Abstract
BACKGROUND & AIMS
The NLRP3 inflammasome induces inflammation in response to organ injury, but little is known about its regulation. Toll-like receptors (TLRs) provide the first signal required for activation of the inflammasome and stimulate aerobic glycolysis to generate lactate. We examined whether lactate and the lactate receptor, GPR81, regulate TLR induction of signal 1 and limit inflammasome activation and organ injury.
METHODS
Primary mouse macrophages and human monocytes were incubated with TLR4 agonists and lactate and assayed for levels of pro-IL1β, NLRP3, and CASP1; release of IL1β; and activation of NFκB and caspase 1. Small interfering (si)RNAs were used to reduce levels of GPR81andARRB2, and an NFκB luciferase reporter transgene was transfected in RAW 264.7 cells. Cell lysates were analyzed by immunoprecipitation with an antibody against GPR81. Acute hepatitis was induced in C56BL/6N mice by administration of lipopolysaccharaide (LPS) and D-galactosamine. Acute pancreatitis was induced by administration of LPS and caerulein. Some mice were given intraperitoneal injections of sodium lactate or siRNA against Gpr81. Activation of NFκB in tissue macrophages was assessed in mice that express a reporter transgene.
RESULTS
In macrophages and monocytes, increasing concentrations of lactate reduced TLR4-mediated induction of Il1B, Nlrp3, and Casp1; activation of NFκB; release of IL1β; and cleavage of CASP1. GPR81 and ARRB2 physically interacted and were required for these effects. Administration of lactate reduced inflammation and organ injury in mice with immune hepatitis; this reduction required Gpr81 dependence in vivo. Lactate also prevented activation of NFκB in macrophages of mice, and when given following injury, reduced the severity of acute pancreatitis and acute liver injury.
CONCLUSIONS
Lactate negatively regulates TLR induction of the NLRP3 inflammasome and production of IL1β, via ARRB2 and GPR81. Lactate could be a promising immunomodulatory therapy for patients with acute organ injury.
Keywords: Innate Immune Response, Pancreas, Immune regulation, mouse model
The inflammatory cascade initiated by sterile inflammation and mediated by the inflammasome is a central component in a wide range of diseases.1–5 Inflammasome activation has a minimal requirement for two signals. Signal 1 is typically provided by activation of TOLL-like receptors (TLRs), and results in transcriptional up-regulation of pro-cytokines and inflammasome components. These receptors include TLR4 and TLR9 which sense a number of ligands including extracellular high mobility group box protein 1 and double-stranded DNA released from damaged host cells, respectively.6, 7 Signal 2 can be provided by diverse stimuli released from damaged cells and results in activation of inflammasome components culminating in activation of caspase-1 (CASP1).8 Active CASP1 in turn proteolytically cleaves and activates Pro-Il1β into a mature form. TLR4, TLR9, IL1β, and the inflammasome components NLPR3 and CASP1 are all strong determinants of inflammation and organ damage in acute injury of the liver and pancreas.4, 9–11 The broad importance of these pathways to tissue injury has made identification of mechanisms that regulate sterile inflammation and their development as therapeutic targets a high priority.
Activation of a pro-inflammatory signaling pathway also stimulates metabolic pathways.12 Specifically, TLR activation in macrophages causes an increase in aerobic glycolysis through many steps, including up-regulation of the enzymes of glycolysis and down-regulation of Krebs cycle enzymes, with a resultant increase in lactate production.13 This switch to glycolysis results in the accumulation of metabolites such a lactate that can be used for the synthesis of immune mediators. Lactate is a carboxylic acid (pKa 3.86), and almost completely disassociates to the lactate anion near a physiologic pH. Lactate has several chemical and metabolic effects including reduce the extracellular pH and conversion back to glucose by the Cori cycle within the liver.14 Recently, lactate has been identified as the ligand for the plasma membrane Gi protein coupled receptor (GPR) GPR81.15 GPR81 is one of a large family of GPRs with low affinity for hydroxy-carboxylic acid structure ligands.16 Several GPRs have recently been identified to negatively regulate TLR mediated inflammation through interactions with intracellular protein arrestin beta-2.17 ARRB2 is known to interact with GPR81 related proteins, specifically GPR40 and GPR120, and is required for their immunomodulatory effects on TLR and NLRP3 signaling through suppression of TAK1 inflammatory signaling and through direct binding of NLRP3, respectively.18,19 Activation of GPR81 on adipocytes inhibits lipolysis, but its actions on macrophages and other immune cells are not known.15,20 Previous in vitro studies differed substantially from the current investigation as they did not interrogate the effects on inflammation of short-term lactate exposure in primary innate immune cells, and no in vivo studies have addressed the effect on inflammation of short-term lactate exposure as occurs in moderately intense exercise.21,22,23 We hypothesized that lactate could signal though GPR81 to down-regulate NLRP3 inflammasome activity in macrophages and thus provide an important negative regulatory feedback to limit sterile inflammation. By extension, we further hypothesized that brief high concentration lactate exposure could activate GPR81 mediated pathways and limit sterile inflammation in vivo in the liver and pancreas.
Materials and Methods
Reagents
Lipopolysaccharide (LPS) (Sigma) was used at 200 ng/mL in peritoneal macrophages, and 1000 ng/mL in RAW 246.7 cells and Kupffer cells and CpG ODN1826 (Invivogen) was used at 1mM in all cell types. In vitro incubations with LPS or CpG were for 3 hours except in ELISA and CASP1 Western blot experiments, in which LPS incubation was for 8 hours. 5 mM adenosine triphosphate (ATP) was added for 15 minutes to the cells, washed out, and then the cells were incubated for an additional 3 hours prior to collection of supernatant for ELISA or cell lysate for CASP1 Western blot. All in vitro experiments with TLR ligands and lactate were conducted in the following filter sterilized buffer: 138 mM NaCl, 5 mM KCl, 1.25 mM CaCl2, 0.5 mM MgCl, 0.4 mM MgSO4, 0.4 mM KH2PO4, 0.3 mM NaH2PO4, 1.7 mM glucose, 10 mM HEPES, pH 7.40 with or without 15 mM lactate. Buffer with or without lactate was added 15 minutes prior to addition of LPS or CpG. In most experiments, lactate 15 mM and the GPR81 agonist 3-chloro-5-hydroxy benzoic acid 100 mM (Santa Cruz Biotech, Santa Cruz, CA) were added15 minutes before TLR ligands.24
Human Cell Isolation
Human peripheral blood mononuclear cells were freshly isolated using Leukosep separation tubes (Greiner bio-one, Monroe, NC) and Histopaque 1077 (Sigma, St. Louis, MO). Cells were maintained in culture for 2 hours prior to use and non-adherent cells removed. All human cell isolation was approved by the Yale University Institutional Review Board for human subject research.
Animals
C57BL/6N male mice five to eight weeks of age were purchased from the National Cancer Institute and Charles River Laboratory, respectively. Mice transgenic for the NF-kB GFP reporter transgene were a kind gift of Dr. Christain Jobin.25 All experiments and animal handling were performed in accordance with Yale University Institutional Animal Care guidelines.
Peritoneal Macrophage Isolation
Sterile 4% thioglycollate broth (Sigma) ip in mice provided primary macrophages. Cells were plated in 24 well polystyrene dishes. Non-adherent cells were removed by washing after 1 hour to enrich for peritoneal macrophages, which were incubated for three hours in Dulbecco’s modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, penicillin, and streptomycin prior to experiments.
Kupffer Cell Isolation
Mice were perfused in situ with collagenase type IV (Worthington Biochemicals, Lakewood Township, NJ) and the digested liver passed through a 100 mm mesh filter to obtain a cell suspension. Non-parenchymal cells were isolated at the interface in a discontinuous gradient of 13% and 18% Optiprep (Sigma) in Hanks buffered salt solution and plated in 48 well polystyrene dishes. Non-adherent cells were removed by washing after 1 hour to enrich for Kupffer cells.26 Adherent cells were then incubated for three hours in DMEM supplemented with 10% hat-inactivated fetal calf serum, penicillin, and streptomycin prior to experiments.
Lactate Measurement
Supernatant was collected from peritoneal macrophages stimulated with LPS in vitro and assessed for lactate content by a fluorometric commercial L-lactate assay kit (abcam, Cambridge, MA). Serum was also collected from animals 15 minutes after intraperitoneal administration of lactate or PBS and assessed for lactate content as above.
Quantitative Polymerase Chain Reaction
Commercially available Taqman primer-probes from Applied Biosystems were used to quantify expression of Pro-Il1b, Nlrp3, Casp1, Pro-Il18, Il10, Gpr81, Arrb2, and Gapdh from reverse transcribed human and mouse RNA samples. Real-time PCR reactions were performed in a LightCycler 480 PCR machine from Applied Biosystems. Expression of target genes was normalized relative to Gapdh. Gapdh transcript levels in cDNA were not significantly affected by LPS or CpG stimulation in vitro or by LPS with galactosamine or caerulein treatment in vivo.
Western Blots and Immunoprecipitation
Peritoneal macrophages were isolated, treated with LPS with ATP as detailed under reagents, and cell lysates run on Western blots which were immunostained with anti-CASP1 antibody (sc-514) and anti-β-actin antibody from Santa Cruz Biotechnology (Santa Cruz, CA). Cell lysates from RAW 246.7 cells were immunoprecipitated with anti-GPR81 antibody (sc-32647) according to the manufacturer’s instructions and Western blots were immunostained with anti-ARRB2 antibody (C16D9) from Cell Signaling Technology (Danvers, MA). Liver levels of GPR81 were determined by quantitation of Western blot immunostained with anti-GPR81 antibody using PC Image Software and an Analyst Imager from FOTODyne (Hartland, WI).
ELISA for IL1β and phospho-NF-κB p65
Peritoneal macrophages and human peripheral blood mononuclear cells were treated with LPS and ATP as described in reagents and supernatant collected. Supernatant, macrophage, pancreatic and liver tissue was assessed for IL1β and phospho-NF-κB p65 (Ser536) using commercially available ELISA kits (eBioscience, San Diego, CA). Supernatant levels of IL1β are expressed as pg per mL, tissue levels of IL1β were normalized to total protein content, and macrophage phospho-NF-κB levels normalized to values in untreated wells.
NF-κB Reporter Construct and Assay
RAW 246.7 cells were transfected with pNF-κB Luc reporter plasmid (Agilent, Englewood, CO) and with Lipofectamine2000 (Invitrogen, Grand Island, NY). After 48 hours, cells were treated with LPS with or without lactate 15 mM for 3 hours. Reporter activity was measured using a Luciferase Reporter kit (Promega, Madison, WI), with fluorometric measurements made in a Synergy Microplate Reader (Biotek, Winooski, VT).
In vitro siRNA
RAW 246.7 cells were plated in 24 well dishes and treated with Silencer select siRNA targeting Gpr81, Arrb2, or scramble siRNA complexed with Lipofectamine from Invitrogen according to the manufacturer’s instructions. The siRNA treatment was repeated twenty four hours later, and the cells used in experiments twenty four hours after the second treatment.
In vivo siRNA
Silencer select siRNA targeting Gpr81 or scramble siRNA from Invitrogen was complexed with Invivofectamine according to the manufacturer’s instructions and administered at 7 μg/g body weight by intraperitoneal injection for two doses 24 hours apart and used in experiments 24 hours after the second dose.
Induction of Immune Hepatitis and Lactate Treatment
C56BL/6N mice were administered LPS (Sigma, St. Louis, MO) at 5 μg/g and d-galactosamine (Calbiochem, Billerica, MA) at 300 μg/g body weight by intraperitoneal injection in sterile PBS in a volume of 10 μL/g. In pretreatment experiments, fifteen minutes after administration of either LPS mice were given an intraperitoneal injection of PBS or150 mM sodium lactate in 5 mM HEPES, pH 7.40 at 30μL/g. In post-injury experiments, one hour after LPS dosing, mice were administered intraperitoneal 150 mM sodium chloride or 150 mM sodium lactate in 5 mM HEPES, pH 7.40 at 30 mL/g. Mice were euthanized at 5 hours after LPS dosing for analysis or earlier if moribund.
Induction of Pancreatitis and Lactate Treatment
Mice were given LPS at 5 μg/g with the first of six hourly intraperitoneal injections of caerulein sulfate (Sigma, St. Louis MO) at 100 pg/g body weight in sterile normal in an injection volume of 10 μL/g. In post-injury experiments, mice were administered subcutaneous 150 mM sodium chloride or 150 mM sodium lactate in 5 mM HEPES, pH 7.40 at at 30 μL/g with the third dose of caerulein. Mice were euthanized one hour after the last injection of caerulein for analysis.
Serum Alanine Aminotransferase (ALT) and Serum Amylase Measurements
Serum ALT levels were determined from mouse serum by the Yale Clinical Core Laboratory. Serum amylase values were determined by Phadebas Amylase Test (Magle Life Sciences, Lund, Sweden).
Caspase 1, Trypsin, and Myeloperoxidase Activity by Fluorometric Assay
Caspase 1 activity was assessed in cell and pancreatic tissue lysates using a fluorometric assay kit (abcam,Cambridge, MA) and normalized to untreated cells in vitro. Trypsin activity was assessed in tissue lysates using a fluorometric substrate Boc-Gln-Ala-Arg-MCA as previously described.3 Myeloperoxidase activity was assessed in tissue lysates by chlorination of the substrate 3′-(p-aminophenyl) fluorescein (APF) using a commercial assay kit (Life Technologies). Pancreatic values were normalized to total amylase content in pancreatic tissue and untreated control samples. All fluorometric measurements were made in a Synergy Microplate Reader.
Histopathology Scoring of Liver Injury
Liver and pancreatic tissue was fixed, embedded, sectioned, and stained with haematoxylin and eosin. Sections were scored in a blinded manner for apoptosis and hemorrhage in five 20× magnified fields. Apoptosis was scored as 0–4 for <1%, 1–5%,5–10%, 10–20%, or over 20% apoptotic hepatocytes per 20× field. Hemorrhage was scored as 0–4 for none, 1–5%, 5–20%, 20–50%, or over 50% hemorrhage per 20× field. Pancreatic sections were scored for edema and inflammation as previously described.3 Necrosis scoring as 0–4 for 1–2%, 2–5%, 5–10%, and over 10% acinar cell necrosis by cellular blebbing and nuclear pyknosis.
Tissue Macrophage NF-κB Activation In Vivo
Mice harboring the NF-kB GFP reporter transgene were administered LPS and galactosamine or LPS and caerulein as above with 150 mM sodium lactate or 150 mM sodium chloride in 5mM HEPES, pH 7.40 dosing by intraperitoneal injection 15 minutes later and by subcutaneous injection concurrently, respectively. Mice in the latter group were given two additional doses of caerulein and all animal sacrificed 3 hours after LPS dosing. Liver, pancreas, and spleen were harvested on ice in Hanks balanced salt solution, 5% heat inactivated fetal bovine serum, 0.1% sodium azide by mincing, gentle trituration with a micropipette, and straining through a 40 micron filter. Cells were immunstained with F4/80 allophycocyanin conjugated antibody (ebioscience), assessed for F4/80 and GFP fluorescence on a FACS Calibur (BD Biosciences, San Jose, CA), with data analyzed by FlowJo software.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel. With regard to small sample sizes, normal distribution was assumed. To analyze differences between two normally distributed groups, a two-tailed t-test was used. P< 0.05 was considered a significant difference. If not stated otherwise, data were taken from 3–5 individual experiments and expressed as means ± s.e.m.
Results
Lactate Suppresses TLR4 and TLR9 Mediated Inflammatory Signaling
We confirmed that LPS treatment induces lactate production in peritoneal macrophages (Figure 1A), and tested if sodium lactate could suppress LPS mediated induction of Pro-Il1β in peritoneal macrophages. There was a dose dependent suppression from 5–10 mM above the baseline serum concentrations of 1–2 mM lactate (Figure 1B). At 15 mM, lactate strongly suppressed LPS mediated induction of Pro-Il1β, Nlrp3, Casp1, and Pro-Il18 but not anti-inflammatory Il10 (Figure 1C). Similar results were found at this dose with regard to TLR9 stimulation in peritoneal macrophages except for no significant change in Pro-Il18 (Supplementary Figure 1). We next identified that lactate suppressed LPS mediated phosphorylation of the NF-kB p65 subunit at Ser536 in cell lysates, a key step in TLR4 mediated NF-κB activation (Figure 1D). We confirmed this lactate suppression of LPS induced NF-κB activity using NF-κB luciferase reporter gene activity in RAW246.7 cells (Figure 1E). Finally, we identified that lactate at 15 mM could suppress LPS induction of Pro-Il1β, Nlrp3, Casp1 in human peripheral blood mononuclear cells (Figure 1F).
Figure 1.
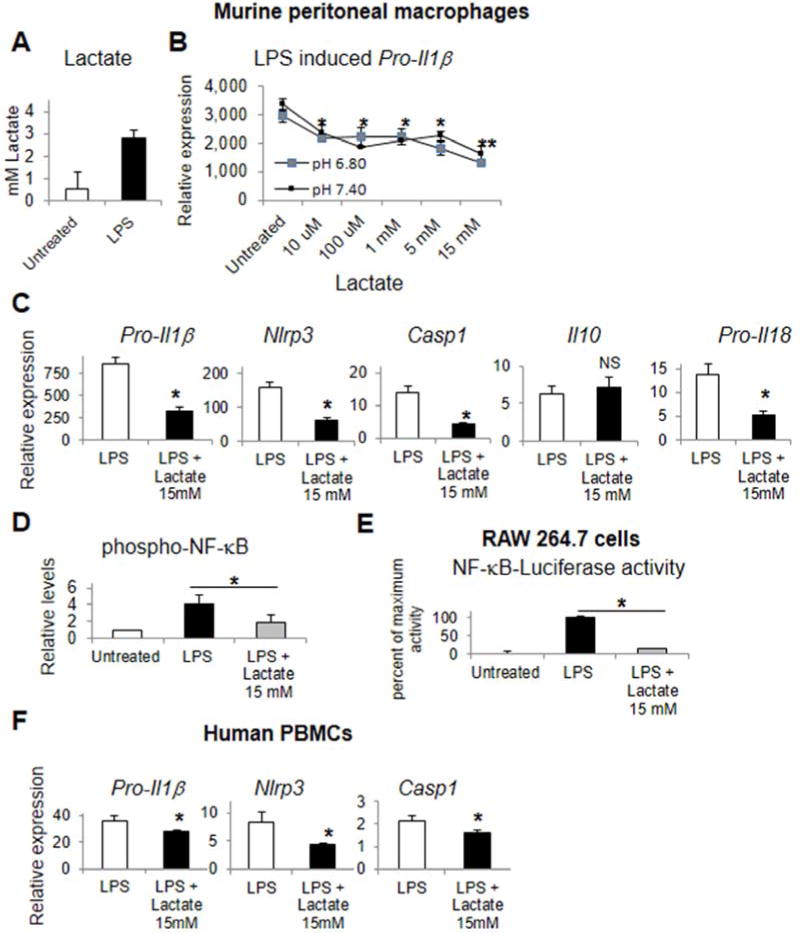
Lactate suppresses TLR4 mediated inflammatory signaling. Murine peritoneal macrophages assessed for LPS mediated lactate production (A), and lactate alteration of LPS induced Pro-Il1β with dose response (B), pro-inflammatory gene transcription (C) and phospho-p65 NF-κB levels (D). NF-κB reporter gene luciferase activity in RAW 264.7 cells in response to lactate and LPS (E). Primary human peripheral blood monocytes assessed for pro-inflammatory gene transcription in response to LPS and lactate (F). Asterisks denote significant difference (P<0.05) between treatment or bracketed groups. Double asterisk denotes significant difference between 5 and 15 mM lactate groups.
Lactate Suppresses TLR Primed NLRP3 inflammasome Activation
Activation of caspase-1 is the central step in sterile inflammation and lactate suppressed CASP1 cleavage, mature Caspase 1 proteolytic activity, and Caspase 1 dependent processing and extracellular release of IL1β (Figures 2A–C). Lactate also suppressed NLRP3 dependent release of IL1β in human peripheral blood mononuclear cells (Figure 2D). Administration of lactate after LPS treatment but shortly before the NLRP3 activation by ATP significantly suppressed release of IL1β in murine macrophages suggesting a modest direct antagonism of NRLP3 activation (Supplementary Figure 2). Additionally, lactate did not induce general cytotoxicity by LDH release or SYTOX vital dye permeability by flow cytometry (data not shown).
Figure 2.
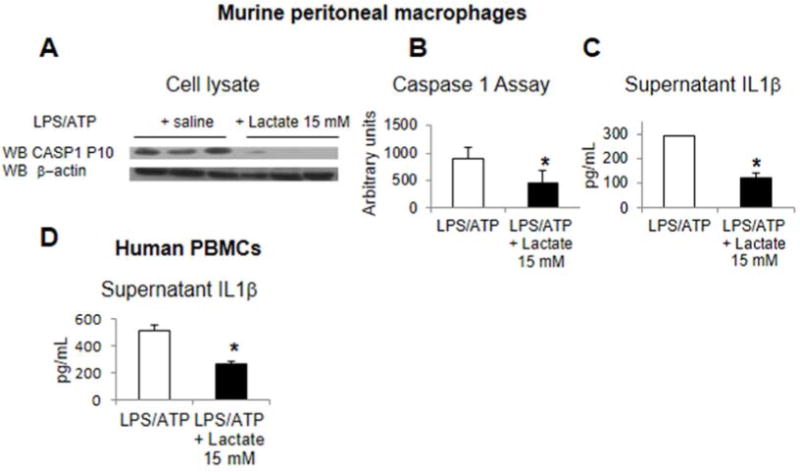
Lactate suppresses TLR primed NLRP3 inflammasome activity. Murine peritoneal macrophages were treated with LPS, ATP, and lactate, and then assessed for CASP1 cleavage to the P10 subunit in Western blots (A), caspase 1 activity (B), and IL1b release into the supernatant (C). Human peripheral blood mononuclear cells treated with LPS, ATP, and lactate and assessed for IL1brelease (D). Asterisks denote significant difference (P<0.05) between treatment groups.
Lactate Suppression of TLR4 Signaling Requires GPR81 and ARRB2
GPR81 has recently been identified as a cell surface receptor for lactate and is expressed in the murine macrophage cell line RAW 264.7 (Figure 1A). Selective silencing of Gpr81 results in loss of lactate mediated suppression of LPS induced Pro-Il1β in RAW264.7 cells (Figures 3A–B). Confirming this finding, the GPR81 selective agonist 3-chloro-5-hydroxy benzoic acid suppressed LPS mediated NF-κB p65 subunit phosphorylation in murine macrophages (Figure 3C).24 ARRB2 was found to physically interact with GPR81 in RAW 264.7 cells in the presence or absence of exogenous lactate by co-immunoprecipitation and immunoblotting (Figure 3E). ARRB2 is also required for lactate immunosuppression as selective silencing of Arrb2 results in loss of lactate mediated suppression of LPS induced Pro-Il1β in RAW264.7 cells (Figure 3D). Of note, lactate immune effects in murine macrophages occurred independent of cAMP and Gi heterotrimeric G protein signaling (Supplementary Figure 3).
Figure 3.
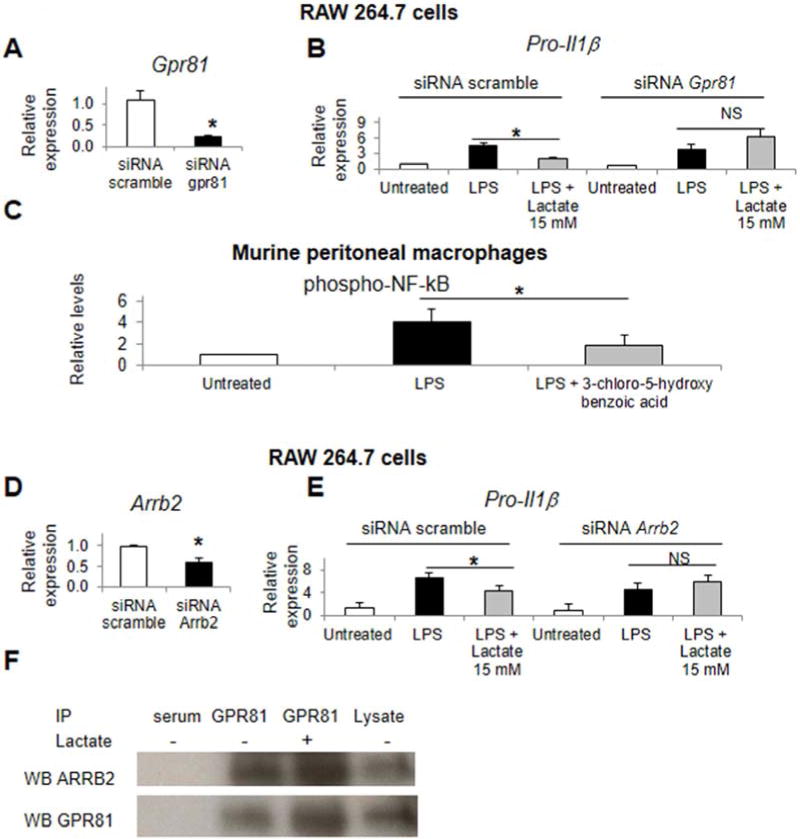
Lactate suppression of TLR4 requires GPR81 and ARRB2. RAW 264.7 cells were treated with siRNA for Gpr81, Arrb2, or scramble siRNA and assessed for Gpr81 and Arrb2 knockdown (A and D).Pro-Il1β induction was measured in siRNA treated cells stimulated with LPS and lactate (B and E). Murine peritoneal macrophages were treated with LPS and the GPR81 agonist 3-chloro-5hydroxybenzoic acid 100 mM and phospho-p65 NF-κB levels assessed (C). RAW 264.7 cell were treated with or without lactate, and cell lysates then immunoprecipitated (IP) with anti-GPR81 antibody or control serum, and immunostained for ARRB2 and GPR81 (F). Asterisks denote significant difference (P<0.05) between treatment or bracketedgroups when marked. NS, not significant.
Lactate Treatment Suppresses Inflammation and Injury in TLR4 and Caspase 1 Mediated Immune Hepatitis
Pretreatment with lactate (n=6) versus PBS (n=6) suppressed hepatic Pro-Il1β, Nlrp3, and Casp1, serum ALT release, serum ALT levels, and liver apoptosis and hemorrhage in LPS and galactosamine induced hepatitis (Figure 4A–C). Lactate bolus administration increased serum lactate levels to 3 mM without inducing liver, kidney, or muscle injury as assessed by serum markers (Supplementary Figure 4). Post-injury with lactate (n=6) versus saline (n=6) also decreased liver apoptosis and hemorrhage and serum ALT levels (Figure 4D, E). Additionally, post-injury with lactate decreased serum IL1β and hepatic IL1β (Figure 4F). Caspase 1 is a strong determinant of liver injury in this LPS and galactosamine model (Supplementary Figure 5), confirming the relevance of our finding to inflammasome mediated liver injury. We also confirmed that lactate pretreatment was protective from acute liver injury mediated by TLR9 in CpG and galactosamine induced acute immune hepatitis (Supplementary Figures 6). Lactate co-treatment decreased hepatic Pro-Il1β and Nlrp3, decreased histologic liver injury, and decreased neutrophil influx into the liver in an established murine model of acetaminophen induced acute liver injury (Supplementary Figure 7).
Figure 4.
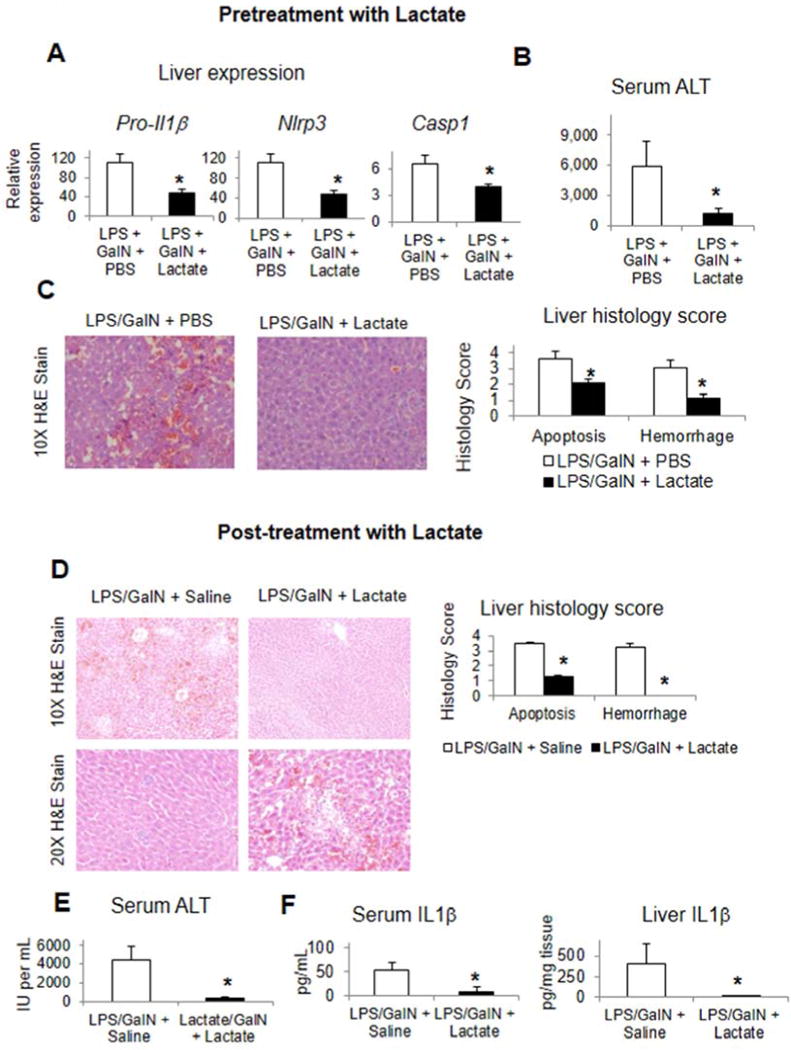
Lactate pre-injury and post-injury suppresses liver inflammation and liver injury in TLR4 mediated immune hepatitis. Lactate or PBS was administered to mice concurrent with LPS and galactosamine and data collected at 5 hours post-injury (A–C). Induction of inflammatory genes in the liver (A),serum ALT levels (B), and liver histology scoring and representative histology (C). Lactate or normal saline was administered as post-injury to mice one hour after administration of LPS and galactosamine and data collected at 5 hours after LPS and galactosamine dosing. Liver histology scoring and representative histology (D),serum ALT levels (E), and serum and liver IL1b levels (F) were determined. Asterisks denote significant difference (P<0.05) between treatment groups.
GPR81 Is Required for Dampening Pro-inflammatory Responses and For Lactate Mediated Immunomodulation in vivo
Gpr81 is strongly expressed in isolated Kupffer cells relative to hepatocytes and whole liver (Figure 5A). Lactate suppresses LPS and CpG mediated induction of Pro-Il1β, Nlrp3, and Casp1 in isolated Kupffer cells (Figure 5A). Selective knock-down of GPR81 with siRNA was achieved in the liver in vivo in mice (Figure 5B) and worsened liver injury in our model. Specifically, knockdown of Gpr81 (n=5) versus scramble siRNA treatment (n=5) resulted in increased hepatic Pro-Il1β and Nlrp3, increased hepatocyte apoptosis and liver hemorrhage, increased serum ALT values (17,200 ± 2503 versus 7,800 ± 282), and induced progression of injury from 0% to100% mortality (Figures 5C–F); of note, these mice received PBS pretreatment as well to control for lactate therapy in the following groups. Lactate pretreatment in mice with Gpr81 knockdown (n=4) versus siRNA scramble (n=4) did not significantly mitigate Gpr81 knockdown induced increases in hepatic Pro-Il1β and Nlrp3 or hepatic apoptosis. Of note, lactate pretreatment in the Gpr81 knockdown background substantially improved liver hemorrhage, modestly improved serum ALT values, and only slightly altered the Gpr81 knockdown induced mortality (75% versus 100% with PBS) (Figures 5D–F).
Figure 5.
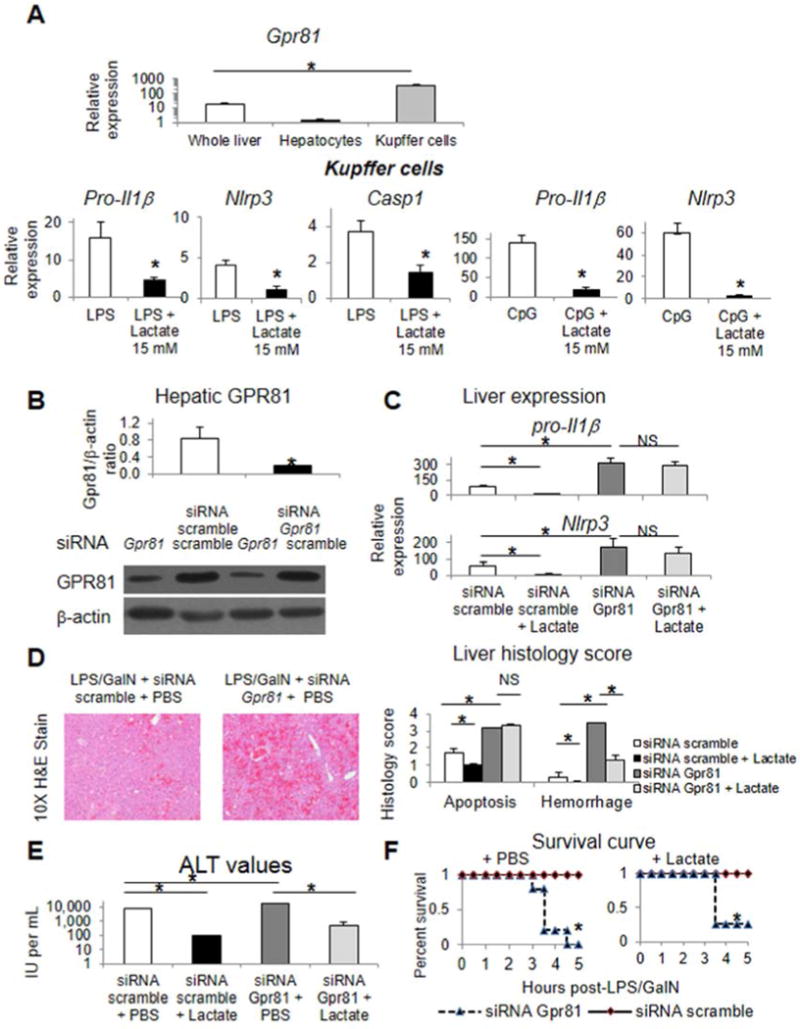
GPR81 is required for dampening pro-inflammatory responses and for lactate mediated immunomodulation in vivo. Gpr81 expression in isolated liver cell populations (A). Inflammatory gene induction in response to LPS, CpG, and lactate in isolated Kupffer cells (A). Western blot and densitometry from liver lysates of mice treated with siRNA scramble or siRNA for Gpr81 probed with anti-GPR81 antibody (B). Mice were treated with siRNA for Gpr81 or siRNA scramble, LPS and galactosamine, and pretreated with lactate or PBS by intraperitoneal injection. Induction of inflammatory genes in the liver (C), liver histology scoring and representative histology (D), serum ALT values (E), and survival data (F). Asterisks denote significant difference (P<0.05) between bracketed groups. NS, not significant.
GPR81 and lactate post-injury mitigate inflammation and injury in severe acute pancreatitis
Gpr81 knockdown was achieved in the murine pancreas (Supplementary Figure 8), and LPS and caerulein induced pancreatitis was more severe in Gpr81 deficiency (n=5) versus scramble siRNA treatment (n=5) as noted by increases in pancreatic histologic injury, serum amylase, and pancreatic trypsin activity (Figure 6A–C). Post-injury with subcutaneous lactate (n=7) versus saline (n=7) substantially mitigates LPS and caerulein induced acute pancreatitis with decreases in pancreatic histologic injury, serum amylase, as well as the activity of pancreatic trypsin, Caspase 1, and myeloperoxidase (Figures 6C–F).
Figure 6.
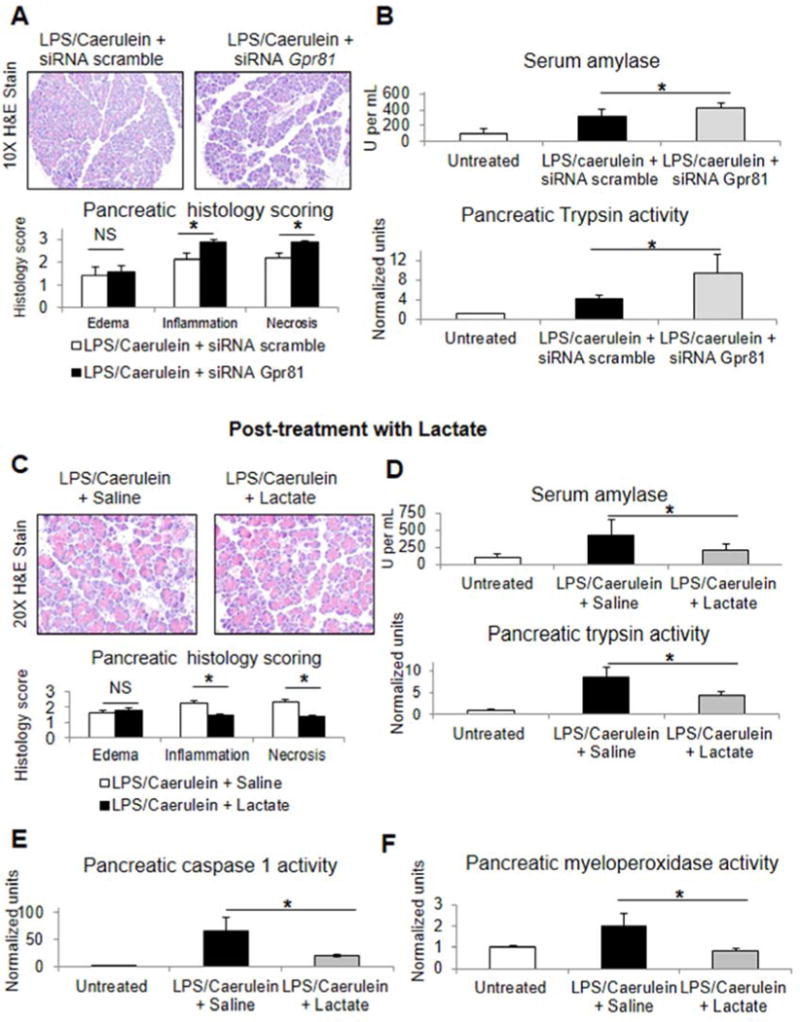
GPR81 and lactate post-injury mitigate inflammation and injury in LPS and caerulein induced severe acute pancreatitis. Mice were treated with siRNA scramble of siRNA for Gpr81 and then administered LPS and six hourly injections of caerulein (A,B). In separate experiments, mice were treated with LPS and caerulein as above and then administeredsubcutaneous lactate or salineconcurrent with thethird intraperitoneal injection of caerulein (C–F). Representative pancreatic histology and histology scoring (A, C), serum amylase and pancreatic trypsin activity (B, D), pancreatic caspase1 activity (E), and pancreatic myeloperoxidase activity (F). Asterisks denote significant difference (P<0.05) between bracketed groups.
Tissue macrophage induction of NF-kB in vivo is suppressed by lactate treatment
Using the NF-κB GFP reporter transgenic mouse, we assessed tissue macrophage activation of NF-κB by GFP expression in vivo by flow cytometry of whole organ cell suspensions gated on F4/80 high positive immunolabeled cell populations. LPS with galactosamine and LPS with caerulein treatment induce GFP expression in liver or spleen and pancreatic macrophages, respectively,and lactate pretreatment decreases this induction (Figure 7).
Figure 7.
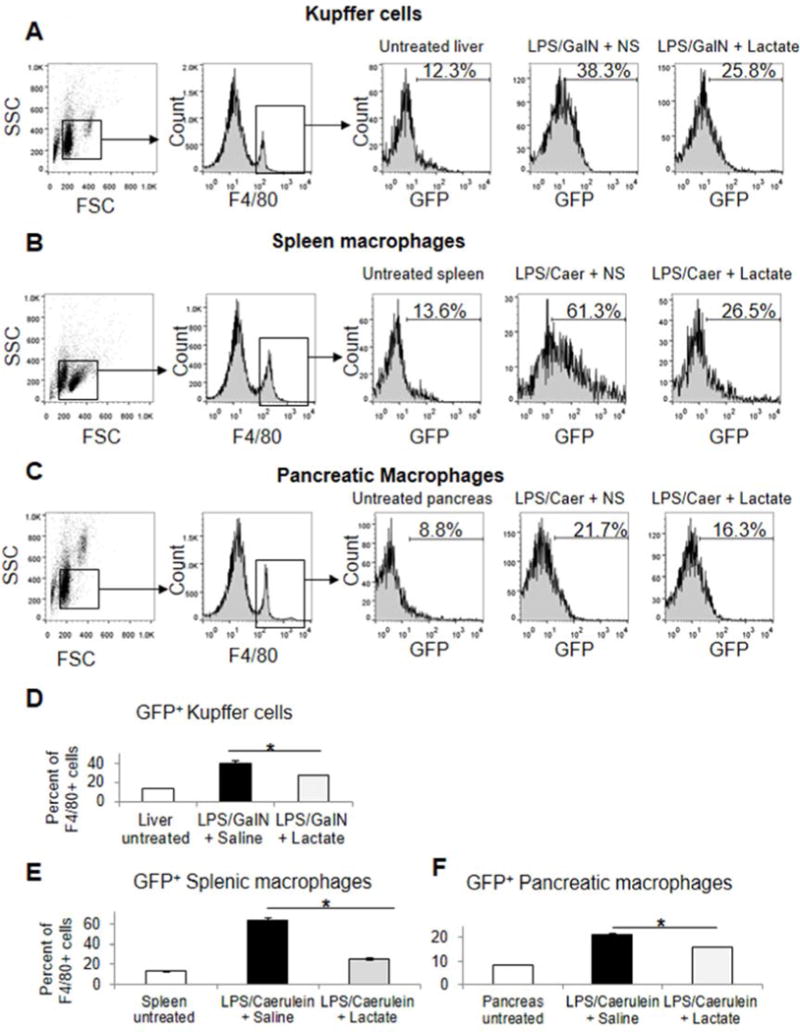
Tissue macrophage induction of NF-kB in vivo is suppressed by lactate pretreatment in experimental acute hepatitis and severe acute pancreatitis. Mice transgenic for the NF-κB GFP reporter gene were treated with lactate or normal saline by subcutaneous or intraperitoneal injection concurrent with administration of LPS and galactosamine (A,D) and LPS or caerulein, (B,C,E,F) respectively. The latter mice received two additional hourly doses of caerulein. Mice were euthanized three hours after LPS dosing, and liveror pancreas and spleen cell suspensions obtained, respectively. Cell suspensions were immunostained for F4/80 and assessed for GFP positive populations on F4/80 gated cells by flow cytometry as described in methods. Asterisks denote significant difference (P<0.05) between bracketed groups. All measured groups were significantly different from untreated groups.
Discussion
Sterile inflammatory pathways triggered by TLRs and NLPR3 activation are major contributors to inflammation in a wide range of tissues. The liver and pancreas however have unique features making sterile inflammatory pathways even more important in both these organs. The liver has a very robust innate immune system with a large resident population of immune cells and greater inflammation than other organs in response to insults such as ischemia reperfusion. The pancreas has a unique composition which can sustain an autocatalytic loop resulting in severe and rapidly progressing inflammation. These features makes the sterile inflammatory response clinically important in both organs, and provides a high threshold for testing new therapeutic modalities.
Activation of TLRs by damage associated patterns is a well recognized trigger for inflammation. TLR ligation results is a large increase in glycolytic metabolism in macrophages and other immune cells12,27, and we hypothesized that the glycolytic metabolite lactate could provide negative regulatory feedback and suppress TLR and NLPR3 mediated signaling in immune cells through the recently identified lactate receptor GPR81. Previous investigation of potential immunomodulatory effects of lactate on macrophages showing pro-inflammatory gene induction were limited to cell lines, high concentration of lactate for many hours, or reduced pH which can independently alter TLR signaling.22,23,28
We confirmed that TLR signaling could induce lactate production in macrophages (Figure 1A), and demonstrated that lactate could suppress TLR4 and TLR9 mediated pro-inflammatory responses such as Pro-Il1β via down regulation of the NF-κB pathway (Figure 1, Supplementary Figure 1). The dose dependence of 5 to 15 mM is consistent with the low affinity ofGPR81 for lactate, with an EC50 for lactate of ~5 mM.15 Serum lactate concentration at rest in humans and mice is 1–3 mM, can transiently increase to about 15 mM with intense exercise, with sustained levels of 5–10 mM in severe ischemic pathology.29,30 The reduction in TLR induced Pro-Il1β and inflammasome components Nlrp3 and Casp1 by lactate predicts that lactate would also reduce CASP1 activity, and IL1β release, which is the case (Figure 2).
The recently identified lactate receptor GPR81 was required for the suppressive effects (Figure 3), but unexpectedly the lactate induced immunosuppressive effects were independent of cAMP signaling (Supplementary Figure 3).20 Instead the cytosolic signaling molecule arrestin beta-2 (ARRB2) was required for these immunosuppressive effects, with a direct interaction between ARRB2 and GPR81 (Figure 3). ARRB2 signaling is known to antagonize both TLR and NLRP3 pathways and may thereby account for these GPR81 effects in macrophages.18,19
IL-1β is an important proximal pro-inflammatory cytokine in a wide range of inflammatory conditions and if lactate was inducing a significant reduction it will have significant therapeutic potential. Pretreatment and post-injury with lactate strongly suppressed liver inflammation and organ injury in a Kupffer cell driven model of fulminant hepatitis mediated by LPS and d-Galactosamine (Figure 4). Of note, this model is also strongly dependent on Caspase 1 (Supplementary Figure 4), and thus allowed interrogation of the effects of lactate on TLR4 and Caspase 1 mediated liver injury. Kupffer cells were the predominant Gpr81 expressing cell type in the liver and displayed lactate mediated suppression of TLR4 and TLR9 pro-inflammatory signaling (Figure 5). GPR81 knockdown strongly augmented hepatic Pro-Il1β and Nlrp3, increased hepatocyte apoptosis and liver hemorrhage, and promoted mortality, highlighting a critical role for GPR81 in endogenous dampening of innate immune response (Figure 5). Of note, lactate treatment in the GPR81 knockdown background did not alter hepatic inflammation, hepatocyte apoptosis, or significantly rescue from lethality, providing evidence that the protective effect of lactate was strongly GPR81 dependent in vivo. Though the lactate concentrations involved in autocrine and paracrine loops remain unclear, the serum lactate concentrations in this hepatitis model are reported to be about 0.2 mM.31 GPR81 knockdown also increased the severity of severe acute pancreatitis induced by LPS and caerulein suggesting a significant endogenous role for GPR81 in dampening inflammation and limiting tissue injury in the pancreas as well (Figure 6). Lactate post-injury mitigated pancreatic inflammation and organ injury, including necrosis, in this severe model (Figure 6). This is especially interesting as lactate therapy has been found to be superior to saline in the management of severe acute pancreatitis and results in less systemic inflammation in a recent well-controlled clinical trial.32
Lactate as administered in these models is rapidly metabolized and it appears that transient engagement of GPR81 with lactate serum concentrations in the range of moderate exercise physiology as measured here at 3–4 mM are sufficient to suppress tissue macrophage NF-kB activation in the our models of fulminant hepatitis and severe acute hepatitis (Figure 7) without inducing measurable liver, muscle, or kidney injury (Supplementary Figure 4). Prolonged exposure to supra-physiologic concentrations of lactate can induce intracellular acidosis, which itself can augment NLRP3 mediated responses, and may account for the findings of increased inflammatory responses in prior studies in vitro.33 Additionally, prolonged elevations in serum lactate are often a marker of significant ischemic injury and any specific benefit of elevated serum lactate levels may be overshadowed in this setting by the many pro-inflammatory signals released by injured cells in ischemic tissue and by intracellular acidosis.
The role of lactate and GPR81 signaling in other innate immune effector cell types remains to be intensely investigated. Human neutrophils express GPR81 and human peripheral blood polymorphonuclear cells have minimal alterations of LPS induced pro-inflammatory gene transcription in response to 15mM lactate. (Supplementary Figure 9).
Collectively, these data show that lactate bolus therapy can engage GPR81 pathways and significantly reduce inflammation and tissue injury in two clinically relevant types of organ injury, specifically acute pancreatitis and hepatitis. The identification of regulators of inflammation with therapeutic potential is a high priority because we already have immunosuppressive agents which can potently suppress the adaptive immune responses but none with high efficacy against inflammation. The ability of lactate to suppress inflammation in vivo, and the large amount of data on the intravenous administration of lactate in humans, means that lactate is uniquely positioned to be investigated as an anti-inflammatory and organ protective agent in a spectrum of inflammatory diseases. Unlike many other ligands for newly identified receptors, lactate is safe, inexpensive and widely available.
Supplementary Material
Acknowledgments
This work was supported by NIH R01DK076674-01A2 and VA Merit (WM), NIH K08DK092281 (RH), DK54021 (FG) and VA Merit (FG) and NIH P30DK34989.
Nonstandard abbreviations used
- GPR81
G-protein coupled receptor 81
- TLR
TOLL-like receptor
- NLRP3
NACHT, LRR, and pyrin domain– containing protein 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
Study concept and design: R. Hoque, F. Gorelick, W. Mehal; data acquisition: R. Hoque, A. Farooq, A. Ghani, and H. Said; data analysis: R. Hoque, A. Farooq; drafting the manuscript: R. Hoque, W. Mehal, F. Gorelick; critical analysis: W. Mehal, F. Gorelick; material support- R. Hoque, W. Mehal; study supervision: R. Hoque, W. Mehal
References
Author names in bold designate shared co-first authorship.
- 1.Gross O, Thomas CJ, Guarda G, et al. The inflammasome: an integrated view. Immunol Rev. 243:136–51. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 2.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 143:1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 141:358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–14. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 33:333–42. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu M, Wang H, Ding A, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 7.Lamphier MS, Sirois CM, Verma A, et al. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci. 2006;1082:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]
- 8.Rock KL, Latz E, Ontiveros F, et al. The sterile inflammatory response. Annu Rev Immunol. 28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoque R, Malik AF, Gorelick F, et al. Sterile inflammatory response in acute pancreatitis. Pancreas. 41:353–7. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–9. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 11.Kamo N, Ke B, Ghaffari AA, et al. ASC/caspase-1/IL-1beta signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 58:351–62. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neill LA. A critical role for citrate metabolism in LPS signalling. Biochem J. 438:e5–6. doi: 10.1042/BJ20111386. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Garcia A, Monsalve E, Novellasdemunt L, et al. Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem. 286:19247–58. doi: 10.1074/jbc.M110.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cori CF, Shine WM. The Formation of Carbohydrate from Glycerophosphate in the Liver of the Rat. Science. 1935;82:134–5. doi: 10.1126/science.82.2119.134-a. [DOI] [PubMed] [Google Scholar]
- 15.Cai TQ, Ren N, Jin L, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008;377:987–91. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–62. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Defea K. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol. 2008;153(Suppl 1):S298–309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Jiang W, Spinetti T, et al. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity. 38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge H, Weiszmann J, Reagan JD, et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res. 2008;49:797–803. doi: 10.1194/jlr.M700513-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Shime H, Yabu M, Akazawa T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–83. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 22.Samuvel DJ, Sundararaj KP, Nareika A, et al. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476–84. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nareika A, He L, Game BA, et al. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289:E534–42. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak CA, Liu CL, Shelton J, et al. Identification of Hydroxybenzoic Acids as Selective Lactate Receptor (GPR81) Agonists with Antilipolytic Effects. Acs Medicinal Chemistry Letters. 2012;3:637–639. doi: 10.1021/ml3000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magness ST, Jijon H, Van Houten Fisher N, et al. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–70. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 26.Hoque R, Sohail MA, Salhanick S, et al. P2×7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 302:G1171–9. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 493:346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 28.Gangloff M. Different dimerisation mode for TLR4 upon endosomal acidification? Trends Biochem Sci. 37:92–8. doi: 10.1016/j.tibs.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beneke R, Hutler M, Jung M, et al. Modeling the blood lactate kinetics at maximal short-term exercise conditions in children, adolescents, and adults. J Appl Physiol. 2005;99:499–504. doi: 10.1152/japplphysiol.00062.2005. [DOI] [PubMed] [Google Scholar]
- 30.Phua J, Koay ES, Lee KH. Lactate, procalcitonin, and amino-terminal pro-B-type natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock. 2008;29:328–33. doi: 10.1097/SHK.0b013e318150716b. [DOI] [PubMed] [Google Scholar]
- 31.Feng B, Wu S, Lv S, et al. Metabolic profiling analysis of a D-galactosamine/lipopolysaccharide-induced mouse model of fulminant hepatic failure. J Proteome Res. 2007;6:2161–7. doi: 10.1021/pr0606326. [DOI] [PubMed] [Google Scholar]
- 32.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–717 e1. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Rajamaki K, Nordstrom T, Nurmi K, et al. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem. 2013;288:13410–9. doi: 10.1074/jbc.M112.426254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


