Abstract
Background
The BARI 2D trial assigned patients with type 2 diabetes to prompt coronary revascularization (REV) plus intensive medical therapy versus intensive medical therapy (MED) alone and reported no significant difference in mortality. Among patients selected for CABG, REV was associated with a significant reduction in death/MI/stroke compared with MED. We hypothesized that clinical and angiographic risk stratification would impact the effectiveness of the treatments overall and within revascularization strata.
Methods and Results
An angiographic risk score was developed from variables assessed at randomization; independent prognostic factors were myocardial jeopardy index, total number of coronary lesions, prior coronary revascularization, and left ventricular ejection fraction. The Framingham risk score for patients with coronary disease was used to summarize clinical risk. Cardiovascular event rates were compared by assigned treatment within high-risk and low-risk subgroups.
No overall MED versus REV outcome differences were seen in any risk stratum. The five-year risk of death/MI/stroke was 36.8% for MED compared with 24.8% for REV among the 381 CABG-selected patients in the highest angiographic risk tertile (p=0.005); this treatment effect was amplified in patients with both high angiographic and high Framingham risk (47.3% MED versus 27.1% REV, p=0.010; Hazard Ratio=2.10, p=0.009). Treatment group differences were not significant in other clinical-angiographic risk groups within the CABG stratum nor any subgroups within the PCI stratum.
Conclusions
Among patients with diabetes and stable ischemic heart disease, a strategy of prompt CABG significantly reduces the rate of death/MI/stroke in those with extensive coronary artery disease or impaired left ventricular function.
Clinical Trial Registration: ClinicalTrials.gov NCT00006305
Keywords: Diabetes mellitus, coronary revascularization, coronary artery disease
INTRODUCTION
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial compared a strategy of prompt coronary revascularization added to intensive medical therapy (REV) to intensive medical therapy alone with deferred revascularization when clinically indicated (MED) in patients with type 2 diabetes and stable coronary disease. The survival rates were similar for both treatment strategies over an average 5.3 years of follow-up.1,2 The intended revascularization procedure, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), was selected before patients were randomized based on the individual physician’s preference resulting in two strata of patients (MED versus PCI and MED versus CABG).3–5 No treatment differences were observed in the rate of all-cause death or myocardial infarction (MI) in the MED versus PCI stratum in BARI 2D1,2 or in COURAGE.6,7 However, in the BARI 2D MED versus CABG stratum, significant reductions in the principal secondary end-point of death/MI/stroke and the end-point of cardiac death/MI were observed with CABG, mainly due to a significant reduction in the rate of myocardial infarction. The explanations for this apparent benefit are multifactorial and have not been well defined.
Patients selected for the CABG stratum had more extensive coronary disease and worse angiographic characteristics than patients selected for PCI and were more likely to have an adverse outcome. In this setting, CABG may provide a more complete and durable revascularization than medical therapy and may be more cardioprotective against future events.1,4 We sought to develop a risk score incorporating angiographic and related variables collected at the time of randomization that would be associated with the risk of major cardiovascular outcomes. We hypothesized that the relative benefit observed with CABG compared with initial medical therapy in reducing the composite end-point of death/MI/stroke would be confined to patients at higher angiographic risk. Since long-term prognosis is also influenced by patient characteristics that may accelerate the atherosclerotic process, a second aim was to assess if high clinical risk would augment the effectiveness of revascularization relative to medical therapy regarding long-term cardiovascular outcomes.
METHODS
Study Population and Treatment Strategies
A detailed description of the BARI 2D study design, protocol, patient characteristics, and consort diagram have been published previously.8,9 Briefly, BARI 2D is a multi-center international randomized clinical trial comparing two major strategies in a 2 × 2 factorial design in patients with type 2 diabetes and stable coronary artery disease: a) prompt coronary revascularization (REV) versus deferred revascularization to be used only if clinically necessary (MED); and b) glycemic control strategy of insulin sensitization (IS) versus insulin provision (IP) therapy to a target HbA1c of <7.0%. The choice of revascularization (PCI or CABG) was determined after coronary angiography by the treating physician, and randomization was then stratified by the type of intended revascularization procedure. Patients selected for CABG generally had more extensive disease, total occlusions, proximal left coronary disease, and greater myocardial jeopardy score than those selected for PCI.4 After randomization, all patients were treated according to current guidelines for lipid and blood pressure management, smoking cessation, physical activity, and weight loss.10 Medication usage and achievement of risk factor targeted therapeutic goals were measured at prespecified intervals during follow-up and treatment was optimized. The protocol was approved by the institutional review board at the University of Pittsburgh and at each participating site. All patients provided written informed consent. The trial was supported by the National Institutes of Health, with additional support from industry.
The trial enrolled 2,368 patients between January 1, 2001 and March 31, 2005.1 Eligible patients had type 2 diabetes and evidence of myocardial ischemia by either angiographically defined coronary artery disease (CAD) with at least one coronary lesion ≥50% stenosis and abnormal noninvasive stress test results or typical angina and at least one coronary lesion that had a ≥70% stenosis. Patients were excluded if they required immediate revascularization, had revascularization within the prior 12 months, had left main coronary disease, a creatinine >2 mg/dL, a glycated HbA1c >13.0%, class III or IV heart failure or significant hepatic dysfunction. Patients were seen in clinic on a monthly basis for the first 6 months and quarterly thereafter. As of November 30, 2008, the vital status was known for 2,283 patients (96%). The mean follow-up interval was 5.3 years (range 3.4–7.8). The primary outcome measure for this analysis is the composite end-point of death/MI/stroke, and the secondary outcome measures of interest are all-cause death and the composite end-point of cardiac death/MI. All acute coronary syndrome events were adjudicated at a core ECG/myocardial infarction laboratory and cause of death was adjudicated by an independent Mortality and Morbidity Classification Committee in both cases masked to treatment assignment.2
To quantify the risk of major cardiovascular outcomes in the BARI 2D patients, we developed a risk score from baseline angiographic and related variables. We then assessed the two treatment strategies (MED versus REV) within the PCI and within the CABG stratum by different levels of angiographic risk. The Framingham Score for recurrent coronary heart disease, as described by D’Agostino et. al.,11 was used to further risk stratify the BARI 2D population at baseline. Outcome differences between MED and REV were assessed according to levels of clinical and angiographic risk.
Statistical Analysis
Baseline demographic, clinical and angiographic characteristics were compared between patients selected for the PCI and the CABG intended revascularization stratum (Table 1). Proportions, means and standard deviations, or medians and ranges are presented, and chi-square statistics are used to compare categorical variables and Wilcoxon rank sum tests for continuous variables.
Table 1.
Baseline Characteristics of BARI 2D Patient Population by Intended Revascularization Randomization Stratum
| Characteristic | Total (N=2365) | PCI stratum (N=1602) | CABG stratum (N=763) | Nominal p-value |
|---|---|---|---|---|
| Age at study entry, mean, SD | 62.4, 8.9 | 62.0, 9.1 | 63.2, 8.4 | 0.0011 |
| Male, % | 70.4 | 67.8 | 75.8 | <0.0001 |
| Race/Ethnicity, % | ||||
| White non-Hispanic | 65.8 | 63.5 | 70.6 | <0.0001 |
| Black non-Hispanic | 16.8 | 19.9 | 10.5 | |
| Hispanic | 12.6 | 11.7 | 14.3 | |
| Asian/Other non-Hispanic | 4.8 | 4.9 | 4.6 | |
| Geographic Region, % | ||||
| USA | 63.3 | 73.7 | 41.4 | <0.0001 |
| Canada | 14.9 | 13.7 | 17.6 | |
| Mexico | 3.6 | 2.1 | 6.8 | |
| Brazil | 15.0 | 7.9 | 30.0 | |
| Czech Republic/Austria | 3.2 | 2.7 | 4.2 | |
| History of MI, % | 32.0 | 30.1 | 36.0 | 0.0039 |
| History of CHF, % | 6.7 | 7.7 | 4.5 | 0.0035 |
| Cerebrovascular accident TIA, % | 9.8 | 10.5 | 8.2 | 0.0703 |
| Peripheral/carotid artery disease, % | 23.7 | 23.8 | 23.5 | 0.8499 |
| Core: HbA1c %, mean, SD | 7.65, 1.61 | 7.60, 1.58 | 7.75, 1.68 | 0.0476 |
| Duration of DM, mean, SD | 10.4, 8.7 | 10.4, 8.8 | 10.5, 8.4 | 0.7420 |
| Currently taking insulin, % | 27.8 | 30.4 | 22.4 | <0.0001 |
| Albumine creatinine ratio, % | ||||
| No albuminuria | 67.4 | 68.4 | 65.4 | 0.2368 |
| Microalbuminuria | 22.9 | 21.8 | 25.1 | |
| Macroalbuminuria | 9.7 | 9.8 | 9.6 | |
| Angina status, % | ||||
| Stable CCS1/2 | 42.5 | 41.4 | 45.0 | 0.0006 |
| Stable CCS3/4 | 8.6 | 7.9 | 10.1 | |
| Unstable angina | 9.5 | 10.8 | 7.0 | |
| No classic angina | 39.3 | 40.0 | 38.0 | |
| Prior revascularization, % | 23.6 | 28.6 | 13.0 | <0.0001 |
| 3 vessel disease, % | 30.7 | 20.3 | 52.4 | <0.0001 |
| Proximal LAD dx, % | 13.2 | 10.3 | 19.4 | <0.0001 |
| Total occlusion, % | 41.1 | 61.2 | 31.5 | <0.0001 |
| Number of lesions, mean, SD | 4.8, 2.3 | 4.4, 2.1 | 5.7, 2.2 | <0.0001 |
| Site LVEF, mean, SD | 57.1, 11.0 | 57.0, 11.0 | 57.3, 10.9 | 0.5455 |
| Site: LVEF < 50%, % | 17.5 | 17.5 | 17.5 | 0.9999 |
| Myocardial jeopardy, mean, SD | 44.5, 24.2 | 37.2, 21.8 | 59.7, 21.7 | <0.0001 |
| Angiographic risk prob. (%)*, | ||||
| mean, SD | 15.5, 6.4 | 14.3, 6.1 | 18.1, 6.1 | <0.0001 |
| median, min-max | 14.3, 6.1–56.9 | 12.9, 6.1–56.9 | 17.1,6.1–41.5 | <0.0001 |
| High angiographic risk, % | 32.9 | 24.8 | 49.9 | <0.0001 |
| Framingham risk prob.(%)**, | ||||
| mean, SD | 15.3, 5.0 | 14.8, 5.2 | 16.1,4.7 | <0.0001 |
| median, min-max | 15.6, 2.9–42.5 | 15.3, 2.9–42.5 | 16.3, 3.0–30.5 | <0.0001 |
| High Framingham risk, % | 33.3 | 30.4 | 39.4 | <0.0001 |
CABG = coronary artery bypass grafting; CCS = Canadian Cardiovascular Society; CHF = congestive heart failure requiring treatment; DM = diabetes mellitus; LAD = left anterior descending; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD =Standard Deviation; TIA = transient ischemic attack;
Probability of death, MI or stroke by 3 years; “high” angiographic risk defined as ≥17.1% probability of Death/MI/Stroke event
Probability of subsequent CVD event by 3 years; “high” Framingham risk defined as ≥17.5% probability of CVD event
The BARI 2D angiographic risk score was based on the predicted probability of experiencing a death MI or stroke by 3 years (by protocol, all patients should have had 3 or more years of follow-up). Stepwise variable selection methods were used to create a logistic regression model, and the following candidate variables were considered: myocardial jeopardy index (MJI), number of diseased vessels with ≥50% stenosis (categorical variables for 2 and for 3 diseased vessels), location of diseased vessels (RCA, LCX, LAD), proximal left anterior descending (LAD) disease ≥50%, presence of one or more proximal lesion ≥50%, total number of lesions ≥20%, history of prior coronary revascularization, left ventricular ejection fraction (LVEF) < 50%, presence of total occlusions, and presence of class C lesions. The myocardial jeopardy index (MJI) is defined as the number of myocardial territories supplied by significantly diseased main coronary arteries or their branches divided by the total number of myocardial territories. The Framingham Score for recurrent coronary heart disease includes sex, age, history of diabetes (which is present in all BARI 2D patients), total cholesterol/HDL, and the additional variables of systolic blood pressure and current smoking in women.11
The angiographic risk score and the Framingham clinical risk score were calculated for each BARI 2D patient based on the individual’s characteristics at study entry. The top tertile of patients based on the angiographic score were considered “high angiographic risk,” and the top tertile of patients based on the Framingham score were considered “high clinical risk.” For each score, the bottom two tertiles were labeled as “low” risk.
Kaplan-Meier estimated cumulative event rates and hazard ratios with 95% confidence intervals from Cox proportional hazards regression models were used to compare the risk of cardiovascular events between the randomized treatment strategies within subgroups defined by individual angiographic factors and by the risk scores according to randomization stratum.12,13 In these analyses, time zero was defined as the date of randomization; event times for composite outcomes were defined as the time to the first event, and if no event occurred, patients were censored at their last valid follow-up. Average follow-up was 5.3 years for death and 4.6 years for other cardiovascular event outcomes. Five-year Kaplan-Meier estimated events rates are presented since this time interval was used for the primary BARI 2D trial treatment comparisons. Cox regression models including randomized treatment, the subgroup variable, and the interaction term between treatment and the subgroup variable were created to evaluate the significance of the risk-treatment interaction. To account for the multiple comparisons conducted in this investigational analysis, a p-value <0.05 was considered marginally significant, a p-value <0.01 statistically significant, and p-value <0.001 highly statistically significant.
RESULTS
Seven hundred sixty three (32.3%) of the 2368 patients enrolled in the BARI 2D trial were intended for CABG if randomized to revascularization. As compared to patients selected for PCI, those intended for CABG had a higher average myocardial jeopardy score and number of lesions, more frequently had 3 vessel disease and proximal LAD disease, and were more often elderly, male and Caucasian (Table 1). A higher proportion of the PCI group had a history of prior revascularization, and left ventricular function was similar. Notable geographic differences were observed in the revascularization choice between PCI and CABG.
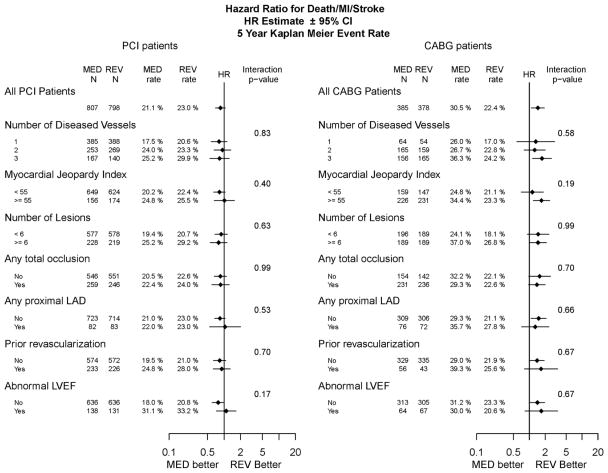
The hazard ratios by treatment assignment (MED versus REV) according to individual angiographic factors are illustrated in Figure 1 for the endpoint of death/MI/stroke in the PCI and CABG strata. Among patients selected for PCI, there were no significant outcome differences between MED and REV in the overall or within any of the individual angiographic subgroups. In contrast, among patients selected for CABG, a significant benefit of prompt revascularization was observed in patients with 3 vessel disease (p=0.0084) and in patients with a high myocardial jeopardy score (p=0.0038)). The interaction between treatment assignment and subgroup level was not statistically significant for any of the designated angiographic subgroup variables.
Figure 1.
The hazard ratio of death/MI/stroke for medical therapy vs. prompt revascularization by baseline angiographic subgroups stratified by intended revascularization. The number of patients and the 5-Year Kaplan-Meier estimated cumulative event rates for patients randomized to medical (MED) and REV in each of the baseline subgroups are reported. The hazard ratio for MED versus REV (diamond) and its 95% confidence interval is plotted using a log scale. The vertical line represents a hazard ratio (HR) of 1 (no randomized treatment effect). The interaction p-value is a test of equality of the HRs among the levels of the subgroup variable. The left panel shows results for patients in the PCI stratum and the right panel shows results for patients in the CABG stratum.
Risk Models
Angiographic risk variables that were identified as independent predictors of death/MI/stroke in the BARI 2D population included MJI, total number of lesions ≥20%, history of prior coronary revascularization, and LVEF <50%, and the logistic regression risk model is shown in Table 2. The same angiographic predictor variables were identified and similar coefficients were obtained when the analysis was restricted to patients assigned to medical therapy or when a Cox regression model for death/MI/stroke based on all follow-up data was created. The probability risk functions for the angiographic and Framingham risk scores are described in the Supplemental Appendix. Based on the angiographic risk function, the predicted probability of death/MI/stroke at 3-years ranged from 6.1% to 56.9% among the BARI 2D participants, and using the Framingham score, the 3-year risk of a coronary event ranged from 3.4% to 42.5%. A third of the patients had an angiographic score of 17.1% or higher and were therefore classified as high angiographic risk (Supplementary Figure 1), and a third of the patients had a Framingham score of 17.5% or higher and were thus classified as high clinical risk. Of note, the majority (55%) of patients classified in the high risk angiographic group had 3 vessel disease and 42% had abnormal LVEF while the majority (51%) of patients classified in the low risk angiographic group had single vessel disease and only 6% had abnormal LVEF. Several patient scenarios are presented in the supplemental on-line appendix.
Table 2.
BARI 2D Angiographic Risk Score Model: the Logistic Regression Model for Death/MI/Stroke at 3 years (n=2,231) used to create the BARI 2D Angiographic Risk Score
| Baseline Coronary Disease Risk Factor | Regression Coefficient | Standard Error | p-value |
|---|---|---|---|
| MJI, per 1 unit | 0.0101 | 0.0028 | 0.0003 |
| Total number of lesions, per 1 lesion | 0.0927 | 0.0292 | 0.0015 |
| Prior Revascularization | 0.2689 | 0.1398 | 0.0544 |
| Abnormal LVEF | 0.5522 | 0.1439 | 0.0001 |
| Missing LVEF | 0.4034 | 0.3005 | 0.1795 |
| Intercept | −2.8334 | 0.1716 | <0.0001 |
Abnormal LVEF = left ventricular ejection fraction < 50%; MJI = myocardial jeopardy index
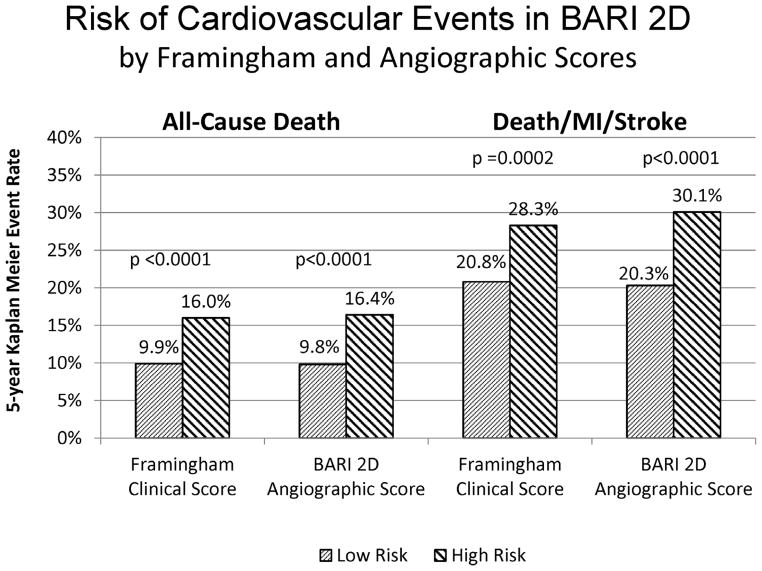
A high-risk Framingham score and a high-risk angiographic score were each associated with a significantly increased risk of all-cause death and death/MI/stroke at five years in the BARI 2D population (Figure 2). In total, the trial was unable to contact 3.6% of the BARI 2D patients at the final vital status sweep in November 2008; the rate of missing data did not differ significantly by treatment assignment, angiographic risk strata or Framingham risk strata.
Figure 2.
Risk of cardiovascular events in BARI 2D by Framingham and angiographic risk score category. The columns on the left represent the 5-year Kaplan–Meier event rate of death for low risk and high risk patient groups as defined by the Framingham clinical score and the BARI 2D angiographic score. The columns on the right represent the 5-year Kaplan-Meier event rate of the composite outcome of death, MI and stroke. The p-values indicate the significance of the log-rank statistic comparing the risk of events in the low and the high risk groups.
The mean angiographic score was significantly higher among patients selected for CABG compared to those selected for PCI (18.1 versus 14.3, p<0.0001, Table 1). Patients with high-risk angiographic scores accounted for 49.9% of the CABG stratum as compared to 24.8% of the PCI stratum (p<0.0001), and a larger proportion of patients in the CABG stratum were categorized as clinically high-risk by the Framingham risk score (Table 1).
Treatment Comparisons by Risk Group
Angiographic Risk Group Comparisons
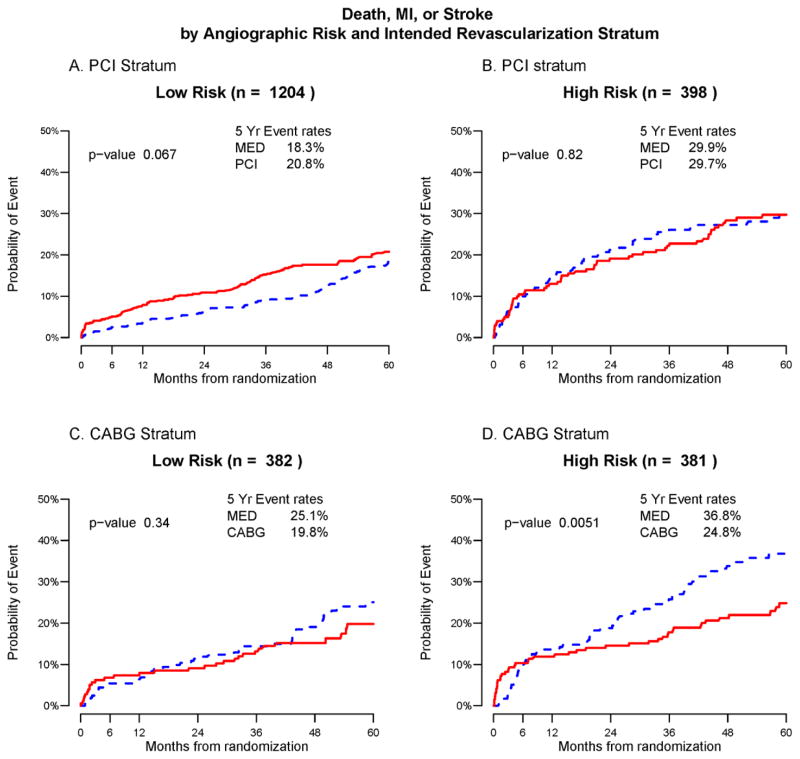
No significant treatment difference was observed in the 382 low-risk CABG patients, or in the 398 high-risk or 1204 low-risk PCI patients (Figure 3A–3C). However, among the 381 CABG stratum patients with high-risk angiographic characteristics, the risk of death/MI/stroke over five years of follow-up was significantly higher for those assigned to MED as compared to REV (36.8% versus 24.8%, p=0.005, Figure 3D). Long term risk of all-cause mortality was comparable between the MED and REV treatment groups within each angiographic risk and revascularization stratum (Supplementary Figures 2) whereas similar patterns favoring revascularization in the angiographically high-risk CABG stratum were noted for the composite end-point of cardiac death/MI and MI alone (Supplementary Figures 3 and 4); no treatment differences were detected for the stroke outcome (Supplementary Figure 5). A breakdown of the spontaneous and peri-procedural status of the first MI and stroke event per patient is shown in Supplementary Table 1.
Figure 3.
Death, MI or stroke by angiographic risk score category and intended revascularization stratum. Each panel shows the Kaplan-Meier event rates for the composite outcome death/MI/stroke for patients randomized to Medical Therapy (MED blue) and Prompt Revascularization (REV red) with the log-rank p-value. Patients are stratified according to intended revascularization stratum and angiographic risk score. Panel a) PCI stratum, low angiographic risk, b) PCI stratum, high angiographic risk, c) CABG stratum low angiographic risk, d) CABG stratum, high angiographic risk
Framingham and Angiographic Risk Group Comparisons
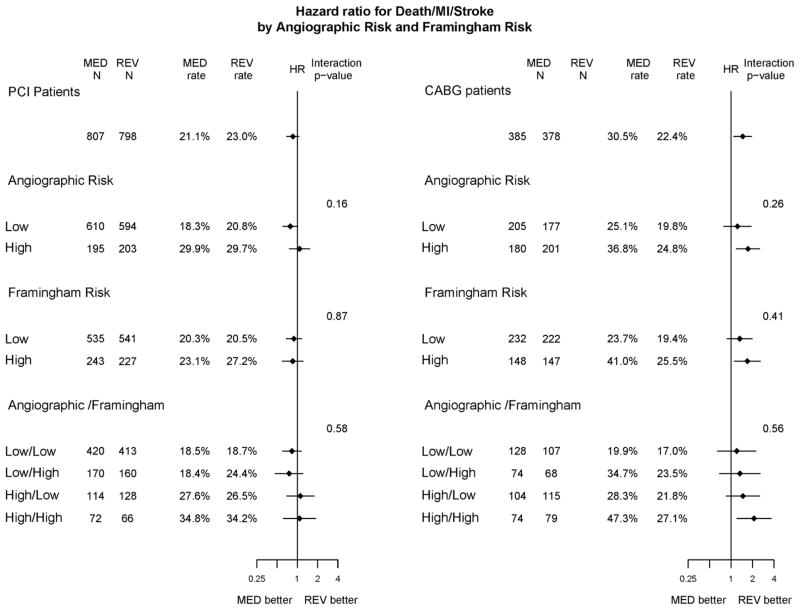
The five-year risk of death/MI/stroke was 47.3% for MED compared with 27.1% for REV in the 211 CABG stratum patients with high-risk Framingham and angiographic risk scores (p=0.010) (Figure 4; Supplementary Table 2). The risks for cardiac death/MI in the MED and REV groups within this CABG cohort were 37.9% and 17.4%, respectively (p=0.011) (Supplementary Table 2). Treatment differences were not statistically or clinically significant in the other clinical risk-angiographic risk groups in the CABG stratum, nor in any of the PCI subgroup comparisons.
Figure 4.
The HR of death/MI/stroke for MED therapy vs. prompt revascularization by angiographic and Framingham risk stratified by intended revascularization. The number of patients and the 5-Year Kaplan-Meier event rates for patients randomized to MED and REV in each of the subgroups are reported. The hazard ratio for MED versus REV (diamond) and its 95% confidence interval is plotted using a log scale. The vertical line represents a HR of 1 (no randomized treatment effect). The interaction p-value is a test of equality of the HR’s among the levels of the subgroup variable. The left panel shows results for patients randomized in the PCI stratum and the right shows results for patients in the CABG stratum.
Based on Cox regression analysis, the hazard ratios for MED versus REV regarding death/MI/stroke and cardiac death/MI were statistically significant in the patients classified as high-risk by the angiographic score in the CABG stratum (HR for death/MI/stroke =1.72, p=0.0049; HR for cardiac death/MI =1.94, p=0.0038; Figure 4), but not in the larger low-risk angiographic group in the CABG stratum. Further stratification by Framingham risk score revealed that the treatment hazard ratio was significant only among patients who were high risk according to both the angiographic and the clinical scale (HR for death/MI/stroke= 2.10, p=0.009; HR for cardiac death/MI= 2.41, p=0.012; HR for MI= 3.76, p=0.0022; Table 3; Figure 4). There were consistent patterns indicating that revascularization was relatively more advantageous among higher risk patients; however, the interactions testing for modification of treatment effect by risk group were not statistically significant when risk was defined by angiographic, clinical or both risk scores. Notably, in the CABG stratum, the treatment effect of MED versus REV did not differ significantly between the two angiographic risk groups for the end-point of death/MI/stroke (interaction p=0.26, Figure 4) nor for cardiac death/MI (p=0.082).
Table 3.
Hazard Ratios for Medical Therapy versus Prompt Revascularization within Subgroups Defined by Framingham and Angiographic Risk
| Hazard Ratio (95% CI) for MED vs REV | p-value | Hazard Ratio (95% CI) for MED vs REV | p-value | |
|---|---|---|---|---|
| DEATH | ||||
| PCI Stratum | Low Angiographic Risk Score | High Angiographic Risk Score | ||
| Low Framingham Risk | 0.85 (0.54 – 1.34) | 0.49 | 1.39 (0.77 – 2.54) | 0.28 |
| High Framingham Risk | 0.86 (0.47 – 1.58) | 0.64 | 0.83 (0.42 – 1.67) | 0.61 |
| CABG Stratum | ||||
| Low Framingham Risk | 1.33 (0.57 – 3.06) | 0.51 | 1.10 (0.55 – 2.23) | 0.79 |
| High Framingham Risk | 1.61 (0.74 – 3.52) | 0.23 | 1.15 (0.58 – 2.29) | 0.68 |
| DEATH/MI/STROKE | ||||
| PCI Stratum | Low Angiographic Risk Score | High Angiographic Risk Score | ||
| Low Framingham Risk | 0.83 (0.60 – 1.14) | 0.25 | 1.11 (0.69 – 1.77) | 0.67 |
| High Framingham Risk | 0.75 (0.47 – 1.21) | 0.24 | 1.08 (0.62 – 1.87) | 0.79 |
| CABG Stratum | ||||
| Low Framingham Risk | 1.20 (0.64 – 2.23) | 0.57 | 1.47 (0.86 – 2.51) | 0.16 |
| High Framingham Risk | 1.33 (0.69 – 2.56) | 0.40 | 2.10 (1.21 – 3.65) | 0.0087 |
| CARDIAC DEATH/MI | ||||
| PCI Stratum | Low Angiographic Risk Score | High Angiographic Risk Score | ||
| Low Framingham Risk | 0.82 (0.55 – 1.23) | 0.33 | 0.85 (0.49 – 1.48) | 0.56 |
| High Framingham Risk | 0.77 (0.39 – 1.51) | 0.44 | 0.88 (0.46 – 1.68) | 0.69 |
| CABG Stratum | ||||
| Low Framingham Risk | 0.98 (0.47 – 2.06) | 0.95 | 1.68 (0.89 – 3.14) | 0.11 |
| High Framingham Risk | 1.13 (0.47 – 2.72) | 0.79 | 2.41 (1.21 – 4.80) | 0.0124 |
| MI | ||||
| PCI Stratum | Low Angiographic Risk Score | High Angiographic Risk Score | ||
| Low Framingham Risk | 0.87 (0.56 – 1.33) | 0.52 | 1.27(0.67 – 2.42) | 0.47 |
| High Framingham Risk | 0.87 (0.42 – 1.83) | 0.71 | 0.97 (0.46 – 2.04) | 0.94 |
| CABG Stratum | ||||
| Low Framingham Risk | 1.12(0.47–2.66) | 0.80 | 1.78(0.84–3.77) | 0.13 |
| High Framingham Risk | 1.49 (0.49–4.54) | 0.49 | 3.76 (1.61 – 8.77) | 0.0022 |
CABG = coronary artery bypass grafting; CI = confidence interval; MED = medical therapy; MI = myocardial infarction; PCI = percutaneous coronary intervention; REV = prompt revascularization
DISCUSSION
The main finding from this report is that the treatment differences observed in the CABG stratum of the BARI 2D trial are confined to the subgroup of patients considered to be at high-risk based on their angiographic profile. In this group of patients, the benefit of revascularization is amplified in those patients considered to be at high clinical risk. Thus, for patients with type 2 diabetes who are suitable candidates for CABG with a greater atherosclerotic risk factor profile and more extensive coronary disease as defined by the angiographic risk score, prompt CABG is a reasonable treatment strategy as compared to medical therapy alone in order to significantly reduce cardiovascular events, in particular spontaneous myocardial infarction, over a 5-year period. The incidence of first spontaneous myocardial infarction during follow-up was 21.1% in the medical therapy group compared to 6.5% in the CABG group without an increased risk of stroke (3.3% for MED versus 2.0% for REV) (Supplementary Table 2). In angiographic high-risk patients with more diffuse disease, CABG provides conduits that bypass obstructive as well as nonobstructive atherosclerotic plaques, possibly offering a cardioprotective effect against future spontaneous plaque rupture. We did not observe a treatment difference between REV and MED for death/MI/stroke over 5 years of follow-up in the angiographically defined high risk patients in the PCI stratum, even in those with a high-risk Framingham score. One potential explanation for this finding is that the high risk PCI patients in BARI 2D had less severe coronary disease than the high risk CABG patients.
The angiographic score we developed included myocardial jeopardy index, total number of lesions ≥20% diameter stenosis, prior coronary revascularization, and LVEF <50% as independent predictors of death/MI/stroke. All baseline coronary angiograms were read at the Stanford angiographic core laboratory. The myocardial jeopardy index integrates the number of myocardial segments supplied by significantly diseased main coronary arteries or their branches. Thus, MJI is a higher for patients with a greater number of diseased vessels, particularly vessels with proximal disease.
We used the Framingham risk score to assign a clinical risk level to individual patients in BARI 2D. The Framingham investigators have developed models for individuals with a history of coronary heart disease or ischemic stroke from the combined experience of the original Framingham cohort and participating offspring and their spouses.11 Our data confirm that the Framingham model for patients with coronary disease can classify patients with established coronary artery disease and type 2 diabetes into higher and lower-risk groups. The Framingham score incorporates well known atherosclerotic risk factors associated with more frequent coronary artery disease progression into a single score. It is possible that other scoring methods that cluster other or additional risk factor groupings may produce different results.14,15 To assess the concept that high clinical risk magnifies treatment differences in the angiographic score risk, we examined the randomized treatment difference using the EuroSCORE.15 The relationship between the benefit of CABG compared to initial intensive medical therapy and risk was no more pronounced using the EuroSCORE strata than the BARI 2D angiographic score (see supplementary appendix) Our report is focused on clinical event outcomes including all cause death, death/MI/stroke, all-cause death, and cardiac death/MI. CABG and PCI have been shown to reduce the likelihood of new or worsening angina16 in this group of patients with type 2 diabetes and stable coronary disease. Risk stratification based on angiographic and Framingham scores may not have the same impact on treatment effect estimates for functional outcomes.
Although the results observed in this study support the concept that the benefit of revascularization with CABG is greater for high risk patients, the interaction tests between the angiographic risk score, the Framingham risk score and prompt revascularization were not statistically significant. The BARI 2D trial was designed to have sufficient power to detect differences between REV and MED in the entire trial population. Moreover, a statistically significant interaction was detected between treatment assignment and intended revascularization stratum indicating that prompt revascularization resulted in lower death/MI/stroke rates in the CABG stratum but not in the PCI stratum (interaction, p=0.01). The BARI 2D trial, however, has limited power to test an interaction between risk scores and treatment assignment within one stratum of the trial. Thus, our finding, that prompt revascularization with CABG is significantly beneficial in the subgroup of high risk patients but not the subgroup of low risk patients, is suggestive but not definitive. Our results are consistent with other revascularization trials17–19 in which CABG is beneficial compared with medical therapy alone for patients with reduced left ventricular function and extensive CAD, whether CAD is measured by number of diseased vessels, myocardial jeopardy, or number of lesions.
Limitations
In addition to the power limitations due to the fixed sample size of the BARI 2D trial, our angiographic risk score has not been validated in other patient populations. It remains to be seen whether these results can be replicated in other stable ischemic heart disease patient populations with or without type 2 diabetes.
It is also important to consider the implications of invasive interventions among those with lower risk scores. A trend favoring intensive medical therapy among those identified as candidates for PCI is evident both in the low Framingham and angiographic risk score groups. We did not routinely use other methods to risk-stratify our patients such as fractional flow reserve (FFR) in patients with borderline lesions (i.e. luminal narrowing 50–70%).20,21 The BARI 2D entry criteria required that patients have myocardial ischemia as evidenced by ≥ 50% luminal narrowing in a major coronary vessel with abnormal noninvasive testing or by typical angina with ≥ 70% luminal narrowing. Perhaps some patients with less severe anatomic disease (50–70% narrowings) and less severe ischemia on noninvasive testing were entered into the trial in whom a coronary revascularization procedure would not be expected to show benefit. During the BARI 2D enrollment period and trial, bare metal stents were more commonly used (56%). However, the rates of bare metal and drug eluting stent usage were similar in the low and high angiographic risk groups who received PCI as their initial procedure.
Other angiographic scoring methods such as the SYNTAX score are useful in assigning levels of angiographic risk.22,23 The BARI 2D trial was initiated and the core angiographic analysis was completed before the SYNTAX score was developed. Therefore, we are unable to compare our angiographic score to the SYNTAX score at this time. Patients with left main coronary disease > 50% were not enrolled in BARI 2D. However, the two scoring methodologies are internally consistent in providing angiographic risk profiles and demonstrate an increased rate of myocardial infarction in patients with more diffuse and extensive coronary disease.24
Conclusions
The BARI 2D trial provides a unique opportunity to study a treatment strategy of prompt revascularization with CABG compared with medical therapy alone in diabetes patients with documented coronary disease. A comprehensive program was used to provide intensive medical therapy to all participants regardless of whether coronary revascularization was performed.1,5,10 Thus, our findings apply to patients in whom a strategy of aggressive atherosclerotic risk factor reduction is performed with the potential to prevent more rapid coronary and cerebrovascular ischemic events. Even in this setting of coordinated medical care, our results suggest that high risk patients with type 2 diabetes and more extensive coronary disease might benefit from prompt coronary revascularization with CABG as compared to a strategy of “watchful waiting” to avoid subsequent myocardial infarction and its long-term consequences.
Supplementary Material
Acknowledgments
FUNDING SOURCES
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) is funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804). BARI 2D receives significant supplemental funding provided by GlaxoSmithKline, Collegeville, PA, Lantheus Medical Imaging, Inc. (formerly Bristol-Myers Squibb Medical Imaging, Inc.), North Billerica, MA, Astellas Pharma US, Inc., Deerfield, IL, Merck & Co., Inc., Whitehouse Station, NJ, Abbott Laboratories, Inc., Abbott Park, IL, and Pfizer, Inc, New York, NY. Generous support is given by Abbott Laboratories Ltd., MediSense Products, Mississauga, Canada, Bayer Diagnostics, Tarrytown, NY, Becton, Dickinson and Company, Franklin Lakes, NJ, J. R. Carlson Labs, Arlington Hts., IL, Centocor, Inc., Malvern, PA, Eli Lilly and Company, Indianapolis, IN, LipoScience, Inc., Raleigh, NC, Merck Sante, Lyon, France, Novartis Pharmaceuticals Corporation, East Hanover, NJ, and Novo Nordisk, Inc. Princeton, NJ.
As an NIH funded trial, we are required to abide by the NIH PubMed Central Policy that we retain the right to provide a copy of the final manuscript to the NIH upon acceptance for publication by your journal, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Brooks has a research grant from Sanofi. Dr. Chaitman is on the speakers bureau for Gilead Pharmaceuticals and is a consultant for Merck, Pfizer, Eli Lilly, Takeda and Roche. Dr. Nesto is a consultant for GSK, Merck, and Sanofi. Dr. Feit is a shareholder of Johnson and Johnson, The Medicines Company, Boston Scientific, and Eli Lilly. Dr. Gersh is a consultant for Ortho-McNeil Janssen Scientific Affairs, Amorcyte, GE Healthcare, St. Jude Medical, Medispec Limited, Merck & Company, and Boston Scientific. Dr. Krone receives honorarium from Boston Scientific, is an expert witness for Sichmeller, Groves, Bowman, Sulak, and is a consultant for Janssen and Rivaroxaban. Dr. Garber has a research grant with Novo Nordisk insulin and liraglutide studies, is on the speakers bureau for Merck, Novo Nordisk, Santarus, Daiichi Sankyo; Honoraria for Nova Nordisk, Merck, Daiichi Sankyo, Takeda, Boehringer Ingelheim, LipoScience, is an expert witness for Nova Nordisk and Astra-Zeneca and serves on the AACE Board of Directors. Dr. Spencer King is on the speakers bureau for Network for continuing medical education and is a consultant for Merck, Wyeth Pharmaceuticals, CeloNova Biosciences, and Contact Surgical. Dr. Davidson is a consultant for Abbott. Dr. Ikeno has received honoraria from Medtronics, Abbott, Terumo Pharmaceuticals, and Asahi Intecc Co.
References
- 1.Frye RL, August P, Brooks M, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TLZ, Molitch ME, Nesto RW, Sako EY, Sobel BE BARI IID Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaitman BR, Hardison RM, Adler D, Gebhart S, Grogan M, Ocampo S, Sopko G, Ramires JA, Schneider D, Frye RL the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study Group. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease. Impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation. 2009;120:2529–2540. doi: 10.1161/CIRCULATIONAHA.109.913111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsness GW, Gersh BJ, Brooks MM, Frye RL. Rationale for the revascularization arm of the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:31G–40G. doi: 10.1016/j.amjcard.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kim LS, King SB, Kent K. Factors related to the selection of surgical versus percutaneous revascularization in diabetic patients with multivessel coronary artery disease in the BARI 2D trial. J Am Coll Cardiol Intv. 2009;2:384–392. doi: 10.1016/j.jcin.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris C, Chaitman BR, Shaw L, Gosselin G, Nawaz Sh, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GBJ, Weintraub WS for the COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 7.Boden WE, O’Rourke RA, Teo KK, Maron DJ, Hartigan PM, Sedlis SP, Dada M, Labedi M, Spertus JA, Kostuk WJ, Berman DS, Shaw LJ, Chaitman BR, Mancini GBJ, Weintraub WS on behalf of the COURAGE trial investigators. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial) Am J Cardiol. 2009;104:1–4. doi: 10.1016/j.amjcard.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 8.Brooks MM, Barsness G, Chaitman BR, Chung SC, Faxon D, Feit F, Frye R, Genuth S, Green J, Hlatky M, Kelsey S, Kennedy F, Krone R, Nesto R, Orchard T, O’Rourke R, Rihal C, Tardif JC. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the BARI 2D Trial. Am Heart J. 2008;156:528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz L, Kip KE, Alderman E, Lu J, Bates ER, Srinivas V, Bach RG, Mighton LD, Feit F, King S, Frye RL the BARI 2D Study Group. Baseline coronary angiographic findings in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial (BARI 2D) Am J Cardiol. 2009;103:632–638. doi: 10.1016/j.amjcard.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Albu J, Gottlieb SH, August P, Nesto RW, Orchard TJ for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial Investigators. Modifications of coronary risk factors. Am J Cardiol. 2006;97:41G–52G. doi: 10.1016/j.amjcard.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PWF, Hartz SC. Primary and subsequent coronary risk appraisal: New results from The Framingham Study. Am Heart J. 2000;139:272–281. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society. Ser B (Methodological) 1972;34:187–220. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 14.Miller TD, Roger VL, Hodge DO, Gibbons RJ. A simple clinical score accurately predicts outcome in a community-based population undergoing stress testing. Am J Med. 2005;118:866–872. doi: 10.1016/j.amjmed.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 15.O’Boyle F, Mediratta N, Fabri B, Pullan M, Chalmers J, McShane J Shaw M, Poullis M. Long-term survival after coronary artery bypass surgery stratified by EuroSCORE. Eur J Cardiothorac Surg. 2012 Jan 4; doi: 10.1093/ejcts/ezr253. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Dagenais GR, Lu J, Faxon DP, Kent K, Lago RM, Lezama C, Hueb W, Weiss M, Slater J, Frye RL Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study Group. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492–500. doi: 10.1161/CIRCULATIONAHA.110.978247. [DOI] [PubMed] [Google Scholar]
- 17.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63:285–299. doi: 10.1161/01.cir.63.2.285. [DOI] [PubMed] [Google Scholar]
- 18.Passamani E, Davis KB, Gillespie MJ, Killip T the CASS Principal Investigators and Their Associates. A randomized trial of coronary artery bypass surgery: survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL for the STICH Investigators. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pijls NH, Fearon WF, Tonino PAL, Siebert U, Ikeno F, Bornschein B, van’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B for the FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease. 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) Study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech J-W, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis. 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 22.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr RW for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 23.Sianos G, Morel M-A, Kappetein AP, Morice M-C, Colombo A, Dawkins K, van den Brand M, van Dyck N, Russell ME, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. Euro Interv. 2005;1:219–227. [PubMed] [Google Scholar]
- 24.Mack MJ, Banning AP, Serruys PW, Morice MC, Taeymans Y, Van Nooten G, Possati G, Crea F, Hood KL, Leadley K, Dawkins KD, Kappetein AP. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg. 2011;92:2140–6. doi: 10.1016/j.athoracsur.2011.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.