Abstract
Convergent research suggests that childhood poverty is associated with perturbation in the stress response system. This might extend to aberrations in the connectivity of large-scale brain networks, which subserve key cognitive and emotional functions. Resting-state brain activity was measured in adults with a documented history of childhood poverty (n=26) and matched controls from middle-income families (n=26). Participants also underwent a standard laboratory social stress test and provided saliva samples for cortisol assay. Childhood poverty was associated with reduced default mode network (DMN) connectivity. This, in turn, was associated with higher cortisol levels in anticipation of social stress. These results suggest a possible brain basis for exaggerated stress sensitivity in low-income individuals. Alterations in DMN may be associated with less efficient cognitive processing or greater risk for development of stress-related psychopathology among individuals who experienced the adversity of chronic childhood poverty.
INTRODUCTION
Childhood poverty is a pervasive problem, with nearly one in four children in the United States currently living below the poverty line (Addy et al, 2011). Children in poverty are subjected to higher levels of multiple psychological and physical stressors, including family disruption, unresponsive parenting, community violence, and exposure to harsh physical environments (for a review, see Evans, 2004). These early-life stressors are associated with poorer physical and mental health in adulthood (Matthews and Gallo, 2011; Shonkoff et al, 2009). However, the effects of early childhood poverty on adult brain functioning have not yet been elucidated.
Growing evidence suggests that poverty impacts multiple biological mechanisms, including hypothalamic–pituitary–adrenal (HPA) axis function (Evans et al, 2012; Miller et al, 2009). One method for assessing HPA axis function is the Trier Social Stress Test (TSST), a standard social challenge protocol that reliably activates the HPA axis leading to a robust increase in glucocorticoid levels including cortisol, the hormonal end-product of the HPA axis (Kirschbaum et al, 1993). Low socioeconomic status (SES) is associated with greater cortisol increase (or cortisol reactivity) in response to TSST in boys (Hackman et al, 2012; Harkness et al, 2011). Lower income is also associated with greater cortisol reactivity among children in response to other laboratory stressors or challenges such as the cold pressor task and neuropsychological testing batteries (Blair et al, 2005; Gump et al, 2009). Thus, childhood poverty may increase the risk for physiological reactivity to stress.
In addition to conferring risk for HPA axis abnormalities, low SES in early life may disrupt functioning in individual brain regions associated with emotion and threat processing, potentially through abnormally elevated glucocorticoid levels. In rodents, chronic stress affects the morphology of the hippocampus, amygdala, and prefrontal cortex in a manner that is consistent with heightened stress sensitivity (see McEwen and Gianaros, 2011). Similar effects on brain structure may also be present in humans. Lower childhood SES has been linked to reduced hippocampal volume both during childhood (Hanson et al, 2011) and into adulthood (Staff et al, 2012). Lower early-life SES is also associated with reduced anterior cingulate cortex (ACC) and caudate volume in adults (Cohen et al, 2006). The effects of SES have also been demonstrated in brain activation studies. In children, low SES is associated with greater reactivity to emotion-related stimuli in amygdala, dorsal medial prefrontal cortex (dmPFC), and right middle frontal gyrus (Muscatell et al, 2012; Sheridan et al, 2012). In adults, low childhood SES is linked to reduced activation in insula, fusiform gyrus, ACC, posterior cingulate cortex (PCC), caudate, pons, and hippocampus (Silverman et al, 2009). Furthermore, lower perceived social standing correlates with both reduced gray-matter volume in perigenual ACC (Gianaros et al, 2007) and increased amygdala reactivity to social threat-related stimuli (Gianaros et al, 2008). Thus, low childhood SES may confer lasting effects on brain structure and function. However, these studies lack clarity about whether such cortical alterations could underlie elevated stress reactivity among persons growing up in poverty.
While prior research has emphasized the contribution of individual brain regions (eg, hippocampus and amygdala) affected by childhood poverty, there is a paucity of data on the functional connectivity of large-scale networks, which is the focus of the current report. There is growing recognition that higher order cognitive and emotional functions depend on networks of distributed brain regions, rather than single regions in isolation. Large-scale intrinsic connectivity networks are distributed, functionally coherent regions that are identified by connectivity methods. These networks interact to coordinate complex behavior and cognitive function, and disruptions in these networks can serve as neural signatures of stress-related psychopathology (Menon, 2011). Some of the key brain networks for which SES may affect functional connectivity are the default mode network (DMN; which includes dmPFC, hippocampus, and PCC) (Menon, 2011; Seeley et al, 2007; Spreng et al, 2009) and the salience network (SN; which includes dorsal ACC, amygdala, and anterior insula). Preliminary evidence linking SES to network function has emerged from reports that perceptions of relative social status may relate to DMN connectivity (Muscatell et al, 2012), and that childhood maltreatment may be associated with intrinsic connectivity network abnormalities in adulthood (Elton et al, 2014; Wang et al, 2013). However, no previous studies have investigated the link between large-scale networks and childhood poverty, which may have long-term effects that are separable from those related to childhood maltreatment. Though childhood maltreatment is more common among families living in poverty (Sedlak et al, 2010), childhood poverty and the multiple factors associated with it (eg, environmental stress, nutrition, parenting styles, and schooling) may have long-lasting effects on brain function, even in the absence of childhood maltreatment.
In turn, the connectivity of these large-scale networks is associated with HPA axis function. For example, SN connectivity is related to greater HPA axis responsivity to aversive stimuli, and pharmacologically dampening adrenergic activity reduces functional connectivity strength (Hermans et al, 2011). Since previous studies of low-income children and adults have reported aberrant activity in brain regions associated with DMN and SN, we hypothesized that a history of childhood poverty would affect DMN and SN functional connectivity in adulthood. Since investigations with this population have also reported alterations in stress response, and since stress response and HPA axis function are linked to large-scale network function, we also hypothesized that individual stress response to TSST challenge would be associated with alterations in DMN and SN. A focus on these two networks was also motivated by the fact that stressors related to SES (including perceived social status and childhood maltreatment) relate to alterations in DMN and SN (Elton et al, 2014; Muscatell et al, 2012). To investigate whether childhood poverty is prospectively associated with adult neural function, independent of current adult income, we controlled for adult income in all our analyses.
MATERIALS AND METHODS
Participants
The participants for this study were part of a longitudinal study of rural poverty and child development. The original sample (mean age=9.18 years) was recruited in rural counties in upstate New York using records from public schools, the Cooperative Extension System of the US Department of Agriculture, the federal Head Start program, subsidized housing, and other antipoverty programs. Fifty-two of these participants with no MRI contraindications (eg, metallic/ferrous materials in their body), no prior or current treatment for any psychiatric disorder (as assessed via clinician-conducted psychiatric evaluation based on the Structured Clinical Interview for DSM-IV (First et al, 2002), and no current neurological condition were enrolled. We selected participants without any history of psychiatric treatment to more precisely study the effects of childhood poverty on adult brain function, without the potential confounds associated with psychiatric illness. Twenty-six of the participants were from low-income backgrounds at age 9 and 26 were from families with incomes of two to four times the poverty line. Demographic information can be found in Table 1. Details about the participants are reported in Supplementary Methods in the Supplementary Material available online. This study was approved by the University of Michigan and Cornell University Institutional Review Boards and was carried out in accordance with the provisions of the World Medical Association Declaration of Helsinki.
Table 1. Demographic Characteristics of Participants.
| Characteristic | Low income (n=26) | Middle income (n=26) | t/χ2 | p |
|---|---|---|---|---|
| Mean age (SD) | 24.4 (1.1) | 23.1 (1.2) | 4.04 | <0.001 |
| Sex (male/female) | 14/12 | 15/11 | 0.08 | 0.78 |
| Race | 1AA, 22C, 3O | 1AA, 25C | 3.19 | 0.20 |
| Marital status (married/single) | 23/3 | 9/17 | 15.9 | <0.001 |
| Education (years) | 14.2 (2.4) | 17.3 (3.3) | 3.53 | <0.001 |
| Age 9 income to needs ratio | 0.78 (0.36) | 2.6 (0.71) | 11.5 | <0.001 |
| Current income to needs ratio | 1.9 (1.9) | 5.3 (4.3) | 3.39 | 0.002 |
| PSS | 19.2 (7.0) | 16.3 (6.7) | 1.52 | 0.14 |
| ABCL anxiety/depression subscale | 0.42 (0.35) | 0.39 (0.26) | 0.31 | 0.76 |
Abbreviations: AA, African American; ABCL, Achenbach Adult Behavior Checklist; C, Caucasian; O, Other; PSS, perceived stress scale.
Trier Social Stress Test
The TSST is a social evaluative threat task that reliably activates the HPA axis and causes a robust increase in serum glucocorticoid concentration (Kirschbaum et al, 1993). It combines a mock job application speech with a cognitively challenging mental arithmetic task, performed in front of an audience structured to create high demand characteristics and low feedback. Further details can be found in Supplementary Methods.
Salivary Cortisol
To measure salivary cortisol, participants were asked to provide saliva samples immediately before the TSST, immediately after the TSST, 15 min after the TSST, and 1.5 h after the TSST. The TSST lasted 15 min; thus, the second saliva sample was collected 15 min after the first sample. Salivary cortisol levels were determined by chemiluminescent enzyme immunoassay (IMMULITE) according to the manufacturer's directions (Siemens Healthcare Diagnostics, Tarrytown, NY).
Self-Report Measures
Participants were administered the Perceived Stress Scale, a 10-item measure of the degree to which situations in one's life are appraised as stressful (Cohen et al, 1983). Items are rated on a scale of 0 (‘Never') to 4 (‘Very Often'). The Perceived Stress Scale has good internal consistency and test–retest reliability (Cohen et al, 1983), and is a widely used measure of subjective stress.
MRI Data
Participants underwent structural (sMRI) and functional (fMRI) scanning that included emotion regulation tasks (Kim et al, 2013; Sripada et al, 2013) and resting-state procedures. MRI scanning occurred on a Philips 3.0 Tesla Achieva X-series MRI (Philips Medical Systems) using a standard 8-channel SENSE head coil. A standard series of processing steps was performed using statistical parametric mapping (SPM8; www.fil.ion.ucl.ac.uk/spm). Additional information about fMRI acquisition and processing parameters can be found in the Supplementary Methods.
Data Analysis
Based on a meta-analysis of DMN studies (Spreng et al, 2009), a 5-mm-radius sphere seed region was placed in the PCC (MNI: −7,−51,31). For SN, a 5-mm-radius sphere was placed in dorsal ACC/supplementary motor area (SMA) (MNI: 8,12,50). This point represents the peak of the cluster with highest negative Z-value obtained from the DMN connectivity map in the supergroup of low- and middle-income participants combined, and was selected to provide maximal contrast with DMN connectivity. Functional connectivity analysis was performed using the ConnTool package developed by author RCW. We extracted the spatially averaged time series from PCC and dorsal ACC/SMA (dACC/SMA) seeds for each participant. Next, linear detrending was performed, followed by nuisance regression. Covariates of no interest included six motion regressors generated from the realignment step noted above, as well as their first derivatives. In addition, we included five principal components of the BOLD time series extracted from white-matter and cerebrospinal fluid masks, which has been demonstrated to effectively remove signals derived from the cardiac and respiratory cycle (Behzadi et al, 2007). In generating the white-matter and cerebrospinal fluid regressors, subject-specific masks were first created using VBM-based segmentation implemented in SPM8. To eliminate border regions of potentially ambiguous tissue-type, white-matter and CSF masks were eroded with an FSL tool (fslmaths) to ensure that no gray matter was included in the masks. The erosion was performed by removing the outermost layer of voxels (ie, any 1 voxel touching a 0 voxel in the mask). The white-matter mask was eroded two times, while the CSF mask was only eroded once. A spatially averaged time series was extracted from these masks, and the first five principal components were included in the regression. The residuals from this regression were then retained for further analysis. Since resting-state functional connectivity measures low-frequency spontaneous BOLD oscillations (0.01–0.10 Hz band) (Fox et al, 2005), the time course for each voxel was band-pass filtered in this range. Next, motion scrubbing was performed. During motion scrubbing, individual frames with excessive head motion were censored from the time series, following Power et al (2012). The target high motion frame, but not those flanking it, was removed in accordance with the findings of Satterthwaite et al (2013). Participants with >60% of their frames removed by scrubbing were excluded from further analysis, as justified by the findings of Fair et al (2012). Additional information can be found in the Supplementary Methods. Pearson product-moment correlation coefficients were calculated between average time courses in the seed regions of interest (ROIs) and all other voxels of the brain, resulting in a 3-dimensional correlation coefficient image (r-image). These r-images were then transformed to z-scores using the Fisher r-to-z transformation.
Whole Brain and ROI Analysis
Z-score images from the individual activation maps were entered into second-level random-effect analyses implemented in SPM8. Low- and middle childhood-income groups were compared via independent samples t-tests, with age and subject-specific motion added as covariates. Current income was significantly greater in the childhood middle-income group than the childhood low-income group (t=3.39, p=0.002); thus, current income was also added as a covariate. Single-group maps were corrected for multiple comparisons using whole-brain family-wise error correction, p<0.001. For the two-group comparisons, we additionally implemented an ROI analysis with small volume correction (SVC) with a priori brain areas identified as key components of DMN (PCC, hippocampus, and vmPFC) and SN (anterior insula, amygdala, and SMA) (Menon, 2011; Spreng et al, 2009). Additional information can be found in the Supplementary Methods. To assess the relationship between large-scale network effects and stress reactivity, functional connectivity beta values were extracted from each region of group difference at a threshold of p<0.01 and correlated with cortisol measurements. Betas were extracted from PCC (k=174), left hippocampus (k=189), and vmPFC (k=434). Correlations were implemented in SPSS, using two-tailed t-tests at a statistical threshold of p<0.05.
RESULTS
Motion and Physiological Variables
There were no movements greater than 3 mm or 3 degrees and no motion differences between low and mid-income groups in mean displacement (p>0.5), mean angle (p>0.5), max displacement (p>0.07), or max angle (p>0.6). There were no group differences in heart rate (p>0.5) or respiration (p>0.3).
FMRI Results
Results of single group t-tests can be found in Supplementary Tables S1 and S2. PCC seed connectivity was consistent with published work on DMN (Menon, 2011; Qin and Northoff, 2011; Spreng et al, 2009; Toro et al, 2008), and dACC/SMA seed connectivity was consistent with SN (Menon, 2011; Seeley et al, 2007).
DMN Connectivity
Controlling for age and current income, the childhood (age 9) low-income group demonstrated weaker DMN connectivity. Compared with middle-income participants, low-income participants showed reduced PCC to hippocampus connectivity (see Table 2; Figure 1). Childhood income was positively correlated with PCC to hippocampus connectivity, PCC to PCC connectivity, and PCC to vmPFC connectivity. Furthermore, the proportion of life lived in poverty between birth and age 9 was negatively correlated with PCC to hippocampus connectivity, PCC to PCC connectivity, and PCC to vmPFC connectivity. None of these regions showed a significant effect in relation to current income.
Table 2. Two-Group Comparisons and Correlations with Childhood Income.
| Contrast map and brain region | Cluster size | MNI coordinates | Analysis (z) |
|---|---|---|---|
| Default mode network (PCC seed) | |||
| Middle income>Low income | |||
| Left hippocampus | 68 | −22 −26 −10 | 2.83 |
| Positive correlations with childhood income | |||
| PCC | 174 | −2 −48 20 | 3.73 |
| Left hippocampus | 189 | −22 −22 −10 | 3.84 |
| vmPFC | 434 | −8 56 −8 | 3.17 |
| Salience network (dACC/SMA seed) | |||
| Negative correlations with childhood income | |||
| SMA | 426 | −12 −4 52 | 3.51 |
Abbreviations: MNI, Montreal Neurologic Institute; PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
Significance set at p<0.05, corrected for multiple comparisons within region of interest.
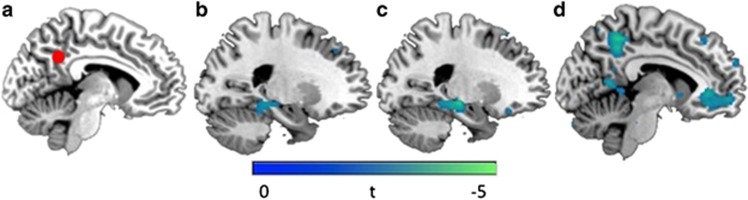
Figure 1.
Compared with childhood middle-income participants, childhood low-income participants showed reduced connectivity between (a) PCC seed and (b) left hippocampus (x=−22). Childhood income was positively correlated with (c) PCC to left hippocampus connectivity (x=−20), (d) PCC to PCC connectivity, and PCC to vmPFC connectivity (x=−7). Images presented at p<0.05, corrected for multiple comparisons within region of interest. PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
SN Connectivity
There were no differences in SN connectivity between low- and middle-income groups. Childhood income was negatively correlated with dACC/SMA to SMA connectivity (see Table 2).
Correlations with Cortisol Response to TSST
Overall, there were no group differences found in cortisol response to TSST, suggesting a comparable level of stress axis activation in low- and middle-income participants on a group level. However, pre-TSST cortisol levels were greater in the childhood low-income group than in the middle-income group (t=3.14, p=0.003). Collapsing across groups, lower connectivity within PCC was associated with higher pre-TSST cortisol (r=−0.299, p=0.049), indicating that reduced within-DMN coupling was associated with higher cortisol in anticipation of social stress.
DISCUSSION
In this study, we investigated the effects of childhood poverty on adult resting-state functional brain connectivity and HPA axis reactivity. Individuals from low-income backgrounds demonstrated reduced DMN connectivity, while demonstrating comparable peripheral level HPA axis response to social stress. These correlates of childhood poverty were independent of adult income levels, suggesting that early financial status may relate to brain function in adulthood, irrespective of subsequent social and economic mobility. Reduced DMN was in turn associated with higher cortisol levels in anticipation of social stress. Our results identify altered connectivity in large-scale networks as a possible mechanism of exaggerated stress sensitivity in individuals growing up in poverty.
Our results suggest that childhood poverty is associated with reduced network connectivity in the DMN, a network associated with stimulus-independent, internally focused thought, including spontaneous cognition, autobiographical memory, prospection, and mind-wandering (Menon, 2011; Qin and Northoff, 2011; Spreng et al, 2009; Toro et al, 2008). Specifically, we found that childhood income predicted reduced connectivity within PCC, reduced connectivity between PCC and hippocampus, and reduced connectivity between PCC and vmPFC. Though few researchers have investigated the relationship between SES and PCC, one study showed that low SES was associated with reduced PCC activation to positive stimuli (Silverman et al, 2009). In addition to reduced connectivity within PCC, individuals with a history of childhood poverty showed reduced DMN connectivity to vmPFC. Previous reports indicate that that lower perceived social standing correlates with reduced gray-matter volume in ACC/vmPFC (Gianaros et al, 2007), and that low childhood SES is linked to reduced activation in ACC/vmPFC in adulthood (Silverman et al, 2009). Thus, individuals from low-SES backgrounds may recruit these regions to a lesser extent across a variety of contexts.
We also found reduced connectivity in the low-income group between PCC and hippocampus. Hippocampal abnormalities have been robustly linked to SES. For instance, one study reported that low childhood SES predicted smaller hippocampal size in a sample of 249 older adults, after controlling for gender, education, mental ability at age 11, and adult SES (Staff et al, 2012). Another study noted that financial hardship (but not childhood poverty) reduced hippocampal volume in middle-aged adults (Butterworth et al, 2012). Decreased connectivity between hippocampus and PCC has also been associated with heightened anxiety in individuals with posttraumatic stress disorder (Chen and Etkin, 2013; Sripada et al, 2012). However, one study of healthy adolescents found that reduced connectivity between hippocampus and DMN was associated with reduced anxiety and cortisol output in the scanner, as well as reduced cortisol during TSST (Thomason et al, 2013). In our sample, reduced functional connectivity within PCC was associated with greater cortisol output in anticipation of social stress, potentially indicating that reduced coupling within DMN is related to greater HPA reactivity. Discrepancies in findings may be due to differences in methodology, or potentially may be partially attributable to maturational changes in DMN structure across the lifespan. Functional integration of DMN increases with age, which might explain divergent results in adolescent and adult samples (Fair et al, 2008).
Though few studies have investigated the relation between DMN and SES, one study reported that perceptions of social status related to DMN activity while encoding social information, such that greater DMN activity was related to lower perceived social status (Muscatell et al, 2012). Thus, the relation between DMN and SES may depend on the task being performed and on the way SES is assessed. Future studies are needed to further illuminate this link. DMN is typically active during task-free contexts such as the resting state (Menon, 2011), and we have previously reported that reduced DMN connectivity during rest is associated with heightened anxiety (Sripada et al, 2012). Thus, reduced resting-state DMN connectivity in adults from low-income backgrounds may represent a risk factor for the development of stress sensitivity and anxiety psychopathology
In the current study, we also investigated the relation between childhood poverty and cortisol response to social stress, as assessed via the TSST. While the childhood low-income group demonstrated greater cortisol levels immediately before TSST, there were no significant differences in TSST-induced stress activation between low- and middle-income groups. Though our finding of comparable HPA activation across groups may seem counterintuitive, in fact, collective TSST investigations demonstrate a stronger link between SES and cortisol reactivity in childhood than in adulthood. In children, neighborhood disadvantage is associated with greater cortisol reactivity during TSST (Hackman et al, 2012; Harkness et al, 2011). Lower income is also associated with greater cortisol reactivity to lab stressors (Blair et al, 2005; Gump et al, 2009). In adults, one study found that low early-life SES was associated with increased daily cortisol output in adulthood (Miller et al, 2009). However, the collected findings on cortisol and adult SES are inconsistent. SES may have no effect on or may in fact diminish cortisol reactivity to stress in adults (Dowd et al, 2009). This mirrors our own finding that cortisol response to TSST did not vary systematically as a function of childhood income. We did, however, find that cortisol levels immediately before TSST were greater in individuals with a history of childhood poverty. Thus, it is possible that individuals with a history of poverty show greater HPA activation in anticipation of stress or in response to novel experimental contexts, rather than in response to acute social stress.
Contrary to our hypothesis, we did not find connectivity differences in SN. Previous findings suggest that low SES is associated with reduced activation in the insula and ACC (Silverman et al, 2009). In addition, healthy adults with a history of adverse childhood events exhibit reduced ACC volume (Cohen et al, 2006). Thus, we predicted that individuals from low-income backgrounds would exhibit alterations in SN connectivity. However, our results did not support this hypothesis. Of note, we also did not find connectivity differences with the amygdala, an SN region of particular interest. Previous research indicates SES-related differences in amygdala morphology and activation. Financial hardship reduces amygdala volume in middle-aged adults (Butterworth et al, 2012), and lower socioeconomic position (as measured by subjective parental socioeconomic position) is associated with increased amygdala reactivity to angry/threatening facial expressions in young adulthood (Gianaros et al, 2008). Based on these previous studies, we hypothesized, though ultimately did not find, that low-SES individuals would show aberrant amygdala functional connectivity. One potential reason for these negative findings is that the resting state may not be the best probe of SN function. The SN is active during homeostatic regulation, interoceptive, autonomic, and reward processing (Cauda et al, 2011; Dosenbach et al, 2007; Seeley et al, 2007; Sridharan et al, 2008), thus differential SN functioning may not be as easy to discern during rest. Indeed, the active fMRI tasks from the parent study have revealed amygdala differences between groups (Kim et al, 2013), suggesting that certain tasks may be more effective in eliciting differential activation in SN than the task-free resting state. Additionally, previous studies revealing amygdala differences, such as Gianaros et al, 2008, used retrospective measures of perceived social inequality, whereas we used prospective measures of income. These methodological differences may also have contributed to the differences in obtained results.
Though we found evidence of DMN and HPA axis differences between low- and middle-income groups, it is important to note that the observed findings need not represent pathology, and in fact could be adaptive for low-SES individuals. This is posited by Chen and Miller's ‘Shift-and-Persist' model, in which the ‘shift' involves adjusting to the environment through cognitive reappraisal of stressors and through emotion regulation, and ‘persist' involves maintaining fortitude by holding onto hopes for the future (Chen and Miller, 2012a). Among a sample of 1207 adults, those with low-SES childhoods who scored high on shift-and-persist strategies had the lowest allostatic load, calculated as the sum of seven physiological system risk indices (sympathetic nervous system, parasympathetic nervous system, HPA axis, cardiovascular, glucose metabolism, lipid metabolism, and inflammation). This strategy had no benefit for adults with high-SES childhoods (Chen et al, 2012b). Thus, it is possible that the observed alterations in DMN connectivity or HPA axis reactivity could in fact reflect the physiological correlates of a shift-and-persist strategy. For instance, greater cortisol release in anticipation of social stress could reflect the ‘shift' strategy of controlling and adjusting the self when dealing with stress, and thus could be considered as a coping mechanism in this setting. More longitudinal studies are needed to investigate this hypothesis.
The present results should be interpreted in light of the following limitations. Our sample size was relatively small, thus our results require replication. Many years transpired between childhood poverty and the subsequent measurements of brain function and stress reactivity. It is difficult to ascertain whether the reported between-group differences are due to childhood poverty alone or were also contributed to by other intervening influences that could have occurred differentially between the groups since childhood (eg, differing paths of education, marriage, and employment), or other intervening influences that could not be controlled for in this study (eg, incidences of childhood maltreatment, and environmental exposure during adolescence). For instance, childhood maltreatment occurs up to five times more frequently in low-SES families than in middle-SES families (Sedlak et al, 2010); however, it is still relatively uncommon (2.2% incidence) and unlikely to account for the reported findings. Controlling for differences in current income allowed us to control for present-day income-associated factors, but does not capture many of these developmental influences. Future work should explore underlying mechanisms that mediate the effects of childhood poverty on brain function and stress reactivity in adulthood.
In conclusion, we found that after controlling for current income, childhood poverty was associated with reduced within-DMN connectivity. These connectivity differences, in turn, were associated with higher cortisol levels in anticipation of social stress. Early childhood poverty may relate to alterations in resting brain function as well as greater peripheral stress reactivity. Future studies are needed to continue to elucidate the neural sequelae of childhood poverty to better understand its lasting neural effects and their relationship to functional outcomes.
FUNDING AND DISCLOSURE
The current study was supported by grants from the National Institute of Health (RC2MD004767) to JES, GWE, and IL, the W.T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health to GWE, and the Robert Wood Johnson Foundation to JES. RKS is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Veterans Affairs Ann Arbor Health Care System, and the Veterans Affairs Serious Mental Illness Treatment Resource and Evaluation Center (SMITREC). The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Addy S, Engelhardt W, Skinner C.2011. Basic Facts About Low-income Children http://www.nccp.org/publications/pub_1074.html National Center for Children in Poverty. Vol. 2013, pp1–8.
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Peters Razza R. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Dev. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Butterworth P, Cherbuin N, Sachdev P, Anstey KJ. The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc Cogn Affect Neurosci. 2012;7:548–556. doi: 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chen AC, Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacol. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE. ‘Shift-and-Persist' strategies: why being low in socioeconomic status isn't always bad for health. Perspect Psychol Sci. 2012;7:135–158. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosom Med. 2012;74:178–186. doi: 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Tripathi SP, Mletzko T, Young J, Cisler JM, James GA, et al. Childhood maltreatment is associated with a sex-dependent functional reorganization of a brain inhibitory control network. Hum Brain Mapp. 2014;35:1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Chen E, Miller GE, Seeman TE. How Poverty Gets under the Skin: A Lifecourse Perspective. Oxford University Press: New York; 2012. [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, JBW Williams. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometrics Research: New York; 2002. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Reihman J, Stewart P, Lonky E, Granger DA, Matthews KA. Blood lead (Pb) levels: further evidence for an environmental mechanism explaining the association between socioeconomic status and psychophysiological dysregulation in children. Health Psychol. 2009;28:614–620. doi: 10.1037/a0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between Income and the Hippocampus. PLoS ONE. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, et al. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60:1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network. Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, et al. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress. US Department of Health and Human Services, Administration for Children and Families: Washington, DC; 2010. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D'Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS ONE. 2012;7:e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Silverman ME, Muennig P, Liu X, Rosen Z, Goldstein MA. The impact of socioeconomic status on the neural substrates associated with pleasure. Open Neuroimag J. 2009;3:58–63. doi: 10.2174/1874440000903010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, et al. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2013;89:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71:653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Tocco MA, Quednau KA, Bedway AR, Carre JM. Idle behaviors of the hippocampus reflect endogenous cortisol levels in youth. J Am Acad Child Adolesc Psychiatry. 2013;52:642–652.e641. doi: 10.1016/j.jaac.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2013;35:1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.