Abstract
Steroid hormones are essential regulators of a vast number of physiological processes. The biosynthesis of these chemical messengers occurs in specialized steroidogenic tissues via a multi-step process that is catalyzed by members of the cytochrome P450 superfamily of monooxygenases and hydroxysteroid dehydrogenases. Though numerous signaling mediators, including cytokines and growth factors control steroidogenesis, trophic peptide hormones are the primary regulators of steroid hormone production. These peptide hormones activate a cAMP/cAMP-dependent kinase (PKA) signaling pathway, however, studies have shown that crosstalk between multiple signal transduction pathways and signaling molecules modulates optimal steroidogenic capacity. Sphingolipids such as ceramide, sphingosine, sphingosine-1-phosphate, sphingomyelin, and gangliosides have been shown to control the steroid hormone biosynthetic pathway at multiple levels, including regulating steroidogenic gene expression and activity as well as acting as second messengers in signaling cascades. In this review, we provide an overview of recent studies that have investigated the role of sphingolipids in adrenal, gonadal, and neural steroidogenesis.
Keywords: steroidogenesis, sphingolipids, CYP, sphingosine-1-phosphate, ceramide
Introduction
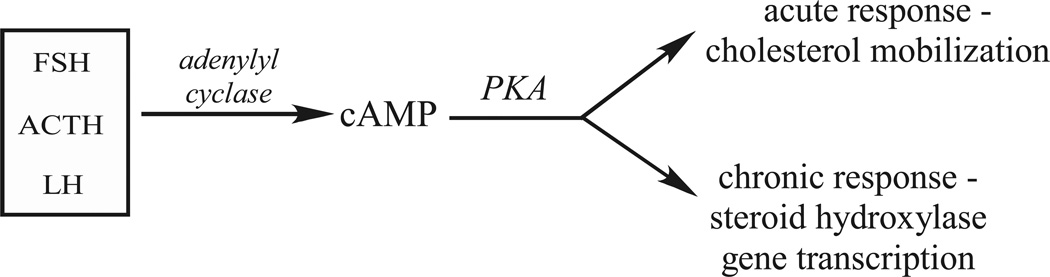
Steroid hormones like testosterone, progesterone, cortisol, aldosterone, and estradiol are important endocrine chemical messengers that are involved in a vast number of physiological processes including metabolism, inflammation, electrolyte and fluid balance, and secondary sex differentiation (Foster, 2004; Ghayee and Auchus, 2007; Newton and Holden, 2007; Williams-Ashman and Reddi, 1971). Steroid hormone synthesis occurs in the gonads, adrenal gland, placenta, intestines (Abdallah et al., 2004; Ghayee and Auchus, 2007; Mueller et al., 2007; Payne and Hales, 2004), and has recently been characterized in brain and peripheral nervous system tissues (Mellon et al., 2004; Mellon, 2007; Tsutsui et al., 2000). Biosynthesis is catalyzed by the sequential activities of cytochrome P450 monooxygenases and hydroxysteroid dehydrogenases that convert cholesterol into the many steroid hormones. Selective expression of these steroidogenic enzymes assures the production of steroid hormones in a tissue-specific manner. Activation of steroidogenesis is initiated by the binding of trophic peptide hormones – adrenocorticotrophin (ACTH), leutenizing hormone (LH), follicle stimulating hormone (FSH) - derived from the anterior pituitary to cognate receptors in target tissues which activates a cAMP/cAMP-dependent protein kinase (PKA) signaling pathway. Activation of cAMP-dependent signaling leads to a rapid increase in cholesterol mobilization and a chronic induction of steroidogenic gene transcription (Figure 1). Although this cAMP-dependent pathway is the main regulator of steroid hormone production, many other signaling systems involving many cytokines and sphingolipids have been reported to modulate steroidogenesis (Sewer et al., 2007).
Figure 1.
Temporal regulation of steroidogenesis by peptide hormones.
Sphingolipids, a family of lipids with a common sphingoid base backbone, have recently been identified as important bioactive molecules involved in a variety of cellular processes, including steroidogenesis (Ozbay et al., 2004; Urs et al., 2007; Ledeen and Wu, 2006; Spiegel and Milstein, 2007; Spiegel and Milstein, 2003b; Zheng et al., 2006). Sphingolipids such as ceramide (Cer), sphingosine (SPH), sphingosine-1-phosphate (S1P), sphingomyelin (SM), and gangliosides (GMs) have been shown to modulate the steroidogenic pathway at multiple levels including regulating steroidogenic gene expression and activity as well as acting as secondary messengers in signaling cascades. In this review, we will provide a summary of recent studies that have investigated the role of sphingolipids in steroid hormone biosynthesis, with special emphasis on the role of sphingolipids in adrenal, gonadal, and neural steroidogenesis.
1. Review of Steroidogenesis
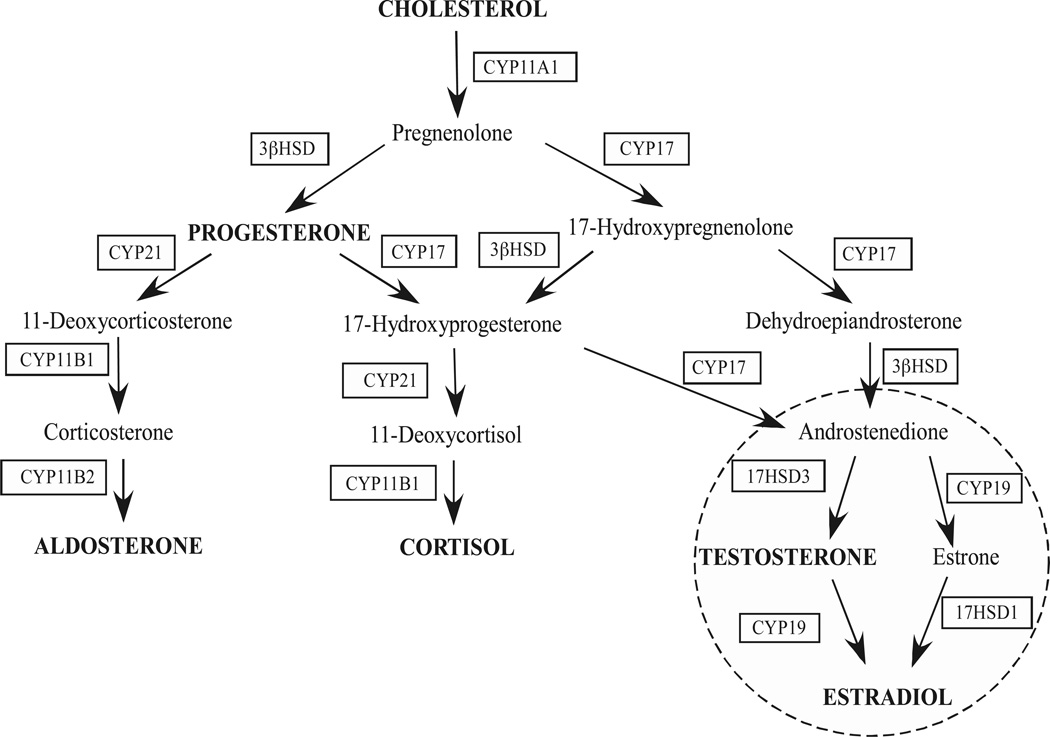
Steroidogenesis is a highly regulated biological process that is required for physiological homeostasis. This process takes place in specialized steroidogenic tissues where the biosynthesis of steroid hormones occurs in a highly synchronized manner via the coordinated activity of a series of steroidogenic enzymes. The primary steroidogenic tissues involved in the de novo steroid hormone biosynthesis include the gonads (ovaries and testis), the adrenal glands, and the placenta. Cholesterol, the substrate for the synthesis of all steroid hormones, is differentially metabolized into steroid hormones via the concerted action of enzymes localized in these steroidogenic centers (Figure 2).
Figure 2.
Steroid hormone biosynthetic pathways.
Activation of steroidogenesis is initiated upon binding of peptide trophic hormones to their cognate G protein-coupled receptors (GPCRs) in the target tissues. As shown in Figure 1, peptide hormones activate two temporally distinct phases of steroid production: a rapid acute response and a slower chronic phase. The acute phase of steroidogenesis involves activation of the steroidogenic acute regulatory protein (StAR) for rapid cholesterol mobilization from the outer to the inner mitochondrial membrane (Miller, 2007; Sewer and Waterman, 2001; Thomson, 1997). Because cholesterol transport to the inner mitochondrial membrane is the rate-limiting step in steroid hormone production, StAR is a key protein necessary for the acute response. In most steroidogenic tissues, StAR expression is mediated by cAMP; in addition, intracellular Ca2+ may also play a role in StAR transcription in the adrenal cortex. In addition to StAR, the peripheral benzodiazepine receptor (PBR) and hormone-sensitive lipase (HSL) are essential proteins for the intracellular transport and production of free cholesterol, respectively (Kraemer et al., 2004; Papadopoulos, 1993). In rodents, the scavenger receptor class B type I (SR-BI) plays a key role in the uptake of cholesterol esters from lipoproteins (Krieger, 1999). The de-esterification of cholesterol esters by HSL is essential for its transport into the mitochondria and therefore its utilization in steroidogenesis (Kraemer et al., 2004). Once cholesterol-laden vesicles are at the outer mitochondrial membrane, a large macromolecular complex containing StAR, PBR, and voltage-dependent anion channel (VDAC) (Hauet T et al., 2002; Liu J et al., 2003; Liu J et al., 2006; Miller, 2007) facilitate import of the substrate into the inner mitochondrial membrane.
The chronic phase of steroidogenesis involves the transcriptional activation of steroidogenic genes that are responsible for cholesterol metabolism. This occurs via activation of adenylyl cyclase and signaling through multiple signaling mechanisms, including a cAMP/PKA pathway, leading to the activation of varied downstream effectors that ultimately promote the binding of transcription factors to the promoters of steroidogenic genes (Arlt and Stewart, 2005; Bassett et al., 2004a; Bornstein et al., 2004; Condon et al., 2002; Jamnongjit and Hammes, 2006; Mendelson et al., 2005; Okamoto et al., 2004; Otis and Gallo-Payet, 2007; Sewer et al., 2007; Sewer and Waterman, 2003; Sirianni et al., 2003). One of the major transcription factors that regulates the expression of most steroidogenic genes in the adrenal gland and gonads of mammals is the nuclear receptor steroidogenic factor-1 (SF1/Ad4BP/NR5A1) (Lala et al., 1992; Morohashi et al., 1992; Parker et al., 2002). The ability of SF-1 to bind to target genes is regulated by post-translational modifications including phosphorylation and acetylation (Chen et al., 2004; Hammer et al., 1999; Ishihara and Morohashi, 2005). More recently, ligand binding has also been implicated in the regulation of SF1 activity (Ishihara and Morohashi, 2005; Krylova et al., 2005; Li et al., 2007; Li et al., 2005; Urs et al., 2006).
1.1 Adrenal steroidogenesis
The adrenal gland is divided into two regions: cortex and medulla. The medulla, which comprises about 10% of the gland, is made up of neuroendocrine cells that synthesize catecholamines. The adrenal cortex, on the other hand, comprises most of the adrenal gland and is the site of adrenal steroid hormone biosynthesis. This region can be further subdivided into 3 distinct zones, each with a characteristic steroidogenic profile: (1) the zona glomerulosa is the outer cortical zone where the mineralcorticoid aldosterone is produced. (2) The middle zone, zona fasciculata, makes glucocorticoids, (3) while the inner zone, zona reticularis, is the site of androgen biosynthesis. Each of the three cortical zones expresses a unique profile of steroidogenic genes, thereby allowing for zone-specific cholesterol metabolism (Bassett et al., 2004b; Rainey, 1999).
Adrenocortical steroid hormones have a vast array of biological functions. Cortisol, the primary human glucocorticoid, regulates the inflammatory response (Newton and Holden, 2007), carbohydrate and lipid metabolism, and stress response (Kassel and Herrlich, 2007). Aldosterone regulates blood pressure by modulating fluid and electrolyte balance (Brizuela et al., 2006; Foster, 2004). In the adrenal cortex, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione are the androgens produced (Havelock et al., 2004; Rainey et al., 2002).
In the zona fasciculata and reticularis of the adrenal cortex, steroidogenesis is mainly regulated by the binding of ACTH to the melanocortin-2 receptor, whereas in the zona glomerulosa, angiotensin II (AngII) directs aldosterone production by binding to angiotensin receptors. As shown in figure 2, there are two classes of steroidogenic enzymes whose transcription is activated in the chronic phase of steroid hormone production: the cytochrome P450 heme-containing proteins (CYPs) and hydroxysteroid dehydrogenase (HSD) enzymes (Payne and Hales, 2004; Sewer and Waterman, 2003). Additionally, the expression of StAR (Caron et al., 1997; Clark and Combs, 1999; Clem et al., 2005; Reinhart et al., 1999), PBR (Besman et al., 1989), HSL (Kraemer et al., 2004), SR-BI (Azhar et al., 1998), and adrenodoxin (Brentano and Miller, 1992; Chen and Waterman, 1992), the iron-sulfur electron transfer protein, are also induced by trophic hormone stimulation. The P450 side chain cleavage enzyme (encoded by CYP11A1) is an inner mitochondria membrane-bound enzyme that catalyzes the first enzymatic reaction in the synthesis of all steroid hormones: cleavage of free cholesterol into pregnenolone. P450c17α is encoded by CYP17 and is localized in the endoplasmic reticulum (ER). P450c17α catalyzes the hydroxylation of progesterone and pregnenolone at the carbon-17 and the conversion of pregnenolone to DHEA in the zona reticularis and the conversion of progesterone into androstenedione in the zona fasciculata. The microsomal 3β-hydroxysteroid dehydrogenase (3βHSD) catalyzes the conversion of pregnenolone, 17α-hydroxypregnenolone, and DHEA into progesterone, 17α-hydroxyprogesterone, and androstenedione, respectively. P45021 hydroxylase, encoded by CYP21, is also microsomal and catalyzes the conversion of progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively. P45011-β-hydroxylase (CYP11B1) is localized at the inner mitochondrial membrane in the zona fasciculata and converts 11-deoxycorticosterone or 11-deoxycortisol into corticosterone or cortisol. In the zona glomerulosa, aldosterone synthase (encoded by CYP11B2) is expressed in the inner mitochondrial membrane and catalyzes the conversion of 11-deoxycorticosterone into aldosterone. As previously discussed, the zone-specific expression of CYP11B1 and CYP17 in the zona fasciculata and reticularis and CYP11B2 in the zona glomerulosa allow for the differential steroid hormone biosynthesis.
1.2 Gonadal steroidogenesis
The primary sites of androgen and estrogen biosynthesis are the testis and ovaries. Analogous to adrenocortical steroidogenesis, tissue-specific steroidogenic gene expression accounts for differential production of sex hormones. Also, gonadal steroidogenesis occurs via the two above-mentioned temporally distinct phases (acute and chronic) that assures proper and controlled steroid hormone output. In the gonads, LH and FSH regulate acute and chronic steroidogenesis. LH and FSH activate an adenylyl-cyclase/cAMP-dependent pathway in the same manner as ACTH in the adrenal cortex (Jamnongjit and Hammes, 2006; Mendelson et al., 2005; Sewer and Waterman, 2003; Tajima et al., 2005). The steroid hormones produced in the gonads -testosterone, estradiol, and progesterone - function primarily in the control of secondary male and female sex characteristics and in embryogenesis.
As in the adrenal cortex, a specific pattern of steroidogenic enzymes expression allow for the production of the gonadal steroid hormones in a cell-specific manner. Some enzymes expressed in the adrenal are also equally expressed in the gonads: CYP11A1 and 3βHSD are expressed in the ovaries and testis and catalyzes the same reactions as in the adrenal cortex (Figure 2). CYP17 is also expressed in the Leydig cells of the testis and theca cells of the ovary but, in contrast to the 17α-hydroxylase reaction that is prevalent in the adrenal cortex, the lyase reaction predominates, resulting in the convertion of pregnenolone into androstenedione. Moreover, additional gonadal-specific enzymes, such as aromatase (CYP19) and 17α-HSD types 1 and 3 direct the production of gonadal-specific hormones. Testosterone biosynthesis is terminated in the Leydig cells of the testis by the activity of 17αHSD3, which catalyses the conversion of androstenedione to testosterone. In ovarian granulosa cells, aromatase catalyzes the conversion of androstenedione or testosterone into estrone or estradiol, respectively, while 17αHSD1 converts estrone to estradiol (Figure 2).
1.3 Neurosteroidogenesis
Adrenal and gonadal steroid hormones have long been known to regulate many important brain functions (Fuxe et al., 1981; McEwen, 1991). In addition, circulating steroids like progesterone, 11-deoxycorticosterone, and testosterone can be converted to neuroactive steroids within the brain (Mellon and Griffin, 2002). More recently, however, many laboratories have reported that nervous tissue is capable of expressing essential steroidogenic enzymes and therefore de novo synthesize steroid hormones, that are at least in part independent from classic steroidogenic tissues (Mellon and Griffin, 2002; Tsutsui et al., 2000). Moreover, the expression of StAR has also been detected in neural tissues (Lavaque et al., 2006; Sierra, 2004). Today, the term neurosteroid refers to both de novo synthesized steroids by nervous cells and circulating steroids that are subsequently converted to neuroactive forms within nervous tissues.
Neurosteroids appear to mainly function as neurotransmitters in a paracrine and autocrine fashion in the modulation of many brain functions including myelination, inhibition of neuronal toxicity and ischemia, behavioral aspects, and neuronal survival, growth, and differentiation (Griffin et al., 2004; Mellon et al., 2004; Mellon, 2007; Mukai et al., 2006). Such neurosteroids include progesterone, pregnenolone, allopregnanolone, DHEA, their sulfate esters, and 5α/5β-tetrahydroprogesterone, some of which were previously viewed as inactive metabolites or steroid precursors (Plassart-Schiess and Baulieu, 2001; Sakamoto et al., 2007).
Glial cells, oligodentrocytes and type I astrocytes, are considered the major neuronal steroidogenic cells (Tsutsui and Ukena, 1999). However, other cell types including Schwann cells, cerebellar Purkinje cells, and neurons have also been reported as capable of steroid hormone production (Mellon and Griffin, 2002; Plassart-Schiess and Baulieu, 2001). Purkinje cells, for example, are one of the major site of de novo progesterone and pregnenolone sulfate production (Tsutsui and Ukena, 1999).
The neurosteroidogenic pathway involves the same group of cytochrome P450 and HSDs enzymes as in classical steroidogenic tissues. Tissue-specific expression of a unique panel of steroidogenic enzymes also occurs in different brain regions and nerve cells (Mellon and Deschepper, 1993). However, some enzymes are expressed at higher levels in the brain than in other steroidogenic tissues and vary during development (Mellon et al., 2004). CYP11A1, 3β-HSD, and CYP17 are expressed in many brain regions including the cortex, cerebellum, and hypothalamus (Mellon and Griffin, 2002). These three brain regions also express CYP11B1 and CYP11B2 as well as StAR (reviewed in ref. (Mellon and Griffin, 2002)).
A significant amount of data relating the function of neurosteroids to nervous system development comes from the Niemann-Pick Type C-1 (NPC-1) knockout mouse model (Griffin et al., 2004; Mellon et al., 2004). This mouse has a mutation in the npc1 gene, which codes for a late endosomal membrane protein that is involved in the trafficking of cholesterol out of late endosomes for steroidogenesis (Blanchette-Mackie, 2000). Mutation of this gene leads to the accumulation of cholesterol and GMs in lysosomes and impaired neurosteroidogenesis (Mellon et al., 2004).
1.4 Other steroidogenic tissues
The liver, placenta, and small intestines have also been reported as steroidogenic centers where a selective set of cytochrome P450 and HSD enzymes are expressed. CYP17 is expressed in the rat liver and this expression fluctuates during development, indicating a pattern of expression exclusively tuned for the needs of this tissue (Vianello et al., 1997). Mueller et al., (Mueller et al., 2007) reported that murine intestinal epithelial cells are capable of producing glucocorticoids utilizing a differently regulated steroidogenic pathway than in adrenocortical cells. cAMP accumulation abrogates glucocorticoid production in this intestinal cells, illustrating a diversification of the classical steroidogenic pathway, which has likely evolved as a result of the different requirements of their environment. The placenta is an important steroidogenic tissue during pregnancy, which secretes human chorionic gonadotropin (hCG) hormone that stimulates progesterone production by the corpus luteum (Pepe and Albrecht, 1995). It has been reported that in addition to the corpus luteum, other nongonadal tissues including the kidney, lungs, pancreas and liver express hCG receptors (Abdallah et al., 2004). Although the role of these receptors is unknown, the expression of steroidogenic genes in these tissues suggests that placental hCG may mediate additional pleiotropic effects in the developing fetus.
2. Review of sphingolipids
Sphingolipids comprise a family of phospholipids and glycolipids that are characterized by the presence of a common sphingoid base (such as SPH) backbone. This family of lipids has a large structural diversity that allows for the existence of many structurally similar moieties yet with crucial differences in biochemical and biophysical properties. The role of this class of lipids in membrane structure is well-established (Goni and Alonso, 2006). Although the precise amount may vary considerably, as high as 30% of lipids that make up plasma membranes are sphingolipids, especially complex sphingolipids such as SM and glycosphingolipids (GSL) (Smith and Merrill, 2002). Furthermore, certain sphingolipids like SM, Cer, and glucosylceramides may aggregate to form higher order domains termed “lipid rafts”, which are believed to be important in cell signaling (Huwiler et al., 2000; Smith and Merrill, 2002; Tani et al., 2007). Significantly, a large body of data has established a role for sphingolipids as key bioactive molecules in a variety of biological processes (Brizuela et al., 2006; Budnik et al., 1999; Cuvillier et al., 1996; Degnan et al., 1996; Gomez-Munoz, 2006; Hannun, 1996; Kihara et al., 2007; Meroni et al., 2000; Rabano et al., 2003; Thon et al., 2005) (Table 1). These processes include cell differentiation, growth, apoptosis, cell-cell interaction, and mediation of signaling pathways and gene expression (Merrill et al., 1999; Zeidan and Hannun, 2007; Zheng et al., 2006). Roles for sphingolipids in vascular function (Lorenz et al., 2007), neurodegeneration (Tamboli et al., 2005), cancer [reviewed in ref. (Ogretmen, 2006)], autophagy (Lavieu et al., 2006; Lavieu et al., 2007), and insulin resistance (Adams et al., 2004; Turinsky et al., 1990) have also been reported, which illustrate the broad spectrum of cellular processes that can be directed or indirectly mediated by these bioactive lipid molecules. In addition, a series of recent studies examining the role of sphingolipids, primarily Cer and S1P, in adrenal and gonadal steroidogenesis (Brizuela et al., 2007; Brizuela et al., 2006; Budnik et al., 1999; Li et al., 2001; Meroni et al., 2000; Ozbay et al., 2006; Rabano et al., 2003) add to a rapidly expanding list of roles for sphingolipids in cellular processes.
Table 1.
Summary of recent data obtained for selected bioactive sphingolipids in steroidogenesis as well as their multiple downstream molecular targets and upstream molecular inducers.
| Sphingolipid | Role in steroidogenesis | Upstream inducers | Downstream targets |
|---|---|---|---|
| Cer | Suppress progesterone and testosterone production; attenuate StAR expression; regulate CYP17α activity; inhibit cAMP production; regulate 11β-HSD1 and CYP19arom activity. | TNF-α, Fas ligand, INF-γ, IL-1β, SMase | ERK1/2, SAPK, p38, c-Jun, caspases, CAPK, CAPP |
| S1P | Upregulate CYP17 and CYP19 expression; increase cortisol, estrogen, and aldosterone secretion; | SK1/2 activity. PKC, ERK2, and external S1PR ligands regulate SK activity. | ERK and PI3K/Akt pathways, PKC, PLD, PLC, S1PR ligand |
| SPH | Antagonist ligand | SF1 | |
| SM | Precursor of ceramide | Acid/neutral/alkaline ceramidase enzymatic activity | |
| GMs | Neurosteroidogenesis: degradation by allopregnanolone | allopregnanolone |
Abbreviations used: tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-1β (IL-1β), sphingosine kinase (SK), protein kinase C (PKC), sphingomyelinase (SMase), extracellular-regulated mitogen-activated protein kinase (ERK), stress-activated protein kinase (SAPK), ceramide activated protein kinase (CAPK), ceramide activated protein phosphatase (CAPP), mitogen activated kinase (MEK), phosphatidylinositol-3-kinase (PI3K), phospholipase C (PLC), phospholipase D (PLD), S1PR (S1P receptor).
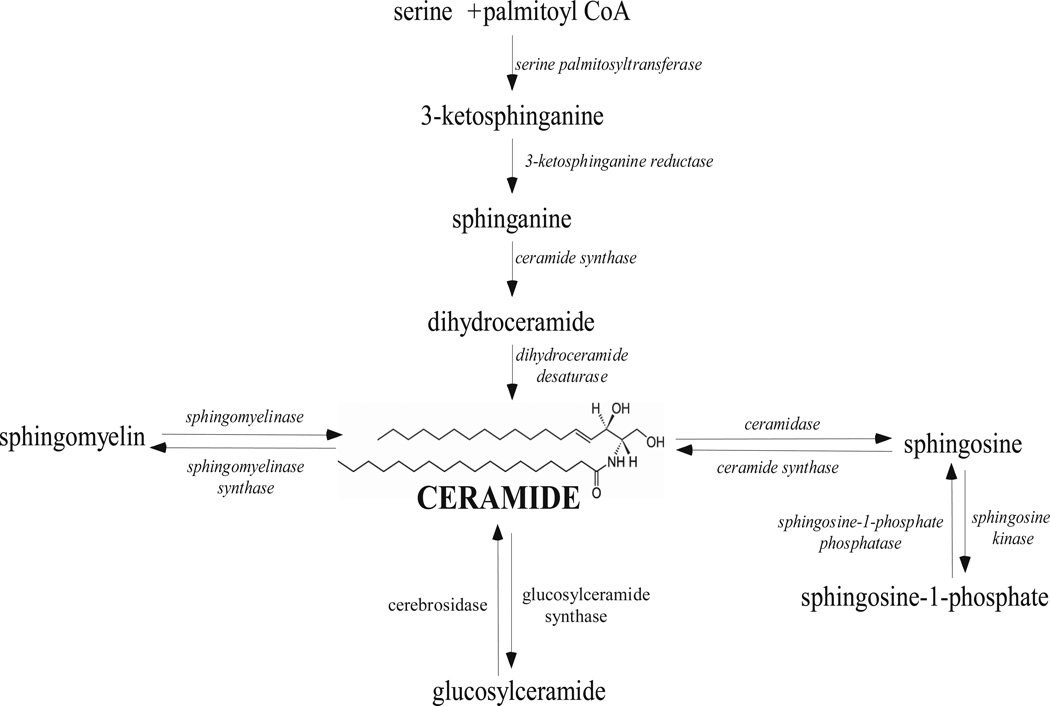
The simplest sphingolipids, SPH ((2S, 3R, 4E)-2-aminooctadec-4-ene-1,3-diol -Ser) and Cer (N-acylsphingosine) shown in Figure 3, constitute the basic structure of higher order sphingolipids like SM, cerebrosides, GMs, and other GSLs (Goni and Alonso, 2006). Complex sphingolipids are synthesized by Cer metabolism. Cer can be produced by the degradation of SM or by de novo biosynthesis. De novo Cer biosynthesis occurs from the condensation of serine and palmitoyl-CoA by the sequential action of the enzymes serine palmitoyltransferase, ketodihydro-sphingosine reductase, and ceramide synthase (Hanada et al., 2003) (Figure 3). SM hydrolysis is the major cellular source of Cer and it can be activated in response to a variety of extracellular signals, e.g. vitamin D3, cytokines, growth factors, and cytotoxic agents (Andrieu-Abadie and Levade, 2002; Huwiler et al., 2000; Okazaki et al., 1989). Alternatively, SPH formed as a product of complex sphingolipid metabolism can be recycled back into Cer via the scavenger pathway (Zeidan and Hannun, 2007).
Figure 3.
Overview of sphingolipid metabolic pathways.
The amounts of different sphingolipid moieties within a cell is controlled by multiple enzymes including sphingomyelinases (SMases) that hydrolyze SM into Cer; ceramidases that convert Cer into SPH and a free fatty acid; ceramide synthase which catalyzes the de novo synthesis of Cer from SPH; sphingomyelinase synthase that catalyses the production of SM; and sphingosine kinases (SKs) and ceramide kinases (Cerk) that phosphorylate SPH and Cer, respectively (Figure 3) (Huwiler et al., 2000; Maceyka et al., 2002; Maceyka et al., 2005; Pettus et al., 2003; Spiegel and Milstien, 2007). All of the above-mentioned enzymes play a central role in regulating the many bioactive sphingolipid types. Because the activity of these enzymes can be regulated by multiple signaling factors (Huwiler et al., 2000; Maceyka et al., 2002; Maceyka et al., 2005; Pettus et al., 2003; Spiegel and Milstien, 2007), the sphingolipid metabolic profile in any given cell at any given point in time is comprised of a unique set of bioactive sphingolipids. Therefore, the amounts and molecular species of the different sphingolipids are in a constant dynamic flux.
Of all known sphingolipid moieties, a large body of research has established physiological roles for SPH, Cer, S1P, and C1P (Adams et al., 2004; Andrieu-Abadie and Levade, 2002; Brizuela et al., 2007; Brizuela et al., 2006; Budnik et al., 1999; Hadizadeh et al., 2007; Kihara et al., 2007; Li et al., 2001; Meroni et al., 2000; Ozbay et al., 2006; Pettus et al., 2003; Rabano et al., 2003). Although these molecules are structurally similar and can be interconverted by one or two-step reactions, they have unique, and sometimes opposing, biological functions (reviewed in ref. (Kihara et al., 2007)). As shown in Figure 3, the dynamic balance of these sphingolipid metabolites is maintained by enzymes such as ceramidases, SKs, and S1P phosphatases whose activity is regulated by a variety of intra- and extracellular signals including TNF-α, Fas ligand, and cytokines (Cai et al., 1997; Cai et al., 2007; Hannun, 1996; Ruvolo et al., 2002; Sawai et al., 1997; Spiegel and Milstien, 2003a).
SM, the most abundant sphingolipid in mammalian cells, has also been implicated as a bioactive sphingolipid (Degnan et al., 1996; Ding et al., 2007; Porn et al., 1991). SMase activity is triggered by diverse stimuli, which results in the cleavage of membrane SM into free Cer. This process, now called the “sphingomyelin cycle”, is a receptor-mediated signaling system that plays an important role in regulating sphingolipids homeostasis (Andrieu-Abadie and Levade, 2002; Hannun, 1996; Ziulkoski et al., 2001). Cytokines such as TNF-α and interleukin 1β (IL-1β) have been found to activate SMase activity (Santana et al., 1996; Zeidan et al., 2006).
Cer, aside from the precursor of complex sphingolipids, is a second messenger molecule involved in a series of cellular events including differentiation, senescence, proliferation, mediation of stress response, cell cycle arrest, and apoptosis (Gomez-Munoz, 2006; Kolesnick, 2002; Pettus et al., 2002). Cytokines and fatty acids have been reported as mediators of intracellular ceramide production and subsequent activity (Lu et al., 2003; Osawa et al., 2005; Zeidan et al., 2006). Conversely, C1P, a major metabolite of Cer produced by Cerk-catalytic phosphorylation of Cer, has been identified as having antagonistic effects than Cer by being an inhibitor of apoptosis and a cell survival inducer (Gomez-Munoz et al., 2004). C1P has also been reported to play a role in inflammation and phagocytosis (Gomez-Munoz, 2006) and in arachidonic acid release (AA) and prostanoid production (Pettus et al., 2003). Since a role for AA in steroid hormone production stimulation has been found (Castilla et al., 2004), C1P may be a regulator of steroidogenesis.
Like Cer, SPH acts as a pro-apoptotic signal (Hung et al., 1999; Sakakura et al., 1998; Sweeney et al., 1998) as well as an inhibitor of protein kinase C (PKC) (Hannun et al., 1986), phospholipase D (PLD) (Natarajan et al., 1994), and calmodulin-dependent kinase (Jefferson and Schulman, 1988) in diverse cell types. SPH has also been shown to activate diacylglycerol (DAG) kinase (Yamada and Sakane, 1993). S1P is involved in cell survival and proliferation (Olivera et al., 1999; Olivera and Spiegel, 1993; Spiegel and Milstien, 2002). In contrast to Cer, which is membrane-bound, S1P can diffuse into the cytosol and be secreted into the extracellular space, where it can exert its signaling properties by binding to cell surface receptors (reviewed in ref. (Kihara et al., 2007)).
S1P is formed by phosphorylation of SPH by SKs. There are two isoforms of SK (1 and 2) whose function and subcellular location are distinct (Liu et al., 2003; Maceyka et al., 2005; Okada et al., 2005). SK2 prevents apoptosis and leads to increase in S1P metabolism back to ceramide (Spiegel and Milstien, 2007). SK1, however, appears to be a critical regulator in the intracellular amounts of S1P and its precursors SPH and Cer. Overexpresion of SK1 leads to accumulation of S1P and tumorgenesis (Le Stunff et al., 2007; Spiegel and Milstien, 2007). Therefore, there is evidence to suggest that these two SK isoforms may have distinct physiological functions and effect on sphingolipid metabolism. External ligands including acetylcholamine, prosaposin, lysophosphatidic acid (LPA), formylmethionine peptide, and even S1P itself have been reported as agonists of SK activity via GPCR signaling (Maceyka et al., 2002). Downstream targets of GPCR signaling are still under investigation, but PKC and ERK2 activation have been shown to regulate SK1 activity (Spiegel and Milstien, 2007) and may be important in regulating the dynamic balance of intracellular S1P, SPH, and Cer.
Given the opposite functions of Cer/SPH and S1P in cell viability, it has been proposed that the balance of these lipid mediators is a key factor in determining cell fate toward proliferation or apoptosis (Cuvillier et al., 1996). As a result of such important role in cell viability, these sphingolipid metabolites are under extensive investigation as potential anti-cancer targets (Mimeault, 2002; Pettus et al., 2002; Thon et al., 2005). Nonetheless, the dynamic balance of these sphingolipid molecules will also affect other biological processes including steroidogenesis.
3. The role of sphingolipids in steroid hormone production
As previously summarized, steroidogenesis involves the binding of the trophic hormones ACTH, LH, and FSH to their cognate receptors which lead to the subsequent activation of a series of cascade pathways (Figure 1), primarily the cAMP-dependent/PKA pathway leading to the activation of many downstream targets. Calcium is also important for maximal steroidogenesis (Gallo-Payet and Payet, 1989). Additionally, cAMP-independent signaling systems play an integral role in the regulation of steroidogenesis (Bornstein et al., 2004; Okamoto et al., 2004; Otis and Gallo-Payet, 2007). Interleukins (IL-3, IL-6, IL-1β, TNF-α) (Budnik et al., 1999; Hedger, 1997; Weber et al., 1997), calmidazolium (Choi and Cooke, 1992), steroidogenic-inducing protein (SIP) (Stocco and Khan, 1992), and chloride ions (Choi and Cooke, 1990; Gallo-Payet et al., 1999; Ramnath et al., 1997) are a few of the many regulators of steroid hormone production.
In addition, recent data suggests that sphingolipids can also act as secondary modulators of steroid hormone production. In the adrenal cortex, for example, ACTH/cAMP rapidly activates sphingolipid metabolism by decreasing intracellular amounts of SM, Cer, and SPH while increasing S1P production via SK activation (Ozbay et al., 2004; Ozbay et al., 2006). Bioactive sphingolipids have been identified as modulators of steroidogenesis by acting at different levels of the steroidogenic signaling pathway (Table 1). Some points of regulation include: (1) regulating cytochrome P450 gene expression, (2) serving as ligands for the major steroidogenic transcription factor SF-1, and (3) participating in secondary signaling cascades and second messenger systems. Some of these key finding are described below.
3.1 Ceramide
Current data suggests that the primary role of Cer in steroidogenesis is as a mediator in cytokine and growth factors signaling pathways, which untimely lead to a change in basal steroid hormone production (Arai et al., 2007; Budnik et al., 1999; Cai et al., 1997; Degnan et al., 1996; Meroni et al., 2000; Santana et al., 1995; Santana et al., 1996). TNF-α, Fas ligand, interferon-γ (INF-γ), and IL-1β modulate intracellular Cer concentrations via the activation of SMases (Cai et al., 1997; Hannun, 1996; Sawai et al., 1997). Of note, although some of the end point results of Cer accumulation in steroid production have been reported (Budnik et al., 1999; Cai et al., 1997; Degnan et al., 1996; Meroni et al., 2000; Santana et al., 1995; Santana et al., 1996), the precise molecular mechanism of action of Cer is still mostly unknown.
Cer has been shown to negatively regulate progesterone production in granulosa cells (Santana et al., 1996). The accumulation of intracellular Cer is a result of activation of SM hydrolysis by IL-1β. Similarly, TNF-α, which has many overlapping cellular effects with IL-1β, was shown to activate SMases and generate intracellular Cer in both MA-10 murine Leydig cells (Degnan et al., 1996) and Jeg-3 human choriocarcinoma cells (McClellan et al., 1997). Cer was also reported to suppress human choriogonadotropin (hCG)-stimulated testosterone production in rat Leydig cells (Meroni et al., 2000) and progesterone production in rat luteal cells in a dose-dependent manner (Li et al., 2001). Budnik et al. (Budnik et al., 1999) reported that regulation of progesterone biosynthesis by TNF-α/Cer signaling occurs via inhibition of StAR expression in MA-10 cells. Attenuation of StAR expression and reduced testosterone secretion by TNF-α and Cer was also seen in rat Leydig cells (Morales et al., 2003). It has been proposed that the apoptotic effect of TNF-α signaling is mediated via SM degradation into Cer (Mimeault, 2002; Thon et al., 2005). It is important to point out that the role of Cer in steroid hormone production is likely to be independent from the role of Cer in cell proliferation and/or apoptosis because in the majority of these studies, apoptosis was not a reported reason for the decrease in steroid hormone production (Son et al., 2004).
Cer can also modulate the activity and/or expression of steroidogenic enzymes. Meroni and colleagues have shown that Cer can regulate P450c17α enzymatic activity as well as inhibit cAMP production (Meroni et al., 2000). In another report, Cer was identified as a novel regulator of 11β-HSD1 in preadipocytes (Arai et al., 2007). Cer was observed to cause an increase in CCAAT/enhancer binding protein-β (C/EBPβ) recruitment to the 11β-HSD1 promoter, and in this way activate gene expression (Arai et al., 2007). Furthermore, Cer can inhibit hCG-induced aromatase activity and estradiol production in granulosa cells (Santana et al., 1995; Son et al., 2004).
Enzymes involved in Cer metabolism may also play a key role in steroidogenic regulation. Cortisol can activate the expression of the acid ceramidase gene (ASAH1) and promote Cer degradation (Lucki and Sewer, unpublished observations), thereby increasing the cellular concentrations of SPH, the antagonist for SF-1 (Urs et al., 2006). The regulation of ASAH1 by glucocorticoids may represent an intra-adrenal feedback mechanism that controls optimal steroid hormone output by repressing the ability of SF-1 to induce the transcription of steroidogenic genes.
3.2 Sphingosine
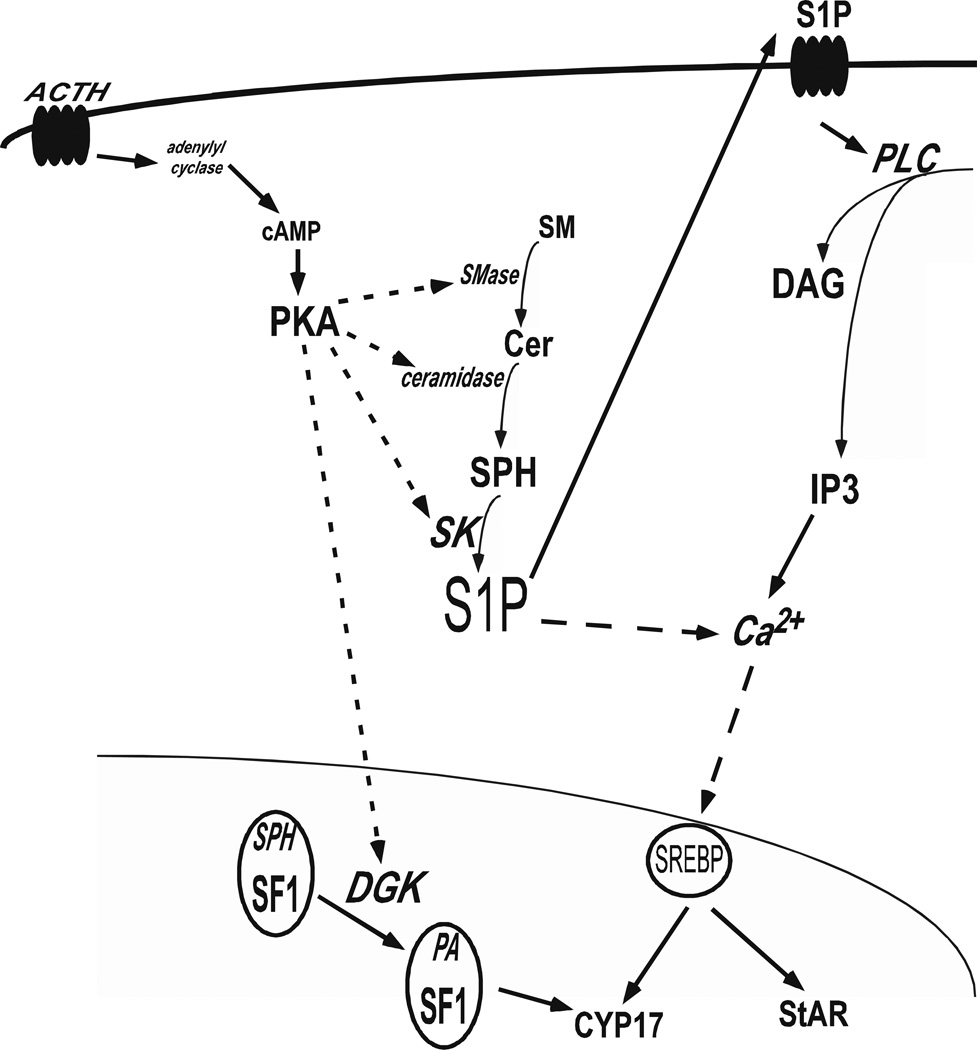
SF-1 was once classified as an orphan nuclear receptor because the identity of the endogenous ligand for the receptor was unknown. However, investigations of the endogenous receptor have identified SPH as a bonafide ligand (Urs et al., 2006) and recent crystallographic studies in bacterially expressed SF-1 confirmed that the ligand binding domain (LBD) of SF-1 is bound by phospholipids (Krylova et al., 2005; Li et al., 2005; Wang et al., 2005). SPH is an antagonist for SF-1 and its binding decreases CYP17 expression (Urs et al., 2006). cAMP stimulation reverses the antagonistic action of SPH possibly by displacing SPH from the SF-1 ligand binding pocket and promoting PA binding (Figure 4). Notably, we have also demonstrated that phosphatidic acid (PA) is an endogenous activating ligand for SF1 in H295R adrenocortical cells (Li et al., 2007). The identification of SPH as a ligand for SF-1 adds yet another level of regulation for sphingolipids in controlling steroid hormone production. Moreover, since SF-1 Also regulates the expression of genes involved in endocrine development and sex differentiation, it is possible that SPH may also control these processes by antagonizing SF-1.
Figure 4.
Relationship between sphingolipid metabolism and steroid hormone biosynthesis in the human adrenal cortex.
3.3 Sphingosine-1-Phosphate
As previously mentioned, S1P is an amphiphilic molecule that can exert its signaling functions both within the cell and extracellularly by binding to specific G protein-coupled receptors (S1PRs) (Kihara et al., 2007). There are 5 S1PRs and each couples to multiple heterotrimeric G protein leading to the activation of specific intracellular targets (An et al., 1997; Im et al., 2000; Lee et al., 1998; Van Brocklyn et al., 2000; Yamazaki et al., 2000). S1PR1 couples to Gi and activates the phospholipase C (PLC), phosphatidylinositol 3-kinase (PI3K), and extracellular regulated kinase (ERK) pathways. S1PR2 and S1PR3 couple to Gi, G12/13, and Gq and activate pathways such as PLC, PI3K, ERK, and Rho. S1PR4 activates the PLC and ERK pathways, while S1P5 has been shown to inhibit adenylyl cyclase and ERK (reviewed in ref. (Kihara et al., 2007). Binding of S1P to its cognate receptor seems to be the major mode of signaling and the PI3K/Akt and ERK pathways have been identified as downstream targets (An et al., 1997; Payne et al., 2004; Spiegel and Milstien, 2002; Spiegel and Milstien, 2003b). Previous reports have found a link between PI3K and ERK activation and steroidogenesis, which supports a role for S1P in steroid hormone production (Meroni et al., 2002; Shah et al., 2005).
As depicted in Figure 4, our laboratory has demonstrated that cAMP-mediated S1P accumulation lead to an increase in CYP17 expression by activating cleavage of the sterol regulatory element binding protein 1 (SREBP1) in H295R human adrenocortical cells (Ozbay et al., 2006). In the zona fasciculata of bovine adrenal cells, S1P was demonstrated to illicit cortisol secretion via binding to a S1PR and subsequent activation of PKC and PLD (Rabano et al., 2003). In addition, Brizuela et al. (Brizuela et al., 2006) reported that S1P stimulate aldosterone secretion in bovine glomerulosa adrenal cells. This aldosterone modulation is mediated by S1P binding to its cognate S1PR and activating the PLD/phosphatidate phosphohydrolase pathway with PKC and extraceullar Ca2+ as possible components of the signaling cascade.
Interestingly, we have also found that SK1 is rapidly translocated to the nucleus of H295R human adrenocortical cells in response to ACTH/cAMP stimulation (Li et al., unpublished observations). Since SPH inhibits steroidogenesis by antagonizing SF-1, it is likely that nuclear import of SK1 facilitates conversion of SPH to S1P and acts to promote activated gene steroidogenic gene transcription. Given the identification of sphingolipids in the nucleus (Ledeen and Wu, 2006), future investigation into the significance of these molecules in nuclear function is likely to reveal novel roles for these bioactive lipids in controlling diverse cellular processes.
Akt and ERK 1/2 have also been identified as direct S1P targets (Brizuela et al., 2007). The finding that inhibiting PI3K and MEK prevents S1P-mediated aldosterone secretion led Brizuela et al. to propose a model for S1P in the regulation of aldosterone production in which stimulation of PI3K/Akt and MEK/ERK pathways activate PLD and ultimately result in aldosterone production. S1P was identified as an inducer of CYP19 expression and estrogen production in granulosa cells by mediating the production of prostaglandin-2 (PGE2), which is an established activator of CYP19 (Cai et al., 1997). Given that S1P induces the expression of liver receptor homologue-1 (LRH-1) (Hadizadeh et al., 2007) and LRH-1 regulates CYP19 expression in breast cancer cells (Clyne et al., 2002), it is likely that estradiol production in both physiological and pathophysiological conditions is controlled by the amounts of S1P.
3.4 Sphingomyelin
SM is present in the plasma membrane and can be hydrolyzed into Cer via the action of SMases. SM hydrolysis is mediated by a series of stimuli including TNF-α, IL-β1, and Fas ligands (Degnan et al., 1996; Hannun, 1996), which, as described above, activate ceramide production. Therefore, SM seems to be a target for a series of external signals that modulate, among other cellular processes, steroidogenesis. S1P and C1P have been reported to repress acid SMase and may perhaps be part of a negative feedback loop that regulates flux through the sphingolipid metabolic pathway (Gomez-Munoz et al., 2003; Gomez-Munoz et al., 2004).
In mouse Leydig testicular cells, SM degradation was shown to be correlated with cholesterol movement from the plasma membrane to the mitochondria and subsequent increase in steroid hormone secretion (Porn et al., 1991). Conversely, SMase activity was shown to inhibit Leydig cell function via degradation of SM and accumulation of the pro-apoptotic sphingolipid Cer (Degnan et al., 1996). In addition, our laboratory has found that lysoSM (sphingosylphosphorylcholine) is able to bind to SF-1 in H295R adrenocortical cells under basal conditions, and that cAMP treatment promotes dissociation of the sphingolipid from SF1 (Urs et al., 2006). Given that different sphingolipid species are in a dynamic balance at any given point within a cell, SM can play an important role in steroidogenesis regulation by serving as a precursor for Cer, lysoSM, and S1P.
3.5 Glycosphingolipids (gangliosides)
Thus far, the roles of bioactive sphingolipids in the modulation of steroidogenesis have been discussed. However, an equally important aspect of the relationship between sphingolipids and steroid hormones involves the regulation of sphingolipid metabolism by steroid hormones. This concept is exemplified in neurosteroidogenesis between the complex sphingolipids, GMs, and the steroid hormone allopregnanolone (Griffin et al., 2004; Mellon et al., 2004). Mellon et al. have demonstrated that allopregnanolone treatment reduces GM accumulation and ameliorates neurodegeneration in the NPC-1 mouse model (Mellon, 2007). Even though the precise molecular mechanism by which these molecules regulate NPC progression is not unclear, these findings indicate the intimate relationship between steroid hormone biosynthesis and sphingolipid metabolism and emphasize the complexity of the regulatory mechanisms that control steroidogenesis. Complex GMs have also been found to be essential for optimal testosterone production and spermatogenesis in mice (Takamiya et al., 1998). Testosterone has also been found to regulate GM levels in rat kidney (Anic and Mesaric, 1998).
Summary and Future Outlook
A significant amount of recent studies have pointed toward an important role for sphingolipids in the regulation of steroidogenesis. These bioactive lipids are key mediators that act at different levels of steroid hormone production including regulation of steroidogenic enzymes expression and activity as well as participating in regulatory signaling cascades. Although the studies discussed in this review significantly increased our understanding of the multiple mechanisms by which sphingolipids control steroidogenesis, it is evident that future studies are necessary to fully elucidate the roles of sphingolipids in steroid hormone biosynthesis. Technologies such as mass spectroscopy, metabolomic profiling, and proteomics are likely to be valuable tools in continued study of the relationship between these two classes of lipids. The identification and characterization of novel bioactive sphingolipids, quantification of flux through both the sphingolipid and steroidogenic metabolic pathways in response to various factors, and the examination of the role of steroid hormones as regulators of sphingolipid production are just a few research avenues that will provide more insight into the roles of sphingolipids in steroid hormone biosynthesis and endocrine function.
References
- Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima ST, Jauniaux E, Rao Ch V. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J Clin Endocrinol Metab. 2004;89:952–956. doi: 10.1210/jc.2003-030917. [DOI] [PubMed] [Google Scholar]
- Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ., 2nd Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- Anic M, Mesaric M. The influence of sex steroid hormones on ganglioside biosynthesis in rat kidney. Biol Chem. 1998;379:693–697. doi: 10.1515/bchm.1998.379.6.693. [DOI] [PubMed] [Google Scholar]
- Arai N, Masuzaki H, Tanaka T, Ishii T, Yasue S, Kobayashi N, Tomita T, Noguchi M, Kusakabe T, Fujikura J, Ebihara K, Hirata M, Hosoda K, Hayashi T, Sawai H, Minokoshi Y, Nakao K. Ceramide and Adenosine 5’-Monophosphate-Activated Protein Kinase Are Two Novel Regulators of 11{beta}-Hydroxysteroid Dehydrogenase Type 1 Expression and Activity in Cultured Preadipocytes. Endocrinology. 2007;148:5268–5277. doi: 10.1210/en.2007-0349. [DOI] [PubMed] [Google Scholar]
- Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 2005;34:293–313. doi: 10.1016/j.ecl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Azhar S, Nomoto A, Leers-Sucheta S, Reaven E. Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J Lipid Res. 1998;39:1616–1628. [PubMed] [Google Scholar]
- Bassett MH, White PC, Rainey WE. Regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004a;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bassett MH, White PC, Rainey WE. A role for the NGFI-B family in adrenal zonation and adrenocortical disease. Endocr Res. 2004b;30:567–574. doi: 10.1081/erc-200043715. [DOI] [PubMed] [Google Scholar]
- Besman MJ, Yanagibashi K, Lee TD, Kawamura M, Hall PF, Shively JE. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci U S A. 1989;86:4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta. 2000;1486:171–183. doi: 10.1016/s1388-1981(00)00055-x. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Rutkowshi H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–141. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Brentano ST, Miller WL. Regulation of human cytochrome P450scc and adrenodoxin messenger ribonucleic acids in JEG-3 cytotrophoblast cells. Endocrinology. 1992;131:3010–3018. doi: 10.1210/endo.131.6.1446636. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Rabano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3K/PKB and MEK/ERK 1/2 pathways. J Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Rabano M, Pena A, Gangoiti P, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine 1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- Budnik LT, Jahner D, Mukhopadhyay AK. Inhibitory effects of TNF alpha on mouse tumor Leydig cells: possible role of ceramide in the mechanism of action. Mol Cell Endocrinol. 1999;150:39–46. doi: 10.1016/s0303-7207(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Cai Z, Bettaieb A, Mahdani NE, Legres LG, Stancou R, Masliah J, Chouaib S. Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-alpha-mediated cytotoxicity. J Biol Chem. 1997;272:6918–6926. doi: 10.1074/jbc.272.11.6918. [DOI] [PubMed] [Google Scholar]
- Cai Z, Kwintkiewicz J, Young M, Stocco C. Prostaglandin E2 increases cyp19 expression in rat granulosa cells: implications of GATA-4. Molecular and Cellular Endocrinology. 2007;263:181–189. doi: 10.1016/j.mce.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ. Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol. 1997;11:138–147. doi: 10.1210/mend.11.2.9880. [DOI] [PubMed] [Google Scholar]
- Castilla R, Maloberti P, Castillo F, Duarte A, Cano F, Maciel FC, Neuman I, Mendez CF, Paz C, Podesta EJ. Arachidonic acid regulation of steroid synthesis: new partners in the signaling pathway of steroidogenic hormones. Endocr Res. 2004;30:599–606. doi: 10.1081/erc-200043765. [DOI] [PubMed] [Google Scholar]
- Chen JY, Waterman MR. Two promoters in the bovine adrenodoxin gene and the role of associated, unique cAMP-responsive sequences. Biochemistry. 1992;31:2400–2407. doi: 10.1021/bi00123a027. [DOI] [PubMed] [Google Scholar]
- Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1) J Biol Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- Choi MS, Cooke BA. Evidence for two independent pathways in the stimulation of steroidogenesis by luteinizing hormone involving chloride channels and cyclic AMP. FEBS Lett. 1990;261:402–404. doi: 10.1016/0014-5793(90)80602-f. [DOI] [PubMed] [Google Scholar]
- Choi MS, Cooke BA. Calmidazolium is a potent stimulator of steroidogenesis via mechanisms not involving cyclic AMP, calcium or protein synthesis. Biochem J. 1992;281(Pt 1):291–296. doi: 10.1042/bj2810291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Combs R. Angiotensin II and cyclic adenosine 3’,5’-monophosphate induce human steroidogenic acute regulatory protein transcription through a common steroidogenic factor-1 element. Endocrinology. 1999;140:4390–4398. doi: 10.1210/endo.140.10.7085. [DOI] [PubMed] [Google Scholar]
- Clem BF, Hudson EA, Clark BJ. Cyclic adenosine 3’,5’-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology. 2005;3:1348–1356. doi: 10.1210/en.2004-0761. [DOI] [PubMed] [Google Scholar]
- Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem. 2002;277:20591–20597. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Degnan BM, Bourdelat-Parks B, Daniel A, Salata K, Francis GL. Sphingomyelinase inhibits in vitro Leydig cell function. Ann Clin Lab Sci. 1996;26:234–242. [PubMed] [Google Scholar]
- Ding T, Li Z, Hailemariam T, Mukherjee S, Maxfield FR, Wu M, Jiang XC. Sphingomyelin synthase (SMS) overexperssion and knockdown: Impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apopotosis. J Lipid Res. 2007;49(2):376–385. doi: 10.1194/jlr.M700401-JLR200. [DOI] [PubMed] [Google Scholar]
- Foster RH. Reciprocal influences between the signalling pathways regulating proliferation and steroidogenesis in adrenal glomerulosa cells. J Mol Endocrinol. 2004;32:893–902. doi: 10.1677/jme.0.0320893. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Gustafsson JA, Wetterberg L. Steroid hormone regulation of the brain. Oxford: Pergamon Press; 1981. [Google Scholar]
- Gallo-Payet N, Cote M, Chorvatova A, Guillon G, Payet MD. Cyclic AMP-independent effects of ACTH on glomerulosa cells of the rat adrenal cortex. J Steroid Biochem Mol Biol. 1999;69:335–342. doi: 10.1016/s0960-0760(99)00079-5. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Payet MD. Excitation-secretion coupling: involvement of potassium channels in ACTH-stimulated rat adrenocortical cells. J Endocrinol. 1989;120:409–421. doi: 10.1677/joe.0.1200409. [DOI] [PubMed] [Google Scholar]
- Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8(4):289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Munoz A. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim Biophys Acta. 2006;1758:2049–2056. doi: 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Gomez-Munoz A, Kong J, Salh B, Steinbrecher UP. Sphingosine-1-phosphate inhibits acid sphingomyelinase and blocks apoptosis in macrophages. FEBS Lett. 2003;539:56–60. doi: 10.1016/s0014-5793(03)00197-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- Goni FM, Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–1921. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Hadizadeh S, King D, Shah S, Sewer MB. Sphingosine-1-phosphate regulates the expression of the liver receptor homologue-1. Molecular and Cellular Endocrinology. 2007 doi: 10.1016/j.mce.2007.11.030. In press, accepted manuscript, available online 5 December 2007. [DOI] [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Loomis CR, Merrill AH, Jr, Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604–12609. [PubMed] [Google Scholar]
- Hauet T, Liu J, Li H, Gazouli M, Culty M, Papadopoulos V. PBR, StAR, and PKA: partners in cholesterol transport in steroidogenic cells. Endocr Res. 2002;28:395–401. doi: 10.1081/erc-120016814. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod. 1997;2:38–47. doi: 10.1530/ror.0.0020038. [DOI] [PubMed] [Google Scholar]
- Hung WC, Chang HC, Chuang LY. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem J. 1999;338(Pt 1):161–166. [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O’Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a Novel Sphingosine 1-Phosphate Receptor, Edg-8. J Biol Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Ishihara SL, Morohashi K. A boundary for histone acetylation allows distinct expression patterns of the Ad4BP/SF-1 and GCNF loci in adrenal cortex cells. Biochem Biophys Res Commun. 2005;329:554–562. doi: 10.1016/j.bbrc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Hammes SR. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle. 2006;5:1178–1183. doi: 10.4161/cc.5.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AB, Schulman H. Sphingosine inhibits calmodulin-dependent enzymes. J Biol Chem. 1988;263:15241–15244. [PubMed] [Google Scholar]
- Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: Molecular aspects. Molecular and Cellular Endocrinology. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kihara A, Mitsutake S, Mizutani Y, Igarashi Y. Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res. 2007;46:126–144. doi: 10.1016/j.plipres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, Azhar S. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–557. doi: 10.1210/me.2003-0179. [DOI] [PubMed] [Google Scholar]
- Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Lavaque E, Sierra A, Azcoitia I, Garcia-Segura LM. Steroidogenic acutre regulatory protein in the brain. Neuroscience. 2006;138:741–747. doi: 10.1016/j.neuroscience.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- Lavieu G, Scarlatti F, Sala G, Levade T, Ghidoni R, Botti J, Codogno P. Is autophagy the key mechanism by which the sphingolipid rheostat controls the cell fate decision? Autophagy. 2007;3:45–47. doi: 10.4161/auto.3416. [DOI] [PubMed] [Google Scholar]
- Le Stunff H, Giussani P, Maceyka M, Lépine S, Milstien S, Spiegel S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–34380. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Wu G. Sphingolipids of the nucleus and their roles in nuclear signaling. Biochim Biophys Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Liu CH, Thompson BD, Hla T. Lysophosphatidic acid stimulates the G-protein-coupled receptor EDG-1 as a low affinity agonist. J Biol Chem. 1998;273:22105–22112. doi: 10.1074/jbc.273.34.22105. [DOI] [PubMed] [Google Scholar]
- Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol Cell Biol. 2007;27:6669–6685. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ni J, Bian S, Yao L, Zhu H, Zhang W. Inhibition of steroidogenesis and induction of apoptosis in rat luteal cells by cell-permeable ceramide in vitro. Sheng Li Xue Bao. 2001;53:142–146. [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu J, Li H, Papadopoulos V. PAP7, a PBR/PKA-RIalpha-associated protein: a new element in the relay of the hormonal induction of steroidogenesis. J Steroid Biochem Mol Biol. 2003;85:576–586. doi: 10.1016/s0960-0760(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- Lu ZH, Mu YM, Wang BA, Li XL, Lu JM, Li JY, Pan CY, Yanase T, Nawata H. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem Biophys Res Commun. 2003;303:1002–1007. doi: 10.1016/s0006-291x(03)00449-2. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- McClellan DR, Bourdelat-Parks B, Salata K, Francis GL. Sphingomyelinase affects hormone production in Jeg-3 choriocarcinoma cells. Cell Endocrinology Metabolism. 1997;9:19–24. [Google Scholar]
- McEwen BS. Steroid hormones are multifuctional messengers in the brain. Trends in Endocrinology Metabolism. 1991;2:62–67. doi: 10.1016/1043-2760(91)90042-l. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Gong W, Griffin LD. Niemann pick type C disease as a model for defects in neurosteroidogenesis. Endocr Res. 2004;30:727–735. doi: 10.1081/erc-200044016. [DOI] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Jiang B, Shelton JM, Richardson JA, Hinshelwood MM. Transcriptional regulation of aromatase in placenta and ovary. J Steroid Biochem Mol Biol. 2005;95:25–33. doi: 10.1016/j.jsbmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Meroni SB, Pellizzari EH, Canepa DF, Cigorraga SB. Possible involvement of ceramide in the regulation of rat Leydig cell function. J Steroid Biochem Mol Biol. 2000;75:307–313. doi: 10.1016/s0960-0760(00)00188-6. [DOI] [PubMed] [Google Scholar]
- Meroni SB, Riera MF, Pellizzari EH, Cigorraga SB. Regulation of rat Sertoli cell function by FSH: possible role of phosphatidylinositol 3-kinase/protein kinase B pathway. J Endocrinol. 2002;174:195–204. doi: 10.1677/joe.0.1740195. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Nikolova-Karakashian N, Schmelz EM, Morgan ET, Stewart J. Regulation of cytochrome P450 expression by sphingolipids. Chemistry and Physics of Lipids. 1999;102:131–139. doi: 10.1016/s0009-3084(99)00081-x. [DOI] [PubMed] [Google Scholar]
- Miller WL. Mechanism of StAR’s regulation of mitochondrial cholesterol import. Mol Cell Endocrinol. 2007;265-266:46–50. doi: 10.1016/j.mce.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- Morales V, Santana P, Diaz R, Tabraue C, Gallardo G, Blanco FL, Hernandez I, Fanjul LF, Ruiz de Galarreta CM. Intratesticular Delivery of Tumor Necrosis Factor-{alpha} and Ceramide Directly Abrogates Steroidogenic Acute Regulatory Protein Expression and Leydig Cell Steroidogenesis in Adult Rats. Endocrinology. 2003;144:4763–4772. doi: 10.1210/en.2003-0569. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267:17913–17919. [PubMed] [Google Scholar]
- Mueller M, Atanasov A, Cima I, Corazza N, Schoonjans K, Brunner T. Differential regulation of glucocorticoid synthesis in murine intestinal epithelial versus adrenocortical cell lines. Endocrinology. 2007;148:1445–1453. doi: 10.1210/en.2006-0591. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology. 2006;84:255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Jayaram HN, Scribner WM, Garcia JG. Activation of endothelial cell phospholipase D by sphingosine and sphingosine-1-phosphate. Am J Respir Cell Mol Biol. 1994;11:221–229. doi: 10.1165/ajrcmb.11.2.8049083. [DOI] [PubMed] [Google Scholar]
- Newton R, Holden NS. Separating transrepression and transactivation: A distressing divorce for the glococorticoid receptor? Molecular Pharmacology. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Takemori H, Katoh Y. Salt-inducible kinase in steroidogenesis and adipogenesis. Trends Endocrinol Metab. 2004;15:21–26. doi: 10.1016/j.tem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem. 2005;280:27879–27887. doi: 10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- Otis M, Gallo-Payet N. Role of MAPKs in angiotensin II-induced steroidogenesis in rat glomerulosa cells. Mol Cell Endocrinol. 2007;265–266:126–130. doi: 10.1016/j.mce.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Ozbay T, Merrill AH, Jr, Sewer MB. ACTH regulates steroidogenic gene expression and cortisol biosynthesis in the human adrenal cortex via sphingolipid metabolism. Endocr Res. 2004;30:787–794. doi: 10.1081/erc-200044040. [DOI] [PubMed] [Google Scholar]
- Ozbay T, Rowan A, Leon A, Patel P, Sewer MB. Cyclic adenosine 5’-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1. Endocrinology. 2006;147:1427–1437. doi: 10.1210/en.2005-1091. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr Rev. 1993;14:222–240. doi: 10.1210/edrv-14-2-222. [DOI] [PubMed] [Google Scholar]
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Payne SG, Milstien S, Barbour SE, Spiegel S. Modulation of adaptive immune responses by sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:521–527. doi: 10.1016/j.semcdb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Plassart-Schiess E, Baulieu EE. Neurosteroids: recent findings. Brain Res Brain Res Rev. 2001;37:133–140. doi: 10.1016/s0165-0173(01)00113-8. [DOI] [PubMed] [Google Scholar]
- Porn MI, Tenhunen J, Slotte JP. Increased steroid hormone secretion in mouse Leydig tumor cells after induction of cholesterol translocation by sphingomyelin degradation. Biochim Biophys Acta. 1991;1093:7–12. doi: 10.1016/0167-4889(91)90131-g. [DOI] [PubMed] [Google Scholar]
- Rabano M, Pena A, Brizuela L, Marino A, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates cortisol secretion. FEBS Lett. 2003;535:101–105. doi: 10.1016/s0014-5793(02)03882-6. [DOI] [PubMed] [Google Scholar]
- Rainey WE. Adrenal zonation: clues from 11beta-hydroxylase and aldosterone synthase. Mol Cell Endocrinol. 1999;151:151–160. doi: 10.1016/s0303-7207(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Ramnath HI, Peterson S, Michael AE, Stocco DM, Cooke BA. Modulation of steroidogenesis by chloride ions in MA-10 mouse tumor Leydig cells: roles of calcium, protein synthesis, and the steroidogenic acute regulatory protein. Endocrinology. 1997;138:2308–2314. doi: 10.1210/endo.138.6.5162. [DOI] [PubMed] [Google Scholar]
- Reinhart AJ, Williams SC, Clark BJ, Stocco DM. SF-1 (steroidogenic factor-1) and C/EBP beta (CCAAT/enhancer binding protein-beta) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol. 1999;13:729–741. doi: 10.1210/mend.13.5.0279. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- Sakakura C, Sweeney EA, Shirahama T, Hagiwara A, Yamaguchi T, Takahashi T, Hakomori S, Igarashi Y. Selectivity of sphingosine-induced apoptosis. Lack of activity of DL-erythyro-dihydrosphingosine. Biochem Biophys Res Commun. 1998;246:827–830. doi: 10.1006/bbrc.1998.8719. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Kawata M, Tsutsui K. Expression, localization and possible actions of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell of the cerebellum: A new insight into the biosynthesis, metabolism and multiple actions of progesterone as a neurosteroid. Cerebellum. 2007 Mar;2:1–8. [Epub ahead of print] [Google Scholar]
- Santana P, Llanes L, Hernandez I, Gallardo G, Quintana J, Gonzalez J, Estevez F, Ruiz de Galarreta C, Fanjul LF. Ceramide mediates tumor necrosis factor effects on P450-aromatase activity in cultured granulosa cells. Endocrinology. 1995;136:2345–2348. doi: 10.1210/endo.136.5.7720683. [DOI] [PubMed] [Google Scholar]
- Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarreta CM, Fanjul LF. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology. 1996;137:2480–2489. doi: 10.1210/endo.137.6.8641202. [DOI] [PubMed] [Google Scholar]
- Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional Regulation of Adrenocortical Steroidogenic Gene Expression. Drug Metab Rev. 2007;39:371–388. doi: 10.1080/03602530701498828. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. Insights into the transcriptional regulation of steroidogenic enzymes and StAR. Rev Endocr Metab Disord. 2001;2:269–274. doi: 10.1023/a:1011516532335. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- Shah BH, Baukal AJ, Shah FB, Catt KJ. Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol Endocrinol. 2005;19:2535–2548. doi: 10.1210/me.2005-0082. [DOI] [PubMed] [Google Scholar]
- Sierra A. Neurosteroids: the StAR protein in the brain. J Neuroendocrinol. 2004;16:787–793. doi: 10.1111/j.1365-2826.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Carr BR, Ando S, Rainey WE. Inhibition of Src tyrosine kinase stimulates adrenal androgen production. J Mol Endocrinol. 2003;30:287–299. doi: 10.1677/jme.0.0300287. [DOI] [PubMed] [Google Scholar]
- Smith WL, Merrill AH., Jr Sphingolipid metabolism and signaling minireview series. J Biol Chem. 2002;277:25841–25842. doi: 10.1074/jbc.R200011200. [DOI] [PubMed] [Google Scholar]
- Son DS, Arai KY, Roby KF, Terranova PF. Tumor necrosis factor alpha (TNF) increases granulosa cell proliferation: dependence on c-Jun and TNF receptor type 1. Endocrinology. 2004;145:1218–1226. doi: 10.1210/en.2003-0860. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003a;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003b;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Khan SA. Effects of steroidogenesis inducing protein (SIP) on steroid production in MA-10 mouse Leydig tumor cells: utilization of a non-cAMP second messenger pathway. Mol Cell Endocrinol. 1992;84:185–194. doi: 10.1016/0303-7207(92)90029-6. [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Inokuchi J, Igarashi Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 1998;425:61–65. doi: 10.1016/s0014-5793(98)00198-7. [DOI] [PubMed] [Google Scholar]
- Tajima K, Yoshii K, Fukuda S, Orisaka M, Miyamoto K, Amsterdam A, Kotsuji F. Luteinizing hormone-induced extracellular-signal regulated kinase activation differently modulates progesterone and androstenedione production in bovine theca cells. Endocrinology. 2005;146:2903–2910. doi: 10.1210/en.2005-0093. [DOI] [PubMed] [Google Scholar]
- Takamiya K, Yamamoto A, Furukawa ZK, Zhao J, Fukumoto S, Yamashiro S, Okada M, Hraguchi M, Shin M, Kishikawa M, Shiku H, Aizawa S, Furukawa K. Complex gangliosides are essential in spermatogenesis of mice: Possible roles in the transport of testosterone. Proc Natl Acad Sci. 1998;95:12147–12152. doi: 10.1073/pnas.95.21.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Barth E, Heneka M, Sandhoff K, Walter J. Inhibition of glycosphingolipid biosynthesis reduces secretion of the beta-amyloid precursor protein and amyloid beta-peptide. J Biol Chem. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Thomson M. Molecular and cellular mechanisms used in the acute phase of stimulated steroidogenesis. Hormone Metabolism Research. 1997;30:16–28. doi: 10.1055/s-2007-978825. [DOI] [PubMed] [Google Scholar]
- Thon L, Mohlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schutze S, Bulfone-Paus S, Adam D. Ceramide mediates caspase-independent programmed cell death. Faseb J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Neurosteroids in the cerebellar Purkinje neuron and their actions (review) Int J Mol Med. 1999;4:49–56. [PubMed] [Google Scholar]
- Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36:261–273. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- Urs AN, Dammer EB, Sewer MB. Sphingosine Regulates the Transcription of CYP17 by Binding to Steroidogenic Factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- Urs AN, Dammer EB, Sewer MB. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- Vianello S, Waterman MR, Dalla Valle L, Colombo L. Developmentally regulated expression and activity of 17alpha-hydroxylase/C-17,20-lyase cytochrome P450 in rat liver. Endocrinology. 1997;138:3166–3174. doi: 10.1210/endo.138.8.5297. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL. The crystal structures of steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci U S A. 2005;102:7505–7510. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MM, Michl P, Auernhammer CJ, Engelhardt D. Interleukin-3 and interleukin-6 stimulate cortisol secretion from adult human adrenocortical cells. Endocrinology. 1997;138:2207–2210. doi: 10.1210/endo.138.5.5239. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman HG, Reddi AH. Actions of vertebrate sex hormones. Annu Rev Physiol. 1971;33:31–82. doi: 10.1146/annurev.ph.33.030171.000335. [DOI] [PubMed] [Google Scholar]
- Yamada K, Sakane F. The different effects of sphingosine on diacylglycerol kinase isozymes in Jurkat cells, a human T-cell line. Biochim Biophys Acta. 1993;1169:211–216. [PubMed] [Google Scholar]
- Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, Okazaki H, Okajima F, Ohta H. Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca(2+) signaling pathway. Biochem Biophys Res Commun. 2000;268:583–589. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]
- Zeidan YH, Hannun YA. Translational aspects of sphingolipid metabolism. Trends Mol Med. 2007;13:327–336. doi: 10.1016/j.molmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, Norris JS, Hannun YA. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J Biol Chem. 2006;281:24695–24703. doi: 10.1074/jbc.M604713200. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AHJ. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochem Biophys Res Commun. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Ziulkoski AL, Zimmer AR, Guma FC. De novo synthesis and recycling pathways of sphingomyelin in rat Sertoli cells. Biochem Biophys Res Commun. 2001;281:971–975. doi: 10.1006/bbrc.2001.4440. [DOI] [PubMed] [Google Scholar]