuronal death or promotes differentiation of DA neurons remains to be determined. Interestingly, however, it has been previously reported that α-synuclein mRNA was not present in engrailed knockout mice that show reduced survival of dopaminergic SN neurons (Simon et al., 2001; 2003) suggesting an involvement of α-synuclein in DA neuron development. It is also not known whether a sudden lack of α-synuclein or loss of its function in SN pars compacta could lead to the death of some DA neurons in adulthood similar to what has been reported for Nurr1-dependent DA neurons (Kadkhodaei et al., 2009). If this was the case it is possible that the loss of function of α-synuclein besides its aggregation could make the α-synuclein-dependent neurons more prone to death in PD. This has to be kept in mind when designing therapies aimed at reducing α-synuclein amount as treatment for PD. On the other hand, a negative effect of α-synuclein overexpression has also been reported in adult neurogenesis (Winner et al., 2004). However, our study is focused on TH+ neurons of the SN and in mice with and without the endogenous protein therefore examining the physiological effect of α-synuclein rather than its overexpression. It might be that the relationship between α-synuclein and dopaminergic cell numbers is dose-dependent where at physiological concentrations α-synuclein leads to their increase whereas at higher, toxic concentrations it inhibits the proliferation of dopaminergic neuron precursors. This would explain the observation that overexpression of α-synuclein inhibits adult neurogenesis (Winner et al., 2004, 2012). The data presented here will be particularly relevant for the interpretation of results on effect/toxicity of treatments in mice with and without the endogenous protein where the initial difference in neurons could lead to misleading interpretation of α-synuclein role in toxicity.
In summary, we have identified an additional and novel role for α-synuclein in the development of a subset of dopaminergic neurons in the SN pars compacta, and demonstrate that this effect takes place between E10.5 and E13.5. Further studies are needed to determine the precise mechanism by which α-synuclein exerts this effect during development and to clarify whether it is specific to dopaminergic neurons or occurs also in other neuronal populations where α-synuclein is present.
Acknowledgment
PGR was funded by an EU Marie Curie Actions Nervous System Repair studentship. This work was supported by Parkinson’s UK (MGS, G-1006 for VLB)), Alzheimer’s Research UK (OA, MGS) and the Welcome Trust (grant 075615/04/z, VLB). This work was partly funded by a Core Award from the Medical Research Council and the Wellcome Trust to the Behavioural and Clinical Neuroscience Institute (MRC Ref G1000183; WT Ref 093875/Z/10/Z, JWD). The authors are grateful to Dr. Michel Goedert and Ms Isabelle Lavenir and the Medical Research Council for the help, advice and financial contribution in generating and breeding the transgenic α-synuclein animals.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Al-Wandi A, Ninkina N, Millership S, Williamson SJM, Jones PA, Buchman VL. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J. Neurogenet. 2008;22:315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Darios F, Ruiperez V, Lopez I, Villanueva J, Gutierrez LM, Davletov B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010;11:528–533. doi: 10.1038/embor.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reitbock P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano P, Tofaris GK, Goedert M, Spillantini MG. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 2010;133:2032–2044. doi: 10.1093/brain/awq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Identifying PD-causing genes and genetic susceptibility factors: current approaches and future prospects. Prog. Brain Res. 2010;183:3–20. doi: 10.1016/S0079-6123(10)83001-8. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, Chandra SS. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M, Thal LJ, Saitoh T, Masliah E. Expression pattern of synucleins (non-Abeta component of Alzheimer’s disease amyloid precursor protein/alpha-synuclein) during murine brain development. J. Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, Chambon P, Lindqvist E, Larsson NG, Olson L, Bjorklund A, Ichinose H, Perlmann T. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Michell AW, Tofaris GK, Gossage H, Tyers P, Spillantini MG, Barker RA. The effect of truncated human alpha-synuclein (1-120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transplant. 2007;16:461–474. doi: 10.3727/000000007783464911. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J. Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DD. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HH, Bhatt L, Gherbassi D, Sgado P, Alberi L. Midbrain dopaminergic neurons: determination of their developmental fate by transcription factors. Ann. N. Y. Acad. Sci. 2003;991:36–47. [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alphaSynuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatton WG, Greenwood CE, Verrier MC, Holland DP, Kwan MM, Biddle FE. Different rates of age-related loss for four murine monoaminergic neuronal populations. Neurobiol. Aging. 1991;12:543–556. doi: 10.1016/0197-4580(91)90086-y. [DOI] [PubMed] [Google Scholar]
- Thomas B, Mandir AS, West N, Liu Y, Andrabi SA, Stirling W, Dawson VL, Dawson TM, Lee MK. Resistance to MPTP-neurotoxicity in α-synuclein knockout mice is complemented by human α-synuclein and associated with increased β-synuclein and Akt activation. PLoS One. 2011;6(1):e16706. doi: 10.1371/journal.pone.0016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitbock RP, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J. Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen A, Zetterstrom RH, Solomin L, Arvidsson M, Olson L, Perlmann T. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp. Cell Res. 1999;253:737–746. doi: 10.1006/excr.1999.4691. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J. Neuropathol. Exp. Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, Regensburger M, Schreglmann S, Boyer L, Prots I, Rockenstein E, Mante M, Zhao C, Winkler J, Masliah E, Gage FH. Role of alpha-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J. Neurosci. 2012;32:16906–16916. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong SC, Luo X, Chen XS, Cai QY, Liu J, Chen XH, Yao ZX. Expression and subcellular location of alpha-synuclein during mouse-embryonic development. Cell. Mol. Neurobiol. 2010;30:469–482. doi: 10.1007/s10571-009-9473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]