Abstract
Fat distribution differs between individuals, and those with visceral fat predominance develop metabolic profiles that increase risk of adverse cardiovascular events. This is due, in part, to the proinflammatory state associated with visceral obesity as well as depot-specific adipogenesis. The insulin-like growth factor (IGF) system is important in adipose tissue development and metabolic function. Pregnancy associated plasma protein-A (PAPP-A) is a novel zinc metalloproteinase that regulates local IGF availability. The first aim of this study was to characterize PAPP-A mRNA and protein expression in primary cultures of human preadipocytes isolated from omental, mesenteric and subcutaneous depots. PAPP-A expression was significantly increased in omental preadipocytes compared to mesenteric and subcutaneous preadipocytes. The second aim was to investigate factors regulating PAPP-A expression, focusing on proinflammatory cytokines and resveratrol that have been shown to have negative and positive effects, respectively, on metabolism and diet-induced obesity. Treatment of cultured primary human preadipocytes with tumor necrosis factor (TNF)-α and interleukin (IL) 1-β led to significant increases in PAPP-A expression. Activated pathways mediating cytokine-induced PAPP-A expression include the nuclear factor (NF) κB pathway and the mitogen activated protein kinase (MAPK) family, particularly c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated kinase. Resveratrol, a polyphenol with beneficial cardiometabolic effects, significantly down-regulated PAPP-A expression under basal and stimulated conditions. Resveratrol appeared to mediate its effects on PAPP-A through pathways independent of silent mating type information regulation 2 homolog 1 (SIRT1) and AMP kinase (AMPK) activation. Depot-specific PAPP-A expression in human preadipocytes may contribute to depot-specific function.
Keywords: PAPP-A, adipose tissue, resveratrol, cytokines, inflammation
INTRODUCTION
The prevalence of obesity is increasing, particularly in westernized countries, and is now considered an epidemic. Distribution of fat may differ between individuals, and metabolic functions of adipose tissue are inherently distinct between the fat depots (Caserta et al. 2001). This is due, in part, to regional variation of preadipocytes with respect to adipocyte development and metabolic function. Obesity is considered a low grade proinflammatory state with increased circulating cytokines, chemokines and growth factors (Lacasa et al. 2007). This adipose tissue inflammation leads to a higher likelihood of adverse metabolic profiles, including diabetes and atherosclerosis, particularly in subjects with visceral fat predominance (Tchkonia et al. 2010). Little is known about the regulatory factors leading to depot-specific function.
Genome-wide expression profiles of primary preadipocytes from human fat depots identified Pregnancy Associated Plasma Protein-A (PAPP-A) as one of the most distinctive genes expressed, with levels in preadipocytes from omental fat greatly exceeding those from subcutaneous fat (Tchkonia et al. 2007). PAPP-A is a zinc metalloproteinase that enhances local IGF action through cleavage of inhibitory proteins that bind IGFs with high affinity, thereby freeing the IGFs in the pericellular environment to bind and activate receptors (Conover 2012). Elevated PAPP-A has been implicated in aging and age-related disease, while PAPP-A knockout mice have a 30% longer lifespan than wild type mice, with resistance to atherosclerotic plaque development (Boldt et al. 2013, Conover et al. 2010, Harrington et al. 2007) and to visceral fat accumulation on high fat diet (Conover et al. 2013).
In this study, we follow up on the DNA microarray data and characterize PAPP-A mRNA and protein expression in primary cultures of human preadipocytes from omental, mesenteric and subcutaneous fat depots. Furthermore, we investigate the factors that regulate PAPP-A expression in human preadipocytes and the underlying mechanisms for their regulation, including proinflammatory cytokines and resveratrol, the latter being a polyphenol shown to have beneficial cardiometabolic effects.
MATERIALS AND METHODS
Materials
Tumor necrosis factor alpha (TNF-α) was purchased from Research Diagnostics Inc. (Flanders, NJ). Interleukin 1- β (IL1-β), interleukin- 6 (IL-6), IL-6 soluble receptor and EX 527 were purchased from R&D Systems (Minneapolis, MN). Antibodies against total and phosphorylated extracellular-regulated protein kinase (ERK) 1/2, c-Jun NH2-terminal kinase (JNK), p38 mitogen-activated kinase, nuclear factor κB (NFκB) [including inhibitory κB (IκB) α and β], phospho-Hsp27, and IGFBP-4 were purchased from Abcam (Cambridge, MA). Antibodies against total and phosphorylated AKT were purchased from Novus Biologicals (Littleton, CO). SB203580, SP600125, rolipram and splitomycin were purchased from Enzo Life Sciences (Farmingdale, NY). Bay 11-7082 was purchased from Calbiochem (San Diego, CA). Resveratrol was purchased from Sigma-Aldrich (St Louis, MO). Reagents for SDS-PAGE, mini-gels and blocking buffer were purchased from Bio-Rad Laboratories (Richmond, CA) and tissue culture supplements and fetal bovine serum were purchased from Life Technologies (Grand Island, NY). A769662 was purchased from Santa Cruz Biotechnology (Dallas, TX).
Cell Culture
Human adipose tissue was obtained through Mayo Clinic IRB-approved protocols. Human preadipocytes isolated from subcutaneous, mesenteric and omental fat depot were cultured in alpha MEM with 10% fetal bovine serum, 100 units/ml penicillin and 100 mg/ml streptomycin as previously described (Tchkonia et al. 2001, 2007). Donor characteristics are summarized in Table 1. Cells of the third to ninth passage were used in experiments. Preliminary dose response and time course experiments were performed to determine effective concentrations and appropriate duration of treatments. For some studies, cells were pretreated for 60 min with resveratrol before the addition of cytokines. At the end of the incubation, conditioned media were collected and cells were harvested for RNA and stored at −80C.
Table 1.
Human Preadipocytes: Donor characteristics
| Gender (15) | 3M/12F |
| Age (15) | 41 ± 2.5 years |
| Body mass index (15) | 51 ± 3.7 kg/m2 |
| Concomitant diseases (9) | |
| diabetes | 2 |
| degenerative joint disease | 3 |
| obesity hypoventilation syndrome | 3 |
| gastroesophageal reflux disease | 2 |
| sleep apnea | 2 |
| other* | |
| Medications | |
| diabetes | 2a |
| heart/blood pressure | 5b |
| heartburn | 5c |
| depression | 4d |
| inflammation/pain | 3e |
| other# |
Numbers in parentheses on the left indicate the number of donors for which information was available.
Numbers for specific diseases and medications on the right indicate the number of donors. Some donors will have more than one.
1 each hypothyroidism, hyperlipidemia, asthma, hypertension, recurrent sinus infection
1 each Carbidopa/Levodopa, Unithroid, Ortho Novum, Intraconozole
Avandia, Metformin, Prandin
Sotalol, Lasix, Digoxin, Avapro, hydrochlorothiazide, Lovenox, Lipitor, Lopid, Verdapamil, Norvasc
Prilosec
Paxil, Zoloft, Celexa, Trazadone, Prozac, Lorazepam
Flonase, Vioxx, Nasalcort, Zyrtec, Vicodin
Real time PCR
Upon harvest, the preadipocytes were immediately suspended in Trizol (Life Technologies, Carlsbad, CA). Total RNA was then isolated, reverse transcribed with the SuperScript® III First-Strand Synthesis System (Life Technologies) and evaluated by quantitative real-time PCR using the iCycler iQ5 Detection System with iQ SYBR green PCR Master Mix (Bio-Rad). Amplification plots were analyzed with iQ5 Optical System Software version 2.1 (Bio-Rad). Primer sequences for specific detection and amplification of PAPP-A as well as assay validations were described previously (Conover et al. 2006).
Western Blotting
Following experimental treatments, cells were lysed, subjected to SDS-PAGE and transferred to polyvinyl difluoride. Filters were blocked with 5% nonfat dry milk in Tris-buffered saline/0.1% Tween or blocking buffer (Bio-Rad) and probed with primary antibody at the recommended dilution. After the reaction, secondary antibody was applied [fluorescently labeled where applicable (LI-COR Lincoln, NE)]. Blots were visualized using enhanced chemiluminescence reagents and autoradiography or captured with LI-COR Odyssey® Imagers.
PAPP-A ELISA
Conditioned medium (CM) from human preadipocytes was collected, cellular debris removed by centrifugation, and supernatant stored at −80 C prior to assay. Protein was quantified using the Pierce BCA Protein assay kit (Thermo Scientific). PAPP-A protein in the CM was quantified by picoPAPP-A ELISA (generously provided by AnshLabs, Webster, TX), a high sensitivity assay designed to detect PAPP-A levels as low as 100 pg/mL with observed CV values routinely < 5.0. Some of the early experiments with conditioned media was performed using the Ultra-sensitive PAPP-A ELISA kit kindly provided by Diagnostic Systems Laboratories, Inc. (Webster, TX) as described previously (Boldt et al. 2011).
Miscellaneous Biochemistries
TNF-α and IL-1β levels in CM were assayed by the Mayo Clinic Immunochemical Core Laboratory.
Statistical analysis
Results are expressed as mean ± SEM for the indicated number of experiments. Number of experiments noted in figure legends is based on primary cultured cells from different donors. We used ANOVA with pairwise comparisons and Bonferroni correction for multiple comparisons. We used two sample t-tests to compare two groups. Significance was set at P < 0.05.
RESULTS
Depot-specific PAPP-A Expression
PAPP-A mRNA and protein expression was determined in primary cultures of human preadipocytes from subcutaneous, mesenteric and omental adipose tissue depots using real time PCR and PAPP-A ELISA respectively. As shown in Figure 1A, PAPP-A mRNA expression was significantly higher (four- fold) in omental preadipocytes compared to subcutaneous preadipocytes. Although abdominal in origin, mesenteric fat is distinct from omental fat with respect to cellular and gene expression properties. In our experiments, PAPP-A mRNA expression in omental fat was two-fold higher than in mesenteric fat. Levels of PAPP-A protein were also significantly higher in conditioned medium from omental preadipocytes compared to mesenteric and subcutaneous preadipocytes (Fig. 1B). Although these primary cultures of preadipocytes came from severely obese donors (Table 1), PAPP-A protein in extracts of fat depots from subjects with BMI < 30 kg/m2 showed the same significant difference (four- to six-fold) between omental and subcutaneous depots (data not shown). Furthermore, concomitant diseases or the variety of medications taken by the donors (Table 1) did not influence this differential expression. Thus, depot-specific PAPP-A expression is highest in preadipocytes from human visceral fat.
Figure 1. PAPP-A expression in human preadipocytes.
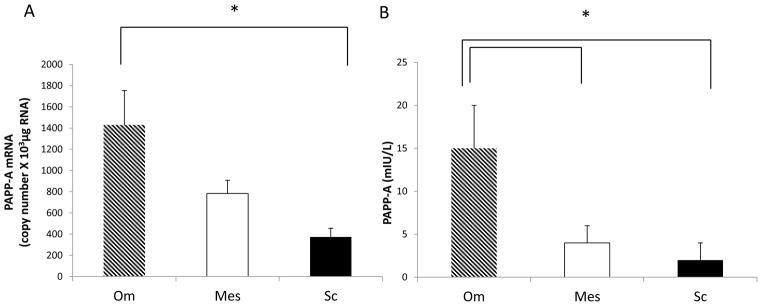
PAPP-A (A) mRNA and (B) protein expression in preadipocytes from omental (Om), mesenteric (Mes) and subcutaneous (Sc) depots were determined using real-time PCR and PAPP-A ELISA, respectively, as described in the Methods section.
Results are mean ± SEM, n = 6–10.
*P < 0.05.
Regulation of PAPP-A Expression by Cytokines
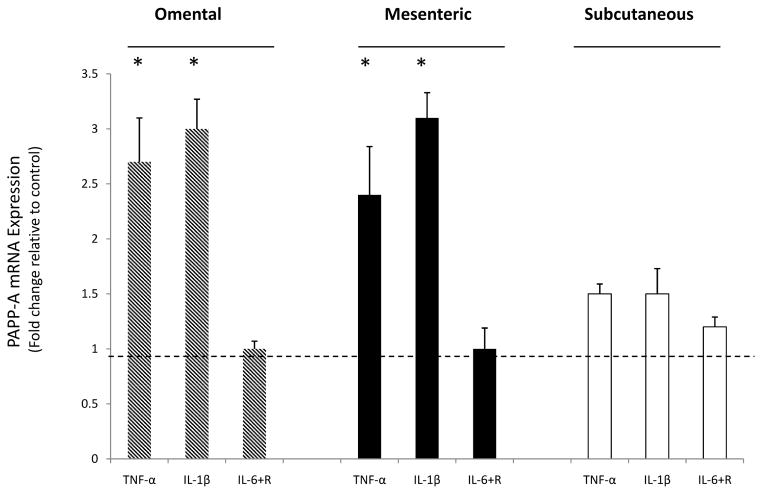
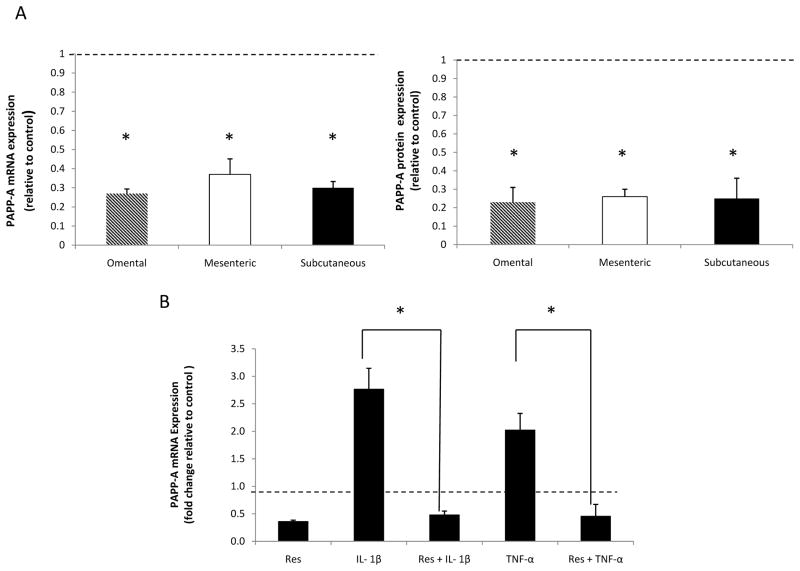
The effects of proinflammatory cytokines, TNF-α, IL1-β and IL-6, on PAPP-A expression were determined in subcutaneous, mesenteric and omental preadipocytes. Cells were treated with both IL-6 and the IL-6 soluble receptor to provide more reliable activation of the IL-6 signaling protein, gp130, in the preadipocytes (Resch et al. 2006). PAPP-A mRNA levels increased in response to TNF-α and IL1-β after 6 hours and remained increased for 24 hours based on time course experiments (data not shown). The effects of these cytokines on PAPP-A mRNA expression are shown in Figure 2. TNF-α and IL1-β led to a significant three-fold increase in PAPP-A mRNA expression in the omental and mesenteric preadipocytes with no significant increase seen in the subcutaneous preadipocytes. No significant effect was seen on PAPP-A expression after treatment with IL-6 and IL-6 with soluble receptor. PAPP-A protein levels in conditioned medium were also increased by TNF-α and IL-1β, with no effect after treatment with IL-6 (data not shown).
Figure 2. Effect of cytokines on PAPP-A expression.
Preadipocytes were stimulated with TNF-α (1 nM), IL1-β (1 nM), and IL-6 (50 ng/ml) with soluble receptor (100 ng/ml) for 24 hours. Upon harvest, RNA was isolated from preadipocytes, reverse transcribed and evaluated by quantitative real-time PCR.
Results (mean ± SEM, n = 3–4) are expressed relative to control (represented by the dotted line).
*P < 0.05.
To determine the intracellular pathways mediating TNF-α and IL1-β stimulation of PAPP-A expression in human preadipocytes, we focused on those commonly associated with stress, apoptosis and proliferation. These included mitogen activated protein kinase (MAPK) family, phosphatidylinositol 3- kinase (PI3K) and NFκB pathways.
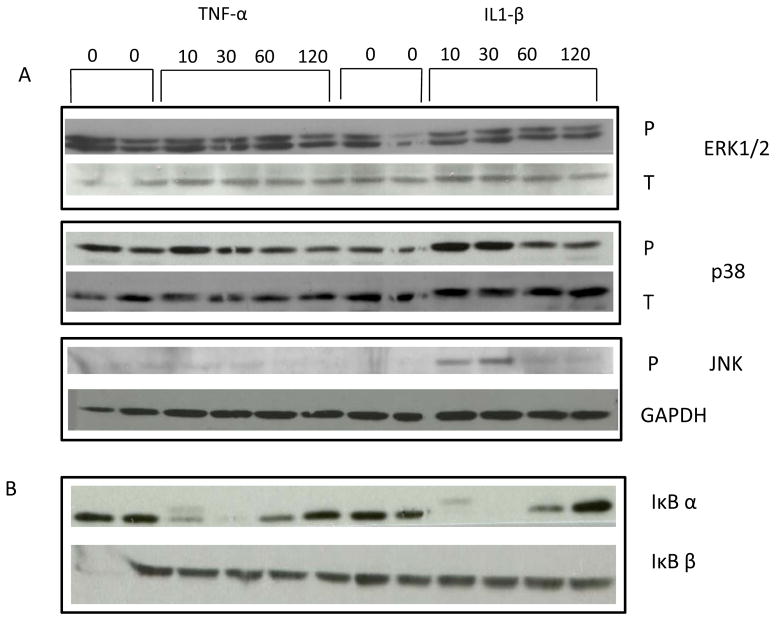
The MAPK family has three main sub groupings: Erk1/2, p38 kinase, and JNK. Western blot analyses were performed using antibodies specific for these pathways. Stimulation with TNF-α and IL1-β had little effect on Erk1/2 phosphorylation but induced p38 phosphorylation at 10 and 30 min with a decrease toward basal at 60 and 120 min. JNK phosphorylation was induced 10 and 30 min after stimulation with IL1-β but returned to baseline at 60 min. IL1-β appeared to be more effective than TNF-α in activating the JNK pathway (Fig. 3A, Fig. 4B).
Figure 3. TNF- α and IL-1β regulation of (A) MAPK and (B) NFκB signaling.
Human preadipocytes from omental fat were treated with TNF (1 nM) or IL-1β (1 nM) for the indicated times. Western blotting was performed for phosphorylated (P) Erk1/2, p38 and JNK, total (T) p38 and ERK1/2, and IκB α and IκBβ as described in the Methods section. GAPDH was used to quantify loading for P-JNK.
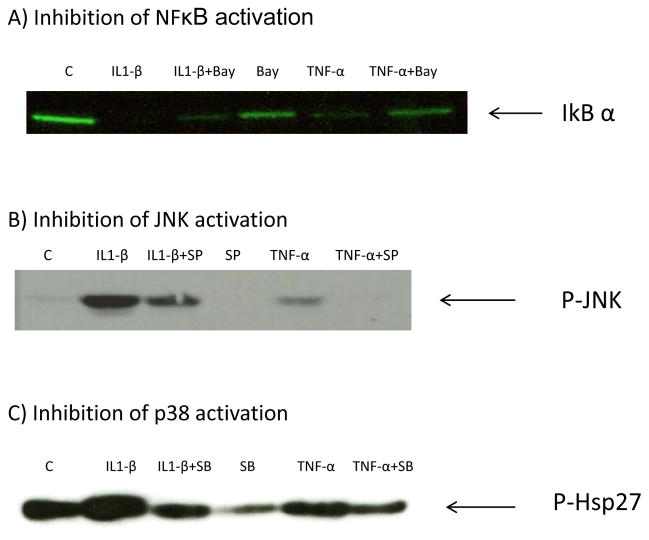
Figure 4. Efficacy of inhibitor concentrations.
Human preadipocytes from omental fat were treated with BAY11–7082 (5 μM), SP600125 (20 μM) or SB203580 (2.5 μM) and cytokines TNF- α (1nM) and IL-1β (1 nM) for 6 hours prior to cell lysis for Western Blotting. (A) Effect of BAY11–7082 on cytokine-induced NFκB activation (IκBα phosphorylation and degradation). (B) Effect of SP600125 on cytokine-induced JNK activation (phosphorylation of JNK). (C) Effect of SB203580 on cytokine-induced p38 kinase activation (phosphorylation of Hsp27). C = Control.
Treatment of human preadipocytes with TNF-α and IL1-β for 10, 30, 60, or 120 min did not result in detectable AKT phosphorylation, a downstream substrate for PI3K. Reprobing with antibody against total AKT indicated that the protein was present (data not shown).
NFκB is usually sequestered in the cytoplasm bound to the IκBs. IκB α and IκB β are prototypes of the inhibitory IκB family. NFκB is activated through phosphorylation of the IκBs which leads to translocation of NFκB into the nucleus where regulation of gene expression occurs (Resch et al. 2006). Western blotting after stimulation with TNF-α and IL1-β led to a rapid loss of IκB α within 10 min, which returned to detectable levels by 60 min. TNF-α and IL1-β had little or no effect on IκB β (Fig. 3B).
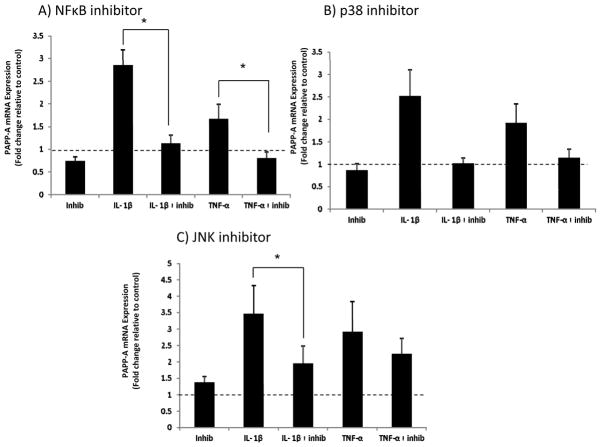
Inhibitors of NFκB (BAY 11–7082), JNK (SP600125), and p38 (SB203580) pathways were evaluated by western blotting to establish effectiveness (Fig. 4). To determine which pathways mediated cytokine-stimulated PAPP-A gene expression, real-time RT-PCR was performed on RNA isolated from human preadipocytes treated with the inhibitors Bay11-7082, SB203580 and SP600125 and then stimulated with IL1-β and TNF-α. Bay 11-7082 (NFκB inhibitor) significantly inhibited IL1-β- and TNF-α-stimulated PAPP-A gene expression (Fig. 5A). SB203580 (p38 inhibitor) showed similar inhibition, but did not reach statistical significance (Fig. 5B). SP600125 (JNK inhibitor) significantly inhibited IL-1β- but not TNF-α–induced PAPP-A expression (Fig. 5C).
Figure 5. Effect of pathway inhibitors on PAPP-A expression.
Real-time PCR was performed on RNA isolated from omental preadipocytes treated with (A) NFκB inhibitor Bay 11-7082 (5 μM) (B) p38 inhibitor SB203580 (2.5 μM) and (C) JNK inhibitor SP600125 (20 μM), and then stimulated with IL1-β (1 nM) and TNF-α (1 nM) for 24 hours.
Results (mean ± SEM, n = 6) are presented relative to control (represented by dotted line).
*P < 0.05.
In summary, the intracellular pathways mediating PAPP-A up-regulation by IL1-β and TNF-α are commonly associated with stress, including NFκB, p38 and JNK, although IL-1β and TNF-α seem to have differing effects on activation of the JNK pathway in human preadipocytes.
Regulation of PAPP-A Expression by Resveratrol
Resveratrol is a polyphenol found in multiple plant derivatives such as grapes and grape products (Baur et al. 2012). There has been much interest in resveratrol over the last decade, due to its potential protective metabolic effects. Pretreatment of human preadipocytes with resveratrol at 50 μM led to a significant reduction in both PAPP-A mRNA and protein expression under basal conditions (Fig. 6A). Resveratrol’s effect on PAPP-A expression was seen in preadipocytes from all three depots. Resveratrol also markedly inhibited IL1-β- and TNF-α- stimulated PAPP-A mRNA expression (Fig. 6B). The effect of stimulating PAPP-A expression in preadipocytes (IL-1β treatment) and of inhibiting PAPP-A expression (resveratrol treatment) on PAPP-A activity, i.e., IGFBP-4 proteolysis, is shown in Figure 7. IL-1β increased IGFBP-4 fragmentation and resveratrol inhibited the appearance of fragments. Addition of a neutralizing PAPP-A antibody confirmed the specificity of PAPP-A-mediated IGFBP-4 proteolysis.
Figure 6. Effect of resveratrol on PAPP-A expression.
(A) Preadipocytes were treated with resveratrol (50 μM) for 24 hours.
(B) Omental preadipocytes were treated with resveratrol (50 μM) alone, resveratrol plus cytokines IL1-β (1 nM) and TNF-α (1 nM) and cytokines IL1-β and TNF-α alone for 24 hours. Preadipocytes were then harvested for real-time PCR.
Results (mean ± SEM, n = 3–10) are presented relative to control (represented by dotted line).
*P < 0.05.
Figure 7. Effect of IL-1β and resveratrol on PAPP-A activity.
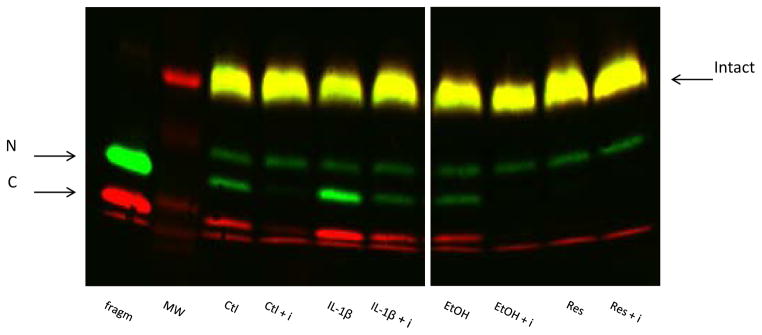
Omental preadipocytes were incubated with IL-1β (1 nM), reveratrol (50 μM), or appropriate vehicle for 24 hours. Conditioned medium was collected and incubated with IGFBP-4 and IGF-II without or with PAPP-A inhibitory antibody (i). Arrows indicate intact and N- and C-terminal fragments of IGFBP-4.
frag: positive control for PAPP-A-mediated IGFBP-4 proteolysis
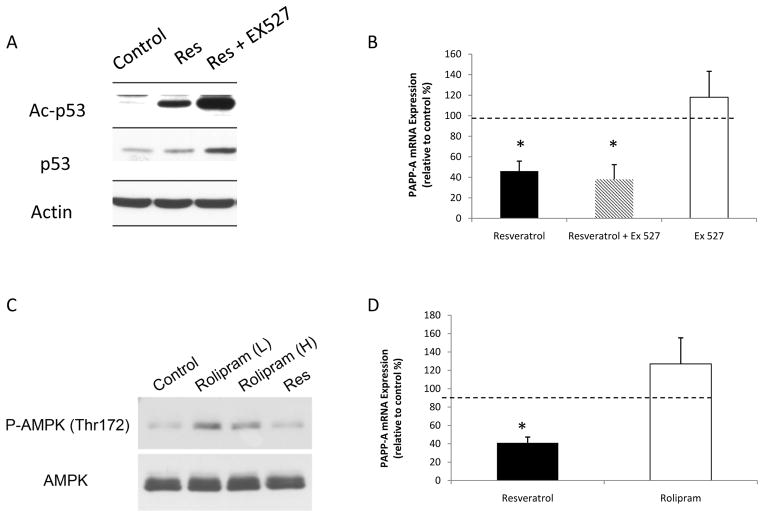
Resveratrol is thought to act through multiple pathways, but primarily through activation of silent mating type information regulation 2 homolog 1 (SIRT1), a mammalian sirtuin that is thought to extend lifespan in a variety of species (Howitz et al. 2003, Wood et al. 2004). Other resveratrol targets include the activation of AMP kinase (AMPK), part of a nutrient sensing pathway, which may also activate SIRT1 (Park et al. 2012). To determine if resveratrol exerts its effects on PAPP-A expression through SIRT1, preadipocytes were treated with the SIRT1 inhibitor, EX 527, plus resveratrol. SIRT1 is a deacetylase with p53 as a native substrate. We confirmed acetylation of p53 in the presence of EX 527 by western blotting (Fig. 8A). Resveratrol effectively down-regulated PAPP-A mRNA expression in the presence of EX 527 (Fig. 8B). We confirmed our findings using two other SIRT1 inhibitors, splitomycin and nicotinamide (data not shown). To determine if resveratrol exerts its effects on PAPP-A expression through AMPK, preadipocytes were treated with resveratrol or AMPK activator, rolipram (Park et al. 2012). AMPK phosphorylation by western blotting was used to confirm activity of rolipram prior to experiments (Fig. 8C). Treatment with rolipram did not reduce PAPP-A mRNA or protein expression (Figure 8D). We confirmed our findings using a second AMPK activator, A769662, with similar results (data not shown).
Figure 8. Effect of SirT1 inhibition and AMPK activation on PAPP-A expression.
(A) Omental preadipocytes were treated with resveratrol (Res) (50 μM) and EX 527 (10 μM) for 24 hours. Western blotting was performed for p53 and acetylated p53.
(B) Omental preadipocytes were treated with resveratrol alone, resveratrol plus SIRT1 inhibitor EX 527 (10 μM) and EX 527 alone for 24 hours. Preadipocytes were then harvested for real-time PCR. Results (mean ± SEM, n = 3) are presented relative to control (represented by dotted line).
(C) Omental preadipocytes were treated with rolipram [25 (L) and 50 (H) μM] for 24 hours. Western blotting was performed for AMPK and phosphorylated AMPK (P-AMPK).
(D) Omental preadipocytes were treated with resveratrol or AMPK activator, rolipram (25 μM), for 24 hours. Preadipocytes were then harvested for real-time PCR. Results (mean ± SEM, n = 4) are presented relative to control (represented as dotted line).
*P < 0.05.
In summary, resveratrol leads to down- regulation of PAPP-A expression in human preadipocytes under both basal and cytokine- stimulated conditions. Resveratrol does not appear to mediate its effects on PAPP-A through AMPK or SIRT1 pathways.
DISCUSSION
PAPP-A is preferentially expressed in human preadipocytes from visceral fat depots compared to preadipocytes from subcutaneous depots
Obesity is commonly associated with health risks, particularly cardiovascular disease. Individuals may preferentially accumulate fat in specific anatomical fat depots including the upper- body subcutaneous fat depot, the lower-body fat depot (intramuscular fat, the subcutaneous leg fat and the gluteal fat), and the intra-abdominal fat depot (mesenteric and omental fat, or visceral fat) (Tchkonia et al. 2010). Adipose tissue in these fat depots may behave differently. The typical “pear” shape of fat distribution (peripheral fat distribution) may lead to fewer cardiovascular risk factors than those with the typical “apple” shape fat distribution (central fat accumulation). Omental fat accumulation, in particular, is associated with adverse metabolic risk profiles including diabetes, insulin resistance, dyslipidemia and atherosclerosis with dysregulation of fatty acid storage and release. Although abdominal in origin, mesenteric fat is distinct from omental fat with respect to cellular and gene expression properties (Tchkonia et al. 2013). Our studies indicate that PAPP-A is preferentially expressed and secreted in human preadipocytes from omental fat compared to subcutaneous fat, despite the variability in donor characteristics. Thus, PAPP-A expression in preadipocytes may be an intrinsic property of the fat depots and may play a role in depot-specific adipogenesis (Tchkonia et al. 2001).
Proinflammatory cytokines IL1-β and TNF-α are potent stimulators of PAPP-A expression in human preadipocytes
The proinflammatory state associated with visceral obesity is thought to be a contributing factor to adverse metabolic profiles (Shah et al. 2008). The stromal-vascular portion of adipose tissue contains many cell types including macrophages, lymphocytes and preadipocytes. Activated macrophages secrete cytokines that can have paracrine effects in adipose tissue (Tchkonia et al. 2013). Omental adipose tissue has been shown to have elevated macrophage infiltration compared to subcutaneous fat, even in lean subjects (Harman-Boehm et al. 2007).
Up-regulation of PAPP-A mRNA and protein expression in preadipocytes was stimulated by cytokines known to be secreted by activated macrophages. [It should be noted that these cultures are essentially pure preadipocytes (Tchkonia et al., 2001, 2007).] IL1-β and TNF-α stimulated a three-fold increase in PAPP-A expression in preadipocytes from the omental and mesenteric depots. Cytokine-induced PAPP-A expression is not unique to preadipocytes. Cytokines also up-regulated PAPP-A in human coronary artery smooth muscle cells (Conover et al. 2006), fibroblasts (Resch et al. 2006) and osteoblasts (Conover et al. 2004). Previous experiments in human coronary artery smooth muscle cells indicate that the effects of IL1-β and TNF-α on PAPP-A gene expression are at the level of transcription (Conover et al. 2008). PAPP-A expression in human preadipocytes was not stimulated by IL-6. There appears to be cell-specific regulation of PAPP-A expression by IL-6. PAPP-A expression was up-regulated by IL-6 in vascular smooth muscle cells (Boldt & Conover 2007), but not in vascular endothelial cells or osteoblasts (Conover et al. 2004, Conover et al. 2008). This is not due to lack of receptor activation, as the IL-6 soluble receptor was added. IL-6 exhibits both pro- and anti-inflammatory properties.
To determine the intracellular signaling mediating cytokine-induced PAPP-A expression, we focused on pathways that are commonly stimulated by cytokines in other cell types (Duronio et al. 1998, Schaefer & Weber 1999, Young 1998, Li & Karin 1999, Landry & Huot 1995, Waskiewicz & Cooper 1995). PAPP-A up-regulation was mediated by pathways typically associated with stress, including the NFκB, p38 and JNK pathways. TNF-α was not as effective as IL1-β in stimulating the JNK pathway, suggesting differing effects of these cytokines in adipose tissue. However, our data using pathway inhibitors (Bay 11-7082, SB203580, SP600125) do not rule out interactive pathways since there are issues with specificity (Bain et al., 2007). It is known that the NFκB pathway is not activated in isolation during a stress response, and activation of the p38 and JNK pathways may occur simultaneously (Herlaar & Brown 1999).
Resveratrol potently inhibits PAPP-A expression in human preadipocytes, independent of its known effects on SIRT1 and AMPK activation
Resveratrol is a polyphenol associated with positive metabolic effects through modulation of cell-signaling pathways, and has been shown to inhibit PAPP-A expression in vascular smooth muscle cells (Conover et al. 2006). Many of resveratrol’s beneficial effects are thought to occur through activation of SIRT1 and/or AMPK. Activation of these pathways has been shown to protect mice against diet-induced obesity and insulin resistance leading to clinical interest in specific SIRT1 analogs (Lagouge et al. 2006). However, not all data convincingly show that resveratrol’s action occurs through the sirtuins alone (Baur et al. 2012). Based on our studies using SIRT1 inhibitors, down-regulation of PAPP-A expression by resveratrol does not seem to be mediated through activation of SIRT1. Resveratrol’s metabolic benefits may also depend on activation of alternative pathways, as mice with tamoxifen-induced deletion of SIRT1 still show a beneficial glucose response to resveratrol (Price et al. 2012). Another of resveratrol’s targets is to inhibit the c-AMP specific phosphodiesterases. Rolipram, a phosphodiesterase inhibitor that leads to activation of AMPK and SIRT1, is thought to mimic resveratrol in that it can reproduce its favorable metabolic effects in mice (Park et al. 2012). In our studies, rolipram did not mimic resveratrol’s effect on PAPP-A. Thus, resveratrol not only down-regulates PAPP-A expression in human preadipocytes under basal conditions, but it is also able to overcome up-regulation of PAPP-A induced by cytokines IL1-β and TNF-α. The effect of resveratrol to inhibit TNF-α or IL-1 β-induced PAPP-A expression is unlikely to explain the effect on basal PAPP-A expression since there was no detectable TNF-α or IL-1 β in the media under these conditions. This striking effect of resveratrol on PAPP-A expression suggests that down-regulation of PAPP-A may be an additional mechanism by which resveratrol exerts its beneficial effects on metabolic profiles. The mechanism behind resveratrol’s ability to down-regulate PAPP-A remains to be determined, but possibilities include inhibition of NFκB activation and attenuation of cell senescence (Holmes-McNary and Baldsin 2000).
Possible Role of PAPP-A in Depot-specific Adipogenesis
PAPP-A proteolyses inhibitory binding proteins, particularly IGFBP-4 (see Fig. 7), allowing increased pericellular IGF availability for activation of the IGF receptor (Boldt & Conover 2007). Therefore, we would expect up-regulation of PAPP-A to increase local IGF-stimulated differentiation, proliferation and survival. However, preadipocytes from human omental fat have increased PAPP-A but are relatively IGF resistant in terms of proliferation and differentiation (Caserta et al. 2001). Our study did not address the specific role of preadipocyte-derived PAPP-A in adipogenesis. However, we speculate that PAPP-A is secreted by the preadipocytes or endothelial cells in the stromal-vascular portion [PAPP-A is not secreted by macrophages (Conover et al. 2007)] with subsequent paracrine effects on stromal-vascular cells as well as mature adipocytes. This is similar to a model proposed for PAPP-A in atherosclerotic plaque progression (Conover 2010). A recent study in mice suggests a novel interactive in vivo setting whereby PAPP-A regulates visceral adipose tissue response to insulin (Conover et al., 2013).
Conclusion
PAPP-A is highly expressed in preadipocytes from omental fat depots. PAPP-A expression in human preadipocytes is up-regulated by proinflammatory cytokines, particularly IL1-β and TNF-α. This up-regulation is mediated through pathways commonly associated with stress including the JNK, p38 and NFκB pathways. PAPP-A mRNA and protein expression is down-regulated by resveratrol, a polyphenol with protective metabolic effects. This appears to be mediated independently from SIRT1 and AMPK. Thus, reduction in PAPP-A expression may be another mechanism responsible for resveratrol’s beneficial effects. Further evaluation of the specific role of PAPP-A in depot-specific adipogenesis is needed, as these findings in human preadipocytes in conjunction with the work in mice (Conover et al. 2013) suggest the potential for PAPP-A to be a therapeutic target in the treatment of visceral obesity.
Acknowledgments
FUNDING
This work was supported by NIH grant AG028141 (to CAC) and by the Minnesota Obesity Center Grant DK50456.
The authors thank Dr. James Kirkland and Nino Giorgadze for providing the human preadipocyte cultures, and Dr. Michael Jensen for providing the human fat samples. All samples were collected under IRB-approved protocols.
Footnotes
DECLARATION OF INTEREST
None
AUTHOR CONTRIBUTION STATEMENT
Caroline Davidge-Pitts. Designed and performed the experiments, analyzed data, wrote the manuscript.
Carlos J. Escande. Contributed to the immunoblotting experiments.
Cheryl A. Conover. Helped with the design of the experiments, discussed the data, reviewed the manuscript.
References
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nature Reviews Drug Discovery. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliot M, Shpiro N, hastie CJ, McLauchlan H, Klevernic I, Arthur JSC, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochemical Journal. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Bale LK, Resch ZT, Oxvig C, Overgaard MT, Conover CA. Effects of mutated pregnancy-associated plasma protein-A on atherosclerotic lesion development in mice. Endocrinology. 2013;154:246–252. doi: 10.1210/en.2012-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Hormone and IGF Research. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology. 2011;152:1470–1478. doi: 10.1210/en.2010-1095. [DOI] [PubMed] [Google Scholar]
- Caserta F, Tchkonia T, Civelek VN, Prentki M, Brown NF, McGarry JD, Forse RA, Corkey BE, Hamilton JA, Kirkland JL. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. American Journal of Physiology Endocrinology and Metabolism. 2001;280:E238–E247. doi: 10.1152/ajpendo.2001.280.2.E238. [DOI] [PubMed] [Google Scholar]
- Conover CA. PAPP-A: a new anti-aging target? Aging Cell. 2010;9:942–946. doi: 10.1111/j.1474-9726.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends in Endocrinology and Metabolism. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. American Journal of Physiology Cell Physiology. 2006;290:C183–C188. doi: 10.1152/ajpcell.00199.2005. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. Journal of Gerontology Biological Sciences. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Chen B-K, Resch ZT. Regulation of pregnancy-associated plasma protein-A expression in cultured human osteoblasts. Bone. 2004;34:297–302. doi: 10.1016/j.bone.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Hormone and IGF Research. 2008;18:213–220. doi: 10.1016/j.ghir.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK, Oxvig C. Surface association of pregnancy associated plasma protein-A accounts for its co-localization with activated macrophages. American Journal of Physiology heart Circulation Physiology. 2007;292:H994–H1000. doi: 10.1152/ajpheart.00798.2006. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harstad SL, Tchkonia T, Kirkland JL. Preferential impact of pregnancy associated plasma protein-A deficiency on visceral fat in mice on high fat diet. American Journal of Physiology Endocrinology and Metabolism. 2013;305:E1145–E1153. doi: 10.1152/ajpendo.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proceedings of the Nutrition Society. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Duronio V, Scheid MP, Ettinger S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cellular Signalling. 1998;10:233–239. doi: 10.1016/s0898-6568(97)00129-0. [DOI] [PubMed] [Google Scholar]
- Franchimont N, Gangji V, Durant D, Canalis E. Interleukin-6 with its soluble receptor enhances the expression of insulin-like growth factor-I in osteoblasts. Endocrinology. 1997;138:5248–5255. doi: 10.1210/endo.138.12.5559. [DOI] [PubMed] [Google Scholar]
- Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. Journal of Clinical Endocrinology and Metabolism. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circulation Research. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Molecular Medicine Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- Holmes-McNary M, Baldsin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Research. 2000;60:3477–3483. [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochemistry and Cell Biology. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB Journal. 1999;13:1137–1143. [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabolism. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch ZT, Oxvig C, Bale LK, Conover CA. Stress-activated signaling pathways mediate the stimulation of pregnancy-associated plasma protein-A expression in cultured human fibroblasts. Endocrinology. 2006;147:885–890. doi: 10.1210/en.2005-0908. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Molecular and Cellular Biology. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. Journal of Parenteral and Enteral Nutrition. 2008;32:638–644. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Deponte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. American Journal of Physiology Endocrinology and Metabolism. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metabolism. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Current Opinion in Cellular Biology. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Young PR. Pharmacological modulation of cytokine action and production through signaling pathways. Cytokine and Growth Factor Reviews. 1998;9:239–257. doi: 10.1016/s1359-6101(98)00011-2. [DOI] [PubMed] [Google Scholar]