Abstract
Patients with Hunter syndrome (mucopolysaccharidosis II) present with skeletal dysplasia including short stature as well as CNS and visceral organ involvement. A previous study on Hunter syndrome indicated an impact on brain and heart involvement after hematopoietic stem cell therapy (HSCT) at an early stage but little impact after enzyme replacement therapy (ERT) (Tanaka et al. 2012). Meanwhile, impact on growth in patients with Hunter syndrome treated with ERT and HSCT has not been compared until now. We recently developed baseline growth charts for untreated patients with Hunter syndrome to evaluate the natural history of growth of these patients compared to unaffected controls (Patel et al., 2014).
To assess impact of ERT and HSCT on growth, clinical data were obtained from 44 Japanese male patients with MPS II; 26 patients had been treated with ERT, 12 patients had been treated with HSCT, and 6 had been treated with both ERT and HSCT. Height and weight were compared to untreated patients and unaffected controls from the previous study.
We demonstrated 1) that MPS II patients, who had been treated with either ERT or HSCT, had increased height and weight when compared to untreated patients, and 2) that HSCT and ERT were equally effective in restoring growth of MPS II patients.
In conclusion, HSCT should be considered as one of the primary therapeutic options for early stage treatment of MPS II, as HSCT has also been reported to have a positive effect on brain and heart valve development (Tanaka et al. 2012).
Abbreviations: DS, dermatan sulfate; ECM, extracellular matrix; ERT, enzyme replacement therapy; GAG, glycosaminoglycan; HSCT, hematopoietic stem cell therapy; HS, heparan sulfate; I2S, iduronate 2-sulfatase; LSD, lysosomal storage disorder; MPS II, mucopolysaccharidosis II
Keywords: Hunter syndrome, Growth impact, Hematopoietic stem cell therapy, Enzyme replacement therapy, Height
Highlights
-
•
Effect on growth of patients with MPS II was evaluated.
-
•
Both ERT and HSCT provided a significant impact on growth.
-
•
No clear difference in growth was observed between therapies.
-
•
Patients showed improved weight gain.
-
•
The reader will understand therapeutic efficacy on growth in patients with MPS II.
1. Introduction
Mucopolysaccharidosis II (MPS II, Hunter syndrome; OMIM #309900) is a lysosomal storage disorder (LSD) caused by a mutation in the X-linked gene IDS. This results in deficiency of the lysosomal enzyme, iduronate 2-sulfatase (I2S), in the metabolic pathway that leads to degradation of the glycosaminoglycans (GAGs), dermatan sulfate (DS) and heparan sulfate (HS) [1]. This enzyme deficiency blocks the stepwise degradation of DS and HS, resulting in the accumulation of DS and HS in lysosomes and extracellular matrix (ECM) of a wide range of tissues. Although the primary result of enzymatic deficiency is accumulation of GAGs and secondary substrates, the mechanism causing the pathogenesis of the disease still remains unknown [2]. MPS II has a prevalence rate between 1:100,000 and 1:170,000 male births [3], [4], and is the most prevalent form of MPS disorders in Asian countries where it accounts for around 50% of all MPS cases diagnosed [5].
Patients with MPS II have a wide range of symptoms caused by the disease that affect multiple different organ systems. The severe phenotype is more than twice as prevalent as the attenuated form of the disease, and is characterized by profound CNS involvement and is usually fatal in early childhood if not treated [6]. Patients with the attenuated phenotype may survive into adulthood without CNS involvement [1], [7].
Inguinal and/or umbilical hernia, course facial features, otitis and nasal obstruction along with recurrent upper respiratory tract infections are some of the early diagnostic cues in MPS II [8]. Extensive and aberrant Mongolian spot is also a characteristic finding of Hunter syndrome in Japan [9]. The skeletal abnormalities in MPS II are similar regardless of clinical phenotype, and are common among other types of MPS disorders. The skeletal abnormalities are characterized in general as a thickening of the long bones with irregular ossification centers [1].
The major causes of morbidity and mortality in patients with MPS II are due to abnormal heart development; 82% of patients have cardiovascular signs and symptoms [1]. Detrimental CNS involvement in MPS II manifests most often as progressive cognitive degeneration, although individuals with the attenuated form of the disease have minimal CNS involvement. Patients may be able to reach early developmental milestones; however, psychomotor delays usually occur during the late infantile period [1]. Surgical procedures to correct inguinal and/or umbilical hernia are often performed before the diagnosis of Hunter syndrome [10].
There are currently two major therapies for patients with MPS II; enzyme replacement therapy (ERT) and hematopoietic stem cell therapy (HSCT).
ERT has been used to treat several types of MPS disorders, and for MPS II a recombinant form of human I2S is used (idursulfase, Elaprase®, Shire Human Genetic Therapies, Inc., Lexington, MA, USA). Clinical trials have shown that ERT decreases urinary GAG levels and improves measures of pulmonary function, walking ability, and visceral organ function [11], [12], [13], [14]. Several limitations for conventional ERT have been noted: 1) limited efficacy for hard connective tissues including bone and heart valves due to avascularity of these tissues, 2) difficulty in compliance due to required 4–5 hour intravenous infusions every week, and 3) the high cost of treatment. Moreover, the enzyme cannot pass through the blood–brain barrier, and conventional ERT will have no effect on the CNS aspects of the disease [1], [2], [15], [16].
Results from the Hunter Outcome Survey (HOS) show that response to treatment is not associated with either clinical phenotype or age at initiation of treatment [11]. MPS II patients treated with ERT continued to grow; however, their growth was not directly compared to untreated patients [11], [17].
HSCT has been shown to be effective in the treatment of several MPS diseases and other LSDs. HSCT has been indicated for MPS II as part of standard care in Japan, leading to the fact that over 50 patients were treated by HSCT until now. The efficacy of HSCT on visceral organs was clear and similar to that of ERT. Tanaka et al. demonstrated that HSCT is effective in relieving both brain (CNS involvement) and heart defects, when treatment is performed at an early stage, before signs of brain atrophy and heart valve issues [18]. However, a systematic description of the impact of HSCT on growth of patients with MPS II has not been reported.
Thus, there remains little knowledge on the relative effectiveness of the two therapies in treating the skeletal deformities and overall growth in patients with MPS II. We recently resolved one of the underlying limitations of such studies by establishing growth charts for untreated patients with Hunter syndrome [19].
In this report we explored the impact of the two therapeutic options on growth of patients with MPS II. We evaluated the therapeutic effect on growth between patients treated with ERT or HSCT, in comparison with untreated patients with MPS II and age-matched healthy controls. We also propose therapeutic options for MPS II considering improvement or attenuation of other signs and symptoms.
2. Materials and methods
2.1. Study subjects
Patients diagnosed with MPS II by enzyme assay were recruited at Gifu University to participate in this study by providing clinical history and growth data. A questionnaire was sent to local medical centers in Japan where patients with MPS II had received ERT and/or HSCT. Informed consent was obtained from the patients and/or their guardians by the attending physicians. The study was approved by the Institutional Review Boards (IRB) at Gifu University and at the Nemours/Alfred I. duPont Hospital for Children. Seventy-two patients treated with ERT and/or HSCT were enrolled in this study. 28 patients were excluded to focus on the 44 Japanese male patients who had started treatment at or before 8 years of age so that we could observe the impact on growth. Twenty-six of these patients were treated with ERT alone, and 12 were treated with HSCT alone. The remaining 6 patients were treated initially with ERT, and then later with HSCT. Their growth data was added to the respective treatment groups during the times when they were receiving treatment. There were 4 sibling cases included in our study.
2.2. Anthropometric measurements
Measurement of height was performed using the health-check system in Japan (infantile health-check at public healthcare centers in local government, health-check in schools and/or in hospitals). Anthropometric measurements were taken using a standard technique and included body length and weight. Until 3 years of age, the lengths of patients were measured in the supine position using a liberometer. After 3 years of age, height was taken in a standing position using a stadiometer, and was fairly objective, although height measurements might be affected due to structural abnormalities. Measurements were made an average of 3 times per patient.
2.3. Treatment
ERT was administered 1 mg/kg weekly. Patients who had received HSCT and had confirmed donor cell engraftment were chosen to participate in this study.
2.4. Statistical analysis
Data used for the construction of growth curves were age (years and months), height (cm), and weight (kg). Body mass index (BMI) was calculated from height and weight data by dividing weight by height squared (kg/m2). Statistical analyses for mean and standard deviation were then performed on the data sets. Student's t-test was performed on the height, weight, and BMI data for patients according to therapy type to determine the statistical relationship between these two patient groups. These data were also compared with the values in untreated patients with MPS II [19]. The standard control data was taken from established Japanese data sets for healthy male controls issued by the Japanese Ministry of Health, Labor, and Welfare. Control data from untreated subjects were obtained from a different study that utilized identical testing within the same laboratory. This study was based upon the data obtained from 111 Japanese male patients with MPS II. On average, height and weight measurements were obtained at 8 time points for each patient [19].
3. Results
3.1. Demographics
The mean age of symptom onset was 24 ± 20 months and the mean age at diagnosis was 37 ± 21 months. Mean age at start of ERT was 4.49 ± 2.35 years, while mean age at start of HSCT was 4.68 ± 1.63 years, suggesting that no significant difference in the age at start of therapy was observed. Of the 44 patients included in our study, 35 had the severe phenotype, and 9 had the attenuated phenotype. One patient diagnosed with the attenuated phenotype was treated with HSCT, while 7 were treated with ERT, and one was treated with ERT then HSCT. Eleven patients with the severe phenotype were treated with HSCT, while 19 were treated with ERT, and 5 were treated first with ERT and successive HSCT.
3.2. Heights
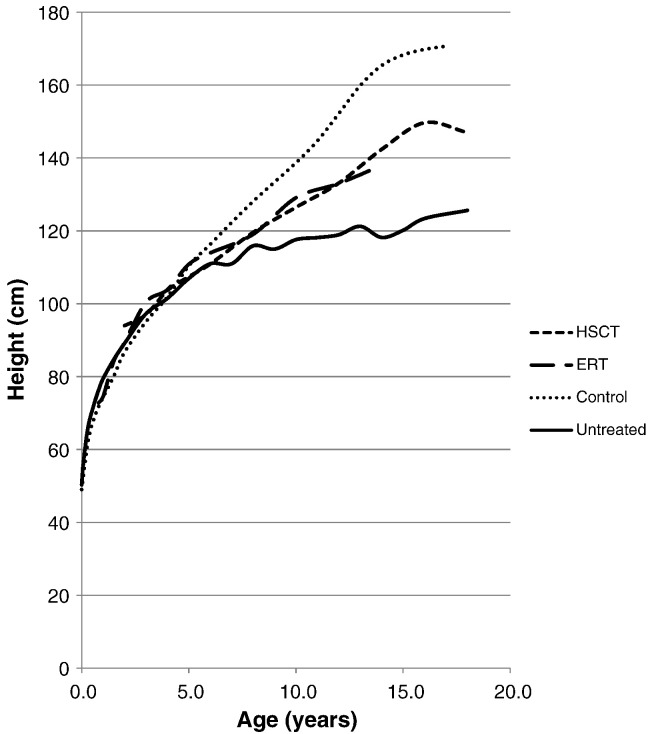
ERT-treated patient heights were not significantly different from untreated patient heights for children younger than 8 years of age, but older treated patients were significantly taller (Table 1, Fig. 1), suggesting that long-term observation is required to see an impact on growth.
Table 1.
Height of patients with MPS II undergoing therapy.
| HSCT (cm) |
t-Test to untreated group |
ERT (cm) |
t-Test to untreated group |
t-Test between two treatment groups |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | n | Mean | SD | p | n | Mean | SD | p | p |
| 9.5 months | 1 | 72.80 | |||||||
| 1 y | 1 | 74.50 | |||||||
| 1.5 y | 4 | 84.25 | 3.96 | 0.83 | |||||
| 2 y | 1 | 94.00 | 4 | 89.38 | 3.12 | 0.88 | |||
| 3 y | 1 | 97.00 | 5 | 100.40 | 4.84 | 0.22 | |||
| 4 y | 4 | 103.65 | 3.03 | 0.25 | 6 | 103.87 | 3.28 | 0.15 | 0.92 |
| 5 y | 9 | 107.42 | 8.02 | 0.87 | 5 | 110.84 | 5.17 | 0.17 | 0.35 |
| 6 y | 12 | 110.92 | 4.95 | 0.97 | 8 | 113.95 | 5.72 | 0.20 | 0.24 |
| 8 y | 9 | 119.57 | 6.00 | 0.13 | 11 | 119.06 | 5.08 | 0.11 | 0.84 |
| 10 y | 10 | 126.47 | 8.25 | < 0.01 | 7 | 129.10 | 5.43 | < 0.005 | 0.44 |
| 12 y | 9 | 133.12 | 10.36 | < 0.005 | 7 | 133.01 | 9.93 | < 0.01 | 0.98 |
| 14 y | 6 | 142.38 | 8.02 | < 0.005 | 1 | 138.00 | – | – | |
| 16 y | 4 | 149.70 | 3.23 | < 0.005 | |||||
| 18 y | 2 | 147.00 | – | – | |||||
Fig. 1.
Mean height for MPS II patients undergoing ERT or HSCT. Dotted line shows the mean heights for normal healthy controls. Solid line shows the mean height for untreated patients.
Similarly, HSCT treated patients were not significantly different from untreated patients for children younger than 8 years of age but were significantly taller than untreated patients from 10 to 18 years of age (Table 1, Fig. 1). The children approaching maturity were 20 cm taller than the age-matched untreated patients.
Heights of treated or untreated patients were similar to normal controls for patients younger than 6 years of age (Fig. 1). After 6 years of age, growth of untreated patients is significantly slowed (19 and Fig. 1). While the growth rate of ERT treated patients is less than that of the controls it is clearly faster than that of untreated patients (Fig. 1).
In this study both treatments showed a similar effect in improving growth curves for MPS II (Table 1, Fig. 1).
3.3. Body weight
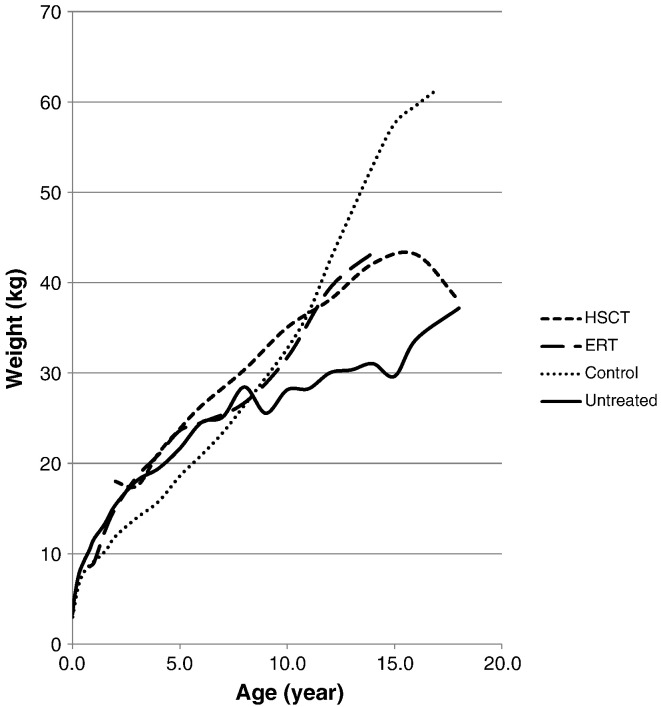
Patients younger than 8 years old appeared to be heavier than age-matched controls, with or without ERT- or HSCT-treatment (Fig. 2). Weight gain in ERT-treated patients younger than 10 years of age was not significantly different to that in untreated patients (Table 2, Fig. 2). After the age of 10, weight gain slows in untreated patients and weight is significantly lower in 12 year old patients compared to the age-matched treated patients (Table 2).
Fig. 2.
Mean weight for MPS II patients undergoing ERT or HSCT. Dotted line shows the mean weights for normal healthy controls. Solid line shows the mean height for untreated patients.
Table 2.
Weight of patients with MPS II undergoing therapy.
| HSCT (kg) |
t-Test to untreated group |
ERT (kg) |
t-Test to untreated group |
t-Test between two treatment groups |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | n | Mean | SD | p | n | Mean | SD | p | p |
| 9.5 months | 1 | 8.56 | |||||||
| 1 y | 1 | 9.04 | |||||||
| 1.5 y | 4 | 12.45 | 1.33 | 0.29 | |||||
| 2 y | 1 | 18.00 | 4 | 15.05 | 1.34 | 0.64 | |||
| 3 y | 1 | 17.50 | 5 | 18.53 | 2.08 | 0.68 | |||
| 4 y | 5 | 21.10 | 2.79 | 0.27 | 6 | 20.94 | 1.48 | 0.08 | 0.91 |
| 5 y | 8 | 23.84 | 3.83 | 0.19 | 5 | 23.63 | 2.03 | 0.12 | 0.90 |
| 6 y | 12 | 26.35 | 6.42 | 0.39 | 8 | 24.51 | 3.76 | 1.00 | 0.43 |
| 8 y | 10 | 30.36 | 7.45 | 0.53 | 11 | 26.68 | 2.41 | 0.39 | 0.16 |
| 10 y | 9 | 35.04 | 9.72 | 0.07 | 7 | 31.70 | 5.13 | 0.15 | 0.39 |
| 12 y | 9 | 38.13 | 8.50 | 0.02 | 7 | 39.41 | 9.61 | 0.04 | 0.79 |
| 14 y | 6 | 42.12 | 8.01 | 0.02 | 1 | 43.30 | – | – | |
| 16 y | 4 | 43.13 | 5.07 | 0.03 | |||||
| 18 y | 2 | 38.00 | – | – | |||||
Similarly, HSCT-treated patients were not significantly heavier than untreated patients up to 10 years of age and older, HSCT-treated patients were heavier than age-matched controls (Table 2). The two 18-year-old HSCT-treated patients were not as heavy as the 16 years old, but the significance of this change for just 2 patients is not clear. Increases in body weight for ERT- and HSCT-treated patients were similar (Table 2, Fig. 2).
3.4. BMI
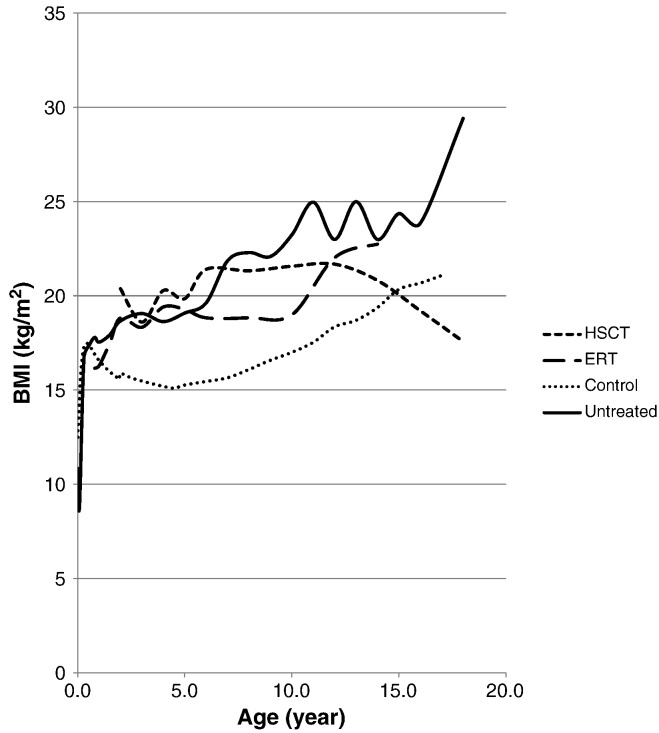
BMI of untreated patients with MPS II is significantly higher than that of controls (Fig. 3). BMI was significantly lower in ERT-treated patients in the 10 year age group and in HSCT-treated patient in the 16 year age group compared to age-matched untreated patients, but were not significantly different in other age-groups (Table 3, Fig. 3).
Fig. 3.
Mean BMI for MPS II patients undergoing ERT or HSCT. Dotted line shows the mean BMI for normal healthy controls. Solid line shows the mean height for untreated patients.
Table 3.
BMI of patients with MPS II undergoing therapy.
| HSCT (kg/m2) |
t-Test to untreated group |
ERT (kg/m2) |
t-Test to untreated group |
t-Test between two treatment groups |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | n | Mean | SD | p | n | Mean | SD | p | p |
| 9.5 months | 1 | 16.15 | |||||||
| 1 y | 1 | 16.29 | |||||||
| 1.5 y | 4 | 17.49 | 0.41 | 0.15 | |||||
| 2 y | 1 | 20.37 | 4 | 18.83 | 1.07 | 0.80 | |||
| 3 y | 1 | 18.60 | 5 | 18.33 | 0.36 | 0.05 | |||
| 4 y | 4 | 20.27 | 3.87 | 0.46 | 6 | 19.40 | 0.95 | 0.19 | 0.69 |
| 5 y | 8 | 19.84 | 2.87 | 0.53 | 5 | 19.27 | 1.73 | 0.85 | 0.66 |
| 6 y | 12 | 21.40 | 4.98 | 0.28 | 8 | 18.83 | 2.09 | 0.43 | 0.13 |
| 8 y | 9 | 21.32 | 5.56 | 0.70 | 11 | 18.83 | 1.42 | 0.06 | 0.22 |
| 10 y | 8 | 21.56 | 6.26 | 0.51 | 7 | 18.99 | 2.70 | < 0.01 | 0.32 |
| 12 y | 9 | 21.68 | 4.96 | 0.56 | 7 | 22.02 | 3.23 | 0.62 | 0.87 |
| 14 y | 6 | 20.81 | 3.98 | 0.39 | 1 | 22.74 | – | – | |
| 16 y | 4 | 19.20 | 1.66 | 0.04 | |||||
| 18 y | 2 | 17.57 | – | – | |||||
3.5. Case reports
3.5.1. Sibling cases
There were 4 sibling pair cases included in our study, one of which gave enough data to compare growth charts. One patient with a severe form was treated with HSCT at 2 years of age. His height and body weight were 89 cm and 12.8 kg at the time of treatment. He was followed up to 16 years of age when his height and weight was 154.0 cm and 47.0 kg. A sibling of this patient with a similar severe form was also treated with HSCT, but at 5.4 years of age. At 18 years of age he reached a height and weight of 149.0 cm and 40.0 kg. The patient treated at the age of 2 was approximately 8.0 cm taller and 5.8 kg heavier than his sibling at ages 6 to 16.
3.5.2. Combined therapies
Six patients were initially treated with ERT before being treated with HSCT. The mean time difference between start of ERT and start of HSCT was 2.08 ± 0.93 years. ERT was discontinued immediately or 2–3 months later after treatment with HSCT. From the limited data from these patients it is not clear that there is any difference in growth characteristics between single or combined therapies.
4. Discussion
In this study, we evaluated the overall growth of patients with MPS II treated with ERT and/or HSCT, when treatment started at or before 8 years of age. Our study has demonstrated 1) that both ERT and HSCT provide a significant positive impact on the growth of patients with MPS II, and 2) that there is no significant difference in growth impact between patients treated with either ERT or HSCT from 4 to 12 years of age. These findings indicate that both treatments are equally effective at improving the overall growth in patients with MPS II.
Although one would predict that the more rigorous treatment in the HSCT-treated group, that includes chemotherapy and radiation, impairs growth, the gain in height and weight of these patients was comparable to that of the ERT-treated patients (Table 1, Fig. 1). In both treatment groups, patients showed initial overgrowth in height, when compared to the healthy control subjects. By 14 years of age, the mean heights of ERT- and HSCT-treated patients were lower than those in the healthy control group (Fig. 1). These findings show that the growth in either treatment is still not restored to that of the age-matched control group, although both treatment groups improved height significantly, when compared to untreated patients.
The overgrowth in weight of young children with MPS II compared to healthy control subjects was not reduced by either treatment. This is not unexpected as most measurements in this age range were from patients who were just beginning treatment. It is not clear whether this overgrowth could be reduced if patients were treated at an earlier age or if this overgrowth is of significance in disease progression. It was noteworthy that although the mean weight of ERT-treated patients fell below that of the healthy control subjects after 8 years of age, the mean weight of ERT-treated patients was not more than one SD below the mean weight in the age-matched control subjects (Fig. 2). Both treatment groups also showed increased body weights compared to untreated patients. Thus improved weight is a clear marker of treatment effect.
The time lag between the start of treatment and its effect on growth could be due to the avascular region of the growth plate, causing the infused or expressed enzyme to have poor access. Even if either of the treatments is able to quickly reduce GAG levels in urine, blood, and visceral organs, it may take more time to correct pathology in bone lesions.
The youngest patient treated with HSCT was 2 years of age, and he reached a height of 154 cm at 16 years of age, showing the most growth among any HSCT- or ERT-treated patients in our study. A sibling of this patient was treated with HSCT at 5.4 years of age, and was on average 8 cm shorter and 5.8 kg lighter than his brother at the same age between 6 and 16 years. This unique case indicates that early intervention with HSCT may provide a major benefit for patients. The mean age at which patients were treated with HSCT in this study was 4.68 years of age. The limited number of patients did not enable a statistical analysis of potential benefit of early treatment, but it is likely that early diagnosis and treatment of patients with HSCT will have a larger impact on growth. Accumulation of more data on growth charts of patients treated with HSCT at a younger age will help to clarify the potential benefit of early treatment on growth and development.
It is of interest that the more significant differences between treated and untreated patients were in height rather than in weight. The less significant changes in body weight resulted in the fact that the BMI of patients remains still higher during treatment compared to normal controls (Table 3, Fig. 3). Increased BMI with age is a hallmark of untreated patients with MPS II (Fig. 3). A high BMI might stem from an overall lower activity in daily life for patients, with or without treatment. In clinical trials, ERT-treated patients showed a significant increase in the 6-minute walk test (6MWT) [20], [21], [22], but this may not have an effect on the daily activity of patients receiving treatment. We recommend that physicians should consider the effect of overweight or obesity to the general health and activity of daily life in patients with Hunter syndrome during the course of treatment and encourage increased activity. More data is required to determine whether treatment can reduce BMI as the children approach adulthood.
In this study, we could not determine whether one treatment was more effective than the other, based on growth charts. Although the diagnosis of phenotype (severe or attenuated) is classified by CNS involvement [6], growth charts for patients with severe or attenuated phenotypes are similar [19]. This is consistent with the observation that patients respond equally to treatment with ERT, regardless of CNS involvement or puberty status [11]. This study had several limitations that impact interpretation. One limitation is the restricted number of patients in each treatment group. Comparisons between the two therapies could not be achieved at all ages, although statistical analysis of patients between 4 and 12 years of age showed no significant difference in effect on growth.
A second limitation is that the starting age of treatment varies in clinical practice. Schulze-Frenking et al. showed that patients who had begun treatment with ERT before 10 years of age had larger improvements in growth than patients who had started treatment after 10 years of age [17]. Therefore, we focused on patients who started therapy before 8 years of age. The mean age at the start of treatment with ERT (4.5 ± 2.4 years) was similar to the mean age at the start of treatment with HSCT (4.7 ± 1.6 years). The limited range of treatment start dates did not allow a detailed analysis of effect of age at treatment on growth even though the staring age is predicted to provide a substantial impact on growth.
A third limitation of our study is that the data collected was from patients from one ethnic background. While this data allows for an accurate tool to track the progression of the disease and any treatments given to Japanese patients, it may not accurately reflect the growth in other ethnicities.
Another limitation is that the accuracy of body length measurements might vary due to contractures, an inability to stand erect, and lack of investigator cooperation in performing the measurements that could skew measurements, particularly for younger patients.
It has been reported that combined treatment with ERT and HSCT has an additive effective in reducing GAG concentrations in kidney, heart, and lung in an MPS II mouse model [23]. Six patients in our study had been given ERT for 2.08 ± 0.93 years before HSCT, but ERT continued only for a short time after HSCT. Consequently, we could not determine whether there was any additive effect of prior or overlapping ERT treatment on the growth.
It has been proposed that HSCT provides a limited effect or no impact to improve the clinical features of MPS II, with a high mortality rate (Table 4). Therefore, HSCT should not have been considered as the first treatment option for MPS II. Recent long-term follow-up studies on MPS I, II, III, IVA, VI, and VII patients treated with HSCT allowed the therapeutic efficacy of HSCT to be re-assessed. Recent data indicate that HSCT does show improvement in brain and bone involvement, while conventional ERT did not provide effectiveness in brain and heart valves [[18], [24], [25], personal communications with Dr. Chinen, Dr. Tanaka, Dr. Kato, Dr. Yabe, and Dr. K. Orii]. Results presented in this study also show that HSCT has a positive effect on growth that is indistinguishable from the effect of ERT.
Table 4.
Advantages and disadvantages of ERT or HSCT for MPS patients.
| ERT | HSCT | References | |
|---|---|---|---|
| Advantage |
|
|
|
| Disadvantage |
|
|
Personal communications
|
Impact on CNS involvement for patients with a severe type is limited by age and HSCT is preferred before the signs and symptoms of CNS disease appear. In this context, the age limitation could be applied to patients with a severe form.
A potential advantage of HSCT for treating MPS II is that marrow-derived donor macrophages can provide a continuous secreting source of enzyme and that these cells can gain access to sites throughout the body where GAGs are stored. The clinical consequence of HSCT relies on 1) the age of the patient at the time of transplantation, 2) the severity of clinical phenotype, 3) the type of donor, and 4) the course of preparative regimen. Tanaka et al. reported long-term efficacy of HSCT on urinary GAG level and improvement/attenuation of CNS and heart involvement for patients with MPS II. They concluded 1) that urinary GAG concentration was remarkably lower in HSCT-treated patients compared to age-matched untreated patients and reduced more compared to ERT-treated patients, and 2) that HSCT showed improvement or stabilization of brain MRI, activity of daily life, and heart valves, compared to ERT-treated patients [18]. Especially, more impact on therapeutic efficacy for CNS involvement was shown to the group of the patients whose clinical signs and symptoms appear after 2 years of age.
Advantages of HSCT over ERT include (Table 4); 1) one time permanent treatment if engraftment is successful, 2) active enzyme secreted from bone marrow can access many tissues including brain, bone, and heart valves, 3) continuous expression of the enzyme during the life-time of the patient, 4) improvement of cognitive function with early treatment, and 5) it is cost-effective (less than the cost of ERT for one year). Disadvantages of HSCT include 1) a chance of mortality during treatment (although the risk has been diminished substantially), 2) age limitation for a severe phenotype of patients with CNS involvement, 3) limited by patient health condition, 4) limited by expertise at medical facility, and 5) requires a rigorous regimen in hospital before and after HSCT for 2–3 months (Table 4). In early studies, the mortality rate of HSCT was approximately 20–25% [26], [27]. With advanced techniques and earlier introduction of HSCT, the survival rate after treatment of MPS II improved to 88.5% during the period from 1990 to 2003 [18]. All eighteen patients with MPS treated by HSCT since 2000 have survived (personal communication with Dr. Yabe, Tokai University). Thus, HSCT is much safer than before, although survival rates could still depend upon the institution and expertise of their staff.
Overall, we propose that HSCT should be considered as a therapeutic option for MPS II, depending on a careful consideration of the risk/benefit ratio. Prospective investigation for improvement or attenuation of CNS involvement is required in order to fully evaluate the impact of HSCT compared with ERT.
One of the reasons why the mortality rate of HSCT was high during initial attempts in 1980–1990 is that patients who underwent HSCT were already at an advanced or even a terminal stage of disease progression. It will not always be suitable for patients with advanced stage disease to complete the rigorous regimen of HSCT. However, with the advanced technology and awareness of the disease, early diagnosis is becoming more feasible, and, therefore, patients with MPS II can receive HSCT when their health condition is favorable at an early stage.
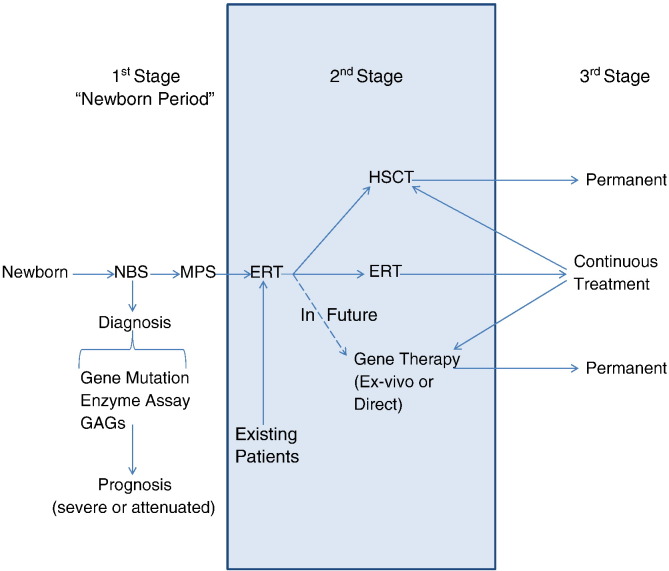
Several models of newborn screening (NBS) for MPS are now under development [28], [29], [30], [31], [32]. Once NBS is established leading to diagnosis of asymptomatic patients, a tailor-made therapeutic approach for the individual patient with MPS can be devised by a well-trained multi-disciplinary team (Fig. 4). Cell therapy including HSCT and/or ex-vivo gene therapy should be one of the primary choices as a permanent therapy. ERT could also contribute to the short-term care to keep patients in a better condition before and after cell therapy. A careful long-term assessment of HSCT, ERT or combination therapy should be made to determine the ultimate guideline of treatment.
Fig. 4.
Model for diagnosis and treatment of patients with MPS II. NBS: newborn screening.
In conclusion, we have evaluated an impact on growth in patients with MPS II treated by ERT and/or HSCT. ERT and HSCT provide a comparable impact on growth, indicating that HSCT can be a recommended option at an early stage in MPS II considering effectiveness towards brain or heart involvement.
Compliance with ethics
The study was approved by the Institutional Review Boards (IRB) at Gifu University and at the Nemours/Alfred I. duPont Hospital for Children.
Conflict of interest
All the authors contributed to the original article and have no conflict of interest with any other party.
Contributions to the project
Pravin Patel has contributed to the planning, data analysis, and reporting of the work described.
Yasuyuki Suzuki is a Principal Investigator for this project and has contributed to the concept, treatment of patients, planning of the project, informed consent, analysis of data, and reporting of the work described. He and his team conducted the project and followed up on the patients.
Akemi Tanaka has contributed to the treatment of patients, data analysis, and reporting of the work described.
Hiromasa Yabe has contributed to the treatment of patients, data analysis, and reporting of the work described.
Shunichi Kato has contributed to the treatment of patients, data analysis, and reporting of the work described.
Tsutomu Shimada has contributed to the data analysis and reporting of the work described.
Kenji E. Orii has contributed to the treatment of patients and the planning, data analysis, and reporting of the work described.
Toshiyuki Fukao has contributed to the treatment of patients and the planning, data analysis, and reporting of the work described. He and his team conducted the project with Dr. Suzuki.
Tadao Orii has contributed to the planning, data analysis, and reporting of the work described.
Shunji Tomatsu is a Principal Investigator for this project and has contributed to the concept and planning of the project, analysis of data, and reporting of the work described. He and his team conducted the project with Dr. Suzuki.
Acknowledgments
This work was supported by grants from the Austrian MPS Society and the International Morquio Organization (Carol Ann Foundation). This work was also supported by the Japanese MPS Family Society. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P20GM103464. The content of the article has not been influenced by the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa of the Nemours/Alfred I. duPont Hospital for Children.
Contributor Information
Yasuyuki Suzuki, Email: ysuz@gifu-u.ac.jp.
Shunji Tomatsu, Email: stomatsu@nemours.org, stomatsu@gifu-u.ac.jp.
References
- 1.Scarpa M. Mucopolysaccharidosis type II. In: Pagon R.A., Adam M.P., Bird T.D., Dolan C.R., Fong C.T., Stephens K., editors. GeneReviews™ [Internet] University of Washington, Seattle; Seattle (WA): 2007 Nov 06. pp. 1993–2013. [updated 2011 Feb 22] [Google Scholar]
- 2.Valayannopoulos V. Enzyme replacement therapy and substrate reduction therapy in lysosomal storage disorders with neurological expression. Handb. Clin. Neurol. 2013;113:1851–1857. doi: 10.1016/B978-0-444-59565-2.00055-1. [DOI] [PubMed] [Google Scholar]
- 3.Nelson J., Crowhurst J., Carey B., Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am. J. Med. Genet. A. 2003;123A:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- 4.Baehner F., Schmiroeskamp C., Krummenauer F., Miebach E., Bajbouj M., Whybra C., Kohlschutter A., Kampmann C., Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J. Inherit. Metab. Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- 5.Lin H.Y., Lin S.P., Chuang C.K., Niu D.M., Chen M.R., Tsai F.J., Chao M.C., Chiu P.C., Lin S.J., Tsai L.P., Hwu W.L., Lin J.L. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A. 2009;149A:960–964. doi: 10.1002/ajmg.a.32781. [DOI] [PubMed] [Google Scholar]
- 6.Wraith J.E., Beck M., Giugliani R., Clarke J., Martin R., Muenzer J., HOS Investigators Initial report from the Hunter Outcome Survey. Genet. Med. 2008;10:508–516. doi: 10.1097/gim.0b013e31817701e6. [DOI] [PubMed] [Google Scholar]
- 7.da Silva E.M.K., Strufaldi M.L.W., Andriolo R.B., Silva L.A. Enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II (Hunter syndrome) Cochrane Database Syst. Rev. 2011;Issue 11 doi: 10.1002/14651858.CD008185.pub2. (Art. No.: CD008185) [DOI] [PubMed] [Google Scholar]
- 8.Wraith J.E., Scarpa M., Beck M., Bodamer O.A., De Meirleir L., Guffon N., Meldgaard Lund A., Malm G., Van der Ploeg A.T., Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur. J. Pediatr. 2008 Mar;167(3):267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochiai T., Suzuki Y., Kato T., Shichino H., Chin M., Mugishima H., Orii T. Natural history of extensive Mongolian spots in mucopolysaccharidosis type II (Hunter syndrome): a survey among 52 Japanese patients. J. Eur. Acad. Dermatol. Venereol. 2007;8:1082–1085. doi: 10.1111/j.1468-3083.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn N.J., Harmatz P., Bodamer O., Burton B.K., Giugliani R., Jones S.A., Lampe C., Malm G., Steiner R.D., Parini R. Hunter Outcome Survey Investigators. Importance of surgical history in diagnosing mucopolysaccharidosis type II (Hunter syndrome): data from the Hunter Outcome Survey. Genet Med. 2010;12:816–822. doi: 10.1097/GIM.0b013e3181f6e74d. [DOI] [PubMed] [Google Scholar]
- 11.Jones S.A., Parini R., Harmatz P., Giugliani R., Fang J., Mendelsohn N.J., HOS Natural History Working Group on behalf of HOS Investigators The effect of idursulfase on growth in patients with Hunter syndrome: data from the Hunter Outcome Survey (HOS) Mol. Genet. Metab. 2013;109:41–48. [Google Scholar]
- 12.Muenzer J., Wraith J.E., Beck M., Giugliani R., Harmatz P., Eng C.M., Vellodi A., Martin R., Ramaswami U., Gucsavas-Calikoglu M., Vijayaraghavan S., Wendt S., Puga A.C., Ulbrich B., Shinawi M., Cleary M., Piper D., Conway A.M., Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 13.Muenzer J., Beck M., Eng C.M., Giugliani R., Harmatz P., Martin R., Ramaswami U., Vellodi A., Wraith J.E., Cleary M., Gucsavas-Calikoglu M., Puga A.C., Shinawi M., Ulbrich B., Vijayaraghavan S., Wendt S., Conway A.M., Rossi A., Whiteman D.A., Kimura A. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet. Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 14.Muenzer J., Beck M., Giugliani R., Suzuki Y., Tylki-Szymanska A., Valayannopoulos V., Vellodi A., Wraith J.E. Idursulfase treatment of Hunter syndrome in children younger than 6 years: results from the Hunter Outcome Survey. Genet. Med. 2011;13:102–109. doi: 10.1097/GIM.0b013e318206786f. [DOI] [PubMed] [Google Scholar]
- 15.Heese B.A. Current strategies in the management of lysosomal storage diseases. Semin. Pediatr. Neurol. 2008;15:119–126. doi: 10.1016/j.spen.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Beck M. Mucopolysaccharidosis Type II (Hunter Syndrome): clinical picture and treatment. Curr. Pharm. Biotechnol. 2011;12:861–866. doi: 10.2174/138920111795542714. [DOI] [PubMed] [Google Scholar]
- 17.Schulze-Frenking G., Jones S.A., Roberts J., Beck M., Wraith J.E. Effects of enzyme replacement therapy on growth in patients with mucopolysaccharidosis type II. J. Inherit. Metab. Dis. 2011;34:203–208. doi: 10.1007/s10545-010-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A., Okuyama T., Suzuki Y., Sakai N., Takakura H., Sawada T., Tanaka T., Otomo T., Ohashi T., Ishige-Wada M., Yabe H., Ohura T., Suzuki N., Kato K., Adachi S., Kobayashi R., Mugishima H., Kato S. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Patel P., Suzuki Y., Maeda M., Yasuda E., Shimada T., Orii K.E., Orii T., Tomatsu S. Growth charts for patients with Hunter Syndrome. Mol. Genet. Metab. Rep. 2014;1:5–18. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn Y.B., Cho S.Y., Park S.W., Kim S.J., Ko A.R., Kwon E.K., Han S.J., Jin D.K. Phase I/II clinical trial of enzyme replacement therapy with idursulfase beta in patients with mucopolysaccharidosis II (Hunter syndrome) Orphanet J. Rare Dis. 2013;8:42. doi: 10.1186/1750-1172-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmatz P., Giugliani R., Schwartz I.V., Guffon N., Teles E.L., Miranda M.C., Wraith J.E., Beck M., Arash L., Scarpa M., Ketteridge D., Hopwood J.J., Plecko B., Steiner R., Whitley C.B., Kaplan P., Yu Z.F., Swiedler S.J., Decker C., MPS VI Study Group Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–475. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F., Eto Y., Orii T. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama K., Shimada Y., Higuchi T., Ohtsu M., Nakauchi H., Kobayashi H., Fukuda T., Ida H., Eto Y., Crawford B.E., Brown J.R., Ohashi T. Enzyme augmentation therapy enhances the therapeutic efficacy of bone marrow transplantation in mucopolysaccharidosis type II mice. Mol. Genet. Metab. 2013;13:00325–00329. doi: 10.1016/j.ymgme.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y., Kato K., Sukegawa K., Tomatsu S., Fukuda S., Emura S., Kojima S., Matsuyama T., Sly W.S., Kondo N., Orii T. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant. 1998;21:629–634. doi: 10.1038/sj.bmt.1701141. [DOI] [PubMed] [Google Scholar]
- 25.Chinen Y., Higa T., Tomatsu S., Suzuki Y., Orii T., Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol. Genet. Metab. Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boelens J.J., Rocha V., Aldenhoven M., Wynn R., O'Meara A., Michel G., Ionescu I., Parikh S., Prasad V.K., Szabolcs P., Escolar M., Gluckman E., Cavazzana-Calvo M., Kurtzberg J. EUROCORD, Inborn error Working Party of EBMT and Duke University. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with hurler syndrome. Biol. Blood Marrow Transplant. 2009;15:618–625. doi: 10.1016/j.bbmt.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Prasad V.K., Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin. Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Tomatsu S., Fujii T., Fukushi M., Oguma T., Shimada T., Maeda M., Kida K., Shibata Y., Futatsumori H., Montaño A.M., Mason R.W., Yamaguchi S., Suzuki Y., Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe B.J., Blanchard S., Sadilek M., Scott C.R., Turecek F., Gelb M.H. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter Syndrome) Anal. Chem. 2011;83:1152–1156. doi: 10.1021/ac102777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller M., Tucker J.N., Lang D.J., Dean C.J., Fietz M.J., Meikle P.J., Hopwood J.J. Screening patients referred to a metabolic clinic for lysosomal storage disorders. J. Med. Genet. 2011;48:422–425. doi: 10.1136/jmg.2010.088096. [DOI] [PubMed] [Google Scholar]
- 31.Meikle P.J., Grasby D.J., Dean C.J., Lang D.L., Bockmann M., Whittle A.M., Fietz M.J., Simonsen H., Fuller M., Brooks D.A., Hopwood J.J. Newborn screening for lysosomal storage disorders. Mol. Genet. Metab. 2006;88:307–314. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Scott C.R., Elliott S., Buroker N., Thomas L.I., Keutzer J., Glass M., Gelb M.H., Turecek F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]