Abstract
As people get older, they tend to remember more positive than negative information. This age-by-valence interaction has been called ‘positivity effect.’ The current study addressed the hypotheses that baseline functional connectivity at rest is predictive of older adults’ brain activity when learning emotional information and their positivity effect in memory. Using fMRI, we examined the relationship among resting-state functional connectivity, subsequent brain activity when learning emotional faces, and individual differences in the positivity effect (the relative tendency to remember faces expressing positive versus negative emotions). Consistent with our hypothesis, older adults with a stronger positivity effect had increased functional coupling between amygdala and medial prefrontal cortex (MPFC) during rest. In contrast, younger adults did not show the association between resting connectivity and memory positivity. A similar age-by-memory positivity interaction was also found when learning emotional faces. That is, memory positivity in older adults was associated with a) enhanced MPFC activity when learning emotional faces and b) increased negative functional coupling between amygdala and MPFC when learning negative faces. In contrast, memory positivity in younger adults was related to neither enhanced MPFC activity to emotional faces, nor MPFC-amygdala connectivity to negative faces. Furthermore, stronger MPFC-amygdala connectivity during rest was predictive of subsequent greater MPFC activity when learning emotional faces. Thus, emotion-memory interaction in older adults depends not only on the task-related brain activity but also on the baseline functional connectivity.
Keywords: aging, emotion and memory, medial prefrontal cortex, positivity effect, resting state
Introduction
Despite the age-related declines seen in many domains of cognitive functioning (Salthouse, 2010), emotional well-being does not decline and in fact improves in some aspects as people get older (Hay & Diehl, 2011). Older adults, compared with younger adults, tend to pay attention to and remember more positive information (Charles, Mather, & Carstensen, 2003; Isaacowitz, Wadlinger, Goren, & Wilson, 2006; Mather & Carstensen, 2003). Older adults also show reduced processing of negative stimuli than younger adults (Grühn, Scheibe, & Baltes, 2007; Wood & Kisley, 2006). This age-by-valence interaction has been called the ‘positivity effect.’ The positivity effect is modulated by other factors, such as level of arousal of stimuli (Kensinger & Leclerc, 2009) and availability of cognitive resources (Mather & Knight, 2005). A recent meta- analysis revealed that the positivity effect is a reliable small-to-medium effect that is stronger when people are allowed to process emotional stimuli in an unconstrained fashion than when they are given explicit task goals, such as memorizing the pictures (Reed, Chan, & Mikel, 2012).
One possible explanation for the positivity effect in attention and memory is that age-related declines in the brain lead the amygdala to activate less to negative stimuli, which results in reduced attention and impaired memory for those stimuli (Cacioppo, Berntson, Bechara, Tranel, & Hawkley, 2011). However, this model cannot explain why older people show reduced amygdala responses selectively to negative stimuli, with preserved responses to other stimuli, such as positive (Mather et al., 2004) or novel stimuli (Moriguchi et al., 2011).
An alternative explanation is that the positivity effect is caused by motivational shifts in aging (Scheibe & Carstensen, 2010). Older adults, relative to younger adults, are more likely to prioritize emotion regulation goals over other goals (Isaacowitz, Toner, Goren, & Wilson, 2008; Riediger, Schmiedek, Wagner, & Lindenberger, 2009). This motivational shift has been hypothesized to result in the positivity effect. Indeed, older adults’ positivity effects do not emerge when they have limited cognitive resources to regulate their emotion (Knight et al., 2007; Mather & Knight, 2005; Petrican, Moscovitch, & Schimmack, 2008). Older adults’ positivity effects are also eliminated when their motivations are manipulated to focus on other goals than emotion regulation (Löckenhoff & Carstensen, 2007), whereas younger adults can be induced to show as much of a positivity preference in memory as older adults by reminding them to focus on their own emotional states (Kennedy, Mather, & Carstensen, 2004; Mather & Johnson, 2000).
Recent neuroimaging studies also provide evidence consistent with the emotion regulation account for the positivity effect (for review see Nashiro, Sakaki, & Mather, 2012). Older adults, compared with younger adults, tend to show increased activity in the medial prefrontal cortex (MPFC) when seeing negative than neutral stimuli (Leclerc & Kensinger, 2008; Roalf, Pruis, Stevens, & Janowsky, 2011; Williams et al., 2006). Older adults’ greater MPFC activity was also observed for positive stimuli (Addis, Leclerc, Muscatell, & Kensinger, 2010; Gutchess, Kensinger, & Schacter, 2007; Kensinger & Schacter, 2008; Leclerc & Kensinger, 2008; Ritchey, LaBar, & Cabeza, 2010). Furthermore, older adults show increased functional coupling between MPFC and the amygdala to emotional materials (e.g., St. Jacques, Dolcos, & Cabeza, 2010). The MPFC and adjacent anterior cingulate (ACC) are known to interact with the amygdala (i.e., a key region for emotion) to regulate emotion across age (e.g., Urry et al., 2006; Winecoff, LaBar, Madden, Cabeza, & Huettel, 2011). For example, the MPFC shows stronger activity when people are told to up-regulate positive emotion and down-regulate negative emotion (Ochsner et al., 2004). Greater MPFC activity was also found when people spontaneously regulate their emotion (e.g., Drabant, McRae, Manuck, Hariri, & Gross, 2009). These results suggest that the increased MPFC activity seen in older adults while processing emotional stimuli reflect their spontaneous efforts to regulate emotion. Thus, it appears that older adults spontaneously recruit the MPFC to engage in emotion regulation when encountering positive stimuli (to up-regulate emotion) and negative stimuli (to down-regulate emotion; Mather, 2012; Nashiro et al., 2012).
However, older adults typically show cognitive decline (Salthouse, 2010), particularly in the prefrontal cortex function (e.g., Allen, Bruss, Brown, & Damasio, 2005). This raises the question of how older adults recruit MPFC to emotional stimuli given their more limited cognitive control resources. One possibility is that older adults are chronically engaged in emotion regulation and have an activated emotion regulation network even at baseline before they encounter any emotional stimuli. This chronically activated emotion regulation network should allow them to use the network easily when encountering emotional events, leading to enhanced MPFC activity and behavioral positivity effects. In contrast, when they do not have an activated emotion regulation network at baseline, they might be unable to recruit the network easily when needed; thus resulting in weaker positivity effects. The current study addressed this hypothesis by examining resting-state functional connectivity in older adults.
Recent studies on resting-state connectivity have revealed coherent patterns of spontaneous low-frequency fluctuations in brain activity. Although these patterns are obtained from brain activity during rest, they appear to represent the functional architecture required to respond to the external world when needed (Smith et al., 2009). For example, resting-state functional networks predict task-induced brain activity (Mennes et al., 2010; Mennes et al., 2011). Resting-state networks also change actively, reflecting dynamic functional networks activated by recent cognitive (Albert, Robertson, & Miall, 2009; Hasson, Nusbaum, & Small, 2009; Tambini, Ketz, & Davachi, 2010; Waites, Stanislavsky, Abbott, & Jackson, 2005) and emotional states (van Marle, Hermans, Qin, & Fernández, 2010). These findings suggest the intriguing possibility that older adults’ baseline functional connectivity during rest is affected by their chronically activated emotion regulation goals and predicts behavioral positivity effects. In the current study, we tested the hypothesis that resting-state functional connectivity at baseline (i.e., before seeing any emotional stimuli) is predictive of older adults’ positivity effects in memory, as well as their brain activity when learning emotional stimuli.
During the study, we first measured resting-state functional connectivity in older and younger adults. After the resting-state scan, participants viewed video clips of positive, negative and neutral faces in an encoding session. Finally, participants’ recognition memory for the faces was tested. To address our hypothesis, participants in each age group were then categorized into those who remembered more positive than negative faces, and those who remembered fewer positive and more negative faces. This within-age-group categorization allowed us to examine how positivity effects in memory are related with resting-state functional connectivity while controlling for overall age-related changes in resting-functional connectivity (e.g., Andrews-Hanna et al., 2007). We also examined whether brain activity to emotional faces during the encoding session is modulated by age and this memory subgroup.
We addressed the following four predictions. The first prediction concerns brain activity during the encoding phase. As discussed above, the MPFC is involved in down-regulation of negative emotion when seeing negative stimuli and up-regulation of positive emotion when seeing positive stimuli (e.g., Ochsner et al., 2004). Given these studies and the emotion regulation account for the positivity effect, we predict that older adults’ positivity effects in memory are related to enhanced MPFC activity to emotional stimuli (both negative and positive stimuli) during the encoding session, as emotion regulation processes should be activated both during viewing of positive and negative stimuli with prefrontal control required to more deeply engage with positive stimuli as well as to disengage from negative stimuli. Thus, greater MPFC activity both during learning positive and negative faces should predict a greater advantage in memory for positive over negative faces especially in older adults.
Second, past studies reported inverse functional coupling between amygdala and MPFC when down-regulating negative emotion (e.g., Urry et al., 2006). Given these findings and evidence that older adults’ positivity effects are associated with their emotion regulation attempts, we expect that memory positivity is associated with stronger inverse coupling between MPFC and amygdala when learning negative faces than neutral faces in older adults.
The third prediction concerns baseline functional connectivity during rest. As mentioned above, we hypothesized that older adults’ positivity effect relies on a chronically active emotion regulation network at baseline. Since amygdala-MPFC interactions are critical for emotion regulation (e.g., Urry et al., 2006), we predict that older adults’ positivity effects in memory are associated with greater functional connectivity between the amygdala and MPFC during rest.
Finally, recent research indicates that a person’s resting-state functional connectivity can predict their task-related brain activity (Mennes et al., 2010; Mennes et al., 2011). Thus, we expect that older adults’ amygdala-MPFC functional connectivity during rest is predictive of their subsequent MPFC activity to emotional faces during the encoding session.
Materials and Methods
Participants
Twenty-one older adults (10 males; age range = 61–78) and 20 younger adults (12 males; age range = 19–37) took part in a 2-day session. They provided written informed consent approved by the University of Southern California Institutional Review Board and were paid for their participation. Prospective participants were screened and excluded for any medical, neurological, or psychiatric illness. Older adults were further screened for their cognitive function using a telephone protocol (TELE: Gatz et al., 1995) which includes 21 questions on cognitive function, such as short-term memory, general knowledge (e.g., current US president), and attention (e.g., count back by 3’s from 20). Data from four participants were excluded: one older adult due to a prior stroke identified by a neuroradiologist who reviewed all structural scans for incidental findings and three younger adults due to technical errors in recording their behavioral responses. The remaining participants included 20 older adults (10 males; Mage = 68.10, age range = 61–78) and 17 younger adults (9 males; Mage = 25.82, age range = 19–37). Participants completed the Wechsler Test of Adult Reading (WTAR) test and several questionnaires including their demographic information, the Positive and Negative Affect Schedule (PANAS: Watson, Clark, & Tellegen, 1988), and the Center for Epidemiological Studies Depression (CES-D) scale.
Materials
We employed 144 videos each of which depicted a face without any sound for 6 sec (72 males and 72 females): 48 videos depicted angry faces, another 48 depicted happy faces, and the remaining 48 depicted neutral faces. They were obtained from the Internet, such as YouTube (www.youtube.com). We did not include any videos of movies or skits. Each video included one person’s face; most of them were looking straight into the camera. The emotional videos varied in terms of facial expressions throughout the 6 sec duration of the clip, but we edited them so that all videos mostly consisted of emotional expressions (either angry or happy). To confirm overall valence, six participants who did not participate in the main study rated each video on a 9-point scale (1: extremely negative-9: extremely positive; Mhappy = 8.11, SD = 0.57; Mneutral = 5.05, SD = 0.43; Mangry = 2.02, SD = 0.56). The amount of motion involved in the videos was also quantified using Matlab (MathWorks; Natick, MA) by averaging the absolute difference in color change (per pixel) between each frame, and then taking the mean of this average across all frames of the video. Videos were chosen such that the average motion of all videos within a valence was equal across the angry, happy, and neutral groups of videos. In each condition, half of the videos were used in the encoding phase, while the other videos were used as foils in the recognition phase; these old/lure video assignments were counterbalanced across participants.
Behavioral Procedures
The experiment followed a 2-day protocol with the second session two days after the first one (see Figure 1).
Figure 1.
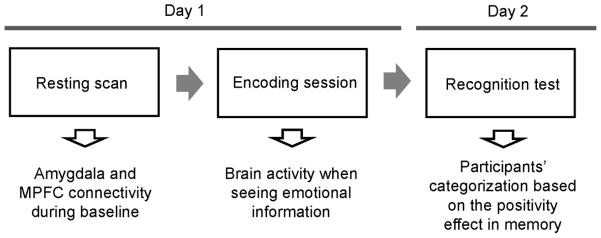
Overview of the current study.
Day 1
First, resting fMRI BOLD (blood oxygenation level-dependent) data were acquired. The resting fMRI scan lasted 5.2 min. Participants were told to lie with eyes closed, to think of nothing in particular, and not to fall asleep. The resting scan was brief to prevent participants from falling asleep. After the resting scan, the experimenters also checked in on the participants; none of our participants self-reported falling asleep during the resting scan.
The resting scan was followed by the encoding session which had four runs. Each run involved six angry, six happy and six neutral videos with a randomized order, irrespective of valence. To examine effects of cognitive resources on the positivity effect (e.g., Mather & Knight, 2005), participants watched videos with (load condition) and without (no-load condition) cognitive distraction in two runs respectively. The order of the four runs was randomized.
On each trial in the load condition (Figure 2), participants first viewed three digits for 1 sec, followed by a 6-sec video. They were told to make a gender judgment about a person in the video. After the video, a blank screen was presented for 3 sec; then, three digits appeared on the screen for 3.5 sec. Participants were asked to press a button to indicate whether these three digits were smaller or bigger than the three digits presented before the video. Following a jittered fixation cross (1.5, 3.5, or 5.5 sec), the next trial started. The procedures in the no-load condition were similar, except that participants saw three nonsense symbols instead of digits both before and after videos. Participants were told to make a gender judgment about the person in each video and to press an arbitrary button when shown the three symbols after each video.
Figure 2.
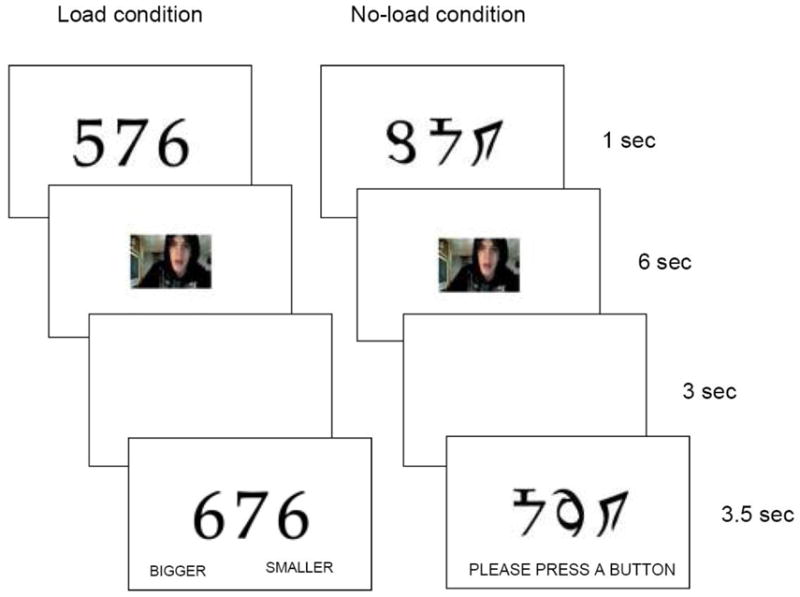
Schematic representations of trials in the load and no-load condition during the encoding session.
Day 2
Two days later, participants’ memory was tested by a recognition task. In the recognition phase, we used shortened 3-sec versions of the videos. Participants viewed each video and made a judgment about whether they saw the video during the encoding phase or not.
Participants’ Categorization by Positivity Effects in Memory
A positivity effect memory score was obtained by subtracting the corrected recognition rate (hit minus false alarm rate) for angry faces from the corrected recognition rate for happy faces. Participants were then categorized into a positive and negative memory group by the median positivity effect memory score in each age group (younger adults: Md = −.09; older adults: Md = −.05). The load manipulation did not have any significant effects on the memory performance (see Behavioral Results); hence the memory group categorization was performed while collapsing the load and no-load conditions. Because the positivity effect is defined as an age-by-valence interaction, the positive group does not necessarily imply people with positivity effect and our main analyses on fMRI data focused on the interaction between age and memory group, rather than the main effect of the memory group.
FMRI Data Acquisition and Image Preprocessing
All scanning was performed on a 3.0-T Siemens MAGNETOM Trio scanner with a 12-channel matrix head coil at the University of Southern California Dana and David Dornsife Neuroimaging Center. The imaging parameters were TR = 2000 ms, TE = 25 ms, slice thickness = 3 mm, interslice gap = 0 mm, and FA = 90°. Data preprocessing were performed using FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl), which included motion correction with MCFLIRT, spatial smoothing with a Gaussian kernel of full-width half-maximum 5 mm, and skull stripping of structural images with BET. Noise components were identified using MELODIC ICA (Beckmann & Smith, 2004) and removed. Registration was performed with FLIRT; each functional image was registered to the participant’s high-resolution brain-extracted structural image and the standard Montreal Neurological Institute (MNI) 2-mm brain. The data from the encoding phase were then temporally filtered using a high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50 sec). Following past research (Roy et al., 2009), we applied both high- (Gaussian-weighted least-squares straight line fitting, with sigma=50 sec) and low- (Gaussian low-pass temporal filtering with a HWHM=2.8 sec) pass temporal filters to the data from the resting scan.
Whole-Brain FMRI Data Analysis during the Encoding Session
Stimulus-dependent changes in BOLD signal were modeled using FSL FEAT. For each valence condition in each participant, we categorized remembered and forgotten faces based on their own recognition performance. The presentation of symbols (no-load condition) and digits (load condition) were also included. The effects of each event type were estimated using a fixed-effects model. Since we were interested in brain activity when learning emotional information (relative to neutral information), signals for happy and angry faces were combined into emotional faces; then we obtained the emotional Dm (difference in memory) effects by contrasting Dm for emotional faces ([remembered emotional > forgotten emotional]) with Dm for neutral faces ([remembered neutral > forgotten neutral]). The data from each participant were entered into a random effects analysis by using FSL’s FEAT (FLAME 1+2) to examine how age and the positivity effect memory subgroup modulated brain activity for the emotional Dm effects. We also performed additional analyses to contrast Dm happy and Dm angry effects to see if there were any differences in MPFC activity across happy and angry faces.
In these and any whole-brain analyses in this paper, we employed cluster-based corrections for multiple comparisons with Gaussian random field theory (Z = 2.3; cluster significance: p = .05-corrected). Locations reported by FSL were converted into Talairach coordinates by the MNI-to-Talairach transformation algorithm (Lancaster et al., 2007). These coordinates were used to provide labels of the nearest gray matter using the Talairach Daemon (Lancaster et al., 2000).
Whole-Brain Functional Connectivity Analysis during the Encoding Session
To address the role of MPFC during the encoding session, a beta-series analysis (Rissman, Gazzaley, & D’Esposito, 2004) was employed. This allowed us to use trial-to-trial variability to characterize dynamic inter-regional interactions. Given that the MPFC is a large structure and is involved in not only emotion regulation but also other cognitive tasks (Heatherton et al., 2006; Van Overwalle, 2008), it is not clear whether the entire MPFC is related with our task. Therefore, the MPFC seed region was defined functionally based on the activated cluster observed in the whole brain analyses described above. First, a new GLM design file was constructed where each trial was coded as a unique covariate, resulting in 72 independent variables. The model also involved additional regressors for the presentation of symbols and numbers, six motion parameters, and global signal. Second, the least squares solution of the GLM yielded a beta value for each trial for each individual participant. Third, mean activity (i.e., mean parameter estimates) was extracted for each individual trial from the seed region. As a fourth step, for each trial type (i.e., remembered and forgotten faces for each valence condition), we computed correlations between the seed’s beta series and the beta series of all other voxels in the brain, thus generating condition-specific seed correlation maps. Correlation magnitudes were converted into z-scores using the Fisher’s r-to-z transformation. Condition-dependent changes in functional connectivity were then assessed using FSL’s random-effects analyses.
Whole-Brain Functional Connectivity Analysis during Rest
The amygdala and MPFC were used as seed regions. The amygdala was defined structurally. For each participant, bilateral amygdalae were segmented using FreeSurfer (surfer.nmr.mgh.harvard.edu) and FSL FIRST. These two segmentations were then visually compared, separately for each hemisphere. The segmentation judged as more accurate was selected and manually corrected based on the anatomical definitions developed in past studies (Allen et al., 2005; Convit et al., 1999; Morey et al., 2009). Since the MPFC is a large structure and involved in multiple cognitive functions (Heatherton et al., 2006; Van Overwalle, 2008), the MPFC seed region was defined functionally based on conjunction analyses between the whole-brain analyses of the encoding phase and the amygdala connectivity analyses during rest.
From each of our seed regions, mean time series were calculated by averaging across all voxels within the region with a command line tool called fslmeants from FSL. Multiple regression analyses were then performed for each participant using FSL FEAT. For each seed region, a regression model was created which included the seed region time series and several nuisance variables: six motion parameters, global signal, signal from a ventricular region of interest (ROI), and signal from a white-matter ROI ventricle (Zhang et al., 2008). This analysis produced individual subject-level maps representing brain areas that had correlations with the seed region. Group-level analyses were then conducted using FSL’s FEAT (FLAME 1+2).
Post-hoc ROI Analyses
The whole-brain analyses described above address interactions between age and the memory subgroups, but do not characterize the direction of the interaction. Therefore, average percent signal change values were extracted from clusters showing significant effects in the whole-brain analyses by FMRIB’s Featquery. For each cluster, a mask image was created for statistically significant voxels, binarised, and registered into each participant’s functional space; the average percent signal change was then obtained relative to the mean intensity of the voxels during the entire scan. In addition, since the amygdala was one of our main regions-of-interest, when the amygdala was included in a significant cluster, we determined the anatomical border of the amygdala within the cluster using the Harvard-Oxford subcortical atlas with probability = .5. Percent signal change values were then extracted from this amygdala area. For most clusters, we used the default interpolation threshold (0.5) in the Featquery. But for the amygdala cluster in the MPFC connectivity analysis during rest, the Featquery failed to transform the mask into some individuals’ functional spaces with the default threshold; therefore, we used 0.2 as the interpolation threshold. Post-hoc 2 (age: old vs. young) X 2 (memory group: positive vs. negative) analysis-of-variances (ANOVAs) were performed on these extracted percent signal changes. Given that MPFC activity can be modulated by individual differences in mood states (e.g., Zald, Mattson, & Pardo, 2002), we included positive and negative affect (measured by PANAS) and depression (measured by CES-D) as covariates in these post-hoc analyses to control for the effects of mood states.
Correlation Analyses between the Encoding and the Resting State
We delineated a 6-mm sphere around the peak voxel in the group-level MPFC cluster in the amygdala’s functional connectivity during rest. Similarly, a 6-mm sphere around the peak voxel in MPFC was determined based on the whole-brain emotional Dm analysis during the encoding phase. Percent signal change values were then extracted from these two spheres using Featquery (with the default interpolation threshold) and used to examine if individual differences in MPFC activity during the encoding phase were correlated with individual differences in MPFC connectivity signals to amygdala during rest.
Results
Behavioral Results
Overall, older adults showed worse corrected recognition rates (M = .28) than did younger adults (M = .44), F (1, 35) = 10.69, R2 = .26, p < .01. Neither the main effect of the load manipulation, nor any interactions involving the load manipulation was significant for recognition performance (ps > .09). Next, we assigned participants to the positive or negative memory group based on their positivity effect memory score. A 2 (age) × 2 (memory group) ANOVA on the positivity effect memory score revealed a significant effect of memory subgroup, F (1, 33) = 59.85, R2 = .57, p < .01, indicating a higher positivity effect memory score in the positive memory group than in the negative memory group. More interestingly, this ANOVA revealed a significant effect of age, F (1, 33) = 8.49, R2 = .05, p < .01, with no significant interaction (p > .20). The significant age effect reflects that the positivity effect memory score was higher in older adults (M = −.01; Mangry = .28, Mhappy = .26) than in younger adults (M = −.11; Mangry = .48, Mhappy = .37), which is consistent with past findings that older adults tend to remember relatively less negative compared with positive materials than do younger adults (Mather, 2012). Other measures of mood and cognitive function did not show any significant effects of age, of memory group, and the interaction between them (Table 1).
Table 1.
Participants’ demographics, cognitive test scores, current mood ratings, depression scores, and memory performance in the face memory task.
| Old | Young | |||
|---|---|---|---|---|
| Neg group | Pos Group | Neg group | Pos Group | |
| Age | 68.50 (1.72) | 67.70 (1.72) | 25.75 (1.92) | 25.89 (1.81) |
| TELE | 18.00 (0.26) | 18.35 (0.26) | -- | -- |
| Positive Affect | 34.20 (2.76) | 34.90 (2.76) | 29.50 (3.08) | 29.56 (2.91) |
| Negative Affect | 11.00 (1.09) | 12.80 (1.09) | 13.63 (1.22) | 11.78 (1.15) |
| CES-D | 5.70 (2.06) | 9.90 (2.06) | 9.13 (2.31) | 11.11 (2.17) |
| WTAR | 43.60 (1.70) | 44.20 (1.70) | 44.00 (1.91) | 41.89 (1.80) |
| Positivity effect memory score | −0.16 (0.03) | 0.13 (0.03) | −0.22 (0.04) | 0.0006 (0.03) |
| CR for happy faces | 0.17 (0.05) | 0.36 (0.05) | 0.31 (0.06) | 0.44 (0.06) |
| CR for angry faces | 0.33 (0.05) | 0.23 (0.05) | 0.53 (0.06) | 0.44 (0.05) |
Note: TELE = telephone protocol to test cognitive functions in older adults (Gatz, et al., 1995). Positive and Negative Affect = measured by the Positive and Negative Affect Schedule (Watson et al., 1998). CES-D = Center for Epidemiological Studies Depression scale. WTAR = Wechsler Test of Adult Reading test. Positivity effect memory score = Corrected recognition rates for happy faces minus corrected recognition rates for angry faces. CR for happy and angry faces = Corrected recognition rates for happy and angry faces. Standard errors are in parentheses.
Functional Imaging Results: Encoding Session
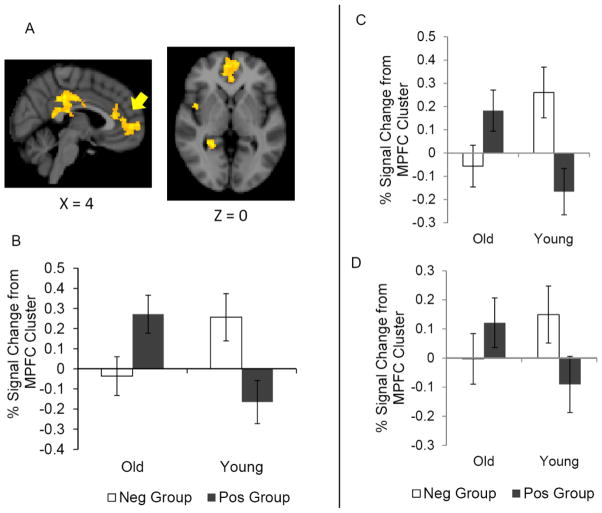
The whole-brain analysis on the emotional Dm effects during the encoding phase revealed a significant age-by-memory group interaction in MPFC and adjacent ACC (Table 2, Figure 3A). To characterize the interaction pattern, a post-hoc ROI analysis was performed on the percent signal change extracted from the significant MPFC/ACC cluster. Confirming the results from the whole brain analysis, this analysis revealed a significant age x memory subgroup interaction, F (1, 28) = 11.79, R2 = .19, p < .01. A simple effect test revealed that, among older adults, the positive memory group showed greater MPFC activity than did the negative memory group (Figure 3B), F (1, 28) = 5.06, p < .05. In contrast, younger adults showed the opposite pattern; the negative memory group showed greater MPFC activity than the positive memory group, F (1, 28) = 6.79, p < .05. Neither the load manipulation (Table 2), nor face valence (Table 3) significantly altered these results. Furthermore, a post-hoc ROI analysis revealed a similar age X memory group interaction for Dm angry (Figure 3C) and Dm happy effects (Figure 3D) in this MPFC cluster, F (1, 27) = 15.44, p < .01, F (1, 28) = 4.71, p < .05. These results indicate that the two age groups showed opposite associations between the MPFC and the positivity/negativity of memory, such that positivity effects in memory were associated with MPFC activity more in older than in younger adults when encoding either positive or negative emotional information.
Table 2.
Brain areas showing significant interaction between age and positivity effect group in emotional Dm effects during the encoding phase.
| Load condition | Contrast | Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||||
| Load+NoLoad | Old(Pos>Neg) vs Young(Pos>Neg) | ||||||||||
| Cingulate Gyrus | R | 31 | 6 | −30 | 36 | 4 | −33 | 34 | 4.31 | ||
| R | 23 | 10 | −24 | 26 | 8 | −26 | 25 | 4.13 | |||
| L | 31 | −2 | −28 | 44 | −3 | −32 | 41 | 3.81 | |||
| Posterior Cingulate | R | 23 | 10 | −28 | 28 | 8 | −30 | 27 | 3.99 | ||
| Occipital Lobe/Lingual Gyrus | R | 30 | 24 | −44 | 0 | 21 | −43 | 1 | 3.91 | ||
|
|
|||||||||||
| Medial Frontal Gyrus | L | 10 | −6 | 58 | 4 | −6 | 52 | 13 | 4.32 | ||
| L | 10 | 0 | 60 | 4 | −1 | 54 | 13 | 4.19 | |||
| L | 10 | −10 | 52 | 6 | −10 | 46 | 14 | 3.82 | |||
| Anterior Cingulate | R | 24 | 12 | 38 | 12 | 10 | 33 | 18 | 3.67 | ||
| L | 32 | −2 | 36 | 20 | −3 | 30 | 25 | 3.66 | |||
|
|
|||||||||||
| Putamen | R | 28 | 8 | −14 | 25 | 7 | −7 | 3.96 | |||
| R | 24 | 22 | −16 | 21 | 20 | −8 | 3.77 | ||||
| Insula | R | 22 | 52 | 10 | −14 | 47 | 9 | −7 | 3.85 | ||
| Claustrum | R | 44 | 4 | −2 | 40 | 2 | 3 | 3.72 | |||
| Inferior Frontal Gyrus | R | 47 | 32 | 8 | −22 | 29 | 7 | −14 | 3.66 | ||
|
| |||||||||||
| Young(Pos>Neg) vs Old(Pos>Neg) | |||||||||||
| No significant results | |||||||||||
|
| |||||||||||
| NoLoad vs.Load | Old(Pos>Neg) vs Young(Pos>Neg) | ||||||||||
| No significant results | |||||||||||
|
| |||||||||||
| Young(Pos>Neg) vs Old(Pos>Neg) | |||||||||||
| No significant results | |||||||||||
Figure 3.
(A) A significant age and memory subgroup interaction emerged in MPFC during the encoding phase. (B) Extracted present signal changes from the MPFC cluster for emotional Dm effects are plotted. Preferential encoding of positive materials is associated with MPFC more in older adults than in younger adults when remembering emotional information. Extracted percent signal changes from the same MPFC cluster for (C) the Dm effects for angry faces and (D) the Dm effects for happy faces also showed the same pattern. Error bars represent standard errors.
Table 3.
Age and positivity effect group effects for differential Dm effects for angry faces than for happy faces ([Remembered angry > forgotten angry] vs. [Remembered happy > forgotten happy]).
| Contrast | Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | Y | z | x | y | z | |||||
| Old(Pos>Neg) vs Young(Pos>Neg) | ||||||||||
| Middle Frontal Gyrus | L | 6 | −14 | 4 | 62 | −15 | −3 | 60 | 3.98 | |
| L | 46 | −50 | 30 | 24 | −48 | 24 | 27 | 3.69 | ||
| Precentral Gyrus | L | 6 | −30 | −8 | 64 | −30 | −15 | 60 | 3.87 | |
| Caudate | L | −18 | 2 | 22 | −18 | −2 | 24 | 3.75 | ||
| Superior Frontal Gyrus | L | 6 | −14 | 24 | 48 | −14 | 16 | 49 | 3.74 | |
|
|
||||||||||
| Occipital Lobe, Cuneus | L | 7 | −6 | −70 | 36 | −7 | −70 | 30 | 4.03 | |
| Superior Temporal Gyrus | L | 39 | −36 | −54 | 34 | −35 | −55 | 29 | 3.57 | |
| Supramarginal Gyrus | L | 40 | −42 | −48 | 36 | −40 | −49 | 31 | 3.56 | |
| Middle Temporal Gyrus | L | 39 | −32 | −56 | 36 | −31 | −57 | 31 | 3.55 | |
| Inferior Parietal Lobule | L | 40 | −32 | −42 | 40 | −31 | −44 | 36 | 3.54 | |
|
|
||||||||||
| Inferior Parietal Lobule | R | 40 | 42 | −50 | 46 | 37 | −53 | 41 | 3.51 | |
| R | 40 | 58 | −28 | 48 | 52 | −32 | 45 | 3.41 | ||
| R | 40 | 42 | −52 | 40 | 37 | −54 | 36 | 3.39 | ||
| R | 40 | 60 | −40 | 46 | 54 | −43 | 43 | 3.36 | ||
|
| ||||||||||
| Young(Pos>Neg) vs Old(Pos>Neg) | ||||||||||
| No significant results | ||||||||||
MPFC Functional Connectivity during the Encoding Session
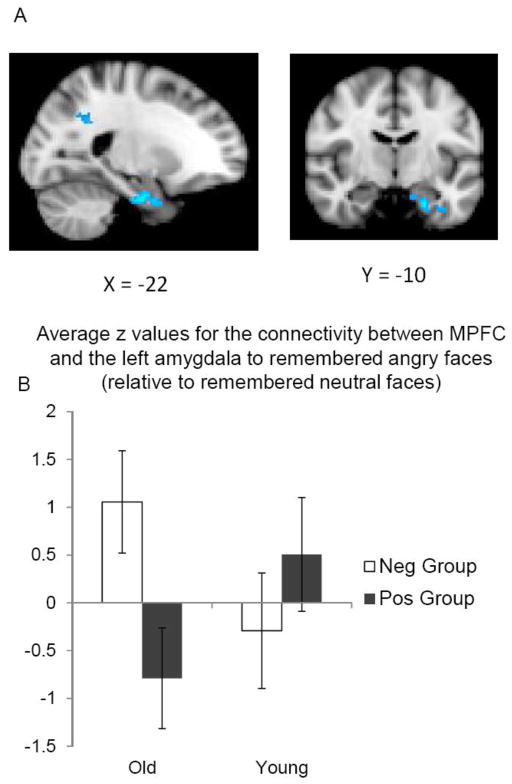
The whole-brain connectivity analysis comparing remembered angry vs. remembered neutral faces revealed a significant memory group effect in the amygdala in older adults but not in younger adults (Table 4; Figure 4A). A post-hoc ROI analysis on the average z value from the left amygdala within the significant cluster (Figure 4B) revealed an age-by-memory group interaction, F (1, 29) = 5.32, R2 = .12, p < .05. In older adults, the positive memory group (relative to the negative memory group) showed a greater negative connectivity between MPFC and left amygdala when remembering angry faces than neutral faces, F (1, 29) = 5.89, p < .05. In contrast, there were no significant differences between the two memory subgroups in younger adults (p > .30). A similar analysis comparing MPFC connectivity to remembered happy vs. remembered neutral faces did not find any effects of the memory group in the amygdala (Table 4). Thus, memory positivity in older adults was associated with greater inverse functional coupling between MPFC and amygdala selectively to negative faces.
Table 4.
Age and positivity effect group effects for MPFC functional connectivity when learning emotional faces compared with neutral faces.
| Contrast | Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | y | z | x | y | z | |||||
| Remembered Angry > Remembered Neutral | ||||||||||
| Old: Positive > Negative | ||||||||||
| No significant results | ||||||||||
|
| ||||||||||
| Old: Negative > Positive | ||||||||||
| Cingulate Gyrus | L | 31 | −18 | −56 | 34 | −18 | −57 | 29 | 3.52 | |
| L | 31 | −14 | −48 | 26 | −14 | −49 | 23 | 3.25 | ||
| Precuneus | L | 31 | −14 | −48 | 30 | −14 | −49 | 26 | 3.36 | |
| Insula | L | 13 | −38 | −40 | 30 | −37 | −41 | 27 | 3.35 | |
|
|
||||||||||
| Parahippocampal Gyrus | L | 35 | −22 | −10 | −28 | −21 | −9 | −22 | 4.14 | |
| L | 34 | −12 | −18 | −28 | −12 | −16 | −23 | 3.69 | ||
| L | 34 | −10 | −16 | −24 | −10 | −15 | −19 | 3.66 | ||
| Amygdala | L | −24 | −2 | −30 | −23 | −1 | −23 | 3.46 | ||
| L | −28 | −6 | −30 | −27 | −5 | −24 | 3.1 | |||
|
| ||||||||||
| Young: Positive > Negative | ||||||||||
| Occipital Lobe, Cuneus | R | 19 | 8 | −86 | 46 | 6 | −86 | 38 | 3.89 | |
| R | 7 | 24 | −76 | 40 | 21 | −76 | 33 | 3.46 | ||
| Precuneus | R | 7 | 24 | −66 | 38 | 21 | −67 | 33 | 3.75 | |
| R | 19 | 28 | −70 | 38 | 24 | −70 | 32 | 3.71 | ||
| R | 31 | 12 | −74 | 34 | 10 | −74 | 28 | 3.47 | ||
|
|
||||||||||
| Occipital Lobe, Cuneus | L | 18 | −2 | −78 | 34 | −3 | −77 | 28 | 3.63 | |
| L | 18 | −2 | −84 | 20 | −3 | −82 | 14 | 3.35 | ||
| L | 18 | −2 | −84 | 28 | −3 | −82 | 22 | 3.17 | ||
| L | 18 | −4 | −72 | 18 | −5 | −70 | 14 | 3.15 | ||
| Occipital Lobe, Lingual Gyrus | R | 18 | 4 | −82 | 10 | 2 | −79 | 6 | 3.33 | |
|
|
||||||||||
| Insula | R | 13 | 46 | 16 | −8 | 42 | 14 | −1 | 3.62 | |
| R | 13 | 48 | 12 | −2 | 43 | 9 | 4 | 3.46 | ||
| Inferior Frontal Gyrus | R | 47 | 48 | 18 | −12 | 44 | 16 | −4 | 3.53 | |
| R | 44 | 56 | 8 | 8 | 51 | 5 | 13 | 3.3 | ||
| Precentral Gyrus | R | 44 | 54 | 12 | 4 | 49 | 9 | 10 | 3.36 | |
|
| ||||||||||
| Young: Negative > Positive | ||||||||||
| Anterior Cingulate | R | 32 | 12 | 48 | −6 | 10 | 43 | 3 | 4.04 | |
| L | 32 | −16 | 46 | −10 | −16 | 42 | −1 | 3.66 | ||
| L | 32 | −6 | 48 | −10 | −6 | 44 | −1 | 3.5 | ||
| L | 32 | −8 | 48 | 4 | −8 | 43 | 12 | 3.48 | ||
| L | 32 | −14 | 50 | −2 | −14 | 45 | 7 | 3.47 | ||
|
|
||||||||||
| Superior Prefrontal Gyrus | L | 6 | −8 | 28 | 56 | −9 | 19 | 56 | 3.99 | |
| L | 6 | −8 | 24 | 56 | −9 | 16 | 56 | 3.61 | ||
| L | 8 | −16 | 28 | 48 | −16 | 20 | 49 | 3.43 | ||
| L | 8 | −20 | 36 | 50 | −20 | 28 | 52 | 3.27 | ||
| L | 8 | −6 | 28 | 50 | −7 | 20 | 51 | 3.26 | ||
|
| ||||||||||
| Old(Pos>Neg) vs Young(Pos>Neg) | ||||||||||
| Superior Prefrontal Gyrus | R | 8 | 12 | 58 | 30 | 10 | 50 | 36 | 3.59 | |
| MPFC | R | 9 | 8 | 48 | 14 | 6 | 42 | 21 | 3.39 | |
| R | 6 | 14 | 36 | 32 | 12 | 29 | 36 | 3.39 | ||
| R | 10 | 8 | 64 | 8 | 6 | 57 | 17 | 2.95 | ||
| Cingulate Gyrus | R | 32 | 12 | 32 | 24 | 10 | 26 | 29 | 3.27 | |
|
| ||||||||||
| Young(Pos>Neg) vs. Old(Pos>Neg) | ||||||||||
| Occipital Lobe, Cuneus | L | 18 | −2 | −78 | 34 | −3 | −77 | 28 | 4.16 | |
| L | 17 | −20 | −82 | 20 | −20 | −80 | 14 | 3.88 | ||
| L | 18 | −4 | −76 | 24 | −5 | −75 | 19 | 3.77 | ||
| R | 7 | 20 | −76 | 40 | 17 | −76 | 33 | 3.73 | ||
| Precuneus | R | 19 | 28 | −72 | 38 | 24 | −72 | 32 | 4.03 | |
|
|
||||||||||
| Superior Temporal Gyrus | R | 22 | 56 | 10 | −6 | 51 | 8 | 0 | 3.37 | |
| R | 22 | 54 | −4 | 0 | 49 | −6 | 5 | 3.27 | ||
| Insula | R | 13 | 48 | 16 | −2 | 43 | 13 | 4 | 3.26 | |
| R | 13 | 46 | 14 | −6 | 42 | 12 | 1 | 3.09 | ||
| Precentral Gyrus | R | 44 | 54 | 12 | 6 | 49 | 9 | 11 | 3.18 | |
|
| ||||||||||
| Remembered Happy vs. Remembered Neutral | ||||||||||
| Old: Positive > Negative | ||||||||||
| No significant results | ||||||||||
|
| ||||||||||
| Old: Negative > Positive | ||||||||||
| No significant results | ||||||||||
|
| ||||||||||
| Young: Positive > Negative | ||||||||||
| Cerebellum | L | −34 | −78 | −32 | −32 | −71 | −32 | 3.8 | ||
| L | −28 | −72 | −36 | −27 | −66 | −35 | 3.6 | |||
| L | −44 | −68 | −30 | −42 | −62 | −29 | 3.5 | |||
| L | −46 | −60 | −44 | −43 | −54 | −41 | 3.22 | |||
| L | −44 | −66 | −34 | −41 | −60 | −33 | 3.13 | |||
|
| ||||||||||
| Young: Negative > Positive | ||||||||||
| No significant results | ||||||||||
|
| ||||||||||
| Old(Pos>Neg) vs Young(Pos>Neg) | ||||||||||
| No significant results | ||||||||||
|
| ||||||||||
| Young(Pos>Neg) vs. Old(Pos>Neg) | ||||||||||
| No significant results | ||||||||||
Figure 4.
(A) In older adults, the MPFC had stronger negative functional coupling with the left amygdala when learning negative faces than neutral faces in the positive memory group compared with the negative memory group. (B) The mean z values for the MPFC-amygdala connectivity signal were plotted. Error bars represent standard errors.
Amygdala Functional Connectivity during Rest
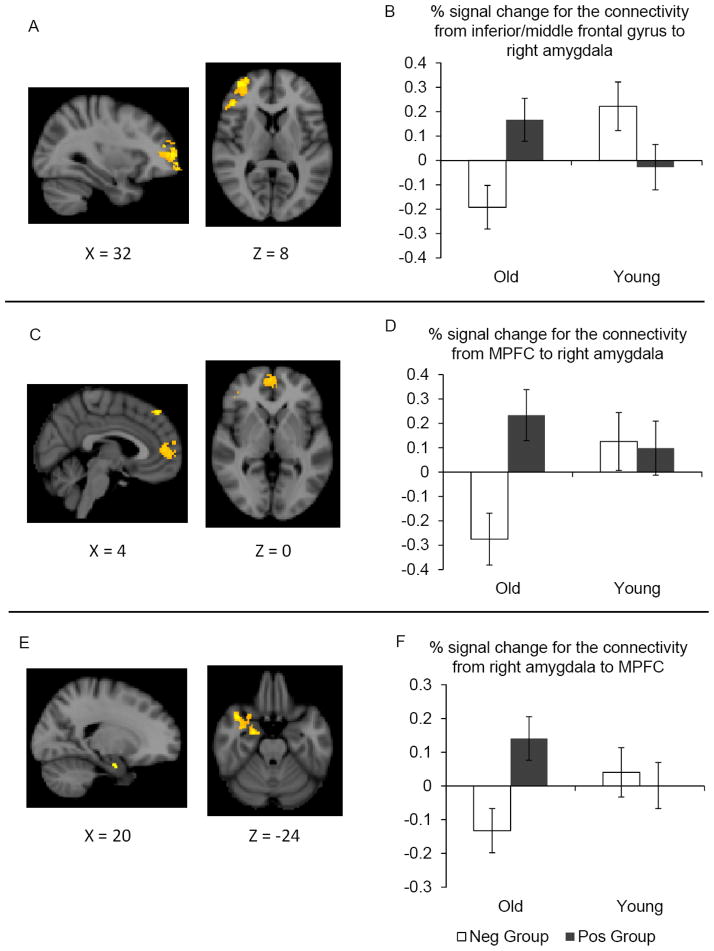
In the whole brain connectivity analyses of the right amygdala during rest, the inferior/middle frontal gyrus showed a significant interaction between age and memory subgroup (Table 5; Figure 5A–B). A subsequent ROI analysis on the percent signal changes confirmed a significant age-by-memory group interaction, F (1, 30) = 10.70, R2 = .26, p < .01. A simple effect test revealed that in older adults, the right amygdala had greater positive functional coupling with the inferior/middle frontal gyrus in the positive than in the negative memory group, F (1, 30) = 8.04, p < .05, whereas the memory group effects were not significant in younger adults (p > .05).
Table 5.
Effects of age and positivity effect groups on the right amygdala functional connectivity during rest.
| Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | Y | z | x | y | z | ||||
| Old(Pos>Neg) vs Young(Pos>Neg) | |||||||||
| Inferior Frontal Gyrus | R | 13 | 46 | 32 | 6 | 42 | 27 | 13 | 4.22 |
| Middle Frontal Gyrus | R | 10 | 38 | 54 | 8 | 34 | 48 | 17 | 4.04 |
| R | 10 | 32 | 50 | 16 | 29 | 43 | 23 | 3.84 | |
| Superior Frontal Gyrus | R | 10 | 30 | 60 | 14 | 27 | 53 | 22 | 4.21 |
| R | 10 | 32 | 62 | −10 | 29 | 57 | 1 | 3.84 | |
|
| |||||||||
| Young(Pos>Neg) vs. Old(Pos>Neg) | |||||||||
| Precuneus | R | 7 | 30 | −66 | 42 | 26 | −67 | 36 | 4.42 |
| Occipital lobe/Cuneus | R | 19 | 30 | −86 | 30 | 26 | −85 | 24 | 3.73 |
| R | 7 | 26 | −80 | 38 | 22 | −80 | 31 | 3.67 | |
| R | 17 | 20 | −78 | 18 | 17 | −76 | 14 | 3.58 | |
| Cingulate Gyrus | R | 31 | 26 | −46 | 32 | 23 | −48 | 29 | 3.65 |
|
| |||||||||
| Middle Temporal Gyrus | L | 21 | −60 | −40 | −2 | −57 | −39 | −2 | 4.26 |
| Sub-Gyral | L | 37 | −50 | −48 | −8 | −47 | −46 | −8 | 4.98 |
| Fusiform Gyrus | L | 37 | −44 | −52 | −10 | −42 | −49 | −10 | 4.16 |
| L | 20 | −46 | −38 | −14 | −44 | −36 | −12 | 3.73 | |
| L | 37 | −42 | −56 | −12 | −40 | −53 | −12 | 3.73 | |
|
| |||||||||
| Old: Positive > Negative | |||||||||
| Inferior Frontal Gyrus | R | 13 | 46 | 32 | 6 | 42 | 27 | 13 | 3.92 |
| R | 45 | 62 | 30 | 12 | 56 | 25 | 18 | 3.51 | |
| R | 46 | 44 | 36 | 2 | 40 | 31 | 10 | 3.35 | |
| Middle Frontal Gyrus | R | 10 | 38 | 54 | 8 | 34 | 48 | 17 | 3.8 |
| Superior Frontal Gyrus | R | 10 | 30 | 60 | 14 | 27 | 53 | 22 | 3.71 |
|
| |||||||||
| Superior Frontal Gyrus | L | 9 | −14 | 56 | 16 | −14 | 49 | 23 | 4.12 |
| L | 9 | −8 | 62 | 20 | −9 | 54 | 27 | 3.67 | |
| L | 9 | −18 | 60 | 26 | −18 | 52 | 32 | 3.33 | |
| L | 10 | −20 | 62 | 8 | −19 | 55 | 16 | 3.25 | |
| Medial Frontal Gyrus | L | 9 | −22 | 38 | 30 | −22 | 31 | 34 | 3.25 |
|
| |||||||||
| Superior Frontal Gyrus | R | 6 | 16 | 28 | 52 | 13 | 20 | 53 | 3.61 |
| R | 6 | 12 | 24 | 54 | 10 | 16 | 55 | 3.28 | |
| R | 8 | 4 | 42 | 50 | 2 | 33 | 52 | 3.55 | |
| L | 8 | 0 | 42 | 50 | −2 | 33 | 52 | 3.4 | |
| L | 6 | −6 | 42 | 50 | −7 | 33 | 52 | 3.34 | |
|
| |||||||||
| Old: Negative > Positive | |||||||||
| Precuneus | R | 7 | 28 | −64 | 42 | 24 | −65 | 36 | 4.49 |
| R | 19 | 28 | −70 | 40 | 24 | −71 | 34 | 3.74 | |
| Occipital lobe/Cuneus | R | 18 | 18 | −80 | 20 | 15 | −78 | 15 | 4.08 |
| Occipital lobe/Lingual Gyrus | L | 17 | −10 | −94 | 0 | −11 | −89 | −4 | 3.72 |
| L | 18 | −2 | −82 | −2 | −3 | −78 | −5 | 3.66 | |
|
| |||||||||
| Precuneus | L | 19 | −32 | −66 | 46 | −31 | −67 | 39 | 3.46 |
| L | 7 | −10 | −62 | 44 | −11 | −63 | 38 | 3.42 | |
| L | 7 | −22 | −68 | 40 | −22 | −68 | 33 | 3.31 | |
| L | 39 | −42 | −68 | 42 | −41 | −69 | 35 | 3.3 | |
| L | 19 | −40 | −70 | 48 | −39 | −71 | 40 | 3.22 | |
|
| |||||||||
| Young: Positive > Negative | |||||||||
| Thalamus | R | 18 | −22 | 4 | 16 | −23 | 6 | 3.83 | |
| R | 8 | −16 | 4 | 6 | −17 | 6 | 3.32 | ||
| Putamen | R | 34 | −8 | 2 | 30 | −10 | 6 | 3.41 | |
| R | 36 | −20 | 4 | 32 | −21 | 6 | 3.4 | ||
| Claustrum | R | 38 | −10 | 6 | 34 | −12 | 9 | 3.35 | |
|
| |||||||||
| Inferior Temporal Gyrus | R | 21 | 68 | −8 | −24 | 62 | −7 | −17 | 4.09 |
| Middle Temporal Gyrus | R | 21 | 54 | 2 | −28 | 49 | 2 | −20 | 3.85 |
| R | 21 | 58 | 2 | −30 | 53 | 2 | −22 | 3.75 | |
| R | 21 | 52 | 8 | −36 | 48 | 9 | −27 | 3.66 | |
| Superior Temporal Gyrus | R | 38 | 48 | 10 | −36 | 44 | 10 | −26 | 3.58 |
|
| |||||||||
| Young: Negative > Positive | |||||||||
| No significant results | |||||||||
Figure 5.
Brain areas showing significant effects in the functional connectivity analyses during rest (left); the average percent signal change extracted from the area for each group was also plotted (right). (A) The right inferior/middle PFC showed a significant interaction between age and memory subgroup in the whole-brain right amygdala connectivity analysis during rest. (B) Older adults showed increased functional coupling between the amygdala and the right inferior/middle frontal gyrus when remembering more positive than negative faces, while younger adults did not show a significant memory group effect. (C) The MPFC also showed a similar memory group effect in older adults. (D) Older adults showed an increased functional coupling between the right amygdala and MPFC in the positive memory group than negative memory group, whereas younger adults did not show a significant memory group effect. (E) Furthermore, the resting connectivity analysis with MPFC showed mirrored patterns to that of the right amygdala: stronger functional coupling between MPFC and the right medial temporal lobe, including the amygdala, in the positive than the negative memory group in older adults. (F) Percent signal changes extracted from the right amygdala within the significant cluster from the MPFC seed region connectivity analysis were plotted. In older adults, MPFC has greater functional connectivity with the right amygdala in the positive than in the negative group. But there were no significant differences by memory group in younger adults. In bar graphs, error bars represent standard errors.
In addition, this whole-brain analysis comparing the two memory groups in older adults revealed greater functional connectivity during rest between the right amygdala and MPFC in the positive than in the negative memory group (Table 5; Figure 5C–D). Interestingly, this MPFC cluster from the amygdala connectivity analysis of resting state data overlapped with the MPFC area showing a significant interaction during the encoding phase (Figure 3A). In contrast, the MPFC area did not show significant differences by memory group in younger adults (Table 5). Thus, preferential encoding of positive faces was associated with greater amygdala-PFC connectivity in older adults but not in younger adults. The left amygdala connectivity map did not show any significant effects of the memory subgroup in MPFC (Table 6).
Table 6.
Effects of age and positivity effect groups on the left amygdala functional connectivity during rest.
| Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| Old: Positive > Negative | |||||||||
| Superior Temporal Gyrus | R | 38 | 48 | 2 | −22 | 44 | 2 | −15 | 4.02 |
| R | 38 | 46 | 14 | −36 | 42 | 14 | −26 | 3.94 | |
| R | 22 | 56 | 8 | −16 | 51 | 7 | −9 | 4 | |
| Inferior Frontal Gyrus | R | 47 | 26 | 12 | −24 | 23 | 11 | −16 | 4 |
| R | 47 | 30 | 14 | −24 | 27 | 13 | −16 | 3.81 | |
|
| |||||||||
| Old: Negative > Positive | |||||||||
| Precuneus | L | 7 | −2 | −78 | 56 | −4 | −79 | 47 | 3.67 |
| R | 7 | 18 | −74 | 42 | 15 | −74 | 35 | 3.48 | |
| Postcentral Gyrus | L | 7 | −2 | −52 | 78 | −4 | −57 | 69 | 3.49 |
| Superior Parietal Lobule | R | 7 | 4 | −64 | 70 | 2 | −68 | 61 | 3.4 |
| Occipital lobe/Cuneus | R | 19 | 4 | −78 | 44 | 2 | −78 | 37 | 3.28 |
|
| |||||||||
| Young: Positive > Negative | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Young: Negative > Positive | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Old(Pos>Neg) vs Young(Pos>Neg) | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Young(Pos>Neg) vs. Old(Pos>Neg) | |||||||||
| No significant results | |||||||||
MPFC Functional Connectivity during Rest
The results reported so far suggest that older adults’ positivity effects in memory are associated with the same MPFC areas in the whole-brain comparisons for the encoding phase (Figure 3) and the whole-brain connectivity analyses with the right amygdala during rest (Figure 5C). Given these results, we identified the MPFC area shared by these two results and used this shared MPFC cluster as a seed region to examine MPFC functional connectivity during rest. The results using this MPFC seed region mirror the patterns seen with the right amygdala seed region (Table 7; Figure 5E); in older adults, the MPFC showed stronger functional connectivity with the right amygdala in the positive memory group than in the negative memory group. In contrast, there were no significant differences in the MPFC resting-connectivity across memory groups in younger adults. A post-hoc ROI analysis on the percent signal change from the right amygdala within the significant cluster also confirmed an age-by-memory group interaction (Figure 5F), F (1, 30) = 5.23, R2 = .12, p < .05; In older adults, the positive memory group showed greater functional connectivity between MPFC and right amygdala than did the negative memory group, F (1, 30) = 8.68, p < .05, whereas there were no significant differences between the two memory subgroups in younger adults (p > .70).
Table 7.
Effects of age and positivity effect groups on MPFC functional connectivity during rest.
| Area | H | BA | MNI | Talairach | Z stat | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| Old: Positive > Negative | |||||||||
| Superior Temporal Gyrus | R | 38 | 42 | 12 | −28 | 38 | 12 | −19 | 4.00 |
| R | 38 | 44 | 6 | −28 | 40 | 6 | −20 | 3.85 | |
| R | 38 | 40 | 20 | −40 | 37 | 20 | −29 | 3.76 | |
| R | 38 | 42 | 10 | −42 | 38 | 11 | −32 | 3.52 | |
| Middle Temporal Gyrus | R | 21 | 56 | 0 | −42 | 51 | 2 | −33 | 3.56 |
| Amygdala | R | 22 | −6 | −24 | 20 | −5 | −18 | 3.51 | |
|
| |||||||||
| Old: Negative > Positive | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Young: Positive > Negative | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Young: Negative > Positive | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Old(Pos>Neg) vs Young(Pos>Neg) | |||||||||
| No significant results | |||||||||
|
| |||||||||
| Young(Pos>Neg) vs. Old(Pos>Neg) | |||||||||
| No significant results | |||||||||
Correlation between Encoding and Resting Phases
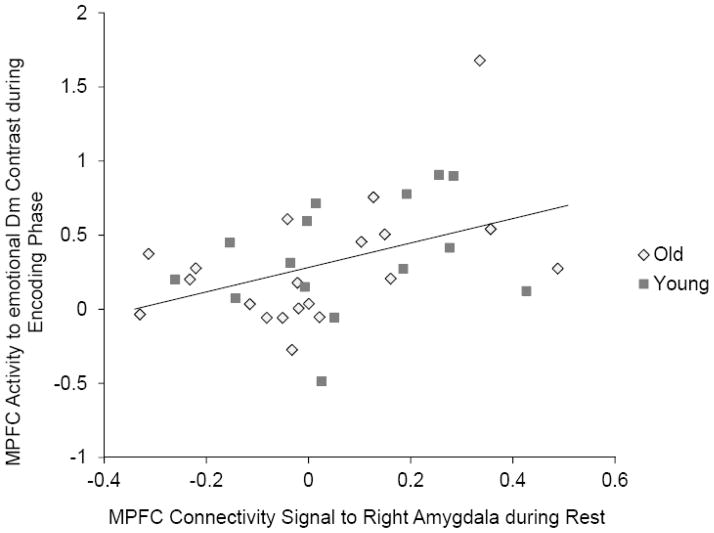
Next, we addressed whether the strength of the connectivity between MPFC and right amygdala during rest was related to MPFC activity for the emotional Dm effects during the encoding phase. Percent signal change values were obtained from a 6-mm sphere around the peak voxel for the age-by-memory group interaction in the emotional Dm effects during the encoding phase. Similarly, we obtained percent signal changes from a 6-mm sphere around the peak voxel in the MPFC cluster showing significant memory subgroup effects in older adults’ right amygdala functional connectivity during rest. Across younger and older adults, there was a significant positive correlation between these two signals (Figure 6), r (35) = .42, p < .05. The correlation magnitude was not significantly different across age groups (p > .60) and across memory subgroups (p > .20). In addition, excluding one potential outlier (an older adult showing the highest MPFC signal in the encoding phase) did not eliminate the significant correlation, r (34) = .35, p < .05. These results indicate that the amygdala-MPFC connectivity during rest is predictive of MPFC activity when learning emotional materials, irrespective of age.
Figure 6.
The MPFC connectivity signal to the right amygdala during rest was predictive of MPFC activity to emotional Dm effects during the encoding phase.
Discussion
The current study examined whether baseline functional connectivity during rest is predictive of older adults’ brain activity when learning emotional faces and their positivity effects in memory. In particular, we were interested in the relationship between MPFC/ACC regions implicated in emotion regulation (e.g., Etkin, Egner, & Kalisch, 2011), amygdala, and the degree to which participants showed positivity effects in memory.
Overall, older adults had poorer memory than younger adults for faces shown in brief video clips. However, consistent with past findings of age-related positivity effects (for reviews see Mather & Carstensen, 2005; Scheibe & Carstensen, 2010), compared with younger adults, older adults had relatively worse memory for the angry faces than the happy faces. Furthermore, dividing the older and younger adult groups into those who showed above and below their group median positivity in memory revealed age differences in the patterns of brain activity that were associated with memory positivity. Older participants who remembered more positive than negative faces had greater MPFC/ACC activity while viewing emotional than neutral faces, as would be expected if they were engaging emotion regulation processes during processing the emotional faces. In contrast, younger adults did not show this positive association between MPFC/ACC activity and the positivity of their memories. These results suggest that prefrontal top-down control processes are more involved in shaping memory positivity in older adults than in younger adults. In addition, memory positivity was associated with inverse functional coupling between amygdala and MPFC when learning negative faces in older adults, but not in younger adults. Together with the notion that the MPFC inhibits the amygdala activity when down-regulating negative emotion (e.g., Urry et al., 2006), these results support the idea that older adults’ positivity effects in memory are related to their emotion regulation attempts.
Furthermore, the same MPFC area showed a significant relationship with memory positivity in older adults’ resting-state amygdala functional connectivity. In older adults, preferential encoding of positive faces was associated with increased functional coupling between the amygdala and MPFC/ACC during rest. In contrast, amygdala-MPFC connectivity during rest was not associated with memory positivity in younger adults. This MPFC cluster revealed in older adults’ amygdala connectivity analysis during rest overlapped with the MPFC cluster showing the significant interaction during the encoding phase described in the previous paragraph. In addition, the amygdala-MPFC connectivity during rest was correlated with MPFC activity when learning emotional faces; the stronger the resting-functional connectivity between the amygdala and MPFC was, the greater MPFC activity was when learning emotional faces than neutral faces. These findings indicate that older adults’ positivity in memory is related not only to MPFC activity during emotional processing, but also to baseline amygdala-MPFC functional connectivity during rest.
Why might there be a relationship between older adults’ memory positivity effects and their resting functional connectivity? One possibility is that older adults with strong emotion regulation goals have emotion regulation networks chronically activated even when they are not consciously regulating emotions. Having emotion regulation networks chronically active should facilitate recruitment of MPFC when emotional stimuli are encountered, and promote preferential encoding processes favoring positive over negative stimuli. Consistent with this idea, we found that stronger amygdala-MPFC functional connectivity at rest predicted better MPFC activity when learning emotional faces. In addition, this MPFC area had inverse functional coupling with the amygdala when learning negative faces especially in older adults with a strong positivity effect.
However, those older people with a strong positivity effect did not show increased functional coupling between MPFC and amygdala to positive faces, as would be expected if MPFC is involved in up-regulating positive emotions. Thus, it is possible that the MPFC activity and its functional connectivity observed in the current study reflect other cognitive processing than emotion regulation. Indeed, recent research suggests that older adults’ positivity effect arises in part from their tendency to process positive information in a self-referential fashion (Kensinger & Leclerc, 2009), and self-referential processing is also associated with MPFC (e.g., Heatherton et al., 2006). In addition, it is also possible that individual differences in anatomical connections (e.g., Andrews-Hanna et al., 2007) or spontaneous emotion regulation during the resting scan mediate the interaction between the behavioral positivity effects and functional connectivity during rest. Future research should include emotion regulation measures as well as anatomical connectivity measures to address these possibilities.
Another issue for future research concerns the opposite patterns in MPFC-amygdala functional connectivity across the encoding and the resting sessions; Older adults’ positivity effect was associated with greater positive connectivity between MPFC and amygdala during rest, but also with greater inverse connectivity between these two regions when encoding negative faces. Recent studies also reported similar opposing connectivity patterns. For example, greater positive functional connectivity between amygdala and MPFC at rest predicted beneficial outcomes in subjective mood (Kim, Gee, Loucks, Davis, & Whalen, 2011), whereas greater inverse functional coupling between the two regions while viewing negative stimuli was associated with better regulation of negative emotion (Lee, Heller, van Reekum, Nelson, & Davidson, 2012). These results suggest the possibility that positive functional connectivity at rest can lead to greater functional connectivity during task, irrespective of whether it is excitatory or inhibitory. But it is also possible that these results are driven by different MPFC subregions that have opposing connectivity patterns with the amygdala (Etkin et al., 2011). Future research with more careful consideration about MPFC structure should help to address this issue.
Another question for future research concerns the opposite relationship between the positivity effect in memory and MPFC activity across younger and older adults. As discussed above, greater MPFC activity when learning emotional faces (relative to neutral faces) was associated with memory positivity in older adults. In contrast, younger adults showed the opposite pattern: greater MPFC activity in the negative than the positive memory group. These results might be due to different emotion regulation strategies employed by younger and older adults. Older adults are more likely than younger adults to ignore negative stimuli, while enhancing attention to and processing of positive stimuli (Bannerman, Regener, & Sahraie, 2011; Emery & Hess, 2011). In contrast, compared with older adults, younger adults are better at reappraising negative emotional stimuli to reduce their emotional impact (Opitz, Rauch, Terry, & Urry, 2012). These different emotion regulation strategies might result in different MPFC effects across age. That is, older adults who recruit MPFC to regulate emotion might preferentially process positive emotional stimuli while ignoring negative stimuli, which should result in better memory for positive than negative stimuli. In contrast, younger adults who recruit MPFC to regulate emotion might pay attention to negative stimuli to reinterpret them, but may not pay attention to positive stimuli very much as they do not need to be reinterpreted. This could result in better memory for negative stimuli than positive stimuli. Future studies examining emotion regulation strategies are needed to address this issue.
Several other questions also remain for future research. First, in the current study, we attempted to manipulate cognitive load, but the load had no significant effects, including no overall impairing effect on recognition memory. Thus, given its lack of potency to reduce cognitive resources, the task was not an effective way to examine how cognitive load might influence amygdala-prefrontal interactions during viewing emotional stimuli. Future studies should use stronger manipulations of cognitive load and/or assess individual differences in cognitive resources to address the relationship between the MPFC-amygdala connectivity and cognitive control abilities. Second, our functional connectivity analyses during rest revealed the memory group effect only in the right amygdala but not in the left amygdala. This result might be consistent with previous notions that the right amygdala is involved in processing of emotional faces more strongly than the left amygdala (Cristinzio, N’Diaye, Seeck, Vuilleumier, & Sander, 2010). However, the MPFC functional connectivity analysis during the encoding session revealed the memory group effect only in the left amygdala. Future research is needed to understand the laterality in the MPFC-amygdala interaction.
In conclusion, the current study revealed that resting-state functional connectivity of the amygdala is predictive of older adults’ positivity effects in memory as well as their subsequent brain activity when learning emotional faces. These results indicate that older adults’ emotional processing relies not only on the task-related brain activity but also on the baseline brain networks. Future studies along these lines should advance understanding of age differences in emotional processing, which may lead to better understanding of how older adults process emotional information differently than younger adults in everyday life.
Acknowledgments
This work was supported by grants from the National Institute on Aging (R01AG025340, and K02AG032309).
References
- Addis DR, Leclerc CM, Muscatell K, Kensinger EA. There are age-related changes in neural connectivity during the encoding of positive, but not negative, information. Cortex. 2010;46:425–433. doi: 10.1016/j.cortex.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Current Biology. 2009;19(12):1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman RL, Regener P, Sahraie A. Binocular rivalry: A window into emotional processing in aging. Psychology and Aging. 2011;26(2):372–380. doi: 10.1037/a0022029. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23(2):137–152. doi: 10.1109/tmi.2003.822821. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Bechara A, Tranel D, Hawkley LC. Could an aging brain contribute to subjective well-being? The value added by a social neuroscience perspective. In: Todorov A, Fiske S, Prentice D, editors. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind. New York: Oxford University Press; 2011. p. 249. [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, et al. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Research: Neuroimaging. 1999;90(2):113–123. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Cristinzio C, N’Diaye K, Seeck M, Vuilleumier P, Sander D. Integration of gaze direction and facial expression in patients with unilateral amygdala damage. Brain. 2010;133(1):248–261. doi: 10.1093/brain/awp255. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery L, Hess TM. Cognitive consequences of expressive regulation in older adults. Psychology and Aging. 2011;26(2):388–396. doi: 10.1037/a0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. International Psychogeriatrics. 1995;7(03):429–438. doi: 10.1017/S1041610295002171. [DOI] [PubMed] [Google Scholar]
- Grühn D, Scheibe S, Baltes PB. Reduced negativity effect in older adults’ memory for emotional pictures: The heterogeneity-homogeneity list paradigm. Psychology and Aging. 2007;22(3):644–649. doi: 10.1037/0882-7974.22.3.644. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social neuroscience. 2007;2(2):117. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proceedings of the National Academy of Sciences. 2009;106(26):10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay EL, Diehl M. Emotion complexity and emotion regulation across adulthood. European Journal of Ageing. 2011;8:157–168. doi: 10.1007/s10433-011-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson HR. Looking while unhappy: Mood-congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19(9):848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006;21(1):40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15(3):208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology. 2009;21(2):192–215. [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience. 2008;20(7):1161–1173. doi: 10.1162/jocn.2008.2008. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7(4):705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBN-152 brain template. Human Brain Mapping. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(2):153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala–prefrontal coupling underlies individual differences in emotion regulation. NeuroImage. 2012;62(3):1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: Motivational manipulations can reduce age differences. Psychology and Aging. 2007;22(1):134–146. doi: 10.1037/0882-7974.22.1.134. [DOI] [PubMed] [Google Scholar]
- Mather M. The emotion paradox in the aging brain. Annals of the New York Academy of Sciences. 2012;1251:33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield SL, Wais PE, Ochsner KN, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Johnson MK. Choice-supportive source monitoring: Do our decisions seem better to us as we age? Psychology and Aging. 2000;15:596–606. doi: 10.1037/0882-7974.15.4.596. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. NeuroImage. 2010;50(4):1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. NeuroImage. 2011;54(4):2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Pannu Hayes J, Wagner HR, II, Lewis DV, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich MR, Dautoff R, Dickerson BC, Wright CI, et al. Differential hemodynamic response in affective circuitry with aging: An fMRI study of novelty, valence, and arousal. Journal of Cognitive Neuroscience. 2011 doi: 10.1162/jocn.2010.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58(2):156–163. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down- and up- regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Opitz PC, Rauch LC, Terry DP, Urry HL. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging. 2012;33(4):645–655. doi: 10.1016/j.neurobiolaging.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events in older adults. Psychology and Aging. 2008;23(3):585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Reed AE, Chan L, Mikel JA. Meta-analysis of the age-related positivity effect. Paper presented at the Paper presented at the annual meeting of the Gerontological Society of America.2012. [Google Scholar]
- Riediger M, Schmiedek F, Wagner GG, Lindenberger U. Seeking pleasure and seeking pain: Differences in prohedonic and contra-hedonic motivation from adolescence to old age. Psychological Science. 2009;20(12):1529–1535. doi: 10.1111/j.1467-9280.2009.02473.x. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ritchey M, LaBar KS, Cabeza R. Level of processing modulates the neural correlates of emotional memory formation. Journal of Cognitive Neuroscience. 2010;23(4):757–771. doi: 10.1162/jocn.2010.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Pruis TA, Stevens AA, Janowsky JS. More is less: Emotion induced prefrontal cortex activity habituates in aging. Neurobiology of Aging. 2011;32(9):1634–1650. doi: 10.1016/j.neurobiolaging.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of International Neuropsychological Society. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2010;65B(2):135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2010;31(2):315–327. doi: 10.1016/j.neurobiolaging.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJF, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2008;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Human Brain Mapping. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.55.1.128. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: Neural basis of improving emotional stability over age. The Journal of Neuroscience. 2006;26(24):6422–6430. doi: 10.1523/jneurosci.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winecoff A, LaBar KS, Madden DJ, Cabeza R, Huettel SA. Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience. 2011;6(2):165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Kisley MA. The negativity bias is eliminated in older adults: Age-related reduction in event-related brain potentials associated with evaluative categorization. Psychology and Aging. 2006;21(4):815–820. doi: 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences. 2002;99(4):2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of Neurophysiology. 2008;100(4):1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]