Abstract
Resistant hypertension is, by definition, a challenge to most physicians treating hypertension. Renal sympathetic denervation has shown promising early results in treating this condition. The SYMPLICITY HTN-3 is the most recent trial to report the effects of this technique on resistant hypertension. This review discusses this study and its surprising neutral results before ending with an overview of key lessons learned.
Keywords: Renal denervation, resistant hypertension, trial design
Introduction
SYMPLICITY HTN-1 1 and SYMPLICITY HTN-2 2 demonstrated that renal denervation (RDN) could achieve a significant reduction in office blood pressure in patients with resistant hypertension. At 36 months, 88 patients in SYMPLICITY HTN-1, a proof-of-concept first-in-man registry, achieved a 32/14 mmHg reduction in blood pressure from baseline. The more robust randomised SYMPLICITY HTN-2 trial showed a 33/11 mmHg drop in blood pressure against control. These positive results, supported by published data on thousands of other patients, added to the enthusiasm for this procedure. 3 There were few who doubted that RDN deserved a place in the hypertension specialist's armoury and thousands of procedures had already been performed mainly in Europe when SYMPLICITY HTN-3 was launched as a prerequisite for registration of renal denervation in the United States (US) according to the stringent standards of the Food and Drug Administration.
Symplicity Htn-3
SYMPLICITY HTN-3 was designed as a prospective, randomised, single-blind, sham-controlled trial. It compared the effect of RDN using the Symplicity™ catheter (Medtronic, Minneapolis, MN-USA) against a sham procedure (renal angiography) in 535 patients. 4 There was unequal randomisation, 2:1 (RDN:control). The study was conducted across 88 centres in the United States between October 2011 and May 2013.
The inclusion and exclusion criteria are illustrated in Table 1. In contrast to SYMPLICITY HTN-2, patients not only had initial evidence of a persistently elevated systolic blood pressure ≥ 160 mmHg in an office measurement but also had an elevated 24hr ambulatory systolic blood pressure ( ≥ 135 mmHg) two weeks later.
Table 1.
Key inclusion and exclusion criteria of the SYMPLICITY HTN-3 trial.
| Inclusion Criteria | Exclusion Criteria |
| Office SBP ≥ 160 mmHg on two clinic visits two weeks apart | Ineligible renal artery anatomy (main renal artery < 4 mm or with < 20 mm treatable length; multiple renal arteries; renal artery stenosis (>50%), previous renal artery intervention) |
| On stable maximally tolerated doses of 3 or more antihypertensive medications including a diuretic | eGFR < 45 ml/min/1.73 m2 |
| 24hr ABPM SBP < 135 mmHg | |
| Symptomatic postural hypotension | |
| Other conditions (including primary pulmonary hypertension, type 1 diabetes, severe valve stenosis, acute coronary syndrome or stroke in last 6 months) |
SBP – systolic blood pressure; eGFR – estimated glomerular filtration rate; ABPM – ambulatory blood pressure monitor.
The primary efficacy endpoint was defined as a change in office systolic blood pressure at 6 months. A primary safety composite endpoint consisting of mortality, end-stage renal failure and need for renal artery intervention amongst others was included. Change in 24hr ambulatory systolic blood pressure was a secondary endpoint. The study was powered to show a superiority margin of 5 mmHg or greater in office systolic blood pressure and 2 mmHg or greater in ambulatory systolic blood pressure.
At baseline, patients were on an average of five anti-hypertensive medications with four of these being at maximally tolerated doses and these proportions did not change significantly at the 6 month follow-up. Blinding was considered to be adequate with an index score >0.5.
Results
There was no difference between the two groups in the change in office systolic blood pressure at 6 months: − 14.13 ± 23.93 mmHg (RDN group) vs − 11.74 ± 25.94 mmHg (sham-procedure) representing a between group change of − 2.39 mmHg (p = 0.26). There was also no difference with respect to ambulatory systolic blood pressure ( − 6.75 ± 15.11 mmHg vs − 4.79 ± 17.25 mmHg, p = 0.98).
Subgroup analysis showed RDN may have been better at lowering blood pressure in non-black individuals and those less than 65 years of age. However, these changes were not found to be significant after adjusting for multiple comparisons.
The study did achieve its primary safety endpoint (composite of death, end-stage renal disease, embolic events resulting in end-organ damage, renovascular complications, or hypertensive crisis at 1 month or new renal-artery stenosis of more than 70% at 6 months), showing no difference between the groups (1.4% vs 0.6%; p = 0.67).
Discussion
SYMPLICITY HTN-3 was the best designed and largest randomised trial of RDN in resistant hypertension to date and demonstrated that RDN achieved a reduction of blood pressure in patients with resistant hypertension that was large in absolute terms but rendered non-significant when compared to the equally large drop of blood pressure in the sham group.
Before we ring the death knell on RDN we must first consider the explanations for this surprising outcome. These explanations fall in to two main camps: the detractors' camp who argues that RDN is truly ineffective at lowering blood pressure and we have been misled by earlier trials in to believing this novel therapy could eradicate resistant hypertension. For the supporters' camp, the argument is that RDN is in principle an effective treatment but that there were factors in this trial which erroneously led to a negative conclusion.
To address the first camp we need to consider the differences between SYMPLICITY HTN-3 and the first two SYMPLICITY HTN trials. SYMPLICITY HTN-3 required that resistant hypertension be confirmed on ambulatory blood pressure monitoring. The value of ambulatory blood pressure monitoring cannot be over-emphasized. In a cohort of 8295 patients classified as having resistant hypertension based on office readings alone, a staggering 37.5% of subjects were reclassified as having white coat hypertension after ambulatory monitoring. 5 SYMPLICITY HTN-2 did not require ambulatory measurements and hence may have recruited individuals with white coat hypertension. The second important difference is that SYMPLICITY HTN-3 included a single-blinded sham procedure where the earlier SYMPLICITY HTN trials did not. Unblinded patients and blood pressure assessors can introduce important biases. Health care professionals assessing office blood pressure may alter the manner in which they measure and record blood pressure to be more optimistic after their patient has undergone RDN and on the patient's part, they may become more compliant with standard medical therapy. 3,6 The combination of these two factors and the recruitment of some white coat hypertensives may have led to such a large drop in blood pressure in SYMPLICITY HTN-2 which would have not have been seen had there been a blinded sham procedure and ambulatory blood pressure monitoring at recruitment. 3
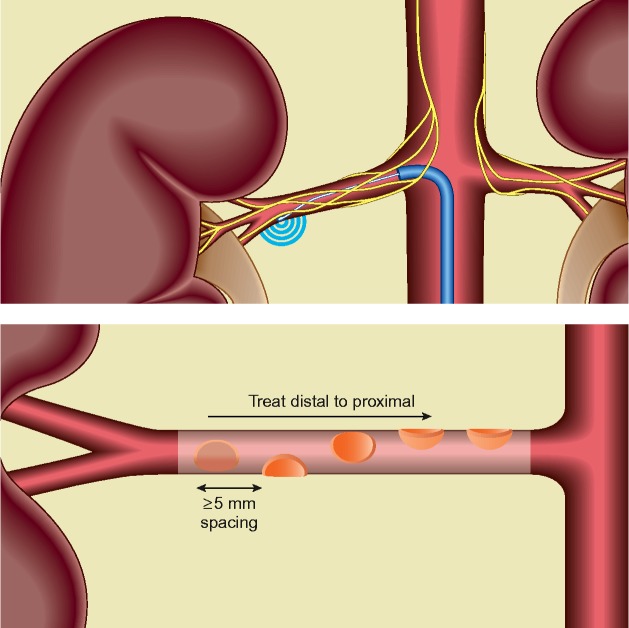
The supporters' camp emphasises the factors in SYMPLICITY HTN-3 which could have falsely lead to a negative result. After all it is not only the earlier SYMPLICITY HTN studies which provided support for this strategy but also the preclinical data showing the role of the renal sympathetic nerves both in hypertension 7 and heart failure. 8 One factor which may have led to a falsely negative study is the uncertainty whether the ablations were technically successful. All three SYMPLICITY HTN trials used the same catheter: the single electrode Medtronic Symplicity™ system (Figure 1). The single electrode system requires manipulation to apply energy in a helical pattern to the superior, inferior and lateral (anterior and posterior) walls of the renal artery (Figure 1). Technically, this can be difficult to achieve, not least because there is no intra-procedure marker of success and it is difficult to distinguish between an anterior and posterior position on fluoroscopy. 9
Figure 1.
Renal Denervation. The Symplicity™ Catheter is abutted to the renal artery wall using fluoroscopic guidance. Ablations are applied as shown in a helical pattern including at least one of each anterior, posterior, superior and inferior positions.
It is possible that the SYMPLICITY HTN-2 trialists (non-US) who had better experience of using the device were able to perform more effective renal denervation than the US operators in SYMPLICITY HTN-3. Only 26 interventionists out of 111 in SYMPLICITY HTN-3 had performed 5 or more denervations. This could explain some of the 19 mmHg discrepancy in the fall of systolic blood pressure in the denervation arms between SYMPLICITY HTN-2 and 3.
The final contribution to the failure of SYMPLICITY HTN-3 is the large drop in blood pressure seen in the sham arm. The SYMPLICITY HTN-2 control arm actually showed an increase in systolic blood pressure of 1 ± 21 mmHg (Figure 2). Thus if it had not been for the large drop in blood pressure in the sham group ( − 11.7 ± 25.9 mmHg), SYMPLICITY HTN-3 would have demonstrated the predicted difference of 10 mmHg. However, we cannot attribute the failure of SYMPLICITY HTN-3 to an “aberrant” drop in blood pressure in the sham arm because although uncommon, such a large decrease in blood pressure in the control arm of a trial is not unique to SYMPLICITY HTN-3. The ASPIRANT study was a randomised, multi-centre, double-blind, placebo controlled trial of spironolactone in patients with resistant hypertension (n = 117). 10 Though it recruited patients whose average blood pressure was lower than that of the SYMPLICITY HTN-3 cohort, it too demonstrated a large placebo effect of − 8.1 ± 14.8 mmHg (Figure 2) but unlike SYMPLICITY HTN-3 it showed that spironolactone significantly lowered systolic blood pressure beyond this placebo effect. Placebo effects do abate with time and conversely RDN effects on blood pressure have been shown to increase with time. 11 SYMPLICITY HTN-3 reported only blood pressure changes at 6 months. It would be of interest to review the 12-month findings. Patient selection is also extremely important. Expecting RDN to be a panacea for patients of all age, race and sex was probably too simplistic and a consequence of our incomplete understanding of the mechanism of the antihypertensive effect of RDN therapy. If black patients were excluded from analysis, the difference in systolic ambulatory blood pressure was greater than 6 mmHg and we know that black patients have a worse response than white hypertensives to beta blockers and ACE-inhibitors and better response to diuretics and vasodilators.
Figure 2.
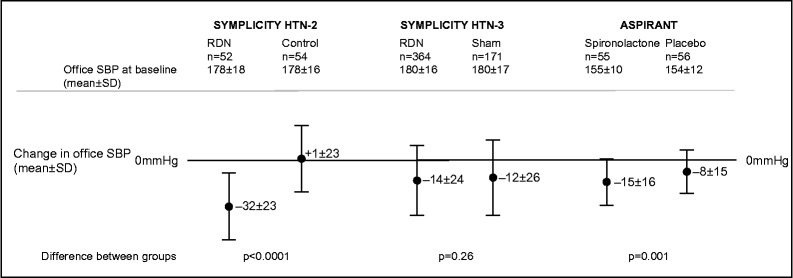
A graph summarising the effect sizes of active and comparator in three contemporary trials of resistant hypertension.
Determining which of these camps is correct is important not only for the future of RDN in hypertension but also in its application to other conditions such as heart failure. If the problem is technical (incomplete denervation) then more effective technology using multi-electrode catheters in experienced hands should lead to better outcomes in future trials of RDN. Another problem may be patient selection; none of the SYMPLICITY HTN trials used evidence of a raised sympathetic tone as an inclusion criterion.
What have we learned?
Despite SYMPLICITY HTN-3 being a well-designed study, a number of uncertainties have arisen which prevent us from asserting that denervation is definitely ineffective at treating resistant hypertension. We can learn some lessons from the design of the earlier SYMPLICITY HTN trials which both promoted the use of RDN but have now left us with some uncertainty. The first simple lesson is the importance of using ambulatory blood pressure to accurately diagnose resistant hypertension and exclude those with white coat hypertension. Another lesson learned is the importance of blinding and a sham procedure even if this carries risk to those in the sham arm because the small risk in a small number of patients early in development can be justified by limiting exposure of a larger number of patients to the procedure later in development if it ultimately fails to show a benefit.
A final comment is on the need to develop a method to confirm incomplete or ineffective renal denervation. This is a critical uncertainty which is essential to determine whether it is the therapy which is ineffective or the technique of achieving denervation is ineffective. Catheters have now been developed with multiple electrodes, balloon mounting and irrigation. If it is ultimately shown that incomplete denervation was the downfall of SYMPLICITY HTN-3 then these new technologies may be the saviour of RDN.
Funding Sources
HP and CH are funded by the NIHR Cardiovascular BRU at the Royal Brompton Hospital, London, UK.
Authors' Contributions
All authors have read and approved the final manuscript.
References
- 1.Krum H, Schlaich M, Whitbourn R, Sobotka P, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham W, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 2.Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 3.Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: insights from meta-analysis of antihypertensive drug trials of 4121 patients with a focus on trial design: the CONVERGE report. Heart. 2013;99:1579–1587. doi: 10.1136/heartjnl-2013-304238. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 5.De la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 6.Howard JP, Cole GD, Sievert H, Bhatt DL, Papademetriou V, Kandzari DE, Davies JE, Francis DP. Unintentional overestimation of an expected antihypertensive effect in drug and device trials: mechanisms and solutions. Int J Cardiol. 2014;172:29–35. doi: 10.1016/j.ijcard.2013.12.183. [DOI] [PubMed] [Google Scholar]
- 7.Patel HC, di Mario C. Renal denervation for hypertension: where are we now? Br J Cardiol. 2013;20:142–147. [Google Scholar]
- 8.Patel H, Rosen S, Lindsay A, Hayward C, Lyon A, di Mario C. Targeting the autonomic nervous system: measuring autonomic function and novel devices for heart failure management. Int J Cardiol. 2013;170:107–117. doi: 10.1016/j.ijcard.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 9.Patel H, Dhillon P, Mahfoud F, Lindsay A, Hayward C, Ersnt S, Lyon A, Rosen S, di Mario C. The biophysics of renal sympathetic denervation using radiofrequency energy. Clin Res Cardiol. 2013;103:337–344. doi: 10.1007/s00392-013-0618-6. [DOI] [PubMed] [Google Scholar]
- 10.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Jarovsky J, Vaclavik T, Husar R, Kocianova E, Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 11.Krum H, Schlaich M, Sobotka P, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler M. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]