Abstract
Initial embryonic determination of artery or vein identity is regulated by genetic factors that work in concert to specify endothelial cell (EC) fate, giving rise to two structurally unique components of the circulatory loop. The Shh/VEGF/Notch pathway is critical for arterial specification, while the orphan receptor nr2f2 (COUP-TFII) has been implicated in venous specification. Studies in mice have shown that nr2f2 is expressed in venous but not arterial ECs, and that it preferentially induces markers of venous cell fate. We have examined the role of nr2f2 during early arterial-venous development in the zebrafish trunk. We show that expression of a subset of markers of venous endothelial identity requires nr2f2, while the expression of nr2f2 itself requires sox7 and sox18 gene function. However, while sox7 and sox18 are expressed in both the cardinal vein and the dorsal aorta during early trunk development, nr2f2 is expressed only in the cardinal vein. We show that Notch signaling activity present in the dorsal aorta suppresses expression of nr2f2, restricting nr2f2-dependent promotion of venous differentiation to the cardinal vein.
Keywords: Nr2f2, COUP-TFII, zebrafish, cardinal vein, dorsal aorta
Introduction
The proper functioning of the circulatory system as a closed loop continually re-circulating blood to and from peripheral tissues depends on the fundamental division of the circulatory system into two distinct and separate yet completely intertwined and interconnected networks of blood vessels – veins and arteries – that direct blood to and from the heart, respectively. Although the existence of these two distinct types of blood vessels has been appreciated for centuries, we have only begun to understand the molecular pathways leading to their differential specification and identity. In the developing zebrafish trunk, Hedgehog signaling regulates arterial differentiation via downstream activation of vascular endothelial growth factor (VEGF) and Notch pathways. Sonic hedgehog (SHH) secreted from the midline axial mesoderm promotes up-regulation and secretion of VEGF in adjacent somitic tissue, which in turn promotes Notch pathway activation and arterial differentiation in the juxtaposed assembling trunk dorsal aorta (Lawson et al., 2001; Lawson et al., 2002). VEGF and Notch signaling have also been shown to promote arterial differentiation in developing mice and other models (reviewed in (Swift and Weinstein, 2009)). While arterial specification is driven by the preferential activation of the Shh/VEGF/Notch pathways, it had been believed that venous differentiation was a default differentiation pathway for endothelial cells (Thurston and Yancopoulos, 2001).
Recently, however, this view was challenged by the identification of genetic factors required for proper venous differentiation. Chicken ovalbumin upstream promoter-transcription factor II (“COUP-TFII;” recently designated “nuclear receptor subfamily 2, group F, member 2,” or “NR2F2” in zebrafish) has been shown to act as a positive mediator of venous specification in mice. The murine COUP-TFII gene is specifically expressed in venous but not arterial ECs, and is required for proper vascular morphogenesis. COUP-TFII knockout mice die at approximately E10.5 following severe hemorrhage and edema due to enlarged blood vessels, improper development of the atria and sinus venosus, and malformed cardinal veins (Pereira et al., 1999). Endothelial-specific targeted disruption of COUP-TFII results in ectopic expression of arterial markers in the cardinal vein, while ectopic pan-endothelial over-expression of COUP-TFII results in fusion of arteries and veins, similar to phenotypes observed in Neuropilin1 -/- or Notch1 -/- mice (You et al., 2005). These and other results from mice and cultured cells have led to the proposal that COUP-TFII promotes venous identity by down-regulating pro-arterial Notch signaling, thereby releasing expression of venous factors from Notch-mediated repression and preventing Notch-mediated activation of arterial gene expression (Chen et al., 2012). However, while knockdown of nr2f2 in zebrafish does result in reduced venous ephb4 and flt4 expression, it does not result in ectopic venous expression of arterial markers grl and efnb2a (Aranguren et al., 2011), suggesting additional investigation is needed to fully elucidate the role of nr2f2 in vascular development and differentiation.
In this manuscript, we further examine the role of nr2f2 during early arterial-venous development in the zebrafish trunk axial vasculature. We show that expression of nr2f2 requires endothelial sox7 and sox18 gene function, while expression of some but not all markers of venous identity requires nr2f2. Importantly, our results also suggest that in addition to promoting arterial gene expression Notch signaling in the dorsal aorta suppresses expression of nr2f2, restricting nr2f2-dependent venous gene expression to the cardinal vein.
Materials and Methods
Zebrafish
Zebrafish lines used and reported elsewhere were wild-type EK, cloche mutant (Stainier et al., 1995), Tg(fli1a:EGFP)y1 (Lawson and Weinstein, 2002), Tg(Tp1bglob:hmgb1-mCherry)h11 (Parsons et al., 2009), and Tg(UAS:N3ICDmyc) (Scheer and Campos-Ortega, 1999). The Tg(efnb2a:eGFP) line was generated using a transgene construct in which cytoplasmic EGFP is driven by 10.2 kb fragment of DNA upstream from the translation start site of efnb2a (efnb The Tg(fli1a:GV-EcRF') line was generated using a transgene construct in which a drug inducible Gal4-VP16 element, GV-EcRF (Esengil et al., 2007), is driven by a minimal fli1a promoter/enhancer element. Zebrafish embryos and strains were maintained as described (Kimmel et al., 1995).
In Situ Hybridization and Immunohistochemistry
Whole-mount RNA in situ hybridization was carried out as previously described (Pham et al., 2007). Dual-color whole mount RNA fluorescent in situ hybridization was carried as previously described (Hauptmann and Gerster, 1994). Antisense probes for kdrl, efnb2a, grl, tbx-20, dab2, ephB4a, fli1a, and cdh5 were prepared as described (Fouquet et al., 1997; Lawson et al., 2002; Siekmann and Lawson, 2007; Thompson et al., 1998; Yaniv et al., 2006). Antisense probes for nr2f2 (full length and short form), sox7, and sox18 were prepared from cDNA using primers listed in supplement table 1. Amplicons were cloned in pENTR-D/TOPO (Invitrogen) vectors. Antisense probes for lyve1 and stab1 were obtained from commercially available clones (Open Biosystems, 7998534 and 8998853). DIG-labeled antisense riboprobes were synthesized using the DIG Labeling Kit (Roche). Immunostaining was performed as described (Yaniv et al., 2006).
Cloning and Transgene Construction
Fulllength nr2f2, sox7, and sox18 were amplified from EK cDNA. The GVEcRF' cassette was amplified from pCS2+GVEcRF' (Esengil et al., 2007). Xenopus laevis Su(H)DBM was amplified from pCS-XSu(H)DBM (Wettstein et al., 1997). Plasmids containing nr2f2 chimeras were made after amplifying a truncated sequence of nr2f2 (aka ‘Ct’) from full length nr2f2. The truncated nr2f2 was subsequently cloned into either pcGlobin2-VP16 (Ro et al., 2004) or ENG-N backbone (Kessler, 1997) to create an activating (referred to as “VCt” in this text) or a repressing (“ECt”) chimera for nr2f2. VCt, ECt, full length nr2f2 (“CF”), sox7, sox18, GV-EcRF', and XSu(H)DBM were cloned into Gateway compatible pME plasmids.
Endothelial specific pI-SceI(kdrl-CeruleanFP-2A-VCt), pI-SceI(kdrl-CeruleanFP-2A-ECt), pI-SceI(kdrl-CeruleanFP-2A-CF), Tol2(fli1a:GVEcRF'), and pI-SceI(kdrl-Su(H)DBM-2A-mCherry) constructs were assembled using Gateway technology (Kwan et al., 2007; Provost et al., 2007; Villefranc et al., 2007).
A list of primers used for cloning can be found in Supplemental Table 1.
Morpholino and Transgene Microinjections
Morpholino (Gene Tools) injections were performed at the described doses into 1- to 2-cell stage embryos. Morpholinos used in this study are as follows: nr2f2 translation blocking MO (translation start site underlined), 5′- AGCCTCTCCACACTACCATTGCCAT-3′; nr2f2 exon1 splice donor MO, 5′-AACAAAAATCCGAATACCTTCCCGT-3′; nr2f2 translation block mismatch control, 5′-AGgCTgTCCACAgTACCATaGCgAT-3′; sox7 translation blocking MO (Cermenati et al., 2008), 5′-ACGCACTTATCAGAGCCGCCATGTG-3′; sox18 translation blocking MO (Cermenati et al., 2008), 5′-TATTCATTCCAGCAAGACCAACACG-3′. Tol2 and pI-SceI plasmid DNA injections were performed as previously described (Grabher et al., 2004; Kawakami et al., 2000). 100pg of Tol2 based constructs and 45pg of pI-SceI plasmids were injected for each experiment.
Microscopy
RNA in situ hybridization images were captured with a ProgRes C14 camera mounted on a Leica MZ12 stereo microscope, or with LAV camera/software on a LM205 stereo microscope. Confocal microscopy of transgenic and immunostained embryos was performed using an Olympus FluoView 1000 microscope.
qRT-PCR Analysis
Total cellular RNA from experimental embryos was isolated using Trizol reagent and treated with DNAse I. For all gene analysis, only trunks excised at the level of the first somite were collected, except for prox1a in which whole embryos were used. Briefly, heads and yolks were removed from 8-10 embryos by scalpel prior to addition of Trizol reagent to the excised trunks (within a given experiment the same number of embryos were used for preparation of cDNA for each sample). Total cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad) and qPCR analysis was carried out using iQ SYBR Green Supermix on a Bio-Rad CFX96 with ef1a used as a reference gene. Bio-Rad CFX Manager software was used to quantify gene expression levels and all analysis conforms with MIQE guidelines (Bustin et al., 2009).
A list of primers used for qPCR can be found in Supplemental Table 2.
Drug Treatment
Dechorionated Tg(Tp1bglob:hmgb1-mCherry)h11 embryos were treated with either 100 uM DAPT (Sigma) in 0.2 % DMSO or in 0.2 % DMSO alone as a control beginning at 6 to 8 hpf. Tg(UAS:N3ICDmyc);Tg(fli1a:GV-EcRF') double transgenic fish were treated with either 100 nM TBF (Sigma) in 0.05% DMSO or in 0.05% DMSO alone as a control beginning at 6 to 8 hpf.
Measuring Notch Reporter Output
For each experimental condition, ImageJ software was used to highlight and count the number of individual mCherry-positive fluorescent cells from separate, standard-sized boxed regions placed over the neural tube and the dorsal aorta. The ratio of measured mCherry-positive cells in the dorsal aorta box versus mCherry-positive cells in the neural tube box was calculated to give the relative vascular notch reporter activity in each animal. Identically sized boxes placed in equivalent positions were used for measuring reporter fluorescence in the neural tube and dorsal aorta in each animal measured. The values reported in the inset numbers in Figure 3G-K are each normalized to the control value (Figure 3G) to provide a final measurement of total notch activation. Five embryos were examined for each condition, with quantitation performed on one representative animal per condition (displayed in the image panels in Figure 3G-K).
Results
nr2f2 is expressed in the posterior cardinal vein during early zebrafish trunk vascular development
We used whole mount ISH to analyze the spatial and temporal expression profile of zebrafish nr2f2. We performed analysis with both a full open reading frame zebrafish nr2f2 probe and with a shorter, highly specific zebrafish nr2f2 probe against only nucleotides 1-240 of the open reading frame. Similar results were obtained with both antisense probes and images presented are from analysis with the full length probe. nr2f2 expression is not detected prior to the 3 somite stage. Expression is detected in the hindbrain rhombomeres and anterior somites can be detected at 3 somites (Supp. Fig. 1). By the 15 and 20 somite stages, expression within the central nervous system is clearly observed in the telencephalon, ventral anterior midbrain, hindbrain rhombomeres as well as in the otic vesicles, pectoral fin buds, and developing spinal cord of the trunk (Supp. Fig. 1). While cranial and spinal cord expression remains robust throughout zebrafish development, weak expression of nr2f2 is first observed in the posterior cardinal vein, but not in the dorsal aorta, at 24 hpf (Figure 1A,B). This expression persists through 48 hpf (Figure 1D-G), in agreement with a previously recorded expression profile of nr2f2 (Aranguren et al., 2011). To confirm posterior cardinal vein-specific expression of nr2f2, we performed two color fluorescent ISH to compare expression profiles of nr2f2 with the pan-endothelial expression of kdrl. While kdrl was observed in both the posterior cardinal vein and dorsal aorta (Figure 1E, G, red), nr2f2 was restricted to the posterior cardinal vein (Figure 1F, G; red and green colocalize in the PCV). Endothelial expression was also confirmed by showing loss of expression in the presumptive ECs of cloche mutant embryos (Stainier et al., 1995) that lack most endothelium (Figure 4D and data not shown).
Figure 1. Expression of nr2f2 in developing veins in the zebrafish.
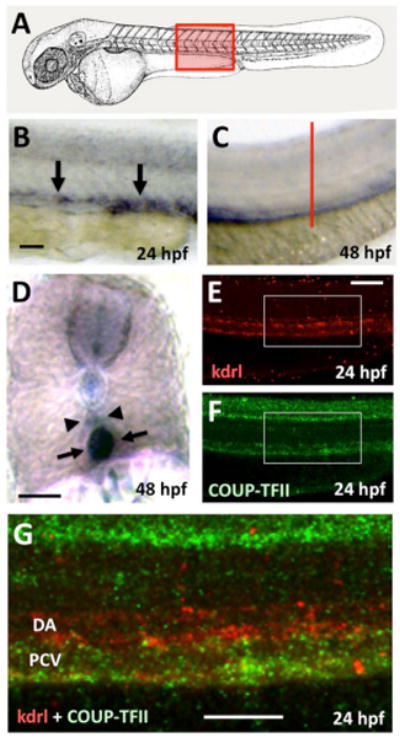
(A) Schematic diagram of a 1 dpf zebrafish (modified from (Kimmel et al., 1995)) with a red box showing the approximate position of images in B-G. (B-D) Whole mount in situ hybridization of the trunks of 24 hpf (B) and 48 hpf (C,D) zebrafish embryos probed for nr2f2. (E-G) Two-color fluorescent in situ hybridization of the trunk of a 24 hpf animal probed for kdrl (in red; panels E,G) and nr2f2 (green; panels F,G). Images are all lateral views of the trunks of whole mount stained animals (anterior to the left) except for panel D, which shows a transverse thick section through the trunk (the red line in panel C indicates the plane of section shown in panel D). Arrows in panels B, D, and G point to posterior cardinal vein expression of nr2f2, while arrowheads in panels D and G note the position of the dorsal aorta. Rostral is to the left and dorsal is up in all image panels. Scale bars, (B,C,E,F,G) 100 μm, (D) 50 μm.
Reduction in nr2f2 results in loss of venous markers without corresponding increase in arterial markers
To analyze the effect of reduction of nr2f2 expression on vascular development, we used antisense morpholino oligonucleotides (MO) that targeted either the ATG start site of nr2f2 translation (ATG MO, MO1), or the exon 1 splice donor site (splice MO, MO2), both of which were effective at reducing normal nr2f2 transcript levels (Supp Fig. 2). Injections of either MO at 5 or 10 ng doses resulted in embryonic lethality after 24 hpf with severe morphological defects and edema, making analysis of vascular development impossible (data not shown). At 2.5 ng most embryos developed with reasonably normal overall morphology and vascular morphology, and displayed only slight developmental delay compared to control fish injected with a control mismatch morpholino (Figure 2A-D). Using this 2.5 ng dose of nr2f2 MO, we performed whole mount in situ hybridization and quantitative RT-PCR to analyze the expression of known arterial, venous, or pan-endothelial markers in the trunk vasculature of nr2f2 deficient 24 hpf animals compared to their control siblings (Figure 2E-M). We observed significant decreases in expression of prox1 and early vein-restricted marker lvye1 in morphant embryos (Figure 2G,M) and modest decrease in vein-restricted marker stab1 (Figure 2F,M), but no change in expression of vein-restricted markers ephb4 and dab2 by qRT-PCR (Figure 2M). We also saw no change in expression of arterial-restricted markers efnb2a, grl, and tbx20 (Figure 2I,J,K,M). The expression of the sox7 and sox18 genes, previously implicated in arterial-venous fate decisions in the zebrafish (Cermenati et al., 2008; Herpers et al., 2008; Pendeville et al., 2008), was also unchanged in nr2f2 deficient animals (Figure 2H,L,M). Other pan-endothelial markers including fli1a, kdrl, and cdh5 were also unchanged (data not shown).
Figure 2. nr2f2 function is required for proper venous gene expression in the zebrafish vasculature.
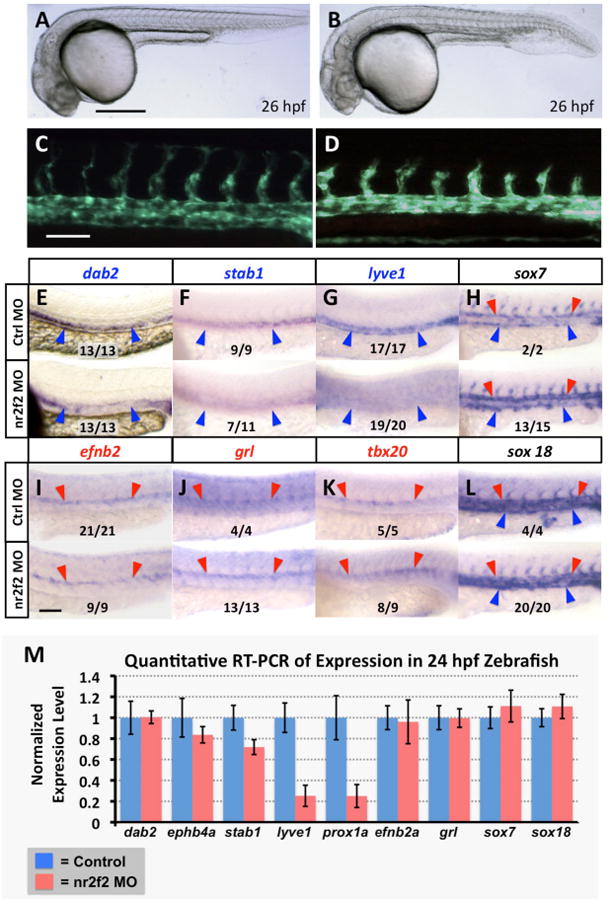
(A-D) Transmitted light (A,B) and green fluorescent light (C,D) images of control (A,C) and nr2f2 morpholino injected (B,D) Tg(fli1a-EGFP)y1 transgenic animals. (E-L) Whole mount in situ hybridization of 24 hpf control (top) and nr2f2 morpholino injected (bottom) zebrafish embryo trunks probed for ephb4a (E), stab1 (F), lyve1 (G), sox7 (H), efnb2a (I), grl (J), tbx20 (K), and sox18 (L). Blue arrowheads note posterior cardinal vein gene expression, while red arrows note dorsal aorta gene expression. The inset numbers in panels F-L show the number of in situ-stained nr2f2 MO-injected embryos exhibiting the phenotype shown in the image panel over the total number of embryos examined. (M) Quantitative RT-PCR measurement of gene expression in excised trunks (see Materials and Methods) of 24 hpf control and nr2f2 morpholino injected animals. Values are all normalized to control gene expression levels, which are set equal to 1. Rostral is to the left and dorsal is up in all image panels. Scale bars, (A,B) 500 μm, (C-L) 100 μm.
Since higher doses of nr2f2 morpholinos were associated with significant developmental abnormalities and lethality, likely due to earlier essential functions of nr2f2 in the nervous system and/or other tissues, we used transgenesis to specifically target endothelial cells for nr2f2 activation or silencing using “activating” or “repressing” nr2f2 chimeras, respectively. Previous reports have described methods for fusing a truncated version of nr2f2 to either the engrailed repressor domain or the VP16 activating domain (Naka et al., 2008). We placed full-length nr2f2 or these chimeric proteins together with cerulean fluorescent protein (to mark expressing cells) under the control of the vascular-specific zebrafish kdrl promoter to generate constructs for endothelial-restricted expression of either wild type full-length nr2f2 (kdrl-CF), “activating” protein containing VP16 fused to an nr2f2 polypeptide with its endogenous transactivation domain removed (kdrl-VCt). or “repressing” protein containing Engrailed fused to an nr2f2 polypeptide with its endogenous transactivation domain removed (kdrl-ECt) (Supp. Fig. 3A-D). When these constructs were injected into zebrafish embryos we found that endothelial expression of “repressing” kdrl-ECt resulted in suppression of venous-specific lyve1 expression similar to that observed in nr2f2 MO-injected animals (Figure 3C,E,Q). In contrast, endothelial expression of either wild type nr2f2 or “activating” kdrl-VCt did not significantly alter lyve1 expression (Figure 3D,F,Q).
Figure 3. nr2f2 does not regulate Notch in the zebrafish vasculature.
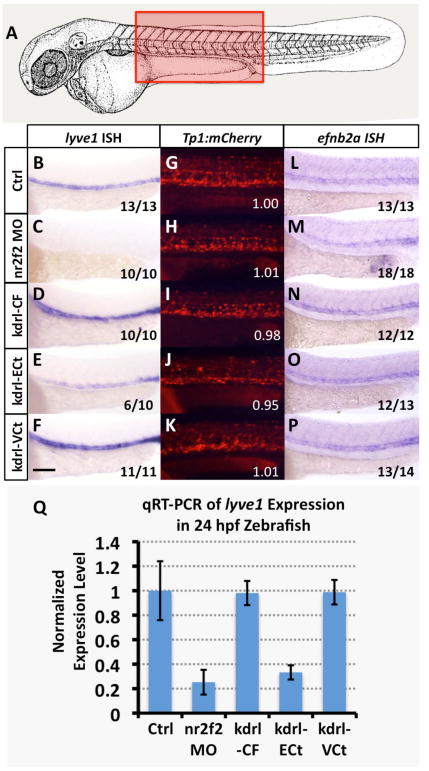
(A) Schematic diagram of a 1 dpf zebrafish (modified from (Kimmel et al., 1995)) with a red box showing the approximate position of images in B-V. (B-F) Whole mount in situ hybridization of 24 hpf zebrafish probed for lyve1. (G-K) Red fluorescent confocal micrographs of 24 hpf Tg(Tp1bglob:hmgb1-mCherry)h11 (“Tp1:mCherry”) transgenic zebrafish. Inset numbers show quantitative measurements of trunk vascular mCherry fluorescence in a single representative injected fish normalized to trunk neural mCherry fluorescence and to values in untreated controls (G). See Materials and Methods for additional information on measurements. (L-P) Whole mount in situ hybridization of 24 hpf zebrafish probed for efnb2a. Animals in image panels B-P were either untreated (B,G,L), injected with nr2f2 MO (C,H,M), injected with kdrl-CF (Full length nr2f2 under the control of the kdrl promoter; D,I,N), injected with kdrl-ECt (chimeric protein with nr2f2 lacking the transactivation domain fused to engrailed repressor domain; E,J,O), or injected with kdrl-VCt (chimeric protein with nr2f2 lacking the transactivation domain fused to VP16; F,K,P). The inset numbers in panels C-F and M-P show the number of in situ-stained embryos exhibiting the phenotype shown in the image panel over the total number of embryos examined. (Q) Quantitative RT-PCR measurement of gene expression in excised trunks of 24 hpf animals (see Materials and Methods). Values are all normalized to control gene expression levels, which are set equal to 1. Rostral is to the left and dorsal is up in all image panels. Scale bar, 100 μm.
nr2f2 does not regulate Notch in the zebrafish vasculature
Previous studies have reported that nr2f2 modulates venous identity in mice by regulating Notch expression in the developing vascular system (Chen et al., 2012; You et al., 2005). To examine the role of nr2f2 in regulating Notch in zebrafish, we injected nr2f2 MO or our vascular nr2f2 chimera-expressing constructs into either a Tg(Tp1bglob:hmgb1-mCherry)h11 (“Tp1:mCherry”) Notch reporter transgenic line (Parsons et al., 2009) or into a Tg(efnb2a:eGFP) Notch responsive transgenic line. We found no change in the level of fluorescent protein expressed in the vasculature in either of these transgenic lines upon injection of nr2f2 MO, or kdrl-CF, kdrl-VCt, or kdrl-ECt constructs (Figure 3G-K and Supp. Fig. 4). Treatment with the Notch inhibitor DAPT did cause strong reduction in Tg(Tp1bglob:hmgb1-mCherry)h11 and Tg(efnb2a:eGFP) transgenic reporter expression, however, confirming that these lines are indeed responsive to reduced Notch signaling (Supp. Fig. 5). We also found no change in expression levels of the Notch–responsive gene efnb2a in nr2f2 morpholino or chimera-injected animals (Figure 3L-Q). Our results indicating that Notch signaling is not regulated by nr2f2 in zebrafish are consistent with previously published results showing that expression of efnb2a and grl are not affected in nr2f2 morphants (Aranguren et al., 2011).
nr2f2 is regulated by sox7 and sox18 in zebrafish
A number of previous publications have reported that the Sry-related HMG box (Sox) genes sox7 and sox18 are involved in regulating arterial-venous specification in zebrafish, although the precise nature of this regulation remains unclear (Cermenati et al., 2008; Herpers et al., 2008;Pendeville et al., 2008). We used the same sox7 and sox18 morpholinos used in Cermenati et al., at the same doses, to determine whether sox7 and/or sox18 function are required for expression of nr2f2. Injection of sox7 or sox18 morpholinos alone did not cause significant change in nr2f2 expression (data not shown), but dual morpholino (DMO) knockdown of both sox7 and sox18 together resulted in strong reduction in nr2f2 expression in the PCV, although neural tube expression was not detectably altered (Figure 4A,B). Quantitative RT-PCR of either whole embryo or excised trunk sections of pooled control and Sox DMO embryos showed that trunk levels of nr2f2 were reduced by approximately 50%, while levels of lyve1 were more strongly reduced (Figure 4C). The 50% reduction in nr2f2 likely reflects reasonably complete loss of the vascular expression of nr2f2, since measurement of excised trunk expression levels by qRT-PCR showed that the reduction in nr2f2 in sox7/sox18 DMO embryos was comparable to that observed in cloche mutant (Stainier et al., 1995) animals that lack virtually all endothelium (but that also retain the neural tube expression of nr2f2; data not shown), and was not further reduced by knocking down sox7 and sox18 in the cloche mutant background (Figure 4D).
Figure 4. Sox gene function is required for nr2f2 expression in the zebrafish vasculature.
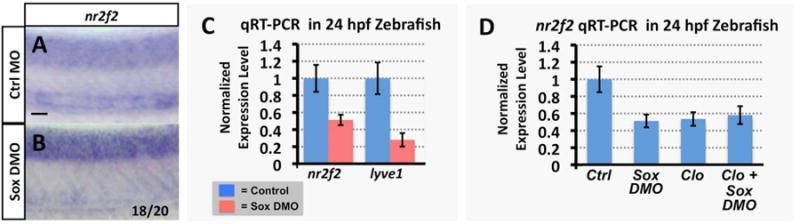
(A,B) Whole mount in situ hybridization of 24 hpf control (A) and sox7/sox18 double morpholino (DMO) injected (B) zebrafish embryo trunks probed for nr2f2. The inset numbers in panels B show the number of in situ-stained embryos exhibiting strongly reduced or absent vascular expression over the total number of embryos examined. (C) Quantitative RT-PCR measurement of nr2f2 and lyve1 gene expression in excised trunks (see Materials and Methods) of 24 hpf control and sox7/sox18 DMO animals. (D) Quantitative RT-PCR measurement of nr2f2 gene expression in excised trunks (see Materials and Methods) of 24 hpf control MO injected, sox7/sox18 DMO injected, cloche mutant, and sox7/sox18 DMO injected cloche mutant animals. All quantitative RT-PCR values are shown normalized to control gene expression levels, which are set equal to 1. Rostral is to the left and dorsal is up in all image panels. Scale bars, 50 μm.
nr2f2 is regulated by Notch in zebrafish
Our results and those of others (Aranguren et al., 2011) suggest that in the zebrafish nr2f2 is not regulating Notch, unlike nr2f2/COUP-TFII in mice. Furthermore, some recent in vitro data suggests that nr2f2 can be a target of Notch signaling in at least some contexts. Ectopic expression of “activated” Notch intracellular domain (ICD) or Notch target transcription factors Hey1 and Hey2 represses nr2f2 expression in cultured lymphatic endothelial cells (Kang et al., 2010). To explore whether Notch acts upstream of nr2f2 in the zebrafish vasculature, we began by examining whether endothelial-specific activation of Notch signaling represses nr2f2 expression, using binary transgenic inducible expression of Notch3 intracellular domain (N3ICD) in endothelium in vivo. We generated fish carrying a vascular-specific, tebufenozide-inducible GAL4 fusion protein (Tg[fli1a:GVEcRF])) and crossed these to fish we have employed previously to inducibly activate Notch signaling (Lawson et al., 2001) that carry a myc-tagged UAS-driven N3ICD transgene (Tg[UAS:N3ICDmyc]) (Supp. Fig. 3E). Tebufenozide-induced activation of N3ICD prior to the onset of vascular development resulted in reduction in nr2f2 expression in the PCV but not in the neural tube in Tg(UAS:N3ICDmyc);Tg(fli1a:GV-EcRF') double transgenic fish (Figure 5A,B). Since ectopic endothelial activation of Notch signaling resulted in reduced nr2f2 expression, we also examined whether suppression of Notch signaling by endothelial-specific expression of a Notch signaling dominant-negative suppressor of hairless DNA binding domain mutant (Su[H]DBM) might cause increased nr2f2 expression. To do this, we generated a construct to drive expression of Su[H]DBM under the control of the kdrl promoter (kdrl:Su[H]DBM-2A-mCherry) (Supp. Fig. 3F) and injected this construct into zebrafish embryos. mCherry-positive endothelial cells co-expressing Su[H]DBM also mis-expressed nr2f2 in the dorsal aorta, although no change was noted in neural tube expression (Figure 5C,D). Binary transgenic inducible expression of N3ICD did not alter expression of sox7 or sox18, indicating that Notch does not regulate nr2f2 expression by modulating expression of these SoxF family genes (Figure 5E-H). Taken together, these findings suggest that Notch works downstream from or in parallel to sox7 and sox18 to repress nr2f2 expression in the dorsal aorta.
Figure 5. Notch negatively regulates nr2f2 in the zebrafish vasculature, but not via Sox.
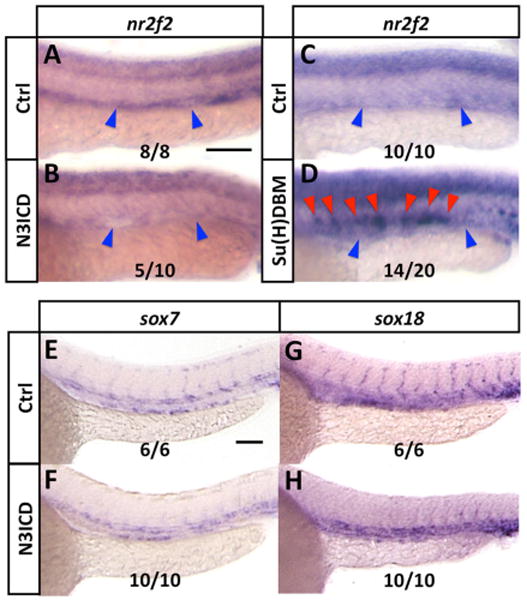
(A,B) Whole mount in situ hybridization of 24 hpf control (A) and Tg[fli1a:GV-EcRF];Tg[UAS:N3ICDmyc] double transgenic (B) animals treated with tebufenozide from 8 hpf to 28 hpf, probed for nr2f2. Blue arrowheads show the position of the posterior cardinal vein. The inset numbers in panel B show the number of in situ-stained embryos exhibiting strongly reduced vascular expression over the total number of embryos examined. (C,D) Whole mount in situ hybridization of 24 hpf control (C) and kdrl:Su[H]DBM-2A-mCherry injected (D) animals, probed for nr2f2. Blue arrowheads show position of the posterior cardinal vein, red arrowheads show ectopic nr2f2 expression in the dorsal aorta. The inset numbers in panel D show the number of in situ-stained embryos exhibiting patches of strong ectopic nr2f2 expression in the dorsal aorta over the total number of embryos examined. (E-H) Whole mount in situ hybridization of 24 hpf control (E,G) and Tg[fli1a:GV-EcRF];Tg[UAS:N3ICDmyc] double transgenic (F,H) animals treated with tebufenozide from 8 hpf to 24 hpf, probed for sox7 (E,F) or sox18 (G,H). The inset numbers in panels F and H show the number of N3ICDmyc- expressing in situ-stained embryos exhibiting sox7 or sox18 expression comparable to that in controls, over the total number of embryos examined. Rostral is to the left and dorsal is up in all image panels. Scale bars, 100 μm.
Discussion
Our results suggest a model for upstream regulation of, and downstream regulation by, nr2f2 during early arterial-venous development in the zebrafish trunk axial vasculature (Figure 6). We show that endothelial expression of nr2f2 requires endothelial sox7 and sox18 gene function, while expression of some but not all markers of venous identity requires nr2f2. Importantly, our results also indicate that in addition to promoting arterial gene expression, Notch signaling in the dorsal aorta suppresses expression of nr2f2, restricting nr2f2-dependent trunk vascular gene expression to the cardinal vein.
Figure 6. A proposed model for the regulation and functional role of nr2f2 in the early zebrafish trunk vasculature.
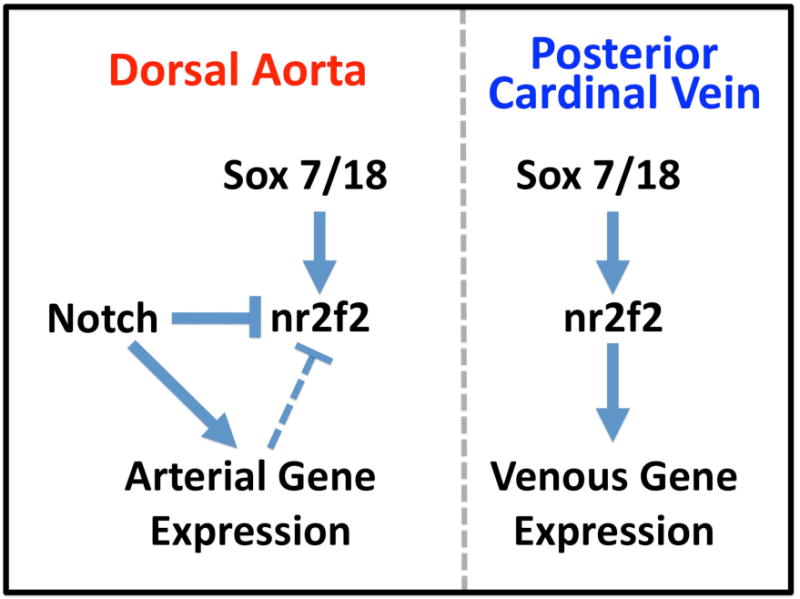
The function of nr2f2 is required for some, but not all, venous gene expression in the posterior cardinal vein (PCV), most likely as a positive-acting or permissive factor. Nr2f2 expression itself is positively regulated by the combined activity of sox7 and sox18, each of which are expressed in both the venous and arterial vasculature. Although Notch does not appear to be regulated downstream from nr2f2 during early zebrafish vascular development, Notch signaling activity suppresses expression of nr2f2 in the dorsal aorta, either directly or indirectly, resulting in restriction of nr2f2 expression to the posterior cardinal vein.
The function of zebrafish nr2f2 is required for proper venous differentiation of the cardinal vein (Figure 2E-G,M). Early expression of prox1 and lyve1 is strongly reduced in nr2f2-deficient animals. However, other venous markers are either modestly reduced (stab1) or unaffected (ephb4a, dab2). These results are similar to those in previously published studies in zebrafish (Aranguren et al., 2011) and mice (You et al., 2005) showing only partial effects on venous gene expression. It should be noted that the translation blocking morpholino used in our study is identical to that used in a previously published study (Aranguren et al., 2011), and the exon 1 splice donor site morpholino nearly identical (shifted 3′ by one nucleotide). Levels of endothelial Ephb4 are only slightly reduced in the cardinal veins of Nr2f2/COUP-TFII knockout mice (You et al., 2005), while only partial reduction is noted in venous expression of flt4 and ephb4a in nr2f2 knockdown zebrafish (Aranguren et al., 2011). These results suggest that factors in addition to nr2f2 may possibly be required for full establishment of venous identity in both mice and zebrafish. Various reports have suggested that nr2f2/COUP-TFII can act as either a repressor or an activator of downstream gene expression (reviewed in (Park et al., 2003)). We examined this in zebrafish using endothelial-specific expression of either wild type nr2f2, or nr2f2-engrailed (repressing) or nr2f2-VP16 (activating) fusion proteins, demonstrating that nr2f2 acts as a cell-autonomous activator required for venous gene expression in the zebrafish, since endothelial expression of an nr2f2-engrailed fusion protein (kdrl-ECt) results in reduced expression of lyve1 in a manner similar to nr2f2 morpholino knockdown, while wild type nr2f2 or an nr2f2-VP16 fusion protein has no significant effect (Figure 3B-F,Q). These results suggest that, at least in this context, nr2f2 is acting primarily as a positive factor for venous gene expression.
Proper arterial-venous differentiation requires restricted expression of not only venous-specific genes but also arterial-specific genes. A variety of previously published studies from our laboratory and others have shown that Notch pathway activation promotes arterial differentiation downstream from hedgehog and VEGF signaling ((Lawson et al., 2001; Lawson et al., 2002), reviewed in (Swift and Weinstein, 2009)). In mice, COUP-TFII has been shown to act as a negative regulator of Notch signaling to suppress arterial gene expression in the cardinal vein (Chen et al., 2012; You et al., 2005). Endothelial-cell specific ablation of COUP-TFII results in the formation of vein-like structures that improperly express arterial markers neuropilin 1 (Np1), Jag1, Hey1, Notch1, and Efnb2, along with the partial reduction in expression of venous marker EphB4 noted above. Conversely, mis-expression of COUP-TFII is developing arteries leads to diminished expression of Np1 and Jag1 coincident with an increase in EphB4 (You et al., 2005). More recently, microarray analysis in HUVEC cells found the expression of several Notch target genes was regulated by COUP-TFII and that COUP-TFII regulates foxc1, Np-1, and Hey2 at the transcriptional level (Chen et al., 2012).
In contrast to these results, we find no change in arterial-specific gene expression in nr2f2-deficient zebrafish. Nr2f2 morpholino-injected animals show neither ectopic expansion of arterial markers into the cardinal vein by in situ hybridization, nor increased trunk expression of these genes as measured by quantitative RT-PCR (Figure 2I-K,M). Again, these results are consistent with a previously published report showing lack of ectopic expression of arterial markers in veins in nr2f2 knockdown zebrafish (Aranguren et al., 2011). In addition to the morpholino knockdown findings, we fail to observe either ectopic venous expression or increased overall expression of arterial marker efnb2a in zebrafish expressing the “repressing” kdrl-ECt fusion protein specifically in the endothelium (Figure 3O,Q), despite strongly reduced expression of the venous marker lyve1 in these same animals (Figure 3E,Q). We used two different Notch-responsive transgenic zebrafish reporter lines to look more directly at the effects of either nr2f2 morpholino knockdown or endothelial-specific expression of nr2f2 fusion proteins on Notch activation. Expression of fluorescent reporters was not affected by functional manipulation of nr2f2 in Tg(Tp1bglob:hmgb1-mCherry)h11 or Tg(efnb2a:eGFP) transgenic animals (Figure 3G-K and Supp. Fig. 4), despite the fact that arterial expression of both reporters is clearly Notch-responsive as indicated by reduced expression upon treatment with DAPT (Supp. Fig. 5). Taken together, these results suggest that nr2f2 is not required to modulate Notch signaling activity or suppress arterial-specific gene expression in the zebrafish cardinal vein.
We examined other potential factors that might be responsible for promoting venous identity or suppressing arterial identity in the cardinal vein. A number of recent studies have reported that the soxF family members sox7 and sox18 modulate arterial-venous differentiation in the zebrafish, although the precise nature of their role remains somewhat unclear, with effects on expression of either arterial genes, venous genes, or both noted in different reports (Cermenati et al., 2008; Herpers et al., 2008; Pendeville et al., 2008). A recent publication suggests that Sox and Notch are required together for full expression of at least on arterial gene, Dll4 (Sacilotto et al., 2013). We find that sox7/18 function promotes expression of at least some of the same genes dependent on nr2f2 (Figure 4). As noted in one of those previous publications (Cermenati et al., 2008;), sox7 and sox18 appear to function redundantly in the regulating vein identity, since double morphants show loss of venous gene expression while single morphants do not. Interestingly, the other two studies (Herpers et al., 2008; Pendeville et al., 2008) reported ectopic expression of the venous marker flt4 in the DA, however these studies did not examine expression beyond a single time point to see if this altered expression persists. We find that sox7/18 function is required for expression of nr2f2 in the cardinal vein, suggesting that sox7/18 function is required upstream of nr2f2 to regulate proper venous differentiation.
While sox7 and sox18 are required for nr2f2 expression and for proper arterial-venous differentiation, it seems unlikely that these genes are acting as “selective factors” for promoting distinct endothelial cell fates in the dorsal aorta and cardina w in. Nr2f2 is expressed only in the cardinal vein, not in the dorsal aorta, while the two sox genes are each expressed in both the cardinal vein and the dorsal aorta during early trunk axial vessel arterial-venous differentiation. The arterial expression of sox7 and sox18 (but not nr2f2) suggests that some other factor(s) in addition to sox7 and sox18 is either necessary to suppress nr2f2 expression in the dorsal aorta or required for nr2f2 expression in the posterior cardinal vein. Surprisingly, we have now found that Notch signaling appears to be acting as an arterial-specific suppressor of nr2f2 expression and venous identity in zebrafish. Using both whole organism and EC-specific gain-of-function experiments to mis-express Notch ICD, we find a consistent and significant loss of nr2f2 expression in the PCV (Figure 5A,B). Mis-expression of “activated” Notch ICD does not result in altered expression of either sox7 or sox18, confirming that Notch does not regulate nr2f2 expression by modulating levels of sox7 or sox18 (Figure 5E-H). Conversely, endothelial-specific suppression of Notch signaling using a dominant negative suppressor of Hairless (Su(H)) DNA binding mutant driven by the kdrl promoter results in ectopic expression of nr2f2 in the dorsal aorta (Figure 5C,D).
Conclusions
Together, these results suggest that Notch serves as an arterial-specific repressor of nr2f2 expression in the zebrafish endothelium (Figure 6). Our findings lead us to hypothesize that Notch signaling functions as a “switch” regulating both arterial and venous gene expression and identity. In the dorsal aorta, the presence of active Notch signaling serves a dual function to simultaneously promote arterial gene expression and suppress nr2f2-driven venous gene expression. In the cardinal vein, absence of Notch signaling prevents arterial gene expression but permits expression of nr2f2 and nr2f2-dependent venous gene expression. Although at least one previous study suggests that Notch may directly regulate activity of the NR2F2 promoter, our data do not establish whether suppression of nr2f2 in the zebrafish dorsal aorta is direct or due to the activity of less proximal downstream genes up-regulated in the dorsal aorta by Notch.
This model is satisfying in terms of explaining how sox7 and sox18, which are both expressed in a pan-endothelial fashion, can be required upstream of nr2f2, which is expressed only in venous but not in arterial venous vessels. However, it is clearly an oversimplification of what is likely to be a more complex regulatory network. nr2f2 and sox7/18 function appear to be required for the expression of only a subset of venous-specific genes, and they also appear to also affect some arterial-specific gene expression (particularly for sox7/18; Herpers et al., 2008; Pendeville et al., 2008). Likewise, the effects of Notch signaling are also not absolute with regard to arterial identity, as previously published results from our laboratory and others have shown that some arterial-specific markers remain unresponsive to manipulation of Notch function (Lawson et al., 2001; Lawson et al., 2002). Nevertheless, our results generally support the role of Notch signaling as a key nexus for the arterial-venous fate decision in the zebrafish, not only through promotion of arterial differentiation but also through suppression of nr2f2-dependent venous differentiation.
Supplementary Material
Highlights.
nr2f2 (aka COUP-TFII) is expressed in the cardinal vein, but not in the dorsal aorta
nr2f2 promotes expression of a subset of venous genes.
sox7/18 gene function is required for nr2f2 expression in the vasculature
Notch signaling suppresses nr2f2 expression in the dorsal aorta
Acknowledgments
We thank members of the Weinstein laboratory for critical comments on this manuscript. We thank Daniel Castranova, Andrew Davis, and Van Pham for technical support and reagents. We also thank Louis Dye of the NICHD Microscopy and Imaging Core for helpful advice and guidance on vibratome use. This research was supported by the intramural program of the NICHD, NIIH and the Leducq Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aranguren XL, Beerens M, Vandevelde W, Dewerchin M, Carmeliet P, Luttun A. Transcription factor COUP-TFII is indispensable for venous and lymphatic development in zebrafish and Xenopus laevis. Biochem Biophys Res Commun. 2011;410:121–126. doi: 10.1016/j.bbrc.2011.05.117. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, Dejana E, Koopman P, Cotelli F, Beltrame M. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111:2657–2666. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- Chen X, Qin J, Cheng CM, Tsai MJ, Tsai SY. COUP-TFII is a major regulator of cell cycle and Notch signaling pathways. Mol Endocrinol. 2012;26:1268–1277. doi: 10.1210/me.2011-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esengil H, Chang V, Mich JK, Chen JK. Small-molecule regulation of zebrafish gene expression. Nat Chem Biol. 2007;3:154–155. doi: 10.1038/nchembio858. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- Grabher C, Joly JS, Wittbrodt J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol. 2004;77:381–401. doi: 10.1016/s0091-679x(04)77021-1. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ Res. 2008;102:12–15. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, Choi I, Otu HH, Shin JW, Dotto GP, Koh CJ, Detmar M, Hong YK. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–150. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci U S A. 2000;97:11403–11408. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann's organizer. Proc Natl Acad Sci U S A. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act uptream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Park JI, Tsai SY, Tsai MJ. Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J Med. 2003;52:174–181. doi: 10.2302/kjm.52.174. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeville H, Winandy M, Manfroid I, Nivelles O, Motte P, Pasque V, Peers B, Struman I, Martial JA, Voz ML. Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Dev Biol. 2008;317:405–416. doi: 10.1016/j.ydbio.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit FG, Salas R, Tsai MJ, Tsai SY. The regulation of COUP-TFII gene expression by Ets-1 is enhanced by the steroid receptor co-activators. Mech Ageing Dev. 2004;125:719–732. doi: 10.1016/j.mad.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost E, Rhee J, Leach SD. Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis. 2007;45:625–629. doi: 10.1002/dvg.20338. [DOI] [PubMed] [Google Scholar]
- Ro H, Soun K, Kim EJ, Rhee M. Novel vector systems optimized for injecting in vitro-synthesized mRNA into zebrafish embryos. Mol Cells. 2004;17:373–376. [PubMed] [Google Scholar]
- Sacilotto N, Monteiro R, Fritzsche M, Becker PW, Sanchez-del-Campo L, Liu K, Pinheiro P, Ratnayaka I, Davies B, Goding CR, Patient R, Bou-Gharios G, De Val S. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci. 2013;110:11893–11898. doi: 10.1073/pnas.1300805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW, 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nature biotechnology. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Thurston G, Yancopoulos GD. Gridlock in the blood. Nature. 2001;414:163–164. doi: 10.1038/35102664. [DOI] [PubMed] [Google Scholar]
- Villefranc JA, Amigo J, Lawson ND. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn. 2007;236:3077–3087. doi: 10.1002/dvdy.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein DA, Turner DL, Kintner C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
- Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


