Abstract
Eosinophilic esophagitis, a recently recognized and growing clinical disorder over the past decade, is characterized by antigen-driven eosinophil accumulation in the esophagus. Symptoms frequently mimic those of gastroesophageal reflux disease (GERD) but the two diseases are quite distinct in terms of their histopathology, genetic signature, response to therapy, hereditary risk and association with allergy. Disease pathogenesis involves the interplay of external and genetic factors, particularly food antigens and the eosinophil chemoattractant eotaxin-3, respectively. Transcript signatures and animal models have uncovered the importance of adaptive T cell immunity involving IL-5 and IL-13 elicited esophageal epithelial cell responses. Notably, symptoms, dysregulated esophageal gene expression and pathology are largely reversible following reduction of specific food antigen exposure, as well as anti-inflammatory therapy, but chronic treatment is necessary to prevent relapse. As such, eosinophilic esophagitis is a disease with the unique features of chronic esophagitis, atopy, immune sensitization to oral antigens, reversibility and familial association.
Keywords: Allergy, Cytokines, Eosinophils, Epithelium and Esophagitis
Clinical Entities Associated with Esophageal Eosinophilia
Unlike all other segments of the gastrointestinal tract, the esophagus is normally devoid of eosinophils,1 so the finding of esophageal eosinophilia denotes pathology. However, the mere presence of esophageal eosinophils is not specific for a particular disorder because eosinophil accumulation in the esophagus occurs in a variety of states including eosinophilic esophagitis (EE), eosinophilic gastroenteritis, gastroesophageal reflux disease (GERD), chronic (non eosinophilic) esophagitis (CE), parasitic and fungal infections, inflammatory bowel disease (IBD), hypereosinophilic syndrome (HES), scleroderma, drug and/or iatrogenic-induced states such as caustic injury,2 multiple convulsive therapy syndrome3 and immunosuppression especially following solid organ transplantation (Table 1).4 As part of a spectrum of eosinophilic gastrointestinal disorders (EGID), it is notable that EE can evolve into and/or be associated with eosinophilic gastritis, enteritis and/or colitis.2 The diagnosis of EE requires elimination of other causes of esophagitis, especially GERD; EE is generally distinguished from GERD by persistent esophageal eosinophilia despite adequate acid neutralization therapy prior to endoscopy.5 EE can be associated with other diseases such as Rubinstein-Taybi Syndrome6 and celiac disease.7–11 The similarities between EE and celiac disease provide insight into possible pathogenic mechanisms that may be operational in EE. Celiac disease is a prototypic T cell-mediated immune disease triggered by an oral antigen (gliadin). While celiac disease has a significant autoimmune component that has not yet been associated with EE, both diseases are associated with immune cell-mediated epithelial cell abnormalities and both are reversible following a food elimination diet yet EE typically does not typically respond to gliadin avoidance. Additionally, the EE transcriptome12 has expression of immune response genes that have been associated with celiac disease including over-expression of the MICA and MICB (the activating ligand for NKG2D) and the cytokine IL-15 that have both been shown to induce intraepithelial lymphocyte activation.13–16 Genetic studies in celiac disease have uncovered the central participation of the adaptive immune system as the HLA Class II genes (DR HLA-DQ2 or DQ8 molecules) are main genetic risk factors, but also a role for environmental factors.17 In addition, a series of other genetic contributions, elucidated by genome wide association studies, have uncovered the participation of genes that regulate immunity including IL18RAP, IL12A, interestingly the eotaxin receptor CCR3, and genetic variants in the IL2/IL21 locus.18, 19 A recent paper has linked blood eosinophilia with several genes including the celiac locus encoding SH2B3 (also known as LNK),20 providing further support for a connection between eosinophils and celiac disease.
Table 1.
Disease States Associated with Significant Eosinphilic Esophagitis
| Caustic injury |
| Collagen vascular disease-including Churg Strauss Syndrome |
| Celiac disease |
| Chronic (non eosinophilic) esophagitis |
| Drug and/or iatrogenic-multiple convulsive therapy syndrome3 and immunosuppression |
| Eosinophilic esophagitis |
| Eosinophilic gastroenteritis |
| Gastroesophageal reflux disease |
| Hypereosinophilic syndrome |
| Infection-parasitic and fungal infections |
| Inflammatory bowel disease |
| Lymphoma and other malignancies |
| Scleroderma |
Symptoms and disease characteristics
EE has many different presentations as patients commonly report difficulty feeding, failure to thrive, vomiting, epigastric or chest pain, dysphagia and food impaction.21–23 These symptoms appear to occur in a progressive order, as they are the presenting symptoms from infancy into adulthood, respectively, 24, 25 suggesting that this is the natural history of EE. Adult patients typically have recurrent dysphagia and food impactions that are refractory to anti-GERD therapy; in fact, recent studies indicate that 10–50% of adult male patients with these symptoms have EE.26, 27 Although a fixed stricture may account for the esophageal dysphagia and food impaction observed in some EE patients,28 evidence is mounting that the esophagus displays impaired smooth muscle function, likely from asynchrony of circular and longitudinal muscle contraction during swallowing.29 A variety of motor disturbances, reversible with disease therapy, have been reported in EE.30, 31 EE patients are predominantly young males,24 have a high rate of atopic disease and normal pH monitoring of the esophagus compared to patients with GERD. Although EE was originally recognized in pediatric patients, it is now appreciated that EE has similar characteristics (including atopic sensitization) and occurrence rates in children and adults;32 in fact, while EE frequently presents during infancy,33 the disease has also been recognized in the tenth decade of life.34 While EE is a chronic disorder with no significant evidence of spontaneous disease remission even over a 14 year follow up period,35 symptoms may have seasonal variability,36 consistent with an etiology related to airborne allergen exposure, at least in some patients.
Epidemiology
EE is a global health disease now reported in all continents except Africa.37–47 Although the incidence of EE has not been rigorously calculated, a mini-epidemic has been noted over the last decade. Liacouras and his group at Children’s Hospital of Philadelphia have found that ∼10% of their pediatric patients with GERD-like symptoms are unresponsive to acid blockade and have EE.48, 49 Furuta and colleagues at Boston Children’s Hospital have reported that 6% of their patients with esophagitis have EE.50 Over a 16-year observation period, Straumann and colleagues have documented a prevalence of ∼1:4,000 adults in Switzerland.51 Croese and colleagues have reported EE to be present in 1:70,000 adults in an Australian provincial city.37 A recent study revealed ∼0.4% (∼4 in 1,000) prevalence in a random adult Swedish population.52 We have noted that EE occurrs 1 in 1,000 children in the Cincinnati metropolitan area over a 9 year time period24 (and unpublished findings). In an outpatient U.S. based military hospital, EE has been found in 6.5% of adult patients undergoing endoscopy.53 The last study was notable for a high frequency of African Americans in the EE patient population, suggesting that the predominance of EE in Caucasians in other studies may be due to patient referral patterns. These epidemiology studies indicate that EE likely has a prevalence at least comparable to inflammatory bowel disease but less than celiac disease.54, 55 Although EE has only been appreciated as a separate disease entity of the past decade, evidence is accumulating that it was previously unrecognized amongst patients diagnosed with reflux esophagitis.56
Eosinophil esophageal levels and histopathology
It is important to emphasize that there is currently no diagnostic test for EE, rather diagnosis depends upon coordinated clinical and pathological data. Although a minimal level of 15 eosinophils/hpf has been proposed to be part of the diagnostic criteria of EE,5 any level has to be interpreted in the context of clinical data. It has been proposed that the number and location of eosinophils is helpful when trying to differentiate EE from GERD. Up to 6 eosinophils/hpf (400x) may be mostly indicative of GERD; whereas more than 20–24 eosinophils/hpf appears to be mostly indicative of EE,48, 57 especially when these levels are encountered while patients are on anti-GERD therapy. The level of eosinophils in the esophagus negatively correlates with response to conventional anti-GERD therapy.48 In particular, esophageal eosinophil levels of >23/hpf have been reported to correlate with lack of responsiveness to anti-GERD therapy. These levels may be diagnostic of EE rather than GERD/CE especially in patients already on anti-GERD therapy. Patients with intermediate levels of eosinophils (7–15/hpf) often present a diagnostic dilemma for several reasons. First, the cutoff value for eosinophil concentrations for the diagnosis of EE has not been vigorously determined. Secondly, EE is often a patchy disease so depending upon the number of biopsies obtained, the maximum level of eosinophils may have great variability.58 A recent expert panel has underscored that EE is a clinicopathological disease that should be considered in patients with (1) symptoms including but not restricted to food impaction and dysphagia in adults, and feeding intolerance and GERD symptoms in children; (2) > 15 eosinophils/hpf; (3) exclusion of other disorders associated with similar clinical, histological, or endoscopic features, especially GERD with sustained esophageal eosinophilia (>15 peak eosinophils/400X hpf) after adequate GERD therapy (e.g. proton pump inhibitors [PPI]s).5 For research studies, a higher threshold (≥24 eosinophils/hpf) has been recommended.5 Based on histological analysis of a large cohort of adult patients and the performance of esophageal pH monitoring, obtaining 1, 2, 3 and 6 esophageal biopsies from the mid and distal esophagus has a sensitivity of 73, 84, 97 and 100%, respectively, using 15 eosinophils/hpf.58 As such, it is recommended to obtain at least three biopsies in order to diagnose EE.5
In addition to high eosinophil levels, other features distinguish EE from GERD (Table 2). The anatomical location of eosinophils in both the proximal and distal esophagus typically denotes EE, while the accumulation of eosinophils mainly in the distal esophagus typical but not specific for GERD.59 In addition, esophageal tissue from patients with EE typically demonstrates a thickened mucosa with basal layer hyperplasia (as assessed by Ki-67 antigen staining) and papillary lengthening,59, 60 and this appears to be more pronounced than in GERD.61 Consistent with increased proliferation of the basal cells in response to allergen provocation rather than acid exposure, the biopsies from EE patients have down regulation of cyclooxygenase 2 (COX2).62 Furthermore, esophageal biopsies from EE patients may have eosinophil surface layering and eosinophilic microabscesses, processes rarely associated with GERD.63 In addition to eosinophils, esophageal biopsies from EE patients have increased levels of dendritic cells and mast cells, at levels generally higher than in GERD.64–66 It has been recently proposed that extracellular deposition of the granule protein eosinophil derived peroxidase (EPO) is readily present in the esophagus of EE patients and may correlate with clinical features even better than eosinophil levels.67 Radiographic and endoscopic studies have shown many findings including strictures, mucosal rings, ulcerations, whitish papules and polyps;23, 68 however, nearly 30% of patients with EE have normal endoscopic appearances,69 necessitating the importance of endoscopic biopsies.
Table 2.
Distinguishing Features between Eosinophilic Esophagitis and Gastroesophageal Reflux Disease
| Characteristic | Eosinophilic Esophagitis | Gastroesophageal Reflux Disease |
|---|---|---|
| Prevalence | 1:1000 | 1:10 |
| Gender | Male>Female | Male=Female |
| Atopy | High | Normal |
| Polysensitization to Food | Yes | No |
| Familial Inheritance | Common | Normal |
| Proximal Esophagitis | Common | Rare |
| pH Probe | Normal | Abnormal |
| Esophageal Eosinophilia | Very High (>15/hpf) | Generally not Very High |
| Esophageal Eotaxin-3 | High | Normal |
Disease Pathogenesis
The pathogenesis of EE is highly linked with atopy based on disease co-occurrence, animal models and the success of allergen avoidance (primarily diet control). The majority of patients have evidence of food and aeroallergen hypersensitivity50 and a concurrent history of respiratory allergy.24 Unlike food anaphylaxis which occurs in ∼15% of EE patients,33 patients with EE have polysensitization to a variety of foods based on skin prick testing.70, 71 The key role of food antigen sensitization is demonstrated by the success of reducing specific food exposures based on provocative skin and patch testing, empiric avoidance of the most common 6 food types or the employment of amino acid based formula, all of which are capable of inducing disease remission.72 Experimental models of EE can be induced in mice by allergen exposure, as well as by overexpression of cytokines derived from type 2 helper T (Th2) cells.73, 74 In particular, repeated intranasal exposure to the aeroallergen Aspergillus fumigatus induces simultaneous eosinophilic airway and esophageal inflammation (without inducing lower gastrointestinal eosinophilia).73 Furthermore, intratracheal delivery of human or mouse IL-13 induces dose-dependent experimental EE;75 this process can be blocked with a therapeutic anti-human IL-13.76 Epicutaneous allergen sensitization potently primes for respiratory allergen-induced experimental EE.77 This latter finding may be particularly important for understanding EE because a large fraction of EE patients suffer from preceding allergic skin disease (atopic dermatitis).22 Collectively, these experimental systems demonstrate an intimate connection between development of eosinophilic inflammation in the respiratory tract and esophagus not only in response to external allergic triggers but also intrinsic Th2 cytokines, and they highlight the potential for sensitization to occur via cutaneous antigen exposure. It is notable that patients with allergic rhinitis have seasonal increases in esophageal eosinophils and patients with EE have seasonal variations in their symptoms,78 providing clinical evidence supporting a contributory role for aero-allergen-driven eosinophil-associated responses in the esophagus.
Experimental modeling in mice has established that Th2 signaling is required for induction of experimental EE. In particular, mice with the targeted deletion of STAT6 are protected (in part) from allergen- and IL-13-induced experimental EE.75, 77 Further, IL-13-deficient mice have impaired allergen-induced EE.77 Notably, IL-13 is overexpressed in the esophagus of EE patients and selectively induces the eosinophil activating and chemoattractant factor eotaxin-3 by a transcriptional mechanism in esophageal epithelial cells.79
There is marked overexpression of ∼1% of the human genome in the esophagus of EE patients compared with normal individuals and patients with CE.12 The EE transcriptome is highly conserved across patient phenotypes including gender, age and familial variants and includes eotaxin-3 as the most highly induced gene.12, 80, 81 Eotaxin-3 mRNA levels in esophageal biopsy tissue present in paraffin blocks can be used to distinguish EE and GERD.82 Comparison of allergic and non-allergic EE patients revealed that the gene transcript signature is markedly conserved across these two major patient phenotypes.12 This demonstrates that the effector phase of the disease is conserved between individuals despite the driving trigger of the inflammation. Interestingly, a marked segment of the EE transcriptome is directly induced by IL-13 treatment of primary esophageal epithelial cells (including eotaxin-3 as the top gene induced by IL-13).79 EE transcriptome genes regulated by IL-13 include periostin (markedly induced by IL-13 and overexpressed in EE)83 and filaggrin (markedly down-regulated by IL-13 and decreased in EE).12 Periostin is a fascilin domain containing extracellular matrix molecule that regulates eosinophil adhesion and promotes eotaxin-induced eosinophil recruitment.83 Filaggrin is a skin structural barrier protein whose loss of function is associated with marked increases in skin permeability and susceptibility to atopic dermatitis in humans84 and atopic sensitization in mice.85 Notably, in contrast to atopic dermatitis that is associated with loss of function genetic variants in the filaggrin gene, EE is associated with a functional impairment in filaggrin expression. Notably, IL-13 down-regulates filaggrin gene expression in skin keratinocytes,86 providing a mechanism by which food antigen-elicited Th2 cell adaptive immunity may impair esophageal barrier function, perhaps propagating local inflammatory processes (including sensitivity to acid) and increased antigen uptake in the esophagus. These processes may be particularly important as there are elevated levels of activated mast cells, B cells and evidence for in situ production of immunoglobulins in the esophagus of EE patients, as demonstrated by histological analysis and transcriptome expression .12, 64, 66, 87
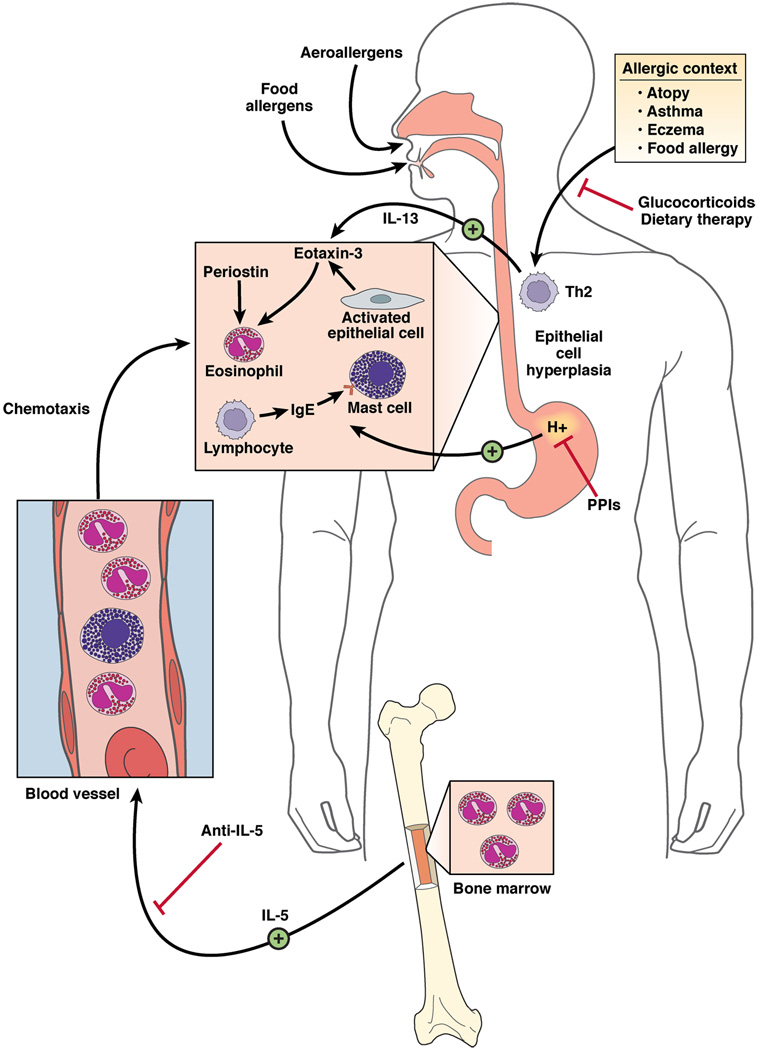
Support for a key role of T cells in the pathogenesis of EE is derived from analysis of lymphocyte deficient mice. T cell but not B cell deficient mice fail to develop antigen-induced EE.88 Notably, CD8 and CD4 cells are largely dispensable for the induction of Aspergillus fumigatus-induced EE,88 highlighting the involvement of a unique component of the adaptive immune system. In addition, strong evidence for the contribution of Th2 cell derived IL-5 in the pathogenesis of EE has accumulated. Overexpression of IL-5 (by pharmacological or transgenic delivery) induces EE and neutralization of IL-5 (with antibodies or gene targeting) blocks allergen- or IL-13-induced EE in mice.73–75 Local IL-5 driven eosinophils have been shown to contribute to esophageal remodeling in experimental EE in mice.89 Notably, IL-5 is overproduced by circulating CD4+ T cells in patients with EE90 and in response to food antigen stimulation in vitro.91 Additionally, preliminary studies with humanized anti-IL-5 antibody in EE patients have shown positive effects, although not in all studies.92–95 A coordinated mechanism for disease pathogenesis is schematically presented in Figure 1.
Figure 1. Molecular and cellular mechanisms involved in EE pathogenesis, eotaxin-3-associated eosinophil recruitment and treatments.
Aeroallergen, food allergen and skin sensitization have been implicated in EE pathogenesis. Elemental diet, glucocorticoids, and anti-IL-5 treatments improve the microscopic features of EE acting at different levels on the disease pathogenesis. Proton pomp inhibitors (PPI) are important in establishing the diagnosis of EE as inflammation should be present on PPIs therapy. Hyperplasic epithelial cells of the esophagus overexpress eotaxin-3 in response to IL-13. Fibroblasts overexpress periostin and downregulate filaggrin likelyin response to IL-13. Eotaxin-3 and periostin overexpression cooperatively chemoattract CCR3+ cells, a process primed by IL-5 which regulates eosinophil responsiveness to eotaxin-3 and the circulating level of eosinophils. Inheritance of EE disease suggests a genetic predisposition. A SNP in the eotaxin-3 gene has been associated with EE. In addition to eosinophils, mast cells and lymphocytes including B cells accumulate in the esophagus of EE patients and likely contribute to the local inflammatory responses.
Genetics of EE
Evidence is accumulating that EE has a strong familial association.24, 96 Nearly 10% of parents of EE patients have a history of esophageal strictures and ∼8% have biopsy proven EE.24 Among 798 pediatric probands recruited into our research databank to date, 27 have at least one sibling or parent with EE. We have recently reported 26 multiplex families with EE and demonstrated primarily conserved clinical, pathological and genetic features compared with simplex EE patients.81 Familial EE typically occurs in siblings or parents; however, three generations of affected distal relatives have also been noted. In addition, Patel and Falchuk have reported three adult brothers with dysphagia who were found to have EE.97
One widely used measure of familial aggregation is the sibling recurrence risk ratio (λS) which compares the sibling recurrence risk vs. the general population prevalence.98 A λS larger than 1 indicates the increased risk of developing the disease among siblings of the proband compared with the general population. Given that the population prevalence for EE is approximately 5 per 10,000, we estimated λS for EE is ∼80.80, 99 Compared with common allergic disorders, such as atopy or asthma (λS is estimated ∼2),99 the considerably higher λS in EE indicates that genetics is likely to have a relatively large role. Our initial genetic study on a SNP (+2496T>G, rs2302009) in the eotaxin-3 gene has shown association with EE by both population-based case-control comparison and family-based transmission disequilibrium testing but the disease-associated allele is only present in 14% of EE patients.12 Clearly, other genes are involved in EE disease risk, phenotype, and prognosis.
Potential role of eosinophils in EE
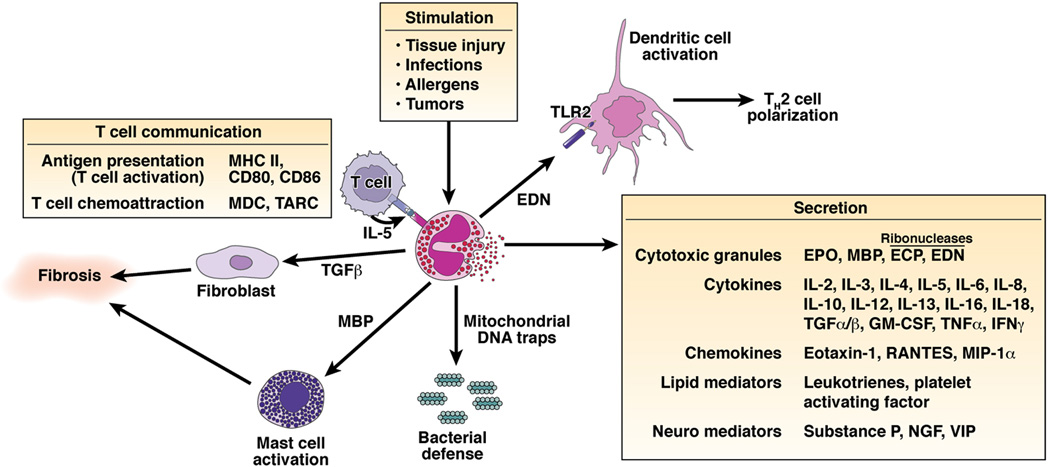
Eosinophil granule constituents are readily detectable in extracellular locations in the esophagus of EE patients and there is strong evidence for in situ eosinophil activation and degranulation.100 In vitro studies have shown that eosinophil granule constituents are toxic to a variety of tissues including intestinal epithelium.101 Eosinophil granules contain a crystalloid core composed of major basic protein (MBP)-1 (and MBP-2), and a matrix composed of eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN) and EPO.102 These cationic proteins share certain pro-inflammatory properties but differ in other ways. For example, MBP, EPO, and ECP have cytotoxic effects on epithelium, in concentrations similar to those found in biological fluids from patients with eosinophilia. Additionally, ECP and EDN belong to the Ribonuclease A superfamily and possess anti-viral and ribonuclease activity.103, 104 EDN has also been shown to be an endogenous ligand of Toll-like Receptor (TLR)2, having capacity to activate myeloid DCs by triggering the TLR2 – myeloid differentiation factor 88 (Myd88) signaling pathway.105 Importantly, EDN polarizes the ability of DCs to promote Th2 responses; as such, EDN represents an endogeneous alarmin that has the propensity to alert the adaptive immune system for preferential enhancement of antigen-specific Th2 immune responses. ECP can insert voltage insensitive, ion-nonselective toxic pores into the membranes of target cells and these pores may facilitate the entry of other toxic molecules.106 MBP directly increases smooth muscle reactivity by causing dysfunction of vagal muscarinic M2 receptors.107 MBP also triggers degranulation of mast cells and basophils. MBP directly binds the extracellular calcium sensing receptor on esophageal epithelial cells, resulting in fibroblast growth factor 9 release and autocrine stimulation of epithelial proliferation.108 Triggering of eosinophils by engagement of receptors for cytokines, immunoglobulins, and complement can lead to the generation of a wide range of inflammatory cytokines including IL-1, −3, −4, −5, −13, GM-CSF, TGF-α, TGF-β, TNF-α, RANTES, MIP-1α, and eotaxin-1, indicating that they have the potential to modulate multiple aspects of the immune response.109 In fact, eosinophil-derived TGF-β is linked with epithelial growth, fibrosis, and tissue remodeling,110, 111 processes that have been shown to occur in EE even in pediatric patients. Notably, a recent study has shown that eosinophils are indeed the chief source of TGF-β1 in pediatric EE patients and their levels correlate with esophageal fibrosis including phosphorylation of the transcription factor SMAD2/3.112 Of interest, eosinophils rapidly release mitochondrial DNA in response to exposure to bacteria, C5a or CCR3 ligands.113 In contrast to neutrophils, eosinophils do not undergo cell death upon release of their DNA; in addition, this process requires free radical production via NADP oxidase. Eosinophil DNA traps contain the granule protein ECP and MBP, and display antimicrobial activity.113 This indicates that eosinophils may have an essential role in innate immunity against bacteria, using a unique mechanism. Perhaps mitochondrial DNA release by esophageal eosinophils contributes to epithelial function and/or innate immunity in EE.114 Additionally, eosinophils can directly activate T cells by antigen presentation as well as regulate T cell recruitment to allergic tissue by controlling the expression of T cell directed chemokines.115, 116 Further eosinophil-mediated damage is caused by toxic hydrogen peroxide and halide acids generated by EPO and by superoxide generated by the respiratory burst oxidase enzyme pathway in eosinophils. Eosinophils also generate large amounts of the cysteinyl leukotriene C4 (LTC4) which is metabolized to LTD4 and LTE4. These three lipid mediators increase vascular permeability and mucus secretion, and are potent stimulators of smooth muscle contraction, which may contribute to the dysmotility in EE and/or peristalsis abnormalities in EGID. Electron microscopy studies have revealed ultrastructural changes including inversion of core-to-matrix densities and lucency of secondary granules (indicative of eosinophil degranulation and mediator release) in esophageal samples from patients with EE.117 In addition, in the intestine, eosinophils are in juxtaposition to nerves and have been shown to participate in axonal necrosis.118 Taken together, eosinophils are pleiotropic cells capable of initiating adaptive immune responses, and sustaining and propagating inflammatory reactions (Figure 2).
Figure 2. Schematic diagram of the multifunctional effects of eosinophils.
Eosinophils are bilobed granulocytes with eosinophilic staining secondary granules. The secondary granules contain four primary cationic proteins designated eosinophil peroxidase (EPO), major basic protein (MBP), eosinophil cationic protein (ECP), and eosinophil derived neurotoxin (EDN). EDN is a ligand for Toll like receptor (TLR)2 and induces the Th2 polarizing ability of dendritic cells. All four proteins are cytotoxic molecules; in addition, ECP and EDN are ribonucleases. Eosinophils respond to diverse stimuli including non-specific tissue injury, infections, allergens and tumors. In addition to releasing their preformed cationic proteins, eosinophils also release a variety of cytokines, chemokines, lipid mediators, and neuro-modulators. Eosinophils directly communicate with T cells and mast cells in a bidirectional manner. Eosinophils are capable of catapulting their mitochrondrial DNA which can serve as extracellular traps for bacteria. Eosinophil derived TGF-β induces fibrosis.
EE therapy
Therapy for EE is based on avoidance diets, anti-inflammatory approaches, and physical dilatation when strictures are present. Dilatation is associated with a relatively high rate of perforation, warranting cautious usage.119 From the onset, anti-GERD therapy is indicated for the initial treatment of EE because acid can trigger esophageal eosinophilia, albeit generally of lower magnitude than that associated with EE.5 Even if pathological reflux is not present, acid exposure has potential to irritate the inflamed esophagus. If anti-GERD therapy is unsuccessful (based on histological assessment), specific food allergen elimination (a restricted diet) or an exclusive elemental (amino acid based) formula is recommended. In a retrospective study conducted on 381 patients over a 10-years period, Laicouras et al demonstrated that the removal of dietary antigens (primarily in the form of elemental diet) significantly improved clinical symptoms and esophageal histology in 98% of patients.23 Although very efficient, dietary eliminations are often unsatisfactory or practically difficult as patients are typically sensitized to multiple food groups including common and uncommon food types. In addition, skin prick testing does not uniformly predict which foods to remove from the diet.33 As such, it has been proposed that skin patch testing to foods may more accurately predict which food eliminations will induce disease remission,120, 121 but this has not been consistently agreed upon or observed.122 While a diet consisting of an exclusive elemental (amino acid based) formula is often effective, this is often not well tolerated (especially in older individuals) because it frequently requires a surgically placed feeding tube, can be financially costly (e.g. thousands of dollars per month) and is unpalatable. Glucocorticoids (systemic or topical) have been used with satisfactory results in some patients. Systemic steroids are often used for acute exacerbations, while topical steroids are used to provide long-term control.123 In a randomized placebo controlled trial, topical therapy with swallowed fluticasone and oral prednisone have comparable efficacy yet both are associated with a high relapse rate upon discontinuation.124 It is notable that the EE transcriptome including overexpression of eotaxin-3 and IL-13 are largely reversible following successful topical glucorticoid therapy.79 However, it has been appreciated that a significant fraction of patients do not respond to swallowed fluticasone, likely due to steroid resistance.60, 65 Smaller body weight and shorter stature increase steroid responsiveness, suggesting dose dependence.65 In addition, it has been appreciated that atopic individuals have reduced responsiveness to glucocorticoid therapy,65 likely due to the ongoing exposure to the triggering antigens. Anti-IL-5 therapy is very effective at ablating the development of experimental EE in murine models,73, 74 and appears to also improve eosinophil infiltration in the human esophagus in select early clinical trials;93 large-scale controlled anti-IL-5 trials for EE are currently underway. In a preclinical trial, anti-human IL-13 has been shown to be useful and it will be of interest to eventually examine the impact of IL-13 blockade in EE patients.79 Other anti-inflammatory approaches such as leukotriene receptor antagonists and anti-TNF agents have been advocated, but they have not been shown to reverse esophageal pathology.125, 126 Preliminary studies with azathioprine and 6-mercaptopurine have suggested their benefit,127 warranting further study. Because IgE effector cells, such as mast cells and basophils, are a source of proinflammatory chemokines, cytokines, and proteases, it is also possible that anti-IgE therapy would have anti-inflammatory effects in EE.128 It is important to note that EE is a chronic disorder that requires ongoing therapy; the disease almost uniformly returns when therapy is discontinued (e.g. glucocorticoids are stopped or diet is liberated).
Conclusion and future directions
Eosinophilic esophagitis is a recently recognized and growing clinical disorder previously misdiagnosed as GERD but the two diseases are quite distinct in terms of their histopathology, genetic signature, response to therapy, hereditary risk and association with allergy. It is now well accepted that atopic disorders, such as asthma and eczema, are complex diseases involving the interplay of multiple genes interacting with environmental factors, ultimately contributing to disease susceptibility. Initial analyses indicate a relatively strong genetic contribution in EE compared with other atopic diseases, indicating that fewer genes might be involved in disease pathogenesis compared with the >100 genes that have now been implicated in respiratory allergy.129 It is now important to examine the association of EE with candidate genes that are biologically relevant in EE (as illustrated in Figure 3). Apart from the candidate gene approach, with the advance of genotyping technology, it is now feasible to conduct a genome-wide linkage analysis to identify the genetic variants associated with the susceptibility to EE. It will be of interest to investigate whether the genetics that underlie atopy, inflammatory bowel disease, celiac disease and EE will be similar, at least in part.
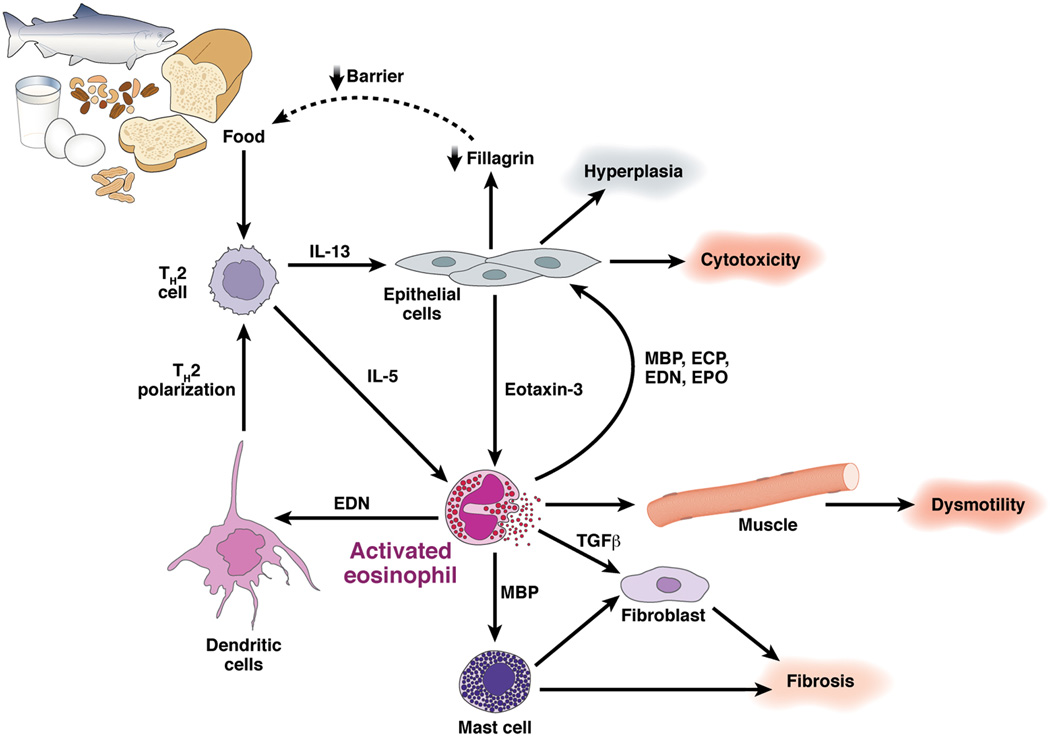
Figure 3. Molecular regulation of the Th2 inflammatory response in EE.
Food antigen triggered T helper type 2 (Th2) cells release IL-5 and IL-13 which target eosinophils and esophageal epithelial cells, respectively. IL-13 triggered responses include marked production of the eosinophil chemoattactrant and activating factor eotaxin-3 by epithelial cells as well as down regulation of filaggrin expression. Activated eosinophils release MBP and EDN which target mast cells and dendritic cells, respectively. Eosinophil derived TGF-β and MBP target fibroblasts, epithelial cells and smooth muscle cells and contribute to cellular hyperplasia, fibrosis and dysmotility. In addition, mast cell activation contributes to fibrosis. Perhaps reduced esophageal barrier function (mediated by decreased filaggrin) perpetuates the process by promoting local food antigen uptake. Genetic variants in regulatory molecules involved in these steps may contribute to EE disease risk.
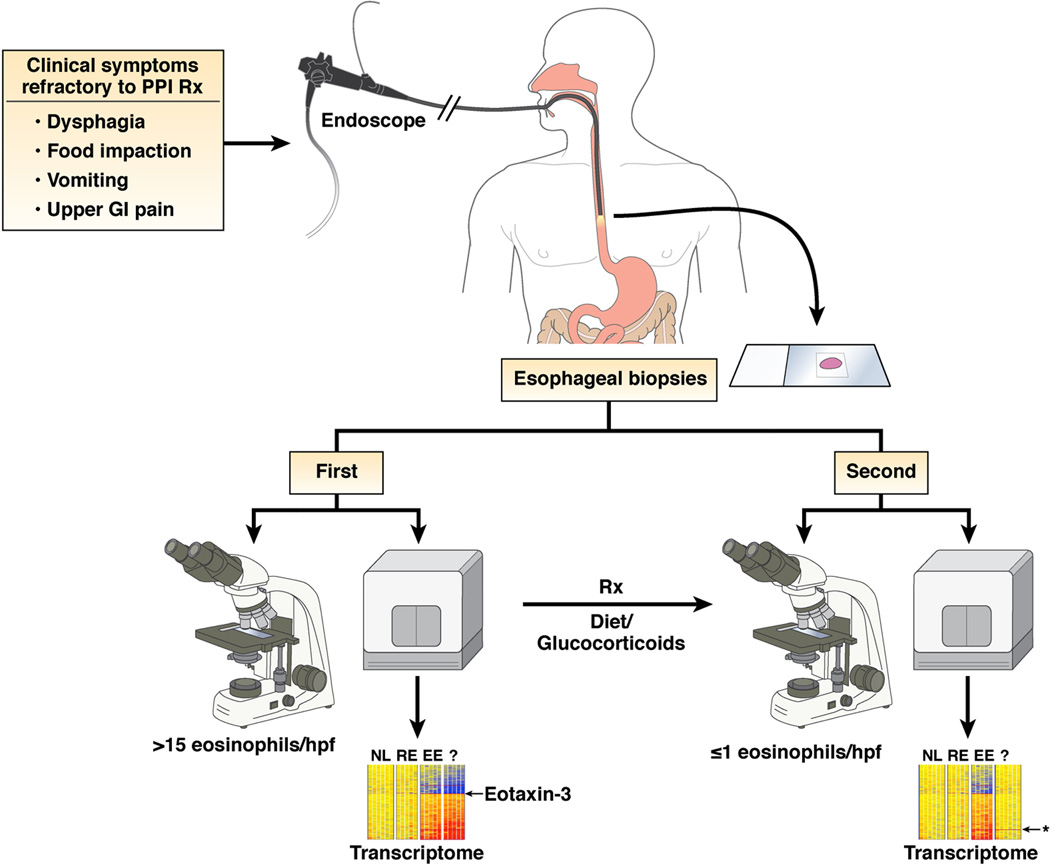
It has been proposed that the cutoff value of esophageal eosinophil concentrations is a peak eosinophil count of 15 cells/hpf.5 While an empiric threshold level of eosinophils may prove useful for disease classification, we recommend that the EE gene transcript signature including overexpression of eotaxin-3 be considered in disease definition. We envision that molecular diagnosticswill be applied to EE and become useful for diagnosis and differentiation from GERD and prediction of therapeutic responsiveness and prognosis (Figure 4). This type of approach is already being taken to classify cancer and is likely to be cost-efficient given improving readily available technology.130 Transcriptome profile analysis may be particularly useful in disease classification in patients on therapy as although the EE transcriptome normalizes with therapy (at the level of 95% correction), there is gene signature that remains persistent and distinct from normal individuals (Figure 4).79
Figure 4. Molecular and histological diagnosis of EE.
Patients with PPI refractory upper gastrointestinal symptoms undergo endoscopic biopsy. Tissue is analyzed microscopically and a minimal level of 15 peak eosinophils per hpf is required for diagnosis. In addition, molecular profiling reveals dysregulated expression of 1% of the human genome including eotaxin-3 overexpression that readily distinguishes biopsies from EE, reflux esophagitis (RE) and normal individuals (NL). Following treatment (Rx) with dietary modification and/or glucocorticoids, endoscopic analysis reveals complete resolution of esophageal eosinophilia and large normalization of the EE transcriptome. Residual abnormal gene expression (*) differentiates treated EE from NL and esophagitis RE patients.
Unlike classic food anaphylaxis (which co-occurs in some patients with EE) that is typically limited to only a select group of foods,131, 132 EE is associated with hypersensitivity to a broad panel of food antigens, indicative of a general breakdown in oral antigen tolerance. Preliminary evidence in mice demonstrate that a limited repertoire of regulatory T cells, may pre-dispose to mucosal Th2 responses including esophagitis.133 The impaired local barrier function in the esophagus of EE patients, combined with the increased level of immunoreactive cells including mast cells, eosinophils, B cells, T cells and dendritic cells likely perpetuates food antigen-driven inflammation and perhaps antigen sensitization locally. While medical management with glucocorticoids is an effective therapy for EE, strategies to curtail upstream antigen-induced inflammation are essential; at present, empiric elimination diets and amino acid based formulas are the primary form of allergen control; however, future trials designed to induce oral antigen tolerance, an approach now underway to treat food anaphylaxis,134 are likely.
Recent attention has been drawn to the importance of adaptive lymphocyte immunity in driving eosinophilic esophageal inflammation; in particular, evidence is accumulating that Th2 cell cytokines are fundamentally important in disease pathogenesis and that their function can be manipulated for therapeutic benefit. Notably, IL-13 expression is both sufficient and necessary for the induction of experimental EE. Furthermore, IL-13 is markedly overexpressed in the esophagus of EE patients and is capable of inducing an esophageal epithelial transcriptome, including eotaxin-3, that overlaps with the esophageal transcriptome expressed in vivo. In parallel, Th2 cell derived IL-5 regulates the pool of circulating eosinophils and their responsiveness to local activating signals, especially eotaxin-3. It is envisioned that therapeutic interventions that target Th2 cell development, Th2 cytokines production and cellular signaling, eosinophils and epithelial/eosinophil interactions (e.g. anti-eotaxin-3, CCR3 antagonists, anti-IL-5 and anti-IL-13) will prove to be successful therapies for EE.
Acknowledgements
The author is indebted to the numerous colleagues, especially those associated with the Cincinnati Center for Eosinophilic Disorders, for their vast contributions to the content of this review. This work was supported by NIH R01 AI45898, R01 DK067255, U19 AI070235, R01 DK076893, R01 AI057803, P30 DK078392, the Campaign Urging Research for Eosinophilic Disorders (CURED), the Food Allergy and Anaphylaxis Network, the Food Allergy Project and the Buckeye Foundation.
Footnotes
Disclosure: Marc Rothenberg is a paid consultant for Centocor, Ception Therapeutics, Merck & Co, Novartis and Nycomed.
References
- 1.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID) J Allergy Clin Immunol. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Balatsinou C, Milano A, Caldarella MP, Laterza F, Pierdomenico SD, Cuccurullo F, Neri M. Eosinophilic esophagitis is a component of the anticonvulsant hypersensitivity syndrome: Description of two cases. Dig Liver Dis. 2007 doi: 10.1016/j.dld.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Noble C, Francis L, Withers GW, Ee LC, Lewindon PJ. Audit of eosinophilic oesophagitis in children post-liver transplant. Pediatr Transplant. 2008 doi: 10.1111/j.1399-3046.2008.01063.x. [DOI] [PubMed] [Google Scholar]
- 5.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Noble A, Drouin E, Faure C. Eosinophilic esophagitis and gastritis in Rubinstein-Taybi syndrome. J Pediatr Gastroenterol Nutr. 2007;44:498–500. doi: 10.1097/MPG.0b013e31802c41cd. [DOI] [PubMed] [Google Scholar]
- 7.Kagalwalla AF, Shah A, Ritz S, Melin-Aldana H, Li BU. Cow's milk protein-induced eosinophilic esophagitis in a child with gluten-sensitive enteropathy. J Pediatr Gastroenterol Nutr. 2007;44:386–388. doi: 10.1097/01.mpg.0000243430.32087.5c. [DOI] [PubMed] [Google Scholar]
- 8.Quaglietta L, Coccorullo P, Miele E, Pascarella F, Troncone R, Staiano A. Eosinophilic oesophagitis and coeliac disease: is there an association? Aliment Pharmacol Ther. 2007;26:487–493. doi: 10.1111/j.1365-2036.2007.03388.x. [DOI] [PubMed] [Google Scholar]
- 9.Verzegnassi F, Bua J, De Angelis P, Dall'oglio L, Di Leo G, Ventura A. Eosinophilic oesophagitis and coeliac disease: is it just a casual association? Gut. 2007;56:1029–1030. doi: 10.1136/gut.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heine RG. Eosinophilic esophagitis in children with celiac disease: new diagnostic and therapeutic dilemmas. J Gastroenterol Hepatol. 2008;23:993–994. doi: 10.1111/j.1440-1746.2008.05514.x. [DOI] [PubMed] [Google Scholar]
- 11.Ooi CY, Day AS, Jackson R, Bohane TD, Tobias V, Lemberg DA. Eosinophilic esophagitis in children with celiac disease. J Gastroenterol Hepatol. 2008;23:1144–1148. doi: 10.1111/j.1440-1746.2007.05239.x. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, Caillat-Zucman S. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 14.van Heel DA. Interleukin 15: its role in intestinal inflammation. Gut. 2006;55:444–445. doi: 10.1136/gut.2005.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caillat-Zucman S. How NKG2D ligands trigger autoimmunity? Hum Immunol. 2006;67:204–207. doi: 10.1016/j.humimm.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Koning F, Gilissen L, Wijmenga C. Gluten: a two-edged sword. Immunopathogenesis of celiac disease. Springer Semin Immunopathol. 2005;27:217–232. doi: 10.1007/s00281-005-0203-9. [DOI] [PubMed] [Google Scholar]
- 17.Stepniak D, Koning F. Celiac disease--sandwiched between innate and adaptive immunity. Hum Immunol. 2006;67:460–468. doi: 10.1016/j.humimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, Wapenaar MC, Barnardo MC, Bethel G, Holmes GK, Feighery C, Jewell D, Kelleher D, Kumar P, Travis S, Walters JR, Sanders DS, Howdle P, Swift J, Playford RJ, McLaren WM, Mearin ML, Mulder CJ, McManus R, McGinnis R, Cardon LR, Deloukas P, Wijmenga C. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet. 2007;39:827–829. doi: 10.1038/ng2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt KA, Zhernakova A, Turner G, Heap GA, Franke L, Bruinenberg M, Romanos J, Dinesen LC, Ryan AW, Panesar D, Gwilliam R, Takeuchi F, McLaren WM, Holmes GK, Howdle PD, Walters JR, Sanders DS, Playford RJ, Trynka G, Mulder CJ, Mearin ML, Verbeek WH, Trimble V, Stevens FM, O'Morain C, Kennedy NP, Kelleher D, Pennington DJ, Strachan DP, McArdle WL, Mein CA, Wapenaar MC, Deloukas P, McGinnis R, McManus R, Wijmenga C, van Heel DA. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40:395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jonsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 21.Orenstein SR, Shalaby TM, Di Lorenzo C, Putman PE, Sigurdsson L, Kocodhid SA. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–1430. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 22.Walsh SV, Antonioli DA, Goldman H, Fox VL, Bousvaros A, Leichtner AM, Furuta GT. Allergic esophagitis in children: a clinicopathological entity. Am J Surg Pathol. 1999;23:390–396. doi: 10.1097/00000478-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, Flick J, Kelly J, Brown-Whitehorn T, Mamula P, Markowitz JE. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–1206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 24.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 25.Noel RJ, Rothenberg ME. Eosinophilic esophagitis. Curr Opin Pediatr. 2005;17:690–694. doi: 10.1097/01.mop.0000184291.34654.be. [DOI] [PubMed] [Google Scholar]
- 26.Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61:795–801. doi: 10.1016/s0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie SH, Go M, Chadwick B, Thomas K, Fang J, Kuwada S, Lamphier S, Hilden K, Peterson K. Eosinophilic oesophagitis in patients presenting with dysphagia--a prospective analysis. Aliment Pharmacol Ther. 2008;28:1140–1146. doi: 10.1111/j.1365-2036.2008.03795.x. [DOI] [PubMed] [Google Scholar]
- 28.Bassett J, Maydonovitch C, Perry J, Sobin L, Osgard E, Wong R. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Dis Esophagus. 2009 doi: 10.1111/j.1442-2050.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 29.Korsapati HR, Babaei A, Bhargava V, Dohil R, Quin AM, Mittal RK. Dysfunction of the Longitudinal Muscles of the Esophagus in Eosinophilic Esophagitis. Gut. 2009 doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 30.Lucendo AJ, Castillo P, Martin-Chavarri S, Carrion G, Pajares R, Pascual JM, Mancenido N, Erdozain JC. Manometric findings in adult eosinophilic oesophagitis: a study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19:417–424. doi: 10.1097/MEG.0b013e328010bd69. [DOI] [PubMed] [Google Scholar]
- 31.Nurko S, Rosen R. Esophageal dysmotility in patients who have eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:73–89. doi: 10.1016/j.giec.2007.09.006. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–535. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Assa'ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, Buckmeier BK, Bullock JZ, Collier AR, Konikoff MR, Noel RJ, Guajardo JR, Rothenberg ME. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, Liacouras CA. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 36.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, Wolfsen H, Raimondo M, Guarderas JC, Achem SR. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009;104:828–833. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 37.Croese J, Fairley SK, Masson JW, Chong AK, Whitaker DA, Kanowski PA, Walker NI. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastroint Endosc. 2003;58:516–522. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 38.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000–1004. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cury EK, Schraibman V, Faintuch S. Eosinophilic infiltration of the esophagus: gastroesophageal reflux versus eosinophilic esophagitis in children--discussion on daily practice. J Pediatr Surg. 2004;39:e4–e7. doi: 10.1016/j.jpedsurg.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–116. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 41.Cantu P, Velio P, Prada A, Penagini R. Ringed oesophagus and idiopathic eosinophilic oesophagitis in adults: an association in two cases. Dig Liver Dis. 2005;37:129–134. doi: 10.1016/j.dld.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Shitrit AB, Reinus C, Zeides S, Braverman D. Eosinophilic esophagitis. Isr Med Assoc J. 2006;8:587. [PubMed] [Google Scholar]
- 43.Fujiwara H, Morita A, Kobayashi H, Hamano K, Fujiwara Y, Hirai K, Yano M, Naka T, Saeki Y. Infiltrating eosinophils and eotaxin: their association with idiopathic eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2002;89:429–432. doi: 10.1016/S1081-1206(10)62047-9. [DOI] [PubMed] [Google Scholar]
- 44.Munitiz V, Martinez de Haro LF, Ortiz A, Pons JA, Bermejo J, Serrano A, Molina J, Parrilla P. Primary eosinophilic esophagitis. Dis Esoph. 2003;16:165–168. doi: 10.1046/j.1442-2050.2003.00319.x. [DOI] [PubMed] [Google Scholar]
- 45.Lucendo Villarin AJ, Carrion Alonso G, Navarro Sanchez M, Martin Chavarri S, Gomez Senent S, Castillo Grau P, Pascual Turrion JM, Gonzalez Sanz-Agero P. Eosinophilic esophagitis in adults, an emerging cause of dysphagia. Description of 9 cases. Rev Esp Enferm Dig. 2005;97:229–239. doi: 10.4321/s1130-01082005000400003. [DOI] [PubMed] [Google Scholar]
- 46.Straumann A, Spichtin HP, Bucher KA, Heer P, Simon HU. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion. 2004;70:109–116. doi: 10.1159/000080934. [DOI] [PubMed] [Google Scholar]
- 47.Molina-Infante J, Hernandez-Alonso M, Perez-Gallardo B, Martin-Noguerol E. The first asian case report of eosinophilic esophagitis in an asymptomatic adult: what about a proton pump inhibitor trial? J Chin Med Assoc. 2009;72:166–167. doi: 10.1016/S1726-4901(09)70046-2. [DOI] [PubMed] [Google Scholar]
- 48.Ruchelli E, Wenner W, Voytek T, Brown K, Liacouras C. Severity of esophageal eosinophilia predicts response to conventional gastroesophageal reflux therapy. Pediatr Dev Pathol. 1999;2:15–18. doi: 10.1007/s100249900084. [DOI] [PubMed] [Google Scholar]
- 49.Markowitz JE, Liacouras CA. Eosinophilic esophagitis. Gastroent Clin N Am. 2003;32:949–966. doi: 10.1016/s0889-8553(03)00047-5. [DOI] [PubMed] [Google Scholar]
- 50.Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis: it's not just kid's stuff. Gastrointest Endosc. 2002;56:260–270. doi: 10.1016/s0016-5107(02)70188-0. [DOI] [PubMed] [Google Scholar]
- 51.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–419. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Bolling-Sternevald E, Lind T, Vieth M, Stolte M, Walker MM, Agreus L. Prevalence of eosinophilia and eosinophilic esophagitis in adults in the community: a random population based study (Kalixanda) Gastroenterology. 2006;130:A575. [Google Scholar]
- 53.Veerappan GR, Perry JL, Duncan TJ, Baker TP, Maydonovitch C, Lake JM, Wong RK, Osgard EM. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol. 2009;7:420–426. doi: 10.1016/j.cgh.2008.10.009. 426 e1-2. [DOI] [PubMed] [Google Scholar]
- 54.Bousvaros A, Morley-Fletcher A, Pensabene L, Cucchiara S. Research and clinical challenges in paediatric inflammatory bowel disease. Dig Liver Dis. 2008;40:32–38. doi: 10.1016/j.dld.2007.07.168. [DOI] [PubMed] [Google Scholar]
- 55.Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol. 2008;24:687–691. doi: 10.1097/MOG.0b013e32830edc1e. [DOI] [PubMed] [Google Scholar]
- 56.Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol. 2009;131:788–792. doi: 10.1309/AJCPOMPXJFP7EB4P. [DOI] [PubMed] [Google Scholar]
- 57.Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108:891–894. doi: 10.1067/mai.2001.120095. [DOI] [PubMed] [Google Scholar]
- 58.Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716–721. doi: 10.1038/ajg.2008.117. [DOI] [PubMed] [Google Scholar]
- 59.Collins MH. Histopathologic features of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:59–71. doi: 10.1016/j.giec.2007.09.014. viii-ix. [DOI] [PubMed] [Google Scholar]
- 60.Noel RJ, Putnam PE, Collins MH, Assa'ad AH, Guajardo JR, Jameson SC, Rothenberg ME. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 61.Steiner SJ, Kernek KM, Fitzgerald JF. Severity of Basal Cell Hyperplasia Differs in Reflux Versus Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:506–509. doi: 10.1097/01.mpg.0000221906.06899.1b. [DOI] [PubMed] [Google Scholar]
- 62.Lewis CJ, Lamb CA, Kanakala V, Pritchard S, Armstrong GR, Attwood SE. Is the etiology of eosinophilic esophagitis in adults a response to allergy or reflux injury? Study of cellular proliferation markers. Dis Esophagus. 2008 doi: 10.1111/j.1442-2050.2008.00896.x. [DOI] [PubMed] [Google Scholar]
- 63.Parfitt JR, Gregor JC, Suskin NG, Jawa HA, Driman DK. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol. 2006;19:90–96. doi: 10.1038/modpathol.3800498. [DOI] [PubMed] [Google Scholar]
- 64.Teitelbaum JE, Fox VL, Twarog FJ, Nurko S, Antonioli D, Gleich G, Badizadegan K, Furuta GT. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–1225. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 65.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier B, Akers R, Cohen MB, Collins MH, Assa'ad AH, Aceves SS, Putnam PE, Rothenberg ME. A randomized double-blind-placebo controlled trial of fluticasone proprionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 66.Lucendo AJ, Navarro M, Comas C, Pascual JM, Burgos E, Santamaria L, Larrauri J. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 67.Protheroe C, Woodruff SA, Depetris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, Sharma VK, McGarry MP, Lee NA, Furuta GT, Lee JJ. A novel histological scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009 doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57:30–36. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 69.Dahshan A, Rabah R. Correlation of endoscopy and histology in the gastroesophageal mucosa in children: are routine biopsies justified? J Clin Gastroenterol. 2000;31:213–216. doi: 10.1097/00004836-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–343. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 71.Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Curr Opin Allergy Clin Immunol. 2007;7:274–278. doi: 10.1097/ACI.0b013e32813aee4a. [DOI] [PubMed] [Google Scholar]
- 72.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, Melin-Aldana H, Li BU. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006 doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. Interleukin-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 75.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096–1103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 77.Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–994. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Onbasi K, Sin AZ, Doganavsargil B, Onder GF, Bor S, Sebik F. Eosinophil infiltration of the oesophageal mucosa in patients with pollen allergy during the season. Clin Exp Allergy. 2005;35:1423–1431. doi: 10.1111/j.1365-2222.2005.02351.x. [DOI] [PubMed] [Google Scholar]
- 79.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, Collins MH, Putnam PE, Wells SI, Rothenberg ME. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibilty with glucocorticoids. J Allergy Clin Immunol. 2007;120:204–214. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 80.Blanchard C, Wang N, Rothenberg ME. Eosinophilic esophagitis: pathogenesis, genetics, and therapy. J Allergy Clin Immunol. 2006;118:1054–1059. doi: 10.1016/j.jaci.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 81.Collins MH, Blanchard C, Abonia JP, Kirby C, Akers R, Wang N, Putnam PE, Jameson SC, Assa'ad AH, Konikoff MR, Stringer KF, Rothenberg ME. Clinical, pathologic, and molecular characterization of familial eosinophilic esophagitis compared with sporadic cases. Clin Gastroenterol Hepatol. 2008;6:621–629. doi: 10.1016/j.cgh.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhattacharya B, Carlsten J, Sabo E, Kethu S, Meitner P, Tavares R, Jakate S, Mangray S, Aswad B, Resnick MB. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol. 2007;38:1744–1753. doi: 10.1016/j.humpath.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 83.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O'Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A, Sundberg JP, Presland RB, Fleckman P, Shimizu N, Kudoh J, Irvine AD, Amagai M, McLean WH. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, Hansen KC, Leung DY. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–2258. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 87.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, Putnam PE, Abonia PJ, Santos J, Rothenberg ME. Local B cells and IgE production in the esophageal mucosa in Eosinophilic Esophagitis. Gut. 2009 doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 89.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, Risma KA, Akers RM, Kirby CL, Buckmeier BK, Assa'ad AH, Hogan SP, Rothenberg ME. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 91.Yamazaki K, Murray JA, Arora AS, Alexander JA, Smyrk TC, Butterfield JH, Kita H. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci. 2006;51:1934–1941. doi: 10.1007/s10620-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 92.Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, Beck LA, Boyce JA, Filipovich AH, Villanueva JM, Sutton SA, Assa'ad AH, Rothenberg ME. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol. 2004;113:115–119. doi: 10.1016/j.jaci.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 93.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–1483. doi: 10.1016/j.jaci.2008.02.033. 1483 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Straumann A, Conus S, Kita H, Kephart G, Bussman C, Belgliner C, Patel J, Byrne M, Simon HU. Mepolizumab, a humanized monoclonal antibody to IL-5, for severe eosinophilic esophagitis in adults: a randomized placebo-controlled double-blind trial. J Allergy Clin Immunol. 2008;121:S44. [Google Scholar]
- 96.Zink DA, Amin M, Gebara S, Desai TK. Familial dysphagia and eosinophilia. Gastrointest Endosc. 2007;65:330–334. doi: 10.1016/j.gie.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 97.Patel SM, Falchuk KR. Three brothers with dysphagia caused by eosinophilic esophagitis. Gastrointest Endosc. 2005;61:165–167. doi: 10.1016/s0016-5107(04)02459-9. [DOI] [PubMed] [Google Scholar]
- 98.Penrose LS. The genetic background of common diseases. Acta Genet Stat Med. 1953:257–265. doi: 10.1159/000150748. [DOI] [PubMed] [Google Scholar]
- 99.Malerba G, Lauciello MC, Scherpbier T, Trabetti E, Galavotti R, Cusin V, Pescollderungg L, Zanoni G, Martinati LC, Boner AL, Levitt RC, Pignatti PF. Linkage analysis of chromosome 12 markers in Italian families with atopic asthmatic children. Am J Respir Crit Care Med. 2000;162:1587–1590. doi: 10.1164/ajrccm.162.4.9909031. [DOI] [PubMed] [Google Scholar]
- 100.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in esophageal mucosa from adult patients with eosinophilic esophagitis (EE). A retrospective comparative pathologic biopsy study. J Clin Pathol. 2006 doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. The cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925. [PubMed] [Google Scholar]
- 102.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 103.Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137:2913–2917. [PubMed] [Google Scholar]
- 104.Rosenberg HF. Recombinant human eosinophil cationic protein: ribonuclease activity not essential for cytotoxicity. Journal of Biological Chemistry. 1995;270:7876–7881. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- 105.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 107.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mulder DJ, Pacheco I, Hurlbut DJ, Mak N, Furuta GT, MacLeod RJ, Justinich CJ. FGF9-induced proliferative response to eosinophilic inflammation in oesophagitis. Gut. 2009;58:166–173. doi: 10.1136/gut.2008.157628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.03.043. in press. [DOI] [PubMed] [Google Scholar]
- 110.Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–4612. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 111.Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis. Int J Mol Med. 1998;1:43–53. [PubMed] [Google Scholar]
- 112.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 113.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 114.Nizet V, Rothenberg ME. Mitochondrial missile defense. Nat Med. 2008;14:910–912. doi: 10.1038/nm0908-910. [DOI] [PubMed] [Google Scholar]
- 115.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Justinich CJ, Ricci A, Jr, Kalafus DA, Treem WR, Hyams JS, Kreutzer DL. Activated eosinophils in esophagitis in children: a transmission electron microscopic study. J Pediatr Gastroenterol Nutr. 1997;25:194–198. doi: 10.1097/00005176-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 118.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, Foster PS, Rothenberg ME. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 119.Lee GS, Craig PI, Freiman JS, de Carle D, Cook IJ. Intermittent Dysphagia for solids associated with a multiringed esophagus: clinical features and response to dilatation. Dysphagia. 2007;22:55–62. doi: 10.1007/s00455-006-9043-6. [DOI] [PubMed] [Google Scholar]
- 120.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 121.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509–511. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 122.Assa'ad A. Detection of causative foods by skin prick and atopy patch tests in patients with eosinophilic esophagitis: things are not what they seem. Ann Allergy Asthma Immunol. 2005;95:309–311. doi: 10.1016/S1081-1206(10)61145-3. [DOI] [PubMed] [Google Scholar]
- 123.Aceves SS, Dohil R, Newbury RO, Bastian JF. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116:705–706. doi: 10.1016/j.jaci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 124.Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, Lim JD, Steiner SJ, Gupta SK. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–173. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Attwood SE, Lewis CJ, Bronder CS, Morris CD, Armstrong GR, Whittam J. Eosinophilic oesophagitis: a novel treatment using montelukast. Gut. 2003;52:181–185. doi: 10.1136/gut.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Straumann A, Bussmann C, Conus S, Beglinger C, Simon HU. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol. 2008;122:425–427. doi: 10.1016/j.jaci.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 127.Netzer P, Gschossmann JM, Straumann A, Sendensky A, Weimann R, Schoepfer AM. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol. 2007;19:865–869. doi: 10.1097/MEG.0b013e32825a6ab4. [DOI] [PubMed] [Google Scholar]
- 128.Stone KD, Prussin C. Immunomodulatory therapy of eosinophil-associated gastrointestinal diseases. Clin Exp Allergy. 2008;38:1858–1865. doi: 10.1111/j.1365-2222.2008.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ober C. Perspectives on the past decade of asthma genetics. J Allergy Clin Immunol. 2005;116:274–278. doi: 10.1016/j.jaci.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 130.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 131.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 132.Lack G. Clinical practice. Food allergy. N Engl J Med. 2008;359:1252–1260. doi: 10.1056/NEJMcp0800871. [DOI] [PubMed] [Google Scholar]
- 133.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci U S A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: implications for future treatment. J Allergy Clin Immunol. 2008;121:1344–1350. doi: 10.1016/j.jaci.2008.02.037. [DOI] [PubMed] [Google Scholar]