Abstract
The aortic valve lies in a unique hemodynamic environment, one characterized by a range of stresses (shear stress, bending forces, loading forces and strain) that vary in intensity and direction throughout the cardiac cycle. Yet, despite its changing environment, the aortic valve opens and closes over 100,000 times a day and, in the majority of human beings, will function normally over a lifespan of 70–90 years. Until relatively recently heart valves were considered passive structures that play no active role in the functioning of a valve, or in the maintenance of its integrity and durability. However, through clinical experience and basic research the aortic valve can now be characterized as a living, dynamic organ with the capacity to adapt to its complex mechanical and biomechanical environment through active and passive communication between its constituent parts. The clinical relevance of a living valve substitute in patients requiring aortic valve replacement has been confirmed. This highlights the importance of using tissue engineering to develop heart valve substitutes containing living cells which have the ability to assume the complex functioning of the native valve.
Keywords: Cells, endothelium, nerves, developmental biology, mechanobiology, nanostructure aortic stenosis, calcification
Introduction
The aortic valve is a sophisticated structure that performs a range of functions resulting in the unidirectional flow of blood out of the left ventricle, the optimising of coronary blood flow, and preservation of myocardial function. For many years the aortic valve was regarded as a passive structure that opens and closes as a result of changes in transvalvular pressures and myocardial contraction/relaxation. It is now widely accepted that the function of the aortic valve is regulated by complex mechanisms that are evident initially during embryonic development, and are present during adaptation of the valve after birth and growth into adulthood. Clinical experience involving the Ross procedure has demonstrated the importance of a “living valve”, 1 and diverse research conducted globally over the past 20 years has increased our understanding of heart valve biology at the molecular, protein, cellular and tissue level. As a consequence, we are now gaining insights into the key factors contributing to the sophisticated function of heart valves, as well as those underpinning the superior performance of living valves compared with other valve substitutes. In addition, this improved understanding is clarifying the role played by specific mediators, certain of which are also involved during valve development, and in the structural and functional changes that lead to disease and, ultimately, valve failure.
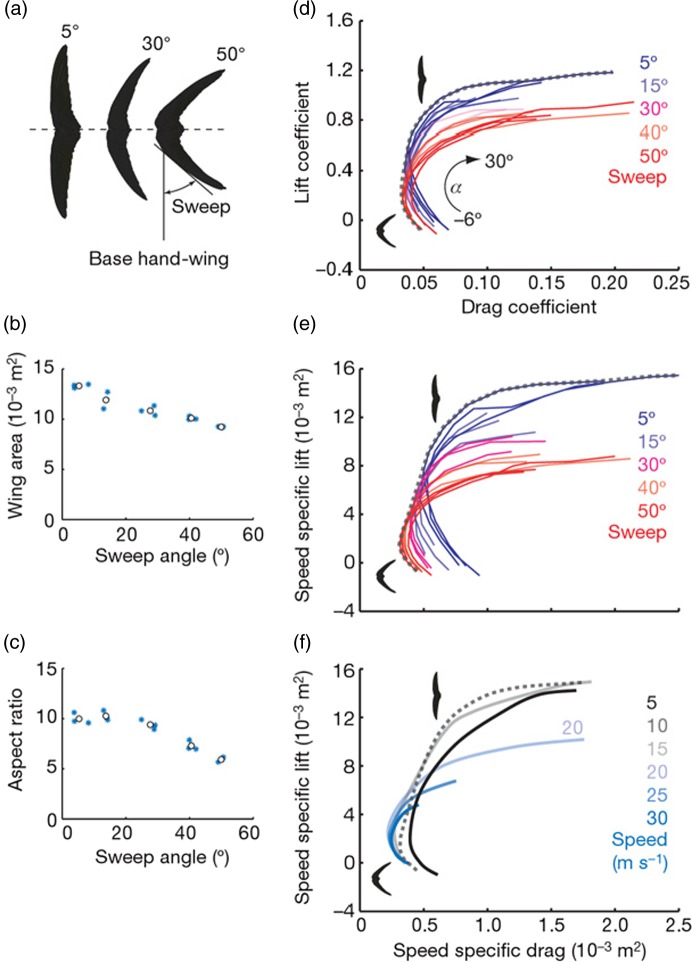
The complexity of aortic valve function was highlighted in 1999 by Yacoub et al. who proposed that it relies on “dynamism and crosstalk”. 2 Dynamism is defined by the ability of the component parts of the valve to move spatially and to change size and shape in a coordinated manner. This principle applies elsewhere in nature, dynamism being used to optimise the function of a specific process. For example, in order to optimise delicate control during flight, swifts are able to change the shape and size of their wings according to whether turning is during a slow or fast glide (Figure 1). 3 In terms of the aortic valve, dynamism may be either passive or active. Passive dynamism relates to the way in which the different components of the aortic root - from the subvalvular regions (LVOT, sub aortic curtain and fibrous trigones) up to the supravalvular regions (sinus of Valsalva and the sinotubular junction) – move in response to the flow of blood throughout the cardiac cycle. An example of passive dynamism is evident from the influence of the shapes of the sinuses on forming the vortices important for valve closure and for maintaining coronary flow during systole. In contrast, active dynamism occurs in cases where structures in each part of the valve (cusp, annulus, sinus, sinotubular junction) change size, shape and stiffness during specific parts of the cardiac cycle. Such changes are necessary to guarantee optimal valve opening, ejection of blood from the left ventricle, rapid valve closure, coaptation of the leaflets to prevent flow of blood back into the left ventricle, and adequate coronary perfusion.
Figure 1.
Relationship between size and shape of the wings of a swift and the speed of lift during flight. 3
Passive dynamism in valve function
The aortic valve is composed of a number of distinct functional units (Figure 2). The entire valve “machinery” is located between the left ventricle and the ascending aorta and is referred to as the “aortic root”. The valve cusps are attached in a semilunar pattern to a crown-shaped annulus. The highest point of the attachment, where the adjacent cusps are closest together (the commissures), marks the boundary between the valve and the ascending aorta, this being identified as a small ridge called the sinotubular junction. At the basal point of attachment of each cusp, the annulus bulges to create three “pockets” called the sinuses of Valsalva. Two of the sinuses of Valsalva have ostia that give rise to the left and right coronary arteries. The sinuses and cusps are named left, right or non-coronary according to which, if any, coronary artery arises from the particular sinus or cusp.
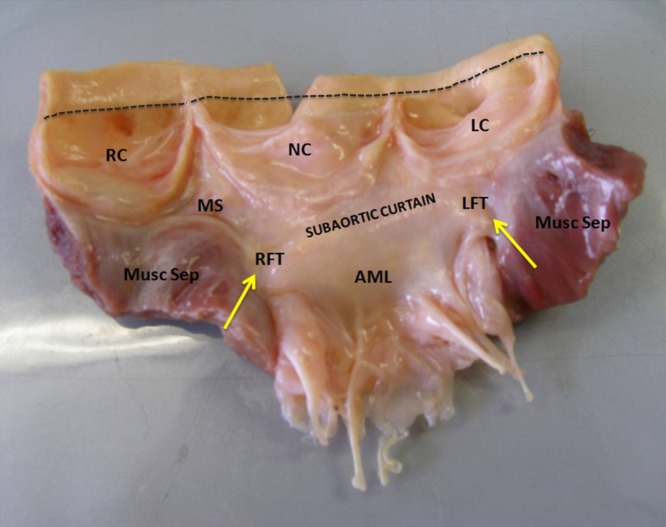
Figure 2.
Photograph of an open aortic root showing its structural components (annulus, cusps, sinuses of Valsalva and sinotubular junction [dashed line]) and their relationship with the left ventricular outflow tract and the mitral valve. AML, anterior mitral leaflet; LC, left coronary cusp; LFT, left fibrous trigone; MS, membranous septum; Musc Sep, muscular septum; NC, non-coronary cusp; RC, right coronary cusp; RFT, right fibrous trigone. Adapted from Yacoub, El-Hamamsy et al. 128
The competent seal of the aortic valve ensures that regurgitation of blood flow is prevented, and it is involved in coapting areas on individual cusps. In the middle of the free edge of each cusp, nodules of Arantius are created from bundles of fibrous tissue. The two crescent-shaped edges of each cusp (known as lunulae), which are located along the free edge between the commissures and the nodule of Arantius, form a competent seal during diastole through contact with the corresponding region of the contiguous cusps. The remaining area of the cusp, apart from the coapting area, is the “belly” region that represents the main load bearing part of the cusp when closed during diastole.
The precise geometry of the valve allows it to be responsive and to function effectively in the face of the different haemodynamic forces operating during the cardiac cycle. It is often the case that differences in this geometry result in alterations in short or long term function. For example, the presence of only two cusps (bicuspid valves) instead of three is associated with accelerated calcific valve disease. 4
The shape of the aortic valve allows for distribution of the stresses to which it is exposed such that the valve will function without complications throughout a lifetime. The cusps of the aortic valve are subjected to a unique stress profile comprising a range of stresses including shear stress, leaflet strain in both the radial and circumferential directions, mechanical pressure during diastole, and bending forces. 5 Importantly, the two sides of the valve are exposed to different forces, particularly in terms of shear stress. The ventricular side of the valve is exposed to a high-velocity, high-shear stress, whereas the aortic side is exposed to a pattern of interrupted low-velocity flow and low-shear stress. 6 These different types of mechanical stress act individually or in combination as stimuli that induce functional responses from the cellular components of the valve. The responses made to these forces by the cells on and within the valve - and similarly to a range of paracrine mediators - represent the regulatory mechanisms that contribute to active dynamism.
Active dynamism in valve function
It has been shown experimentally that the different parts of the valve move in a co-ordinated fashion. Using a closed-chest ovine model with radiopaque markers placed at key points in the aortic root, Dagum et al. studied aortic root deformations during the cardiac cycle. 7 Interestingly, changes in aortic root shape are asymmetric and dictated by both structure and changes in transvalvular pressure (Figure 3). For instance, during the early isovolumic contraction phase, circumferential expansion of the base was greatest in the left annular region and least in the non-coronary annular region that is continuous with the anterior mitral annulus (aortic-mitral continuity and right fibrous trigone) and composed primarily of fibrous tissue. Importantly, this expansion was accompanied by a simultaneous increase in circumferential diameter at the level of the commissures. This conformational change is proportional to the end-diastolic volume. In other terms, before the aortic valve opens, the aortic root prepares to accommodate the large volume of blood exiting from the left ventricle, thereby improving transvalvular hemodynamics and minimising any turbulent damage to the aortic cusps. 7,8
Figure 3.
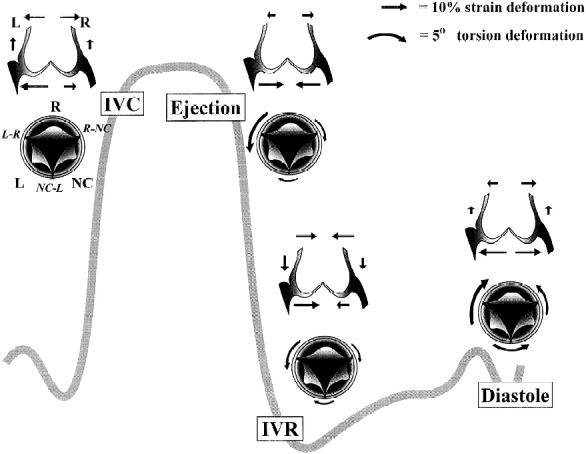
Aortic annular deformation of left (L), right (R), and non-coronary cusp (NC) sectors of aortic annulus throughout different stages of the cardiac cycle (from Dagum et al. 1999). 7
It is known that aortic root expansion is asymmetric but, importantly, precise changes in tilt angle are generated. The aortic root tilt angle (the angle between the basal and the commissural planes) is ∼16° degrees at end-diastole (the angle being oriented posteriorly and to the left). 8 During systole, it is reduced by ∼7°, aligning the left ventricular (LV) outflow tract with the ascending aorta. This reduction in tilt angle results in a straight cylinder that undoubtedly facilitates ejection. During diastole the tilt angle increases, this probably reducing leaflet stress.
Of particular importance are the dynamics of aortic valve opening and closing. Instantaneous movements of the valve, especially the closing velocity during end-systole, can impose major stresses on the cusps. It has been demonstrated clearly that the aortic valve cusps initiate the closure movement during the second half of systole. From a dynamic perspective, the aortic root undergoes a conformational change from a conical shape that generates a velocity gradient to a cylindrical shape that negates any such gradient. In terms of fluid dynamics, as the velocity gradient dissipates, the pressure across the aortic valve reverses with the deceleration in blood flow. Deceleration of flow implies a negative value for δv/δt; consequently, a positive pressure gradient (δp/δz) must exist across the valve from left ventricle to aorta. An effect of this gradient is that the leaflets begin to move towards closure. If the velocity gradient across the aortic root persists, deceleration of flow is offset by this and a pressure reversal will occur later in ejection, meaning that the leaflets will begin to close later in systole. As the leaflet edges must traverse the same distance within a shorter time period, the leaflets close at a faster rate, thereby increasing the closing stress on the cusps. Furthermore, the generation of vortex patterns of flow in the sinuses of Valsalva early in systole generates a momentum that allows timely initiation of valve closure during systole. 9–11
Superimposed on the movements of the valve apparatus are the regulatory influences that comprise active dynamism. Central to the phenomenon of active dynamism is the fact that the valve is a “living” structure containing a number of key “players” that orchestrate the response of the valve to its mechanical environment, whether this is an immediate response or adaptation to chronic changes in the surrounding environment.
Stimuli, cells and mediators involved in active dynamism
The ability of different parts of the valve to change in size, shape and stiffness is due to the communication that occurs between cells and the extracellular matrix in response to exposure of the valve to a range of stresses (shear stress, bending forces, loading forces, and strain). These stresses vary in intensity and direction throughout the cardiac cycle. 12 Due to improved imaging techniques and the application of research disciplines including genomics, proteomics and cell physiology we are beginning to comprehend the vital role played by the living components of heart valves in valve development, valve adaptation, maintenance of valve integrity, modulation of the movements of specific parts of the aortic valve and root throughout the cardiac cycle, and in optimising the function of the valve throughout life.
Valve development
Specification of valve forming fields
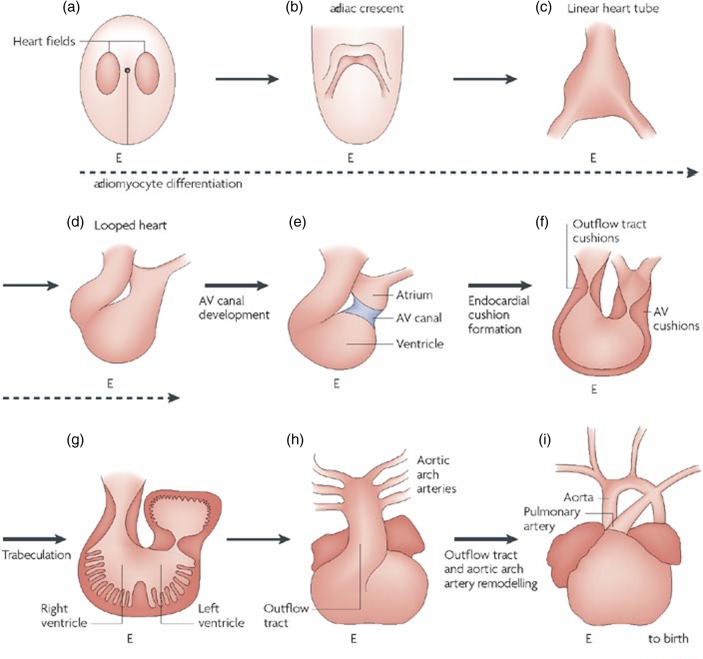
The embryonic heart originates as bilaterally symmetric fields of Nkx2.5+ cardiac progenitors. The primary and secondary heart forming fields resemble a horseshoe (mammal) or butterfly (avian) shape, with the primary heart field forming nearly all of the atria and ventricles, while the secondary field contributes substantially to the outflow tracts, great vessels, and part of the right ventricle. 13–18 These fields fuse medially to form a linear tube that begins contractions virtually simultaneously with the onset of blood flow. 19 The presumptive cardiac segments are arranged with the putative atria most caudal, through a singular contractile ventricular segment, and then finally the outflow most cranial. Shortly after the initiation of a rudimentary linear conduction sequence, the heart bends rightward into a characteristic “C” loop and through differential growth of the ventricular segment arrives at a figure of eight shape with the ventricular segment now caudal to the atrial segment. 20 It is in this configuration that the cardiac valves originate (Figure 4). At this point the heart tube comprises an inner ring of endocardial cells and an outer ring of cardiomyocytes. Between them lies a gelatinous, hyaluronic acid-rich substance called the “cardiac jelly” that is synthesized by the myocardium and is present throughout the heart tube. 21,22 For reasons that are not completely understood, specific fields of endocardial cells located at the junction between the atrial and ventricular segment (the atrioventricular canal), and along the length of the outflow region just distal to the ventricular segment, are induced to undergo an endocardial to mesenchymal transformation (EMT) process. Recent evidence supports a Wnt-Notch axis for constraining a bone morphogenetic protein (BMP) inductive signal to these presumptive regions within the myocardium; disruption of this signal can result in the EMT process occurring in aberrant locations. 23 The daughter mesenchymal progenitors invade and migrate within the cardiac jelly, and further proliferate into amorphous swellings called the cardiac cushions that provide the first valve action in the embryonic heart. Many vertebrate species, for example the zebrafish, chick, and mouse, share this same localization of EMT, although in the zebrafish an activated ring of hypertrophied endocardium is formed that invaginates into a valve leaflet shape and this undergoes an EMT process much later. 24
Figure 4.
Overview of cardiac development and formation of heart valves (from High & Epstein, 2008). 176
Molecular regulation of EMT
Most that is known about EMT and valvulogenesis has been gained from studying animal models, most notably the avian and mouse, and from using the atrioventricular canal as a domain analogous to the outflow tract. More than 115 genes have been identified and characterized for their roles in the EMT process and many reviews on the subject have been published. 25,26 Synthesis of the hyaluronic acid-rich cardiac jelly is essential for valve formation, and requires a local restriction of myocardial vascular endothelial growth factor (VEGF) signaling and an increase in the secretion of BMP2/4. 21,27 This BMP ligand binds to the BMPR1 receptor on the endocardial surface and activates an autocrine/paracrine TGFβ signaling loop. 28 Each of the three TGFβ ligands participates in the EMT process in a unique and redundant manner, there being notable differences according to the species in which the process is occurring. 29–31 In mouse, the TGFβ2 isoform is the ligand particularly responsible for the invasion component of EMT, whereas for chick it is the TGFβ3 isoform. 32 These ligands act promiscuously through a number of TGFβ/Alk receptors, which in turn activate Smad-dependent transcriptional cascades. 33 These transcription factors activate mesenchymal signaling programs (including migratory, contractile, and proliferative), while simultaneously restricting endocardial programs including the disassembly of tight junctions and the digestion of basement membrane substances. Newly transformed mesenchymal cells initially separate from their endocardial neighbors and then invade the cardiac jelly. This process involves the upregulation of contractile filaments such as αSMA, the expression of integrins, and the secretion of matrix metalloproteinases. 34 As the mesenchymal cells invade, they secrete a number of proteoglycans and matricellular proteins, including chondroitin sulphate, versican, and periostin, that further condition the matrix environment for more cellular invasion. 35–37 Genetic tracking studies have established that only 20–30% of the surface endocardial cells undergo EMT, the remainder presumably proliferating and migrating to maintain a confluent monolayer. 38 The transformed mesenchyme is also highly proliferative, this explaining the rapid colonization and expansion of the cardiac jelly into cellularized cushions.
Cushion expansion and valve action
Continued proliferation of the cushion mesenchyme, combined with the secretion of matrix proteins and proteoglycans, has the effect of further expanding the endocardial cushions into the flow field. The cushions are initially wispy swellings that perform a valve-like function through a treadmill-like wave action. 39 Ventricular suction draws the cushions into the lumen of the chamber, where they abut to block blood flow. This plane of apposition then translates cranial to caudal, with linear conduction through the atrioventricular canal. The cushions then sink back in profile as a new wave initiates at the level of the atrium. Although the molecular mechanisms have not yet been studied extensively, a similar process has been found to occur with the outflow tract cushions; within 48 hours, however (in avian and mouse), these cushions are significantly larger and have acquired a much more rigid structural composition. 40 The few mediators of cushion cell proliferation and expansion that have been identified include members of the BMP, FGF, and TGFβ ligand families. 41 At this stage, valve action no longer occurs by a treadmill-like process, rather the cushions are pushed together as a result of ventricular contraction. The cross section of the atrioventricular canal lumen thereby resembles a “dog bone” shape, with flow increasing in the head regions and decreasing in the central shaft. 42 Through a mechanism that currently is not well understood, blood flow ceases between the cushions, which then become fused medially. The intermediating endocardium is removed, leaving a fully mesenchymal septal wall to which the rising ventricular septum soon connects from below, and the protruding dorsal mesocardium from above. 43 This completes the septation of the heart into left and right sides. The outflow tract cushions undergo a similar septation process but with notable differences. The outflow tract contains several cushions that extend almost its entire length, but these can be classified broadly into proximal and distal domains. 44 Although research has recently begun into the molecular and cellular mechanisms specifying these two domains and the resulting tissues formed, currently far more is known about the atrioventricular canal. The outflow cushions also receive cells from the secondary heart field and cardiac neural crest lineages. 45 It appears that the neural crest derived progenitors within the outflow cushions form a boundary delineating where the valves will eventually form. In addition, the aorticopulmonary septum originates as a protrusion of neural crest cells from the aortic sac, which then drives caudally through the outflow tract while twisting to create the separate but twinned pulmonic and aortic outlets. 46 The septum exhibits a two pronged fork-like appearance that drives into the outflow cushions as it moves, dividing the cushions in such a way that two sets of three cushions are created, one set in each of the outflow lumens. 47 The septal wall arrests near the level of the proximal outflow cushions which, unlike the distal cushions, do not form valves but instead fuse to form a septum known as the pulmonary infundibulum that subsequently becomes muscularized. 48 At this point, the ventricular and outflow channel anatomy is largely defined.
Cushion remodeling into valves
Once fused, the lateral edges of the cushions are remodeled into the valves recognized postnatally. In the atrioventricular canal, two additional cushions are initiated around the time of cushion fusion to create what are eventually the mural leaflets of the atrioventricular valves. Both the atrioventricular and outflow cushions undergo a process of condensation and extension into leaflets, the molecular mechanisms underpinning this process currently being unknown. 49 A number of genetic mutations in mouse have been associated with a failure to condense, these including TGFb2, TACE, and NFATc. 27,50,51 In the atrioventricular canal, the left septal leaflet appears to remain free of ventricular connection while it is condensing and elongating. The septal leaflet of the tricuspid valve, and to some degree the mural leaflets, extends along the atrioventricular myocardial substrate and delaminates from the ventricular wall. 52 Interestingly, the mural leaflets are unique in retaining a significant number of cells that are positive for cardiac myosins; this feature was found recently to be associated with the leaflets being populated with a high proportion of epicardially-derived progenitor cells (EPDC). 53 The formation of outflow valve cusps in the outflow tract is somewhat different. The cushions are progressively invaginated from the cranial surface, there being effectively a scooping away of tissue to form their characteristic crescent shape. 54 The process of elongation/extension of the cushion appears to require expression of VEGF by the endocardium, presumably both to enhance its proliferation to cover the increase in valve surface area and to abate the EMT process. 55 Tissue condensation is a cell traction mediated process, involving both BMP and TGFb ligands. 56 Concomitant with tissue morphogenesis is a transition in phenotype of the resident mesenchymal cells from a highly activated myofibroblast-like progenitor toward a more quiescent fibroblast phenotype. Transcriptional profiling has identified a pre-cartilage-like phenotype for these early cushion progenitors, which also express a capacity to undergo osteogenic-like differentiation in vitro and in vivo. 57 Indeed, Smad6-null mice have valves that are heavily ossified, and mutations in other important valve signaling components, such as Notch1, can predispose valves to early calcification. 58 Studies suggest a program by which progenitors begin with transcriptional activity driven by sox9 and twist1 that becomes progressively restricted in favor of scleraxis-mediated transcription. 59–61 Expression of earlier pro-osteochondrogenic signaling components has been reported in calcified postnatal aortic valves, but whether these represent a reactivation of embryonic signaling programs and what the clinical importance may be is still being debated. 62,63
Role of hemodynamics in valvulogenesis
The process of valve formation cannot be disassociated from the rapidly changing and demanding hemodynamic environment in which the process occurs. Until recently, the small size and complex geometry of the embryonic heart has limited our ability to quantify and test the effects of mechanical forces on heart and valve development. While the heart tube can form and even loop independent of any blood flow, 64 the formation of valves appears to require hemodynamic stimuli. Obstruction of either the inflow or outflow of the heart results in no activation of a valvulogenic program. 65 Computational simulations reveal that the intracardiac zones where EMT occurs most prominently are associated with the highest wall shear stresses. 42 In zebrafish, the presence of reversing blood flows was responsible for activating endocardial ring formation mediated by klf2. 66 Shear stress was sufficient to induce EMT in cultured embryonic endothelial cells in vitro through a TGFb/Alk2 mediated pathway, but the specific effect of shear on the valvular endocardium was not addressed. 67 It is likely that hemodynamics influence valve remodeling post EMT. While the inflow surfaces of the atrioventricular cushions (and ventricularis of the outflow cushions) experience the highest shear stress in the entire cardiac lumen, the fibrosa surfaces of each cushion reduce significantly as the cushions expand and remodel. 42 We identified strong flow oscillations and vorticity in these regions, which correlated significantly with separation of the regions from the myocardial wall and condensation. Growth simulations were conducted based solely on the external hemodynamic environment and it was determined that the changing surface shear profile was sufficient to generate the growth and transition of the hemispherical endocardial cushions into a “shark fin”-like valve shape. 68,69 This phenomenon was also demonstrated using isolated cushions subjected to pulsatile fluid flow in vitro, 70 and it was suggested that the remodeling was controlled in part by activity of the small GTPase RhoA. However, it is unclear where in the process this GTPase acts and whether or not there are other contributing pathways. 71 Microsurgical alteration of the blood flow environment can result in malformed valves. For instance, partial ligation of the left atrium restricts blood flow into the left ventricle, leading to a delayed and stunted cushion morphogenesis. 72–75 Conotruncal banding, on the other hand, increases ventricular afterload and may accelerate valve elongation and condensation. Recently, we developed an innovative non-invasive microsurgical approach using two photon microscopy guided femtosecond pulsed laser ablation to specifically localize internal tissue cuts without affecting any other tissue zone. 76 We showed that local cushion ablation creates profound regurgitation that reduces mesenchymal cell proliferation, EMT, and cushion remodeling. While these initial findings indicate that mechanical signaling plays important roles in valve development, much work remains to establish causality and the potential to control the effects of hemodynamic signaling on embryonic and fetal valve growth and remodeling.
Cellular components of the valve
Endothelial cells
The endothelium forms a monolayer of cells throughout the body that separates blood components from underlying microstructural elements of various organs. For many years, endothelial cells from different locations were considered to be similar both at the structural and functional levels. Consequently, from research carried out on the vascular endothelium – as the most widely studied type of endothelium – assumptions were made about endothelial cells in other locations. However, it has now clearly been demonstrated that the endothelium is a superfamily of cells sharing several common features but also expressing many differences that have an impact on function. 77 In light of these findings, aortic valve endothelial cells have generated significant interest in recent years both in terms of understanding normal valve physiology and, more importantly, in an attempt to elucidate the mechanisms of valvular disease. Such interest has stemmed from a paradigm of the pathophysiology of aortic valve disease which suggests that aortic valve endothelial dysfunction is the first step in a series of events leading to valve calcification, similar to the process that occurs in the vasculature during development of atherosclerosis. 78 On this basis, the role played by the endothelium is understood to be central to aortic valve function.
Until recently, aortic valve endothelial cells were thought to play only a minor role in valve physiology as valves were considered passive structures that opened and closed in response to changes in transvalvular pressure. More recently, several studies focusing on the structural and functional properties of aortic valve endothelial cells suggest that valvular endothelium possesses unique properties that distinguish it from other endothelial beds, particularly the endothelium lining the aorta with which it lies in direct continuity. The most striking difference between valvular and vascular endothelial cells is cell alignment with regards to the orientation of flow (Figure 5). Whereas the vascular endothelium throughout the body aligns with the long axis of the cell parallel to flow (except in areas of turbulent flow), 79,80 aortic valve endothelial cells are aligned perpendicular to the direction of flow. 81 This property of valve endothelial cells was reported originally by Deck et al. on the basis of electron microscopic analysis of explanted aortic valves with further validation from in vitro studies. 81,82 The response of cultured porcine aortic valve endothelial cells to unidirectional non-pulsatile laminar flow at 20 dynes/cm2 was compared with that of aortic (vascular) endothelial cells from the same animal. Whereas the vascular cells were found to be aligned parallel to flow after 24 hours, the aortic valve endothelial cells were aligned perpendicular to flow even without the presence of an aligned substrate. 81 These adaptations were found to be dependent on cytoskeletal reorientation, a process involving different mechanotransduction pathways in each type of endothelium. Laminar flow induced activation of Rho-kinase, phosphatidylinositol-3-kinase and calpain pathways in vascular endothelial cells, whereas activation of the latter was not required for cytoskeletal reorganization in valve endothelial cells. In addition, the proliferation rate was found to be higher in aortic valve endothelial cells than in vascular endothelial cells. 83
Figure 5.
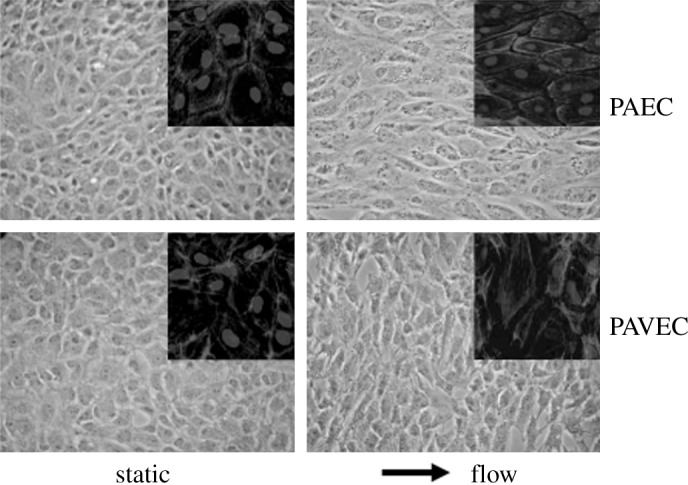
Porcine aortic valve endothelial cell (PAVEC) and porcine aortic endothelial cell (PAEC) morphology in static and fluid flow environments. Insets depict F-actin filament organization; arrow shows the direction of flow (from Butcher & Nerem, 2004). 81
Differences in flow pattern between the two sides of the valve are sensed by a thin layer of glycoproteins on the luminal surface of endothelial cells (the glycocalix), which communicates with the cytoskeleton and can activate several signaling pathways in response to flow. 84–87 One of these responses is the secretion of nitric oxide in response to shear stress. 86 In addition to nitric oxide, other bioactive molecules such as prostacyclin and endothelin-1 are expressed and released by flow-independent mechanisms. 88–90 These substances can interact with both the blood and the underlying valve interstitial cells and have a range of effects on the valve. However, the glycocalix has not yet been identified or characterized on the surface of aortic valves.
Studies on vascular endothelial cells have demonstrated that differences in shear stress correspond with the activation of different gene expression and signaling pathways, as evident from atherosclerotic plaques in areas of the vasculature characterized by low wall shear stress, such as the inner curvature of the aortic arch and arterial bifurcation points. 91,92 A study of the transcription profiles of 847 genes present in the valve and vascular endothelium revealed the common expression of 55 activated genes but differential activation of a further 48 genes.. Among those genes with a higher activation in the valvular endothelial cells were transcription factors associated with a higher proliferation rate, such as jun D and protein kinase C. 83 Notably, both vascular endothelium and valve endothelial cells expressed markers linked with calcification, including osteonectin, and bone morphogenic protein-7 and -9 (BMP-7 and BMP-9). Differences in gene expression profile between vascular and valvular endothelium were similarly revealed in a study of the response of cultured porcine aortic endothelial cells or aortic valve endothelial cells to shear stress stimulation. 93 In this study, Butcher et al. demonstrated a preferential expression of genes associated with chondrogenesis by aortic valve endothelial cells, whereas vascular endothelial cells expressed more genes associated with osteogenesis. Shear stress was found to reduce the expression of osteogenic genes.
Interstitial cells
The matrix of aortic valve cusps is rich with a mixed population of cells known as valve interstitial cells. Phenotypic studies suggest the presence of at least two distinct populations of cells within the matrix: a smooth muscle α-actin (SMA)-positive population of cells, and fibroblasts. SMA-positive cells are themselves considered to include pure smooth muscle cells and an “activated” subset of fibroblasts that express contractile proteins, called myofibroblasts. Myofibroblasts are thought to be involved in certain of the pathological mechanisms involving aortic valves, such as valve calcification or myxomatous valve degeneration. 94,95 This dual cell population confers on valve interstitial cells two main properties: the ability to contract and the capacity to synthesize extracellular matrix (ECM) components, thereby permitting a constant remodeling of the valve. Valve interstitial cells differ from cells derived from other sources. We have shown previously that valve-derived fibroblasts have different properties from dermal fibroblasts or pericardial cells. 96 In addition, valve interstitial cells express a range of specific skeletal and non-muscle cell markers such as β-myosin heavy chain and markers of the troponin complex. 97
Valve interstitial cells represent a dynamic population of cells that can move from one phenotype to another during the lifespan of a cell. 98 It has been proposed that valve interstitial cells comprise five identifiable phenotypes that define their molecular structure-function relationships (Table 1) 99 and include embryonic progenitor endothelial/mesenchymal cells, quiescent VICs (qVICs), activated VICs (aVICs), progenitor VICs (pVICs), and osteoblastic VICs (obVICs). These phenotypes exhibit a plasticity that allows them to convert from one form to another. Indeed, recent work from our laboratory has demonstrated the capacity of valve interstitial cells to transdifferentiate into other cell phenotypes including osteoblasts, adipocytes and chondrocytes. 100
Table 1.
Phenotypic-functional classification of aortic valve interstitial cells. Adapted from Liu et al. 99
| Cell type | Location | Function |
| Embryonic progenitor endothelial/mesenchymal cells |
Embryonic cardiac cushions | Give rise to qVICs through an activated stage or EMT |
| qVICs | Valve leaflet | Maintain valve structure and function and prevent angiogenesis |
| pVICs | Bone marrow, blood, valve leaflet | Enter valve or are resident Provide aVICs for valve repair May be CD34-, CD133- and/or S100+ |
| aVICs | Valve leaflet | SMA-containing VICs with activated cellular repair processes (proliferation, migration, remodeling) Respond to valve injury and abnormal hemodynamic or mechanical forces |
| obVICs | Valve leaflet | Calcification, chondrogenesis and osteogenesis in the leaflet. Secrete ALP, osteocalcin, osteopontin and bone sialoprotein |
Valve interstitial cells respond actively to their local cellular, matrix and hemodynamic environment through a range of specific molecules that allow them to adapt to their environment. Studies by Merryman et al. showed that valve interstitial cells isolated from different valves have different mechanical properties, cells from the left side of the heart being significantly stiffer. 101 This finding emphasises the direct correlation between the structure and function of a cell. Cell-cell communication at the interstitial cell level occurs via cell-cell junction molecules such as cadherins, desmosomal junctions and gap-junction proteins (connexin-26 and -43). 102 Cell-matrix communication is thought to be a major component of valvular responses to changing mechanical conditions. Integrins are present in high concentrations in valve interstitial cells and are thought to contribute to the transmission of force between the ECM and the cellular components of the valve. 103
The interstitial cells within the valve respond both to the mechanical environment and to bioactive substances present in the circulation or released locally by adjacent endothelial cells. Typically, these responses are involved in the regulation of the extracellular matrix, the tension within the valve, and the renewal of the cell population by cell proliferation. It has been shown, for example, that mechanical stretch can stimulate the production of collagen, secretion of the matrix remodeling enzymes MMPs and TIMPs, and the production of growth factors. 104–106
The extracellular matrix
The aortic valve is a thin structure measuring 300–700 μm in thickness. Despite a delicate appearance the valve cusps by necessity are extremely strong. This strength is due to the precise arrangement and composition of the extracellular matrix of the valve. Histologically, the valve is composed of three distinct superposed layers: the fibrosa, spongiosa and ventricularis (Figure 6). The fibrosa lies on the aortic side of the cusp and the ventricularis on the ventricular-facing side with the spongiosa between these two layers. The fibrosa represents about 45% of the thickness of the cusp and is rich in circumferentially oriented collagen fibers that provide structural strength to the cusps. In contrast, the ventricularis (20% of the thickness) is composed mainly of radially aligned elastin fibers, these conferring elasticity on the leaflets. The spongiosa layer (35% of the thickness) is rich in proteoglycans and glycosaminoglycans which have a high hydrous content so allowing smooth sliding of the fibrosa and ventricularis during the various phases of the cardiac cycle, thereby minimizing the repeated microtrauma associated with valve deformation. 107
Figure 6.
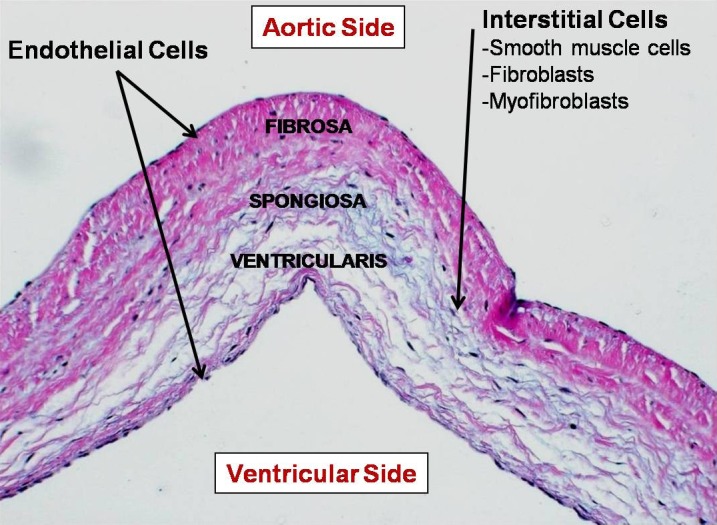
Microstructure of aortic valve cusps showing the characteristic trilaminar architecture.
In addition to defining physical characteristics of the valve cusps the extracellular matrix also provides a framework on and within which the cellular components of the valve reside. Both surfaces of the valve cusp are covered by a monolayer of endothelial cells that lie in direct continuity with the aortic endothelium on one side and the ventricular endocardium on the other, while the body of the cusp is populated by the interstitial cells. Thus, the relationship between the extracellular matrix and the valve cells is fundamental to the longevity of the valve. As outlined above, the valve interstitial cells provide a source of matrix proteins and remodeling enzymes that help sustain the integrity of the matrix during the lifetime of the valve. The matrix itself is responsible for transmitting to the cells the mechanical forces experienced by the valve. The integrin molecules that represent the anchoring points of the cells to the extracellular matrix are fundamental to the communication between these two components of the valve. It has been shown that valve interstitial cells express α1, α2, α3, α4, and α5 integrins to varying degrees, and predominantly β1 integrin but not β3 or β4 integrin. 108 Integrin binding is involved not only in mediating cellular attachment to the ECM but has also been shown to mediate transduction of other biological signals. These biological cues are important during development and throughout the life of the tissue in maintaining specialised cellular function and tissue regeneration. It has been demonstrated that integrins regulate proliferation, differentiation and action of growth factors in other non-valve cell types. 109–111 Currently, few studies have been made of the expression and functional role of integrins in valve cells.
Neuronal supply to the valve
In addition to mechanical stimuli, the valve also receives chemical signals through a rich and highly preserved network of nerves. The precise function of this neural network has yet to be elucidated, but there is increasing evidence that the network plays an active role in normal valve physiology as well as potentially playing a contributory role in valve disease. 112 Marron et al. have completed the most comprehensive anatomical study of the neural distribution in human heart valves to date. 113 These researchers showed that aortic valve innervation arises from ventricular endocardial plexuses, while some fibers originate from the aortic adventitial wall penetrating the leaflets through the raphe. 113,114 These nerves are localized predominantly in the ventricularis (the lower part of the leaflet). The entire length of the leaflets is covered with nerves except for the coapting edge, which is devoid of any nerve terminals. The left and right coronary cusps are equally innervated, whereas the non-coronary cusp is less extensively so. Due to its non-juxtaposition to the ventricular endocardium, the non-coronary cusp receives its nerve endings from both contiguous cusps (the right and left), which may account for the uneven nerve density. Although the mitral valve (which lies in continuity with the left and non-coronary cusps) is more densely innervated than the aortic valve, none of its nerve fibers extend into the aortic cusps. 114 Thus, each valve appears to be innervated and controlled independently. Few of the aortic valves studied have possessed large nerve terminals. Rather, groups of thin nerve terminals originate from large individual nerves that cross the entire leaflet. These thin nerves have a dot- and brush-like ending whereas the thicker proximal nerves appear to have a varicose-like structure. 114,115 According to De Biasi et al., these thicker nerves follow a circumferential distribution within the leaflets, mirroring the orientation of muscle fibers in the valve. 116,117 Nerve terminals present on the aortic valve express immunoreactivity for tyrosine hydroxylase and neuropeptide Y (NPY), which are both markers of postganglionic sympathetic nerves as well as acetylcholinesterase (AchE) activity (Figure 7). 113,118 Using double immunostaining techniques, nerve endings can be shown to be in close proximity to SMA-positive interstitial cells in the matrix, suggesting a direct role of these nerves on valve function. Electrical field stimulation of isolated porcine aortic valve has demonstrated the function of nitric-oxide containing nerves which were shown to mediate a relaxation (loss of tension) in cusp tissue. In contrast, similar experiments with portions of annular tissue resulted in contractile (increased tension) responses. 118 Interestingly, the responses to nerve stimulation were evident only in the left- and non-coronary annular regions, and not in the right-coronary portion of the annulus.
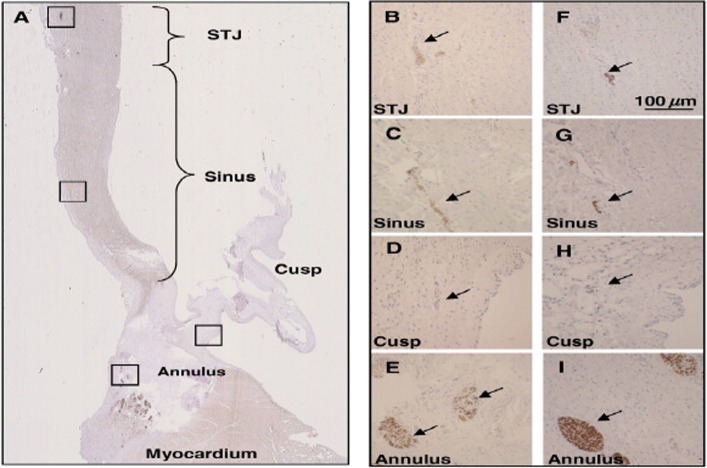
Figure 7.
Presence of sympathetic nerves in the aortic root. Panel A shows the whole aortic root (stained for tyrosine hydroxylase), Panels B–E (stained for tyrosine hydroxylase) and F–I (stained for neurofilament protein) show nerve bundles at a higher magnification in the sinotubular junction (STJ) sinus area, hinge region of the cusp, and in the annular region (from Chester et al., 2008). 118
Regulation of valve mechanics
Stiffness of the aortic valve cusps can be measured by deriving the Young's modulus from the stress-strain relationship of the tissue. To date, studies examining the mechanical properties of aortic valves have determined that the aortic valve cusps possess properties typical of viscoelastic materials. 119–121 Another important property of aortic valve cusps is anisotropy. Anisotropy concerns the possession of mechanical properties that are directionally dependent which, in the case of the aortic valve, refers to the radial and circumferential axes of the cusps. Indeed, it has been shown that aortic valve cusps are significantly stiffer in their circumferential axis than in the radial one. This could be due to the specific content and alignment of ECM proteins or cells in each axis. As both axes of the aortic valve are part of a single unit, inevitably they will influence one another in an inter-dependent manner. Nevertheless, reports published to date have focused predominantly on the use uniaxial tensile testing methods to analyze aortic valve cusps one axis at a time. Furthermore, most studies have focused on the baseline mechanical properties of the cusp, with no regard to the potential contribution of its cellular components. As it forms a monolayer on the surface of the aortic valve cusps the endothelium is exposed to all mechanical stimuli including shear stress. It is now well accepted that valve endothelial cells have unique signaling and gene expression profiles, different from those of endothelial cells in other parts of the body, including the aortic-derived vascular endothelial cells. 81,122 While in the vasculature endothelial cells are responsible for regulating vascular tone, the role of valve endothelial cells on valve mechanical properties remains undetermined.
Findings from a recent study provide evidence for the critical role of endothelial-derived nitric oxide and endothelin-1 in regulating the stiffness of the aortic valve cusp under conditions of physiological loading. 123 This work also demonstrated direct communication by paracrine mechanisms between endothelial-derived nitric oxide and underlying valve interstitial cells, in particular, SMA-positive cells. These findings represent the first evidence of the presence of active cell-mediated pathways regulating the mechanical properties of valves (Figure 8).
Figure 8.
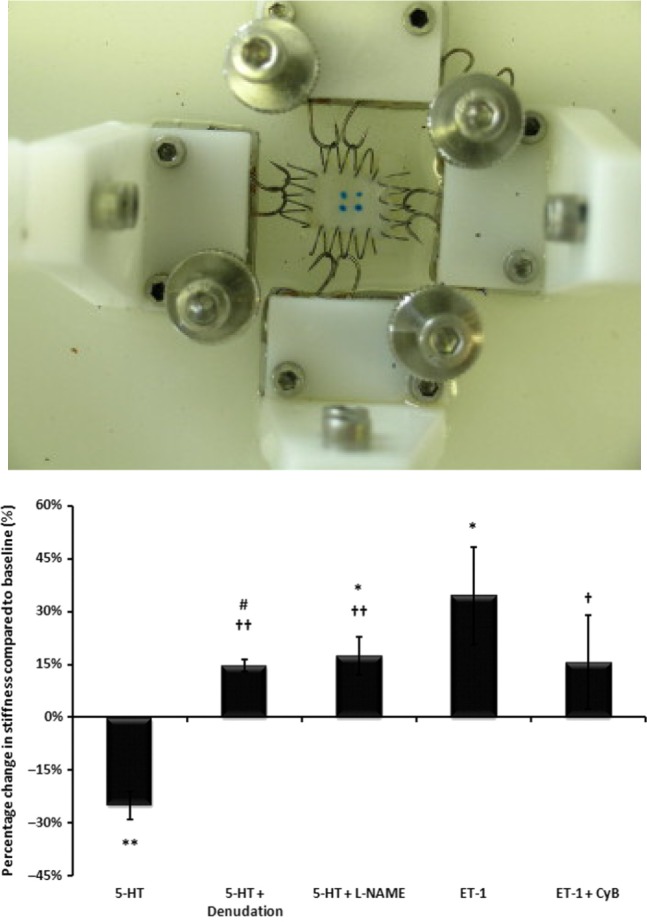
Porcine aortic cusp mounted on biaxial micromechanical testing device (top panel) and changes in the stiffness of the tissue induced by 5-HT, endothelial denudation, nitric oxide synthase inhibitor (L-NAME), endothelin-1, and cytochalasin B (CyB), an actin depolymerizing agent (bottom panel) (from El-Hamamsy et al., 2009). 123
The precise physiological significance of changes in the stiffness of the aortic valve is yet to be fully determined. It is without doubt, however, that the capability of a living valve to adapt its structure and function to the surrounding environment is of major clinical importance, both in terms of patient quality of life and survival, and the ability of the cusps to withstand major hemodynamic forces over many years. Changes in stiffness can optimize stress distribution across the surface of the cusps. Although the belly region is considered to be the major load-bearing region, in the absence of any regulation of the mechanical properties of the cusps, changes to the may result in the load experienced by the cell within the valve, with consequential alterations to the function of the cells. Indeed, it may be that the early valve calcification observed in some patients with bicuspid aortic valves could be due in part to a deficiency in eNOS, which renders the cusps incapable of adapting their stiffness to changing hemodynamic and humoural conditions. 124,125 Changes in the mechanical properties of the valve can also be beneficial in hypertensive patients with high diastolic pressure. An increased stiffness of the cusps could maintain an adequate surface of coaptation and ensure valvular competence despite the elevated afterload.
Together, the aortic annulus, the sinuses of Valsalva, the sinotubular junction and the aortic valve cusps comprise the component parts of the aortic root. These different parts operate in harmony to ensure adequate function of the aortic root, thereby minimizing ventricular workload and optimizing coronary flow. 2,126 Because of the extreme mechanical forces in the aortic root region, tissue stress is distributed between the sinuses and the cusps. 9–11,127 As a consequence, the stiffness of the cusps could have a direct impact on the function of the sinuses of Valsalva and on the magnitude of stresses at that level, which in turn could contribute to changes in structure, such as those observed in bicuspid aortic valve disease. 128 Indeed, Grande-Allen et al. showed that with every change in leaflet dimension, elastic modulus and surface of coaptation, the distribution of strains over the aortic leaflet is significantly altered with a marked increase at the free edge. 129 This increase in strain is thought to contribute to the aortic regurgitation observed in these patients.
An important consideration for in vivo studies of mechanical properties is that the half-life of NO in the cellular environment is short and so it may have an impact on the beat-to-beat regulation of valve biomechanics. On the other hand, ET-1 has a much longer duration of action and is more likely to affect the general tone of the valve, allowing it to adapt to its environment over a longer period of time. In addition, other endothelial-derived mediators, such as prostacyclin and endothelium-independent mediators, undoubtedly have an impact on valve mechanics. The true functional significance of such findings will be fully understood only once four dimensional functional imaging techniques are available as these will allow tracking of the instantaneous movements of the cusps and changes in Young's modulus.
The regulation of valve mechanics by the cellular components of the valve introduces a new paradigm into the role of the endothelium and contractile mechanisms in aortic valve pathology. It is well accepted that endothelial damage or dysfunction – through the loss of anti-inflammatory and anti-oxidant mediators – is an early occurrence in the cascade of events leading to structural valve disease. 130 The ability of the endothelium to influence valve mechanics suggests that endothelial dysfunction may result in changes in the mechanical responses to different stimuli, which potentially could contribute to the pathophysiology of valve disease. Following pharmacological inhibition or mechanical denudation of the endothelium, changes in the valve elastic modulus in response to endogenous mediators were found to be significantly altered. As explained previously, this may expose specific regions of the cusps to abnormally high stresses, so increasing the risk of local microtrauma which in turn will lead to further endothelial damage and structural degeneration. It is known that the formation of calcific lesions on the valve are associated with localised areas of high stress. 131 A loss of the capacity of aortic valves to adapt to their mechanical environment could lead to localised areas of altered stress and thereby initiate or accelerate structural valve degeneration.
Molecular mediators of valve mechanobiology
Biological sensing of mechanical forces is critically important for a proper acute response and longer term adaptation, and arguably especially so in valves where the mechanical environment is demanding and unavoidable. The basic mechanotransduction process involves the conversion of a mechanical stimulus into an electrical or chemical signal that can be propagated towards cellular responses. 132 Two major pathways of mechanotransduction have been classified: centralized and decentralized. Centralized mechanotransduction involves the direct interaction of the mechanical force on a surface receptor and its transmission into the downstream signaling cascades. Several examples of centralized mechanoreceptors have been proposed. Primary cilia are external microtubule-like structures commonly found on epithelial cell types including endocardial and epithelial cells. Recent work suggests that embryonic endocardial cells require intact cilia to respond to changes in fluid flow, presumably by the deflection of the cilia within the flow field. 67 The specific downstream mechanisms of cilia in valve endothelium are not known, but studies suggest the involvement of Alk5-mediated Smad phosphorylation. Despite being located in the highest shear stress domains of the heart tube, the valvulogenic endocardium appears to have no primary cilia, so the means by which cilia transduce cellular responses in the valve endothelium, remains uncertain. 133 In contrast, integrins reside on the basal surface of the endothelium and throughout the interstitium. Mechanosensation from integrins is often achieved through the development of focal adhesion complexes, including accessory proteins such as vinculin, talin, and focal adhesion kinase. 134 Actin filaments are tethered directly to the intracellular ends of focal adhesions via filamin A. 135 Mutations in filamin-A have been associated with mitral valve dysfunction, but a role in aortic valve disease has yet to be identified. 136 Integrins are heterodimeric adhesion receptors essential for cellular interaction with the extracellular environment. Recent studies suggest that integrin base adhesion helps control cellular tone and overall leaflet tension (Chester, unpublished observations), although whether this is an active contractile process or a passive mechanical connectivity remains unclear. The study of integrin-based mechanotransduction is challenging because of the high promiscuity and redundant cellular functions of the integrins. 103 However, signal transduction downstream of focal adhesion complexes is more streamlined. Integrin-based signaling can activate several secondary messengers, including small GTPase proteins such as RhoA, Rac1, and Src. These messenger molecules can affect many cellular functions, including filipodia protrusion, cell contractility, migration, and cell stiffness. In particular, RhoA-based signaling has been investigated for a variety of cellular processes in valve endothelial and interstitial cells. Inactivation of RhoA disrupts endothelial alignment to flow, 81 while inhibition in interstitial cells has been found to prevent nodule formation in culture. 137 In contrast, decentralized mechanotransduction is characterized by a lack of a central signaling pathway, rather a localized deformation of the cytoplasm due to mechanical stimuli induces biological changes. 138 In this case, the cell is viewed as a tensegrity structure with all the internal components being suspended within an equilibrium of tensed and contracted elements. 139 The heterogeneous distribution of components creates local unpredictable maxima and minima of mechanical stress within the cell that may initiate signaling to the nucleus. 138 Although attractive because it lacks any requirement for a specific receptor, this mechanotransduction hypothesis is challenging experimentally due to the difficulties associated with identifying the internal signal initiators. The nucleus, however, is mechanically connected to the cytoplasm and deforms as the cell deforms, supporting the observation that transcriptional activity can be affected rapidly. 140 Recent studies have identified a class of nuclear membrane proteins called lamins that are mechanosensitive and involved in a number of clinical conditions, including progeria and cancer. 141 Recently, we stretched mouse valve leaflets ex vivo and followed local cell and matrix fiber deformations in combination with global tissue stretch. 142 We established that local cell deformation and fiber reorganization occur to different degrees depending on the magnitude of global tissue stretch. In general, cellular deformation lags fiber alignment. Cellular deformation in response to the matrix environment was found to be dependent on the composition of the matrix, and this effect was established as age-dependent in valves. Interstitial cells from fibrillin-1 deficient valves deformed under global stretch in a manner similar to those from immature valves, which could be explained partly by the less mature composition of the tissue matrix, but occurred in a quite different hemodynamic environment. These findings allowed three different modes of mechanotransduction failure to be identified. First, the tissue might not be stretched adequately, resulting in a reduced or elevated stimulus that could adversely affect cell response. Second, cellular adhesion within its local ECM environment may be abnormal, resulting in greater or lesser than normal deformation under the same global stretch. Third, the mechanosensory apparatus in the cell may be inappropriately tuned such that it responds in a muted or overactive way to a specific mechanical stimulus. It is possible that more than one of these components could be adversely affected in valve disease, with obvious consequences for the identification of an effective ameliorating strategy. Further compounding the challenge is the fact that the natural stretch environment in vivo is strongly anisotropic and heterogeneous. 143 We and other researchers have demonstrated that endothelial and interstitial cells respond differently to different patterns of cyclic stretch and shear stress, which exist in a localized geometry. 101,144–147 Further work is required to gain an understanding of the way in which each mechanotransduction pathway participates in valve homeostasis and disease. However, it is clear that any clinically relevant disease mechanism and/or therapeutic strategy must accommodate the reality of mechanical signaling.
Aortic valve disease
Aortic valve calcification is a major clinical problem in elderly patients and those with bicuspid aortic valve disease for whom surgical replacement of the valve remains the only viable treatment. In recent years, it has become increasingly clear that aortic valve calcification is an active cell-driven process that shares some similarities with atherosclerosis, such as early endothelial dysfunction and activation of inflammatory mechanisms. However, despite these similarities, calcification of the aortic valve is recognised as a disease entity in its own right. 130
The structural changes seen in aortic valve disease were believed initially to be due to progressive degeneration of the valve due to “wear and tear”. However, it is now accepted that the onset and progression of aortic valve disease is an active process. Alterations to any of the mechanisms that constitute the passive and active dynamism of the valve can result in initial loss of regulation of cell phenotype and function; his in turn precipitates structural and eventually functional changes to the sophisticated mechanisms that the valve relies on for optimal function. In this regard, interstitial and endothelial cells of the aortic valve have recently been the focus of much attention because of the important role they are considered to play in valve disease. Some authors suggest that the behavior of valve interstitial cells is the main orchestrator of valve pathobiology. Studies from our group and others have shown that human aortic valve interstitial cells are involved directly in the calcification process of aortic valves through the expression of osteoblastic cell markers in response to mediators such as TGFβ. 100,148,149 In addition, it is now recognised that damage to the endothelium results in a loss of protective mechanisms that suppress changes in phenotype and function of the interstitial cells. However, the precise mechanism by which calcification occurs remains unclear. Some studies suggest that following apoptosis of aortic valve interstitial cells, calcium accumulates in the matrix vesicles that are released by these apoptotic bodies, leading to mineralization; other studies have identified a role for oxidant stress and an impaired responsiveness of platelets to nitric oxide, which was attributed to the effects of increased oxidant stress on the platelets. 150,151 Other groups propose a phenotypic transdifferentiation of aortic valve interstitial cells into osteogenic-like cells, which are capable of producing mineralized bone, and suggest that this process may be exacerbated by the stiffness of the valve and/or increased mechanical strain. 100,149,152–156 Yet others have suggested changes to the secretion of paracrine mediators from the endothelium, or the presence of resident stem cells within the valve matrix, which are capable of undergoing differentiation and bone formation, and could be the main culprits in valve disease. 157–159 Although not yet fully understood, the combined evidence suggests that a repertoire of mechanisms exist and contribute to the onset and progression of the disease, and these mechanisms involve the active participation of cells residing within the valve.
Role of valve cells in aortic stenosis
Histologically, aortic valve calcification occurs almost exclusively on the aortic side of the valve, which suggests there are differences in the protective capacity of the cells that cover the two surfaces of the valve. Simmons et al. developed an innovative technique to analyse separately the gene expression of aortic valve endothelial cells from each side of the valve. 160 The authors used this technique to identify the differential expression in situ of 584 genes, 161 and established that cells on the aortic surface expressed a more ‘pro-calcific’ repertoire of genes compared with cells on the ventricular surface of the valve. The differential expression of microRNAs, of which 30 were found to be shear-sensitive and three side-specific, may also contribute to the susceptibility of the aortic surface of the valve to calcification by Holliday et al. 146
In common with the vasculature, the valve endothelium is a rich source of paracrine mediators. These molecules are involved in the active dynamism of the valve, which promotes optimal function and provides protection against pro-calcifying factors. It is unsurprising, therefore, that changes to the functionality of the endothelium of the valve will result in loss of the protective capacity of these important cells. As outlined above, the valve endothelium plays an important role in modulating the stiffness of valve tissue. Risk factors such as high cholesterol, diabetes or smoking, all of which may increase oxidant stress on the endothelium, may induce changes in the function of the endothelium, and this may disrupt the balance between dilator and constrictor factors. Any such disruption would lead to localised alterations in the biomechanics and stress distribution of the valve. It has been established that calcific lesions on the surface of the valve occur preferentially at areas of high stress. However, whether or not a change in the secretory function of endothelial cells in these areas of the valve would have a contributory negative effect is yet to be determined.
One important mediator that has received much attention is nitric oxide. Production of nitric oxide in the valve endothelium is catalyzed by the enzyme type III nitric oxide synthase, and the principal stimulus for its release is the shear force exerted on cells by the passage of blood over the surface of the valve. Due to the difference in the types of blood flow experienced by the two sides of the valve (high velocity flow on the ventricular side, and low velocity, interrupted flow on the aortic side) a differing volumes of nitric oxide are released from each side of the valve. 158 It has been shown using in vitro models of valve calcification that drugs capable of generating nitric oxide (so called nitric oxide donors) are effective in ameliorating the effects of calcification. 159 On this basis, it is apparent that a reduction in the amount of nitric oxide released in response to flow, particularly on the aortic surface of the valve, would have the effect of reducing, or removing, the protective effect of this paracrine mediator. NO achieves its effects by causing an increase in the levels of cyclic guanosine monophosphate (cGMP). Another mediator expressed by valve endothelial cells, which also activates cGMP, is C-type natriuretic peptide (CNP). 162 This peptide also has anti-calcification effects on valve interstitial cells due to its activity at natriuretic peptide receptor-b. 163 In addition, it has been established that expression of CNP is reduced in the valves of patients with aortic stenosis. 162 The paracrine effects of NO and CNP suggest a role for a cGMP-dependent mechanism in the interstitial cells that suppresses the differentiation of cells within the valve towards an osteoblast/calcifying phenotype. However, the precise regulation of valve interstitial cell phenotypes remains unclear, as does the pathway followed by quiescent valve interstitial cells as they differentiate towards calcifying valve cells.
During development and in the post-natal period there are changes to the phenotype of the cells within the valve, the number of myofibroblasts present increasing significantly. 98 These myofibroblasts are gradually lost and subsequently the valve contains a more stable population of quiescent valve interstitial cells that maintain the structure and durability of the valve, as well as preventing angiogenesis. The role of these cells in the calcification process has recently been reviewed. 164 The changes observed during the process are associated with activation of inflammatory signalling pathways, one of which centres on nuclear factor-kB (NF-kB), a highly conserved transcription factor which translocates to the nucleus when activated, where it triggers the production of pro-inflammatory molecules. 165,166 Expression of this transcription factor has been observed in stenotic valves. 167 In addition, there is the revival of a number of mechanisms that are involved in the development of the valve, such as the Wnt/B-catenin pathway and the action of bone morphogenetic proteins (BMPs), principally BMP 2 and 4. 55 A key regulator in the activation of these pathways is TGFβ1, which has been shown to be present in diseased cardiac valves. 168 TGFβ1 stimulates the quiescent interstitial cells to transdifferentiate into an osteoblast-like cell through activation of the transcription factor RUNX2. 156 TGFβ1 may achieve this by stimulating BMPs, which in turn can activate Wnt signaling. 154,169 The precise pathway followed by the cells as they differentiate into a calcifying osteoblast-like cell is unclear. Some reports suggest that activation into a myofibroblast is a key step, while other studies suggest that these cells are not involved. An alternative hypothesis centres on the recruitment of bone marrow-derived cells that populate the valve following injury. We have recently obtained evidence of the involvement of smooth muscle cells in the calcifying processes. These cells, which are phenotypically, structurally and functionally distinct from myofibroblasts, can be found in calcified regions of human valves. 170 The study by Latif et al. also showed that the expression of specific smooth muscle cell markers could be induced in cultured valve interstitial cells by TGFβ1.
Treatment of aortic stenosis
Currently, it is thought that patients with mild forms of aortic valve disease are those with the early stages of the disease. Nevertheless, considering the various roles of the endothelium on valve physiology, the true impact of any therapy may not be realized unless treatment is administered in very early stages. Such early intervention brings with it important issues of cost and ethics that are beyond the scope of this article. Nevertheless, if predictive biomarkers or genetic screening tests can identify subjects at high risk of developing aortic valve disease, the real benefit of medical therapy aiming to restore the mechanisms that regulate active dynamism may be achieved only if administered early in order to prevent the long-term slow progression of local endothelial and aortic valve dysfunction.
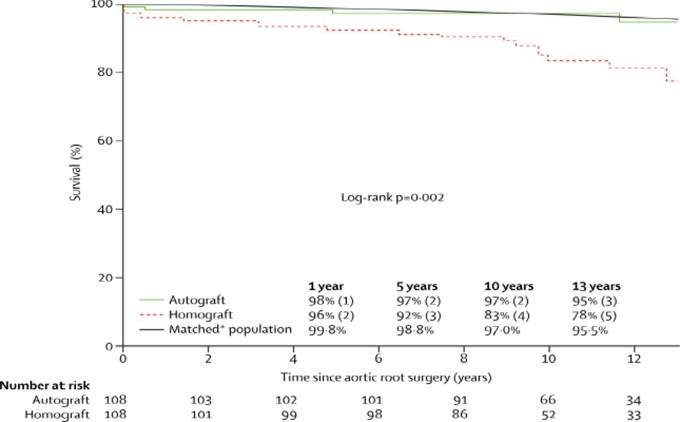
The only means to alter the natural history of aortic valve disease, at present, remains surgical intervention to replace the diseased valve. Studies of patients undergoing aortic valve replacement reveal an improvement in patient survival but not one that matches that of the general population. The first prospective randomized trial to demonstrate restored survival following aortic valve replacement to levels matching an age- and gender-matched population has recently been published. 1 The patients underwent a Ross procedure, whereby their own pulmonary valve was used to replace their diseased aortic valve and a homograft valve was then placed in the pulmonary position. The excellent results as reported are thought to be due to the long-term preserved viability of aortic valve cusps cells, which allows them to adapt to their changing local mechanical and hemodynamic conditions, and to ensure long-term valve durability as well as normal root physiology (Figure 9).
Figure 9.
Actuarial survival after Ross procedure (autograft) compared with homograft aortic root replacement. Survival over a 12 year period after a Ross procedure is indistinguishable from that of an aged matched population (from El-Hamamsy et al., 2010). 1
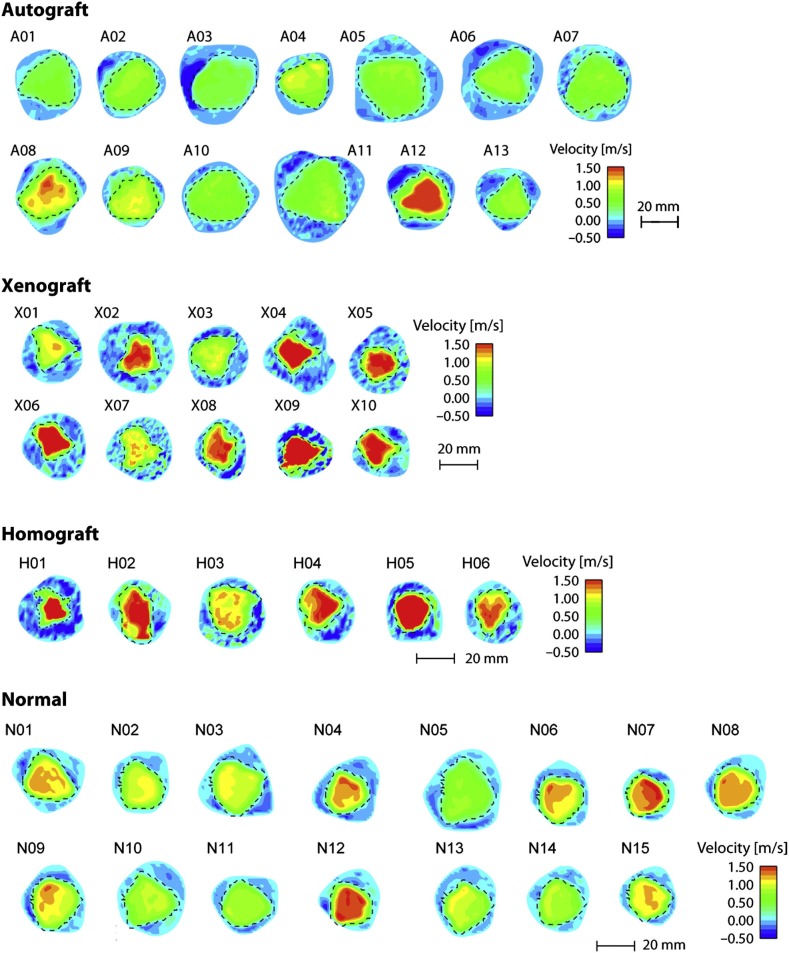
Compared with homografts, the Ross procedure has been found to result in enhanced survival following aortic root replacement in young adults. In addition, the procedure is associated with a lower likelihood of re-operation and an improved quality of life. It has also been shown that peak flow velocities are similar for normal valves and pulmonary autografts despite the larger root dimensions in the autograft (Figure 10). 171 This supports the hypothesis that implanting a living valve in the aortic position can result in significantly improved clinical outcomes. Although homographs, and some bioprosthetic valves, retain the same shape and ECM composition as the native valve, what they lack is living cells. The Ross procedure provides the only aortic valve substitute that contains living cells. The ability of these cells to continually provide a source of paracrine mediators and ECM proteins helps to explain the superiority of the Ross procedure over other valve replacement strategies.
Figure 10.
Effective orifice area. Velocity maps (color contours) and effective orifice areas (broken lines) visualized on cross sections above for autograft, xenograft, homograft native (normal) valve in the aortic position visualised at peak. Images are derived from 2-dimensional cine phase contrast magnetic resonance velocity mapping of valves approximately 10 years after implantation (from Torii et al., 2012). 171
Future directions
Unlike blood vessels, aortic valves are not tubular structures, the pattern of local blood flow is complex and highly variable, and no surrogate imaging or biomarkers of whole aortic valve dysfunction or valve endothelial dysfunction have yet been developed. In addition, computer simulations of valve motion based on high resolution imaging techniques also represent a significant challenge because of the highly dynamic nature of the aortic root. As shown by Dagum et al., the aortic annulus undergoes torsional deformations during the cardiac cycle as well as a significant longitudinal excursion which makes motion tracking necessary but complex. 7 To date, few groups have managed to adjust for dynamic aortic annular motion. Tracking the cusps constitutes an even greater challenge as their motion is non-linear, they undergo important conformational changes during the cycle (particularly bending changes), and the movements of the cusps dictate changes in the volume of blood flowing through them, this adding a particular element of difficulty. Consequently, descriptive findings on aortic valve physiology inevitably precede in vivo functional validation but, nevertheless, these offer important guidance concerning the level of detailed imaging required if we are to understand fully valve function in health and disease.
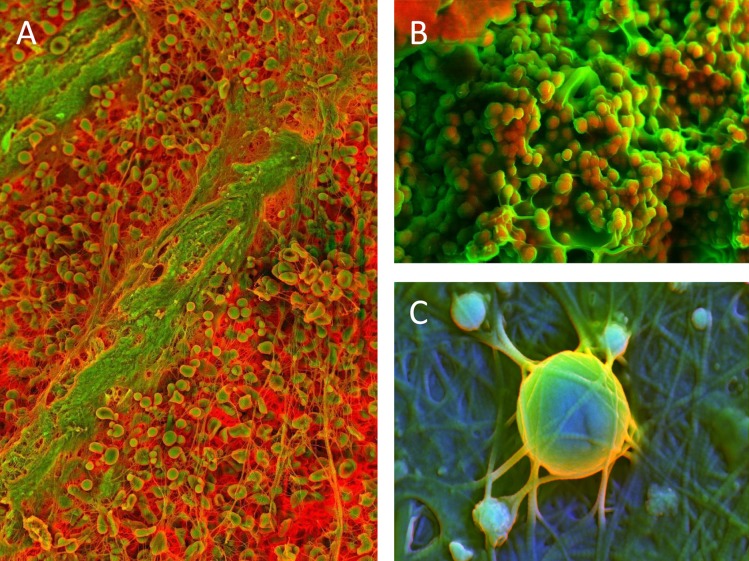
Molecular imaging represents an exciting area of research that has shown promise in characterizing the early inflammatory changes in diseased vessels. This approach has been used to examine the proteolytic and osteogenic markers in aortic valve disease. 172 Novel methods of electron microscopy, such as density-dependent colour scanning electron microscopy (DDC-SEM), have produced images that reveal the very early (sub-clinical) stages of calcific lesion formation. Calcified spherical particles are the first structures to be identified in these lesions (Figure 11A); these particles have also been found in calcific lesions at later stages, in which other nano/micro structures, such as calcified fibres (Figure 11B) and compact calcification, are also present (Figure 11C). 173 It is now clear that the application of molecular and nano-analytical imaging could have a significant effect in advancing our understanding of the mechanisms of aortic valve disease and the origins of calcific lesions. The ultimate objective behind research on the aortic valve is to alleviate the burden of disease in the population. Aortic valve disease remains a major source of morbidity and mortality in the developed world and its incidence is expected to increase significantly over the next 20–30 years. 174 Clinical trials aimed at improving endothelial function in patients have been discouraging and have consistently represented a vital gap between laboratory findings and clinical reality. However, few studies have focused on aortic valve disease, partly because it had long been considered a passive, “degenerative”, “wear and tear” disease. The largest trials, including studies in patients with mild forms of disease, have focused mainly on the role of statins in halting the progression of aortic valve disease and have produced disappointing results. Although not certain, it is likely that the absence of real observed benefit relates to the timing of treatment. However, it is now well established that signaling pathways and mechanisms involved in early morphogenesis remain operative in adult life and can contribute to pathophysiology of disease. Therefore, it is possible that the absence of observed benefit from the various therapeutic strategies aimed at improving endothelial function may be linked directly to the timing of initiation of treatment.
Figure 11.
Density-dependent color scanning electron micrographs of human aortic valve (AV) tissue. Orange color identifies denser material (calcium phosphate) and less dense structures are in green color (extracelular matrix). Micrographs acquired by secondary and backscatter electron detectors were combined in post-processing. A) Surface of AV with spherical calcified particles. B) Surface of AV with spherical calcified particles and calcified fibers. C) Surface of AV with spherical calcified particles and compact calcification. Scale bar = 2 μm.
Conclusions
Evidence that the aortic root performs several sophisticated functions that allow its constituent parts to change shape and function during the different parts of the cardiac cycle continues to accumulate. 7,175 Such changes can influence left ventricular workload and possibly coronary flow as well as stress distribution on the cusps. 9,126 In addition, aortic cusps can modify their stiffness in response to humoral and endothelial signals, allowing them to adapt to varying hemodynamic conditions. 123 An understanding of exactly how and why the living components of the valve contribute to its function will facilitate optimisation of current approaches for surgical replacement of the valve and, importantly, will guide development of the new generation of tissue engineered “living” valves that currently are the focus of numerous studies worldwide.
References
- 1.El-Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L, Melina G, Takkenberg JJ, Yacoub MH. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524–531. doi: 10.1016/S0140-6736(10)60828-8. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub MH, Kilner PJ, Birks EJ, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg. 1999;68(3):S37–S43. doi: 10.1016/s0003-4975(99)00745-6. [DOI] [PubMed] [Google Scholar]
- 3.Lentink D, Müller UK, Stamhuis EJ, de Kat R, van Gestel W, Veldhuis LLM, Henningsson P, Hedenström A, Videler JJ, van Leeuwen JL. How swifts control their glide performance with morphing wings. Nature. 2007;446(7139):1082–1085. doi: 10.1038/nature05733. [DOI] [PubMed] [Google Scholar]
- 4.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation. Cardiovasc Pathol. 2011;20(3):168–176. doi: 10.1016/j.carpath.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arjunon S, Rathan S, Jo H, Yoganathan AP. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng. 2013;41(7):1331–1346. doi: 10.1007/s10439-013-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sucosky P, Padala M, Elhammali A, Balachandran K, Jo H, Yoganathan AP. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng. 2008;130(3):035001. doi: 10.1115/1.2907753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagum P, Green GR, Nistal FJ, Daughters GT, Timek TA, Foppiano LE, Bolger AF, Ingels NB, Jr, Miller DC. Deformational dynamics of the aortic root: modes and physiologic determinants. Circulation. 1999;100(19):II54–II62. doi: 10.1161/01.cir.100.suppl_2.ii-54. [DOI] [PubMed] [Google Scholar]
- 8.Lansac E, Lim HS, Shomura Y, Lim KH, Rice NT, Goetz WA, Duran CM. Aortic root dynamics are asymmetric. J Heart Valve Dis. 2005;14(3):400–407. [PubMed] [Google Scholar]
- 9.Katayama S, Umetani N, Sugiura S, Hisada T. The sinus of Valsalva relieves abnormal stress on aortic valve leaflets by facilitating smooth closure. J Thorac Cardiovasc Surg. 2008;136(6):1528–1535. doi: 10.1016/j.jtcvs.2008.05.054. 35 e1. [DOI] [PubMed] [Google Scholar]
- 10.Robicsek F, Thubrikar MJ. Role of sinus wall compliance in aortic leaflet function. Am J Cardiol. 1999;84(8):944–946. doi: 10.1016/s0002-9149(99)00475-0. A7. [DOI] [PubMed] [Google Scholar]
- 11.Thubrikar MJ, Nolan SP, Aouad J, Deck JD. Stress sharing between the sinus and leaflets of canine aortic valve. Ann Thorac Surg. 1986;42(4):434–440. doi: 10.1016/s0003-4975(10)60554-1. [DOI] [PubMed] [Google Scholar]
- 12.Butcher JT, Simmons CA, Warnock JN. Mechanobiology of the aortic heart valve. J Heart Valve Dis. 2008;17(1):62–73. [PubMed] [Google Scholar]
- 13.Abu-Issa R, Kirby ML. Patterning of the heart field in the chick. Dev Biol. 2008;319(2):223–233. doi: 10.1016/j.ydbio.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281(1):78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Rodriguez RA, Krug EL, Reyes L, Villavicencio L, Mjaatvedt CH, Markwald RR. Bidirectional fusion of the heart-forming fields in the developing chick embryo. Dev Dyn. 2006;235(1):191–202. doi: 10.1002/dvdy.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238(1):97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 17.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313(5795):1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336(2):137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Cruz MV, Markwald RR, Krug EL, Rumenoff L, Sánchez Gómez C, Sadowinski S, Galicia TD, Gómez F, Salazar García M, Villavicencio Guzman L, Reyes Angeles L, Moreno-Rodriguez RA. Living morphogenesis of the ventricles and congenital pathology of their component parts. Cardiol Young. 2001;11(6):588–600. doi: 10.1017/s1047951101000932. [DOI] [PubMed] [Google Scholar]
- 21.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8(8):850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 22.Bernanke DH, Markwald RR. Effects of hyaluronic acid on cardiac cushion tissue cells in collagen matrix cultures. Tex Rep Biol Med. 1979;39:271–285. [PubMed] [Google Scholar]
- 23.Luna-Zurita L, Prados B, Grego-Bessa J, Luxán G, del Monte G, Benguría A, Adams RH, Pérez-Pomares JM, de la Pompa JL. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest. 2010;120(10):3493–3507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135(6):1179–1187. doi: 10.1242/dev.010694. [DOI] [PubMed] [Google Scholar]
- 25.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 27.Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118(5):649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Yamagishi T, Nakajima Y, Miyazono K, Nakamura H. Bone morphogenetic protein-2 acts synergistically with transforming growth factor-beta3 during endothelial-mesenchymal transformation in the developing chick heart. J Cell Physiol. 1999;180(1):35–45. doi: 10.1002/(SICI)1097-4652(199907)180:1<35::AID-JCP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185(1-3):146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 30.Holifield JS, Arlen AM, Runyan RB, Tomanek RJ. TGF-beta1, -beta2 and -beta3 cooperate to facilitate tubulogenesis in the explanted quail heart. J Vasc Res. 2004;41(6):491–498. doi: 10.1159/000081805. [DOI] [PubMed] [Google Scholar]
- 31.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248(1):170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 32.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208(2):530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 33.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283(5410):2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 34.Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn. 2004;229(1):42–53. doi: 10.1002/dvdy.10465. [DOI] [PubMed] [Google Scholar]
- 35.Bernanke DH, Markwald RR. Effects of two glycosaminoglycans on seeding of cardiac cushion tissue cells into a collagen-lattice culture system. Anat Rec. 1984;210(1):25–31. doi: 10.1002/ar.1092100105. [DOI] [PubMed] [Google Scholar]
- 36.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302(1):256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102(7):752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou B, Wu B, Tompkins KL, Boyer KL, Grindley JC, Baldwin HS. Characterization of Nfatc1 regulation identifies an enhancer required for gene expression that is specific to pro-valve endocardial cells in the developing heart. Development. 2005;132(5):1137–1146. doi: 10.1242/dev.01640. [DOI] [PubMed] [Google Scholar]
- 39.Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai HJ, Hove JR, Fraser SE, Dickinson ME, Gharib M. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312(5774):751–753. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- 40.Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100(10):1503–1511. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 41.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev Biol. 2003;258(2):252–263. doi: 10.1016/s0012-1606(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 42.Yalcin HC, Shekhar A, McQuinn TC, Butcher JT. Hemodynamic patterning of the avian atrioventricular valve. Dev Dyn. 2011;240(1):23–35. doi: 10.1002/dvdy.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007;236(5):1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat. 2003;202(4):327–342. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest. 2011;121(1):422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220(4601):1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 47.Qayyum SR, Webb S, Anderson RH, Verbeek FJ, Brown NA, Richardson MK. Septation and valvar formation in the outflow tract of the embryonic chick heart. Anat Rec. 2001;264(3):273–283. doi: 10.1002/ar.1162. [DOI] [PubMed] [Google Scholar]
- 48.van den Hoff MJ, Moorman AF, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, Wessels A. Myocardialization of the cardiac outflow tract. Dev Biol. 1999;212(2):477–490. doi: 10.1006/dbio.1999.9366. [DOI] [PubMed] [Google Scholar]
- 49.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230(2):239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 50.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392(6672):186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 51.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22(11):2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Lange FJ, Moorman AF, Anderson RH, Männer J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95(6):645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- 53.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366(2):111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colvee E, Hurle JM. Maturation of the extracellular material of the semilunar heart values in the mouse. A histochemical analysis of collagen and mucopolysaccharides. Anat Embryol (Berl) 1981;162(3):343–352. doi: 10.1007/BF00299977. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95(5):459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu YN, Norris RA, Mahler G, Recknagel A, Butcher JT. Transforming growth factor beta, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering. Tissue Eng Part A. 2010;16(11):3375–3383. doi: 10.1089/ten.tea.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakraborty S, Combs MD, Yutzey KE. Transcriptional regulation of heart valve progenitor cells. Pediatr Cardiol. 2010;31(3):414–421. doi: 10.1007/s00246-009-9616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24(2):171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 59.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106(4):712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Jr, Kadler KE, Lincoln J. Scleraxis is required for cell lineage differentiation and extracellular matrix remodeling during murine heart valve formation in vivo. Circ Res. 2008;103(9):948–956. doi: 10.1161/CIRCRESAHA.108.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MP, Yutzey KE. Twist1 directly regulates genes that promote cell proliferation and migration in developing heart valves. PLoS One. 2011;6(12):e29758. doi: 10.1371/journal.pone.0029758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292(2):292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 63.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012;52(3):689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec. 2000;259(3):248–262. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 65.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 66.Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, Fraser SE. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 2009;7(11):e1000246. doi: 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res. 2011;108(9):1093–1101. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buskohl PR, Jenkins JT, Butcher JT. Computational simulation of hemodynamic-driven growth and remodeling of embryonic atrioventricular valves. Biomech Model Mechanobiol. 2012;11(8):1205–1217. doi: 10.1007/s10237-012-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biechler SV, Potts JD, Yost MJ, Junor L, Goodwin RL, Weidner JW. Mathematical modeling of flow-generated forces in an in vitro system of cardiac valve development. Ann Biomed Eng. 2010;38(1):109–117. doi: 10.1007/s10439-009-9824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goodwin RL, Nesbitt T, Price RL, Wells JC, Yost MJ, Potts JD. Three-dimensional model system of valvulogenesis. Dev Dyn. 2005;233(1):122–129. doi: 10.1002/dvdy.20326. [DOI] [PubMed] [Google Scholar]
- 71.Tan H, Biechler S, Junor L, Yost MJ, Dean D, Li J, Potts JD, Goodwin RL. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev Biol. 2013;374(2):345–356. doi: 10.1016/j.ydbio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 72.Hu N, Christensen DA, Agrawal AK, Beaumont C, Clark EB, Hawkins JA. Dependence of aortic arch morphogenesis on intracardiac blood flow in the left atrial ligated chick embryo. Anat Rec (Hoboken) 2009;292(5):652–660. doi: 10.1002/ar.20885. [DOI] [PubMed] [Google Scholar]
- 73.Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93(1):77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 74.Sedmera D, Hu N, Weiss KM, Keller BB, Denslow S, Thompson RP. Cellular changes in experimental left heart hypoplasia. Anat Rec. 2002;267(2):137–145. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- 75.Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec. 1999;254(2):238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 76.Yalcin HC, Shekhar A, Nishimura N, Rane AA, Schaffer CB, Butcher JT. Two-photon microscopy-guided femtosecond-laser photoablation of avian cardiogenesis: noninvasive creation of localized heart defects. Am J Physiol Heart Circ Physiol. 2010;299(5):H1728–H1735. doi: 10.1152/ajpheart.00495.2010. [DOI] [PubMed] [Google Scholar]
- 77.Yano K, Gale D, Massberg S, Cheruvu PK, Monahan-Earley R, Morgan ES, Haig D, von Andrian UH, Dvorak AM, Aird WC. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood. 2007;109(2):613–615. doi: 10.1182/blood-2006-05-026401. [DOI] [PubMed] [Google Scholar]
- 78.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ’degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 79.Imberti B, Seliktar D, Nerem RM, Remuzzi A. The response of endothelial cells to fluid shear stress using a co-culture model of the arterial wall. Endothelium. 2002;9(1):11–23. doi: 10.1080/10623320210714. [DOI] [PubMed] [Google Scholar]
- 80.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101(8):2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24(8):1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 82.Deck JD. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res. 1986;20(10):760–767. doi: 10.1093/cvr/20.10.760. [DOI] [PubMed] [Google Scholar]
- 83.Farivar RS, Cohn LH, Soltesz EG, Mihaljevic T, Rawn JD, Byrne JG. Transcriptional profiling and growth kinetics of endothelium reveals differences between cells derived from porcine aorta versus aortic valve. Eur J Cardiothorac Surg. 2003;24(4):527–534. doi: 10.1016/s1010-7940(03)00408-1. [DOI] [PubMed] [Google Scholar]
- 84.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM. The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol. 2009;296(5):H1451–H1456. doi: 10.1152/ajpheart.00894.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355(1):228–233. doi: 10.1016/j.bbrc.2007.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 88.Ku DD, Nelson JM, Caulfield JB, Winn MJ. Release of endothelium-derived relaxing factors from canine cardiac valves. J Cardiovasc Pharmacol. 1990;16(2):212–218. doi: 10.1097/00005344-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 89.Misfeld M, Morrison K, Sievers H, Yacoub MH, Chester AH. Localization of immunoreactive endothelin and characterization of its receptors in aortic cusps. J Heart Valve Dis. 2002;11(4):472–476. discussion 6-7. [PubMed] [Google Scholar]
- 90.Pompilio G, Rossoni G, Sala A, Polvani GL, Berti F, Dainese L, Porqueddu M, Biglioli P. Endothelial-dependent dynamic and antithrombotic properties of porcine aortic and pulmonary valves. Ann Thorac Surg. 1998;65(4):986–992. doi: 10.1016/s0003-4975(98)00075-7. [DOI] [PubMed] [Google Scholar]
- 91.Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(2):158–161. doi: 10.1161/ATVBAHA.108.166736. [DOI] [PubMed] [Google Scholar]
- 92.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49(25):2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 93.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26(1):69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 94.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 95.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 2004;95(3):253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 96.Taylor PM, Allen SP, Yacoub MH. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J Heart Valve Dis. 2000;9(1):150–158. [PubMed] [Google Scholar]
- 97.Brand NJ, Roy A, Hoare G, Chester A, Yacoub MH. Cultured interstitial cells from human heart valves express both specific skeletal muscle and non-muscle markers. Int J Biochem Cell Biol. 2006;38(1):30–42. doi: 10.1016/j.biocel.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 98.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13(5):841–847. [PubMed] [Google Scholar]
- 99.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114(1):I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 101.Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290(1):H224–H231. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 102.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Brand N, Yacoub MH. Characterization of molecules mediating cell-cell communication in human cardiac valve interstitial cells. Cell Biochem Biophys. 2006;45(3):255–264. doi: 10.1385/CBB:45:3:255. [DOI] [PubMed] [Google Scholar]
- 103.Latif N, Sarathchandra P, Thomas PS, Antoniw J, Batten P, Chester AH, Taylor PM, Yacoub MH. Characterization of structural and signaling molecules by human valve interstitial cells and comparison to human mesenchymal stem cells. J Heart Valve Dis. 2007;16(1):56–66. [PubMed] [Google Scholar]
- 104.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296(3):H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PubMed] [Google Scholar]
- 105.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH, Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71(3):548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 106.Merryman WD, Lukoff HD, Long RA, Engelmayr GC, Jr, Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc Pathol. 2007;16(5):268–276. doi: 10.1016/j.carpath.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118(18):1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 108.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Molecules mediating cell-ECM and cell-cell communication in human heart valves. Cell Biochem Biophys. 2005;43(2):275–287. doi: 10.1385/CBB:43:2:275. [DOI] [PubMed] [Google Scholar]
- 109.Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127(9):1961–1969. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- 110.Mawatari K, Liu B, Kent KC. Activation of integrin receptors is required for growth factor-induced smooth muscle cell dysfunction. J Vasc Surg. 2000;31(2):375–381. doi: 10.1016/s0741-5214(00)90167-8. [DOI] [PubMed] [Google Scholar]
- 111.Tsuji T, Waga I, Tezuka K, Kamada M, Yatsunami K, Kodama H. Integrin beta2 (CD18)-mediated cell proliferation of HEL cells on a hematopoietic-supportive bone marrow stromal cell line, HESS-5 cells. Blood. 1998;91(4):1263–1271. [PubMed] [Google Scholar]
- 112.El-Hamamsy I, Yacoub MH, Chester AH. Neuronal regulation of aortic valve cusps. Curr Vasc Pharmacol. 2009;7(1):40–46. doi: 10.2174/157016109787354088. [DOI] [PubMed] [Google Scholar]
- 113.Marron K, Yacoub MH, Polak JM, Sheppard MN, Fagan D, Whitehead BF, de Leval MR, Anderson RH, Wharton J. Innervation of human atrioventricular and arterial valves. Circulation. 1996;94(3):368–375. doi: 10.1161/01.cir.94.3.368. [DOI] [PubMed] [Google Scholar]
- 114.Kawano H, Kawai S, Shirai T, Okada R. Morphological study on vagal innervation in human atrioventricular valves using histochemical method. Jpn Circ J. 1993;57(8):753–759. doi: 10.1253/jcj.57.753. [DOI] [PubMed] [Google Scholar]
- 115.Kawano H, Shirai T, Kawano Y, Okada R. Morphological study of vagal innervation in human semilunar valves using a histochemical method. Jpn Circ J. 1996;60(1):62–66. doi: 10.1253/jcj.60.62. [DOI] [PubMed] [Google Scholar]
- 116.De Biasi S, Vitellaro-Zuccarello L. Intrinsic innervation of porcine semilunar heart valves. Anat Embryol (Berl) 1982;165(1):71–79. doi: 10.1007/BF00304584. [DOI] [PubMed] [Google Scholar]
- 117.Steele PA, Gibbins IL, Morris JL. Projections of intrinsic cardiac neurons to different targets in the guinea-pig heart. J Auton Nerv Syst. 1996;56(3):191–200. doi: 10.1016/0165-1838(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 118.Chester AH, Kershaw JD, Sarathchandra P, Yacoub MH. Localisation and function of nerves in the aortic root. J Mol Cell Cardiol. 2008;44(6):1045–1052. doi: 10.1016/j.yjmcc.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Billiar KL, Sacks MS. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II–A structural constitutive model. J Biomech Eng. 2000;122(4):327–335. doi: 10.1115/1.1287158. [DOI] [PubMed] [Google Scholar]
- 120.Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp–Part I: Experimental results. J Biomech Eng. 2000;122(1):23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 121.Stella JA, Liao J, Sacks MS. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J Biomech. 2007;40(14):3169–3177. doi: 10.1016/j.jbiomech.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12(4):905–915. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 123.El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, Yoganathan AP, Chester AH. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53(16):1448–1455. doi: 10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 124.Aicher D, Urbich C, Zeiher A, Dimmeler S, Schafers HJ. Endothelial nitric oxide synthase in bicuspid aortic valve disease. Ann Thorac Surg. 2007;83(4):1290–1294. doi: 10.1016/j.athoracsur.2006.11.086. [DOI] [PubMed] [Google Scholar]
- 125.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation. 2000;101(20):2345–2348. doi: 10.1161/01.cir.101.20.2345. [DOI] [PubMed] [Google Scholar]
- 126.Davies JE, Parker KH, Francis DP, Hughes AD, Mayet J. What is the role of the aorta in directing coronary blood flow? Heart. 2008;94(12):1545–1547. doi: 10.1136/hrt.2008.144808. [DOI] [PubMed] [Google Scholar]
- 127.Grande-Allen KJ, Cochran RP, Reinhall PG, Kunzelman KS. Re-creation of sinuses is important for sparing the aortic valve: a finite element study. J Thorac Cardiovasc Surg. 2000;119(4 Pt 1):753–763. doi: 10.1016/S0022-5223(00)70011-0. [DOI] [PubMed] [Google Scholar]
- 128.El-Hamamsy I, Yacoub MH. A measured approach to managing the aortic root in patients with bicuspid aortic valve disease. Curr Cardiol Rep. 2009;11(2):94–100. doi: 10.1007/s11886-009-0015-y. [DOI] [PubMed] [Google Scholar]
- 129.Grande KJ, Cochran RP, Reinhall PG, Kunzelman KS. Mechanisms of aortic valve incompetence: finite element modeling of aortic root dilatation. Ann Thorac Surg. 2000;69(6):1851–1857. doi: 10.1016/s0003-4975(00)01307-2. [DOI] [PubMed] [Google Scholar]
- 130.Owens DS, Otto CM. Is it time for a new paradigm in calcific aortic valve disease? JACC Cardiovasc Imaging. 2009;2(8):928–930. doi: 10.1016/j.jcmg.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 131.Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58(3):304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 132.Ingber DE. The mechanochemical basis of cell and tissue regulation. Mech Chem Biosyst. 2004;1(1):53–68. [PubMed] [Google Scholar]
- 133.Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235(1):19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 134.Dumbauld DW, Michael KE, Hanks SK, Garcia AJ. Focal adhesion kinase-dependent regulation of adhesive forces involves vinculin recruitment to focal adhesions. Biol Cell. 2010;102(4):203–213. doi: 10.1042/BC20090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Y, Bismar TA, Su J, Xu B, Kristiansen G, Varga Z, Teng L, Ingber DE, Mammoto A, Kumar R, Alaoui-Jamali MA. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med. 2010;207(11):2421–2437. doi: 10.1084/jem.20100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, Roy E, McGregor L, Lynch SA, Newbury-Ecob R, Tran V, Young I, Trochu JN, Le Marec H, Schott JJ. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115(1):40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- 137.Gu X, Masters KS. Role of the Rho pathway in regulating valvular interstitial cell phenotype and nodule formation. Am J Physiol Heart Circ Physiol. 2011;300(2):H448–H458. doi: 10.1152/ajpheart.01178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Helmke BP, Davies PF. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30(3):284–296. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- 139.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 140.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 141.Lammerding J. Mechanics of the nucleus. Compr Physiol. 2011;1(2):783–807. doi: 10.1002/cphy.c100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gould RA, Sinha R, Aziz H, Rouf R, Dietz HC, 3rd, Judge DP, Butcher J. Multi-scale biomechanical remodeling in aging and genetic mutant murine mitral valve leaflets: insights into Marfan syndrome. PLoS One. 2012;7(9):e44639. doi: 10.1371/journal.pone.0044639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weiler M, Yap CH, Balachandran K, Padala M, Yoganathan AP. Regional analysis of dynamic deformation characteristics of native aortic valve leaflets. J Biomech. 2011;44(8):1459–1465. doi: 10.1016/j.jbiomech.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, Butcher JT. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8(5):1710–1719. doi: 10.1016/j.actbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kural MH, Billiar KL. Mechanoregulation of valvular interstitial cell phenotype in the third dimension. Biomaterials. 2014;35(4):1128–1137. doi: 10.1016/j.biomaterials.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301(3):H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Metzler SA, Digesu CS, Howard JI, Filip To SD, Warnock JN. Live en face imaging of aortic valve leaflets under mechanical stress. Biomech Model Mechanobiol. 2012;11(3-4):355–361. doi: 10.1007/s10237-011-0315-1. [DOI] [PubMed] [Google Scholar]
- 148.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83(3):946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 149.Mohler ER, 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8(3):254–260. [PubMed] [Google Scholar]
- 150.Ngo DT, Sverdlov AL, Willoughby SR, Nightingale AK, Chirkov YY, McNeil JJ, Horowitz JD. Determinants of occurrence of aortic sclerosis in an aging population. JACC Cardiovasc Imaging. 2009;2(8):919–927. doi: 10.1016/j.jcmg.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 151.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52(10):843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferdous Z, Jo H, Nerem RM. Strain magnitude-dependent calcific marker expression in valvular and vascular cells. Cells Tissues Organs. 2013;197(5):372–383. doi: 10.1159/000347007. [DOI] [PubMed] [Google Scholar]
- 153.Mohler ER, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 154.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Jr, Fullerton DA. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138(4):1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 155.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29(6):936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 156.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am J Pathol. 2010;177(1):49–57. doi: 10.2353/ajpath.2010.090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174(3):1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richards J, El-Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, Butcher JT. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182(5):1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kennedy JA, Hua X, Mishra K, Murphy GA, Rosenkranz AC, Horowitz JD. Inhibition of calcifying nodule formation in cultured porcine aortic valve cells by nitric oxide donors. Eur J Pharmacol. 2009;602(1):28–35. doi: 10.1016/j.ejphar.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 160.Simmons CA, Zilberberg J, Davies PF. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann Biomed Eng. 2004;32(10):1453–1459. doi: 10.1114/b:abme.0000042360.57960.2b. [DOI] [PubMed] [Google Scholar]
- 161.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96(7):792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Peltonen TO, Taskinen P, Soini Y, Rysa J, Ronkainen J, Ohtonen P, Satta J, Juvonen T, Ruskoaho H, Leskinen H. Distinct downregulation of C-type natriuretic peptide system in human aortic valve stenosis. Circulation. 2007;116(11):1283–1289. doi: 10.1161/CIRCULATIONAHA.106.685743. [DOI] [PubMed] [Google Scholar]
- 163.Yip CY, Blaser MC, Mirzaei Z, Zhong X, Simmons CA. Inhibition of pathological differentiation of valvular interstitial cells by C-type natriuretic peptide. Arterioscler Thromb Vasc Biol. 2011;31(8):1881–1889. doi: 10.1161/ATVBAHA.111.223974. [DOI] [PubMed] [Google Scholar]
- 164.Chester AH. Molecular and cellular mechanisms of valve calcification. Aswan Heart Cent Sci Pract Ser. 2011;4:19. [Google Scholar]
- 165.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18(5):527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 166.Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J. 2007;21(13):3553–3561. doi: 10.1096/fj.06-8059com. [DOI] [PubMed] [Google Scholar]
- 167.Steinmetz M, Skowasch D, Wernert N, Welsch U, Preusse CJ, Welz A, Nickenig G, Bauriedel G. Differential profile of the OPG/RANKL/RANK-system in degenerative aortic native and bioprosthetic valves. J Heart Valve Dis. 2008;17(2):187–193. [PubMed] [Google Scholar]
- 168.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75(2):457–465. doi: 10.1016/s0003-4975(02)04312-6. ; discussion 65-6. [DOI] [PubMed] [Google Scholar]
- 169.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. J Mol Med (Berl) 2009;87(1):17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 170.Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2014 doi: 10.1093/eurheartj/eht547. In Press. [DOI] [PubMed] [Google Scholar]
- 171.Torii R, El-Hamamsy I, Donya M, Babu-Narayan SV, Ibrahim M, Kilner PJ, Mohiaddin RH, Xu XY, Yacoub MH. Integrated morphologic and functional assessment of the aortic root after different tissue valve root replacement procedures. J Thorac Cardiovasc Surg. 2012;143(6):1422–1428. doi: 10.1016/j.jtcvs.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 172.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 173.Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12(6):576–583. doi: 10.1038/nmat3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–61. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 175.Lansac E, Lim HS, Shomura Y, Lim KH, Rice NT, Goetz W, Acar C, Duran CM. A four-dimensional study of the aortic root dynamics. Eur J Cardiothorac Surg. 2002;22(4):497–503. doi: 10.1016/s1010-7940(02)00405-0. [DOI] [PubMed] [Google Scholar]
- 176.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9(1):49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]