Abstract
Designer TALEs (dTALEs) are chimeric transcription factors that can be engineered to regulate gene expression in mammalian cells. Whether dTALEs can block gene transcription downstream of signal transduction cascades, however, has yet to be fully explored. Here we tested whether dTALEs can be used to target genes whose expression is controlled by Wnt/β-catenin signaling. TALE DNA binding domains were engineered to recognize sequences adjacent to Wnt responsive enhancer elements (WREs) that control expression of axis inhibition protein 2 (AXIN2) and c-MYC (MYC). These custom DNA binding domains were linked to the mSin3A interaction domain (SID) to generate TALE-SID chimeric repressors. The TALE-SIDs repressed luciferase reporter activity, bound their genomic target sites, and repressed AXIN2 and MYC expression in HEK293 cells. We generated a novel HEK293 cell line to determine whether the TALE-SIDs could function downstream of oncogenic Wnt/β-catenin signaling. Treating these cells with doxycycline and tamoxifen stimulates nuclear accumulation of a stabilized form of β-catenin found in a subset of colorectal cancers. The TALE-SIDs repressed AXIN2 and MYC expression in these cells, which suggests that dTALEs could offer an effective therapeutic strategy for the treatment of colorectal cancer.
Keywords: TALE, SID, Wnt, β-Catenin, AXIN2, MYC
1. Introduction
Advances in genome-editing technologies have dramatically improved the ability to manipulate expression of endogenous genes in mammalian cell lines. One technology involves the use of transcription activator-like effectors (TALEs) from the plant pathogenic bacteria of the genus Xanthomonas [1]. TALEs contain a central DNA binding domain that can be engineered to recognize specific DNA sequences within the mammalian genome. Designer tales (dTALEs) tether transcriptional activation or repression domains to TALE DNA binding domains. Effective dTALEs that target distal enhancer elements, proximal promoter regions, non-coding DNA regions and exons have been described [2,3,4]. The mammalian mSin3A interaction domain (SID) has been shown to be an effective transcriptional repressor domain for use in dTALEs [2]. The SID, first characterized from studies of the Mad transcription repressor, is a small amphipathic alpha helix that recruits the mammalian mSin3A/HDAC corepressor complex [5,6]. Whether dTALEs can be used to modulate expression of genes downstream of signaling pathways is an area of open research.
The Wnt/β-catenin signaling pathway is a critical regulator of tissue homeostasis, cellular proliferation, and stem cell biology [7]. A central component of this pathway is the β-catenin transcription coactivator and its levels and sub-cellular localization are tightly regulated. In the absence of extracellular Wnt ligand, cytosolic ß-catenin associates with a multi-protein “destruction complex” that coordinates its phosphorylation and subsequent degradation by the proteasome. Under these conditions, T-cell factor transcription factors (TCFs) bound to Wnt responsive DNA elements (WREs) recruit transducin like enhancer (TLE) corepressor complexes to repress target gene expression [8]. In the presence of Wnt, the destruction complex is inactivated and β-catenin is translocated into the nucleus where it displaces TLE. β-Catenin/TCF complexes recruit additional chromatin modifying complexes to activate gene expression [8]. Mutations in components of the Wnt/β-catenin signaling pathway are found in approximately 90% of colorectal cancers (CRCs) [9]. These mutations cause accumulation of β-catenin in the nucleus and aberrant target gene expression.
AXIN2 and MYC are two well-characterized Wnt/β-catenin target genes [10,11,12,13,14]. AXIN2 is a component of the destruction complex and it thus serves in a negative feedback loop to control the duration of the Wnt response. The WREs that control AXIN2 expression map to the 5’ promoter and regions downstream of the transcription start site [11,12,13,15,16]. MYC is a transcription factor that primarily activates expression of genes whose products drive cellular proliferation [17]. The WREs that control MYC expression are proximal to gene boundaries and also map several hundred thousand kilobases away from the transcription start site [10,14,18,19].
Here, we describe the generation and characterization of three TALE-SID fusion proteins targeting known WREs that control AXIN2 and MYC gene expression. We demonstrate that the TALE-SIDs bind their targeted sequences and repress gene expression in HEK293 cells. Using a stable HEK293 system that mimics oncogenic Wnt/β-catenin signaling, we demonstrate that the TALE-SIDs also repress target gene expression in this setting. Together, these findings indicate that dTALEs can be used to modulate gene expression downstream of oncogenic Wnt/β-catenin signaling.
2. Materials and Methods
2.1 Cell Lines
The HEK293FT and Flp-In T-REx 293 cell lines were purchased from Invitrogen and maintained according to the manufacturer's guidelines.
2.2 Plasmids
The pGL3-basic and pGL3-promoter luciferase reporters were purchased from Promega, pME18-LEF was a gift from D. Ayer (University of Utah), and the YAP1 luciferase reporter and the pcDNA3-β-cateninS45F construct were previously described [20,21]. The TALEN plasmids that target AXIN2 were obtained from Addgene (deposited by Dr. Keith Joung). The MYC TALE DNA binding domain was constructed using the TALE assembly kit (Addgene, deposited by Dr. Keith Joung) following the detailed instructions provided. The TALE1 and TALE2 plasmids were generated by removing the FokI nuclease as a BamHI-AgeI restriction fragment, filling in the 5’ overhangs with Klenow polymerase and ligating the blunt ends. Four copies of the SID were PCR-amplified from pUC57-SID4X (Addgene, deposited by Dr. Feng Zhang) and the products were sub-cloned into BamHI-AgeI digested TALE plasmids to generate the TALE-SIDs.
The AXIN2 luciferase reporter plasmid was generated by PCR-amplifying a 787-bp fragment of the AXIN2 gene from genomic HCT116 DNA that includes the TALE binding sites. The PCR product was sub-cloned into the pGL3 basic vector as a KpnINheI fragment. To generate the pcDNA5/FRT/TO-β-cateninS45F-estrogen receptor (ER) expression plasmid, β-cateninS45F cDNA was PCR-amplified from pcDNA3-β-cateninS45F. The ER cDNA was amplified from pBabepuro-myc-ER (Addgene, deposited by Wafik El-Deiry). The resulting β-cateninS45F and ER PCR products were ligated and cloned into the pcDNA5/FRT/TO expression plasmid (Invitrogen) using the restriction enzymes BamHI, KpnI, and ApaI. The MYC luciferase transgene, which contains approximately 8.6-kb of genomic DNA that encompasses MYC, was generated stepwise with two PCR fragments amplified from a bacterial artificial chromosome (BAC) that harbors human MYC. The firefly luciferase gene was inserted in-frame into the second exon of MYC. See supplementary materials for additional details and oligonucleotide sequences used in plasmid construction.
2.3 Generation of β-cateninS45F-ER HEK293 cell line
These cells were generated using the Flp-In T-REx system (Invitrogen). Approximately 5×106 Flp-In T-REx 293 cells were transfected with 9 μg pOG44 (Invitrogen), encoding the Flp recombinase, and 1 μg pcDNA5/FRT/TO-β-cateninS45F ER using calcium phosphate transfection. After 48 h, the transfected cells were selected with 100 μg/ml hygromycin for two weeks. The resistant cells were pooled and maintained in media containing 100 μg/ml hygromycin and 15 μg/ml blasticidin. To induce β-cateninS45F-ER expression, the cells were treated with 1 μg/ml doxycycline (DOX) overnight. The following day, the cells were treated with 1 μM 4-hydroxytamoxifen (4-OHT) for 24 h to stimulate β-cateninS45F-ER translocation into the nucleus.
2.4 Co-immunoprecipitation
Immunoprecipitations were performed as previously described [22] on 5 Χ 106 HEK293 cells transfected with 5 μg of the indicated TALE plasmids. Samples were pre-cleared with Protein A beads and 6 μg rabbit anti-mouse IgG (Jackson ImmunoResearch, 315-005-003) for 1 h at 4°C. The samples were then were incubated with 3 μg anti-FLAG antibodies (Sigma, F1804) for 1 h at 4°C followed by an overnight incubation with 6 μg rabbit anti-mouse IgG at 4°C.
2.5 Cellular fractionation and Western blot analysis
Nuclear, cytoplasmic, and whole cell protein lysates were prepared as previously described [21]. Western blot analysis was performed as described [15] using the following primary antibodies: anti-HDAC1 (Abcam, ab7028, 1:1000), anti-FLAG (Sigma, F1804, 1:1000), anti-AXIN2 (Santa Cruz, sc-20784, 1:250), anti-α-tubulin (Sigma, T9026, 1:1000), and anti-histone H3 (Upstate, 06-755, 1:25,000).
2.6 Luciferase assays
Luciferase assays were conducted as described previously [14]. HEK293 cells were seeded in quadruplicate wells and transfected by calcium phosphate precipitation with each well receiving 2 ng pLRL-SV40 Renilla luciferase (Promega), 100 ng of firefly luciferase reporter plasmid, 150 ng TALE plasmid or pcDNA3 (Invitrogen), and pBlueScript SK+ (Agilent Technologies) to a final DNA concentration of 1 μg. Where indicated, transfection reactions included 50 ng pcDNA3-β-cateninS45F and 50 ng pME18-LEF.
2.7 Chromatin immunoprecipitation (ChIP)
ChIP was conducted as described previously [14,15] on 5×106 HEK293FT 48 h or 72 h after the cells were transfected with 5 μg pcDNA3 or the TALE-SIDs, using calcium phosphate. For β-cateninS45F-ER cells, ChIP was performed on 5×106 cells following treatment with or without DOX and 4-OHT. To precipitate the cross-linked chromatin, 3 μg of anti-FLAG (Sigma, F1804) or 5 μl of anti-histone H3K4me3 (Active Motif, 39159) antibodies were added to the samples. Oligonucleotide sequences for qPCR are available in supplementary materials.
2.8 Quantitative and real-time reverse-transcription PCR (qRT-PCR)
A total of 5×106 HEK293FT or β-cateninS45F-ER cells were transfected with 10 μg pcDNA3 or 5 μg each of TALE-SID plasmids. Total RNAs were isolated and cDNAs were synthesized 48 after transfection in HEK293FT cells or 72 h after transfection in β-cateninS45F-ER cells as previously described [20]. Transcripts were measured by qRT-PCR as described previously [15,20] and the data was analyzed using the 2-ΔΔCT method. Oligonucleotide sequences used for qRT-PCR are available in supplementary materials.
2.9 Immunohistochemistry
β-CateninS45F-ER expression was detected by staining fixed cells with anti-FLAG antibodies (Sigma, F1804, 1:1000) for 1 h at room temperature as previously described [20].
2.10 Statistics
Each experiment was repeated at least three times and statistical significance was calculated using the Student's t-test.
3. Results
3.1 Generation of designer TALEs that target AXIN2
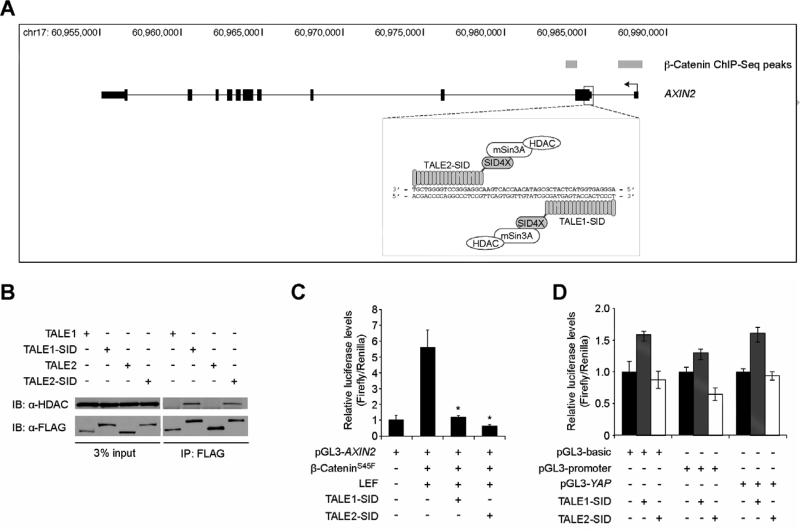
A common strategy to regulate expression of a gene of interest using designer TALEs is to target well-characterized DNA enhancer elements that control expression of that particular gene [2,3,4]. In this respect, AXIN2 is an inherently difficult gene to target because its expression is potentially controlled by numerous enhancer elements in response to Wnt/β-catenin signaling [11,12,15,16]. In a previous study, we mapped regions across the genome of a human colorectal cancer cell line that bound β-catenin using ChIP-Seq [15]. We identified two highly enriched β-catenin-bound regions that mapped to the AXIN2 transcription start site and first exon (Fig. 1A). We therefore reasoned that the region between these sites would serve as an appropriate target sequence for which to develop AXIN2-specific dTALEs. In addition, Reyon et. al. generated a pair of FLAG-tagged TALE nucleases (TALENs) that targeted the first exon of AXIN2, which is positioned between the two β-catenin binding regions [23]. We obtained these TALENs, and replaced the FokI nuclease domain with 4 copies of the SID. We refer to these AXIN2-specific dTALEs as TALE1-SID and TALE2-SID (Fig. 1A).
Fig. 1.
TALE-SIDs that bind AXIN2 associate with HDAC1 and repress AXIN2-luciferase reporter activity. (A) Schematic of the AXIN2 gene locus with the transcription start site on the right side of the figure. Grey rectangles indicate the ß-catenin-bound regions identified in a β-catenin ChIP-Seq screen [15]. A diagram of the TALE-SIDs is boxed below and shown is their target sequence within the first exon of AXIN2. (B) Western blot analysis of protein lysates prepared from HEK293 cells transfected with the indicated FLAG-tagged TALE constructs and immunoprecipitated with anti-FLAG antibodies. (C) Luciferase reporter assays in HEK293 cells transfected with a pGL3-basic plasmid that contains a 787-bp fragment of the AXIN2 gene encompassing the TALE binding sites inserted upstream of the luciferase gene. Where indicated, cells were co-transfected with plasmids encoding β-cateninS45F, LEF, TALE1- SID, and TALE2-SID. Error is SEM (* P < 0.05). (D) Luciferase reporter assays in HEK293 cells transfected with the indicated pGL3 luciferase plasmids and plasmids encoding TALE-SIDs.
3.2 The TALE-SIDs repress AXIN2 gene expression
Because the SID recruits the mSin3A/HDAC corepressor complex [5,6], we tested whether the TALE-SIDs interacted with endogenous HDAC1. Plasmids encoding TALE1-SID and TALE2-SID, or the TALE backbones as controls, were independently transfected into HEK293 cells and the expressed proteins were immunoprecipitated with anti-FLAG antibodies. In a Western blot analysis, we found that HDAC1 co-precipitated with TALE1- and TALE2-SIDs indicating that these chimeric proteins were capable of interacting with endogenous mSin3A/HDAC (Fig. 1B).
To determine whether the TALE-SIDs could regulate AXIN2 gene expression, we initially conducted a series of luciferase reporter assays. A 787-bp fragment of AXIN2 that contains the TALE-SID binding sites was inserted upstream of the luciferase gene in the pGL3-basic plasmid. This fragment also contains the TCF consensus-binding site, ATCAAAG. The AXIN2 reporter was transfected into HEK293 cells with and without plasmids that express human Lef1 (LEF) or a β-cateninS45F protein that contains a serine to phenylalanine substitution at amino acid 45 found in a subset of colon cancers [24]. β-CateninS45F and LEF activated expression of the pGL3-AXIN2 reporter relative to control (Fig. 1C). Co-transfection of plasmids encoding either TALE1-SID or TALE2-SID repressed ß-cateninS45F/LEF-dependent luciferase activity (Fig. 1C). In contrast, the TALE-SIDs only modestly affected luciferase gene expression in control experiments conducted with luciferase reporters that lacked the AXIN2 recognition element (Fig. 1D) [20].
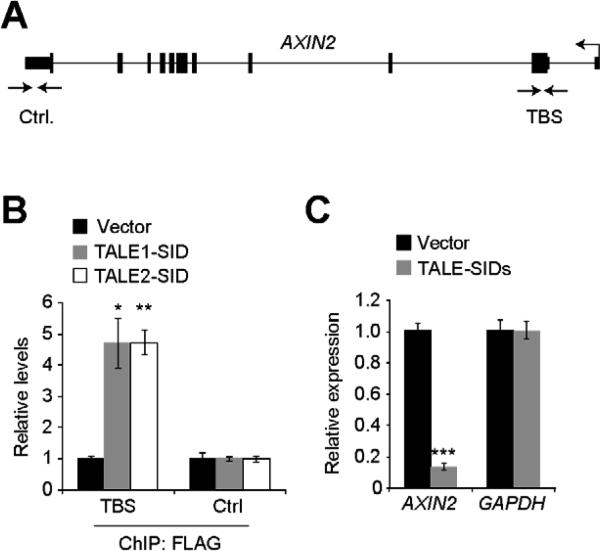
We next determined whether the TALE-SIDs could repress expression from the endogenous AXIN2 gene in HEK293 cells. First, we determined whether the TALE-SIDs bound their expected target site. We conducted ChIP assays with FLAG antibodies in transiently transfected cells and found that both TALE1-SID and TALE2-SID displayed enriched binding to their target sites within AXIN2 relative to a control site that mapped within the 3’ untranslated region (Fig. 2A, B). A qRT-PCR analysis of transcripts found that cells transfected with both TALE-SIDs contained 5-fold lower levels of AXIN2 expression relative to control (Fig. 2C). There was no difference in the levels of GAPDH transcripts in this experiment indicating that the TALE-repressors did not indiscriminately repress gene transcription. Together, these results indicate that the TALE-SIDs can access their target sites and repress AXIN2 expression from its chromosomal locus.
Fig. 2.
The TALE-SIDs repress AXIN2 gene expression. (A) Diagram of the AXIN2 gene locus with the positions of the primer sequences used in the ChIP assays indicated by the opposing arrows. TBS; TALE binding site, Ctrl.; control. (B) ChIP analysis using anti-FLAG antibodies of HEK293 cells transfected with plasmids encoding FLAG-tagged TALE1-SID, TALE2-SID, or pcDNA3 as a control. The precipitated DNA was measured using qPCR analysis with AXIN2-specific oligonucleotides. (C) Analysis of AXIN2 and GAPDH transcript levels in HEK293 cells transfected with the TALE-SIDs or pcDNA3 as a control. In (B) and (C) error is SEM (* P < 0.05, ** P < 0.01, *** P < 0.001).
3.3 The TALE-SIDs repress AXIN2 expression induced by oncogenic β-catenin
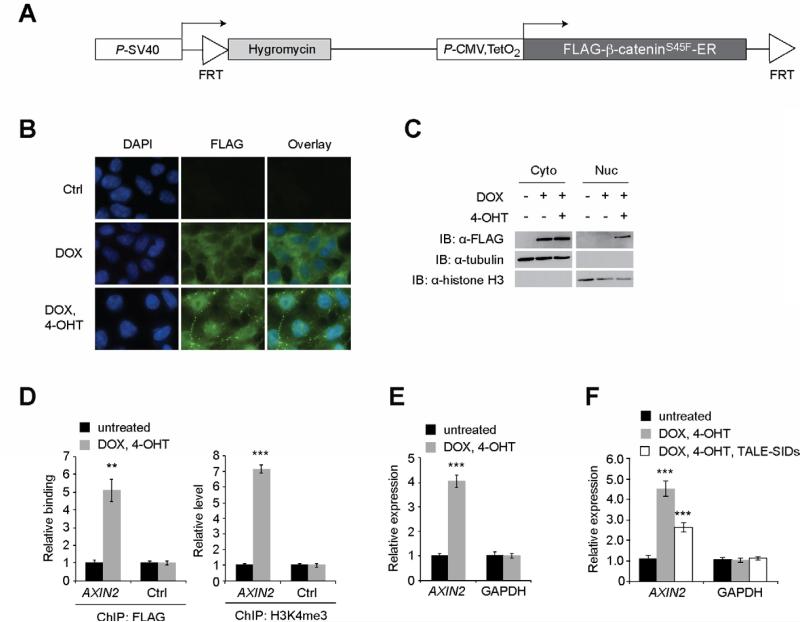
Due to the difficulties of transfecting human CRC cell lines at high efficiency, we established a clonal HEK293 cell line that expresses β-cateninS45F in a doxycycline (DOX)- and tamoxifen (4-OHT)-dependent manner. Using a homologous recombination-based system (see methods), we inserted a cDNA into a single chromosomal locus that encodes a FLAG-tagged β-cateninS45F-estrogen receptor (ER) fusion protein downstream of a CMV promoter under control of two tetracycline operator sequences (Fig. 3A). Treating these cells with DOX induces β-cateninS45F-ER expression; however, the ER sequesters it in the cytoplasm (Figs. 3B, C). When cells are treated with DOX and 4-OHT, 4-OHT binds the ER receptor causing a conformational change and subsequent translocation of β-cateninS45F into the nucleus (Figs. 3B, C). Using FLAG antibodies in ChIP assays, we found that nuclear β-cateninS45F bound the AXIN2 gene promoter and induced levels of trimethylated histone H3 on lysine 4 (H3K4me3), which is a histone modification that correlates with actively transcribed genes (Fig. 3D) [25]. Nuclear accumulation of β-cateninS45F also increased expression of AXIN2 indicating that the β-cateninS45F-ER fusion protein was functioning appropriately (Fig. 3E). Finally, we found that transfection of the TALE-SIDs reduced AXIN2 mRNA levels in these cells indicating that they are capable of repressing AXIN2 expression driven by oncogenic β-catenin (Fig. 3F).
Fig. 3.
The TALE-SIDs repress AXIN2 expression induced by oncogenic β-catenin. (A) Diagram of the transgene inserted into Flp-In T-REx HEK293 cells. FRT; Flprecombinase recognition target. TetO2; Two copies of the tetracycline regulated operator sequence. (B) Indirect immunofluorescence analysis of HEK293-β-cateninS45F ER cells that were treated with DOX or DOX and 4-OHT. β-CateninS45F-ER expression was detected using anti-FLAG antibodies. (C) Western blot analysis of protein lysates prepared from cytoplasmic (Cyto) and nuclear (Nuc) compartments of HEK293-β-cateninS45F-ER cells treated with DOX and 4-OHT as indicated. (D) ChIP analysis of FLAG-tagged β-cateninS45F-ER binding and H3K4me3 levels at the AXIN2 promoter, and the AXIN2 3’ UTR as a control, following the indicated treatments. (E) Analysis of AXIN2 and GAPDH expression following β-cateninS45F-ER induction and nuclear translocation. (F) As in (E) except cells were transfected with the TALE-SID plasmids where indicated. In (D), (E), and (F), error is SEM (** P < 0.01, *** P < 0.001).
3.4 Targeting MYC expression with a TALE-SID
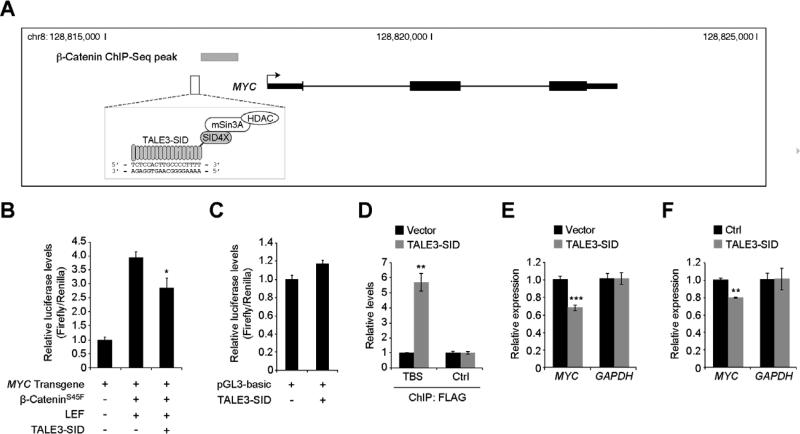
To determine whether this strategy can also be used to modulate expression of an additional Wnt/β-catenin target gene, we designed TALE3-SID which recognizes a sequence adjacent to the promoter proximal MYC 5’ WRE (Fig. 4A) [10]. TALE3-SID specifically repressed β-cateninS45F/LEF-regulation of a MYC-luciferase reporter transgene (Fig. 4B, C). In addition, using ChIP assays and qRT-PCR analysis, we found that TALE3-SID bound its target sequence and repressed MYC transcription in transiently transfected HEK293 cells (Fig. 4 D, E). Finally, TALE3-SID reduced levels of MYC expressed in HEK293 β-cateninS45F-ER cells that were treated with DOX and 4-OHT (Fig. 4F). These results indicate that as was the case for AXIN2, MYC gene expression can also be targeted using a custom dTALE.
Fig. 4.
A TALE-SID that targets MYC. (A) Diagram of the MYC genomic locus with a β-catenin ChIP-Seq peak represented by the gray rectangle and the TALE3-SID binding site indicated below. (B) Luciferase reporter assays conducted in HEK293 cells using a plasmid that contains a 8.6-kb MYC transgene with the firefly luciferase gene inserted into the MYC second exon. Where indicated, cells were co-transfected with plasmids encoding β-cateninS45F, LEF, and TALE3-SID. (C) Control luciferase reporter assays using the promoter-less pGL3-basic construct. (D) ChIP analysis using anti-FLAG antibodies of HEK293 cells transfected with plasmids encoding FLAG-tagged TALE3-SID, or pcDNA3 as a control. The precipitated DNA was measured using qPCR analysis and MYC-specific oligonucleotides that annealed to the TALE binding site (TBS) or a control region (Ctrl.). (E) Analysis of MYC and GAPDH transcripts in HEK293 cells transfected with TALE3-SID or pcDNA3 as a control. (F) As in (E) except HEK293 β-cateninS45F-ER cells were used that were treated with DOX and 4-OHT. In (B), (D), (E), and (F), error is SEM (* P < 0.05, ** P < 0.01, *** P < 0.001).
4. Discussion
TALE proteins can be engineered to bind specific sequences in the mammalian genome [1]. By linking the TALE DNA binding protein to well-characterized and portable transcription regulatory domains, designer TALEs can be used to specifically modulate target gene expression [2,3,4]. In agreement with Cong et. al., our findings here indicate that the SID is a potent and effective transcription repression domain that is suitable for use in mammalian systems [2].
One limitation of designer TALEs is that their efficacy can vary depending on whether they target promoter regions, enhancer elements or non-coding DNA regions. Cong et. al. found that TALE-SIDs that bound proximal promoter regions led to a 3-fold decrease and a 4-fold decrease in SOX2 and CACNA1C expression, respectively [2]. Our TALE-SIDs that targeted the first exon of AXIN2, resulted in a 5-fold decrease of AXIN2 expression in HEK293 cells. However, for MYC the TALE3-SID designed to target the proximal promoter caused only a 30% decrease in MYC gene expression in these cells. It is probable that the strategy of using multiple independent dTALES simultaneously would lead to greater target gene repression. Additional work is needed to optimize dTALE technology to achieve gene-silencing capabilities typically seen when using siRNA and shRNA-based systems.
Using a novel HEK293 cell line that we generated, we found that TALE-SIDs were capable of repressing AXIN2 and MYC expression that is induced by oncogenic β-catenin. However, in this system, the TALE-SIDs caused only a 2-fold decrease and a 20% decrease in AXIN2 and MYC expression, respectively. One possible explanation for this observation is that nuclear ß-catenin is binding several enhancer elements that have been shown to regulate AXIN2 and MYC and that the TALE-SIDs are unable to fully counteract this transcriptionally permissive state [10,11,12,13,14,15,16,19]. Future experiments will use the HEK293-β-cateninS45F-ER cell line and additional TALE-effector proteins such as those linked to the LSD-1 histone demethylase [26], to evaluate how epigenetic regulatory mechanisms and TALE proteins interface to regulate target gene expression.
In summary, our results support the versatility of TALE-based strategies to target genes whose expression is elevated by oncogenic Wnt/β-catenin signaling. With the advance of hierarchical ligation-based assembly methods and fast ligation-based automatable solid-phased (FLASH) systems, custom TALE DNA binding domains can readily and rapidly be designed to recognize virtually any DNA sequence in the mammalian genome [23,27]. Thus, TALE technology should be pursued as a potential therapeutic strategy for the treatment of diseases such as colorectal cancer.
Supplementary Material
Highlights.
We designed TALE-SID fusion proteins to target AXIN2 and MYC
TALE-SIDs bound the chromosomal AXIN2 and MYC genes and repressed their expression
TALE-SIDs repress β-cateninS45F-dependent AXIN2 and MYC transcription
Acknowledgements
We thank members of the Yochum laboratory for helpful discussions and this work was supported from funds provided by the NIH (R01DK080805, to G.S.Y).
Abbreviations
- TALE
transcription activator-like effector
- SID
mSin3A interaction domain
- AXIN2
axis inhibitor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bogdanove AJ, Voytas DF. TAL effectors: customizable proteins for DNA targeting. Science. 2011;333:1843–1846. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- 2.Cong L, Zhou R, Kuo YC, Cunniff M, Zhang F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat Commun. 2012;3:968. doi: 10.1038/ncomms1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao X, Yang J, Tsang JC, Ooi J, Wu D, Liu P. Reprogramming to Pluripotency Using Designer TALE Transcription Factors Targeting Enhancers. Stem Cell Reports. 2013;1:183–197. doi: 10.1016/j.stemcr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Xiang D, Heriyanto F, Gao Y, Qian Z, Wu WS. Dissecting the Roles of miR-302/367 Cluster in Cellular Reprogramming Using TALE-based Repressor and TALEN. Stem Cell Reports. 2013;1:218–225. doi: 10.1016/j.stemcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayer DE, Lawrence QA, Eisenman RN. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 6.Eilers AL, Billin AN, Liu J, Ayer DE. A 13-amino acid amphipathic alpha-helix is required for the functional interaction between the transcriptional repressor Mad1 and mSin3A. J Biol Chem. 1999;274:32750–32756. doi: 10.1074/jbc.274.46.32750. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 9.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 10.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 11.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 13.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yochum GS, Cleland R, Goodman RH. A genome-wide screen for beta-catenin binding sites identifies a downstream enhancer element that controls c-Myc gene expression. Mol Cell Biol. 2008;28:7368–7379. doi: 10.1128/MCB.00744-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bottomly D, Kyler SL, McWeeney SK, Yochum GS. Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res. 2010;38:5735–5745. doi: 10.1093/nar/gkq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, Versteeg R, Cuppen E, van de Wetering M, Clevers H, Stunnenberg HG. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28:2732–2744. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Tuupanen S, Turunen M, Lehtonen R, Hallikas O, Vanharanta S, Kivioja T, Bjorklund M, Wei G, Yan J, Niittymaki I, Mecklin JP, Jarvinen H, Ristimaki A, Di-Bernardo M, East P, Carvajal-Carmona L, Houlston RS, Tomlinson I, Palin K, Ukkonen E, Karhu A, Taipale J, Aaltonen LA. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet. 2009;41:885–890. doi: 10.1038/ng.406. [DOI] [PubMed] [Google Scholar]
- 19.Yochum Multiple Wnt/ beta-catenin responsive enhancers align with the MYC promoter through long-range chromatin loops. PLoS ONE. 2011:e18966. doi: 10.1371/journal.pone.0018966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konsavage WM, Jr., Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yochum GS, Cleland R, McWeeney S, Goodman RH. An antisense transcript induced by Wnt/beta-catenin signaling decreases E2F4. J Biol Chem. 2007;282:871–878. doi: 10.1074/jbc.M609391200. [DOI] [PubMed] [Google Scholar]
- 22.Yochum GS, Ayer DE. Pf1, a novel PHD zinc finger protein that links the TLE corepressor to the mSin3A-histone deacetylase complex. Mol Cell Biol. 2001;21:4110–4118. doi: 10.1128/MCB.21.13.4110-4118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 25.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 26.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013 doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.