Abstract
Parabiosis experiments indicate that impaired regeneration in aged mice is reversible by exposure to a young circulation, suggesting that young blood contains humoral “rejuvenating” factors that can restore regenerative function. Here, we demonstrate that the circulating protein Growth Differentiation Factor 11 (GDF11) is a rejuvenating factor for skeletal muscle. Supplementation of systemic GDF11 levels, which normally decline with age, by heterochronic parabiosis or systemic delivery of recombinant protein, reversed functional impairments and restored genomic integrity in aged muscle stem cells (satellite cells). Increased GDF11 levels in aged mice also improved muscle structural and functional features and increased strength and endurance exercise capacity. These data indicate that GDF11 systemically regulates muscle aging and may be therapeutically useful for reversing age-related skeletal muscle and stem cell dysfunction.
Skeletal muscle is a highly specialized tissue composed predominantly of contractile, multi-nucleated fibers whose regeneration after injury depends on the activity of a specialized subset of muscle fiber-associated mononuclear stem cells called “satellite cells” (1, 2). Satellite cells can be isolated by Fluorescence Activated Cell Sorting based on their unique surface marker profile (CD45−Sca-1−CD11b−CXCR4+β1-integrin+), which effectively distinguishes them from non-myogenic cells and more differentiated myoblasts within the muscle (3, 4).
Aged muscle exhibits decreased satellite cell number, impaired satellite cell function and reduced regenerative potential (2, 5–9). To evaluate satellite cell function in aged muscle on a per cell basis, we performed clonal myogenesis assays (5, 9), and found that CD45−Sca-1−CD11b−CXCR4+β1-Integrin+ satellite cells (Fig. S1) from aged mice formed up to 4-fold fewer colonies compared to young cells (Fig.S2A; (5, 9)). To investigate the molecular basis of this reduced satellite cell activity in aged muscle, we examined DNA integrity in young and aged satellite cells using single cell gel electrophoresis assays. Freshly sorted satellite cells showed a marked increase in DNA damage with age (Fig. S2B, C), with ~60% of aged cells exhibiting severely compromised DNA integrity (red bars, Fig.S2B). Likewise, nearly 60% of satellite cells sorted from aged muscle (Fig. S2D, E) or identified by Pax7-staining on isolated muscle fibers (Fig. S3), showed increased immunoreactivity for the phosphorylated form of histone H2AX (pH2AX), a marker of DNA damage (10). In contrast, 40% of freshly isolated young satellite cells were devoid of detectable DNA damage by gel electrophoresis assay (Fig. S2B, C), and young satellite cell nuclei rarely contained more than two pH2AX foci when assayed after cell sorting (Fig. S2D, E) or on single myofibers (Fig. S3). Induction of DNA damage by X-irradiation reduced the myogenic function of young satellite cells in transplantation assays (Fig. S4), suggesting that increased DNA damage could cause impaired regeneration in aged muscle.
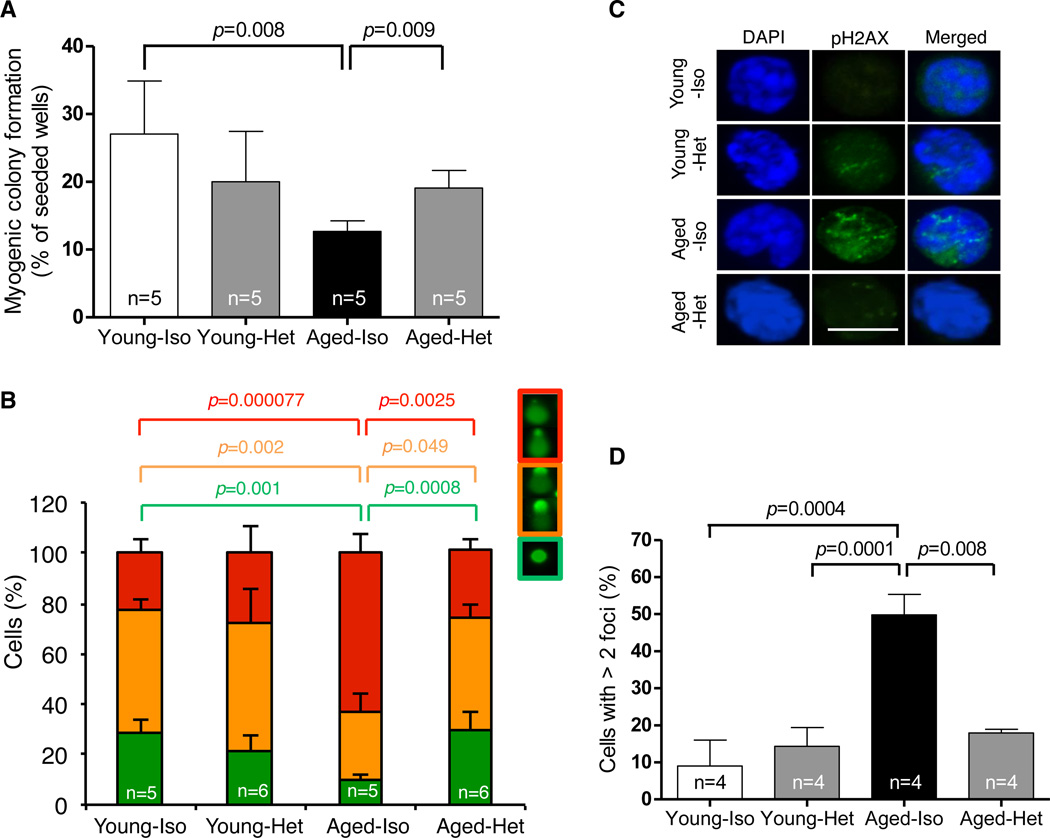
Prior studies demonstrate that impaired regeneration in aged muscle can be reversed by heterochronic parabiosis, which exposes aged tissues to a youthful systemic environment and restores injury-induced satellite cell activation by upregulation of Notch signaling (11). To determine whether this intervention also restores the function and genomic integrity of aged satellite cells, we generated heterochronic parabionts (Fig. S5), joining young C57BL/6 males (2-months of age) with aged partners (22-months of age), and compared these to isochronic (young-young or aged-aged) parabiotic controls. Strikingly, satellite cells sorted from aged mice joined to young partners (referred to hereafter as “aged-heterochronic mice”) showed improved myogenic colony-forming activity when compared to satellite cells from aged-isochronic controls (Fig. 1A). Satellite cells from aged-heterochronic mice also exhibited restored genomic integrity, with DNA damage scores that were indistinguishable from those of young-isochronic mice (Fig. 1B) and reduced numbers of pH2AX foci as compared to aged-isochronic mice (Fig. 1C, D). Interestingly, this restoration of genomic integrity was not accompanied by detectable changes in satellite cell proliferation or proliferative history, as assessed by BrdU incorporation (Fig S6A).
Figure 1. Rejuvenation of muscle stem cells by heterochronic parabiosis.
(A). Frequency of clone-sorted satellite cells from isochronic (Iso) or heterochronic (Het) mice forming colonies after 5 days in culture. All colonies showed characteristic morphology of muscle lineage cells. (B) DNA damage in freshly sorted satellite cells assessed by single cell gel electrophoresis under alkaline conditions. Damage was quantified using a visual scoring metric (25) (key at top) and represented by color-coding: no damage (green), moderate damage (orange), maximal damage (red). (C) Representative images (confocal z-stacks) of freshly sorted satellite cells stained with DAPI (blue) and anti-pH2AX (green); data are quantified in (D). All graphs represent mean ± SD, with p-values, calculated by Mann-Whitney analysis. “n=” indicates number of mice used for each analysis. Scale bar = 10µm.
Several growth factors and cytokines have been studied as potential regulators of muscle growth and repair, including transforming growth factor-β1 (TGF-β1), myostatin, and Wnt-like molecules (7, 11). We recently reported a decline in aged mice in the systemic levels of Growth Differentiation Factor 11 (GDF11), a member of the TGF-β superfamily with homology to myostatin (MSTN) (12). In contrast to GDF11, MSTN levels are unchanged and TGF-β1 increased in the plasma of aged mice (Fig. S7A, B, left panels). GDF11 levels decline in the muscle of aged mice as well (Fig. S7C), whereas TGF-β1 and MSTN levels are unaltered (Fig. S7A, B, right panels).
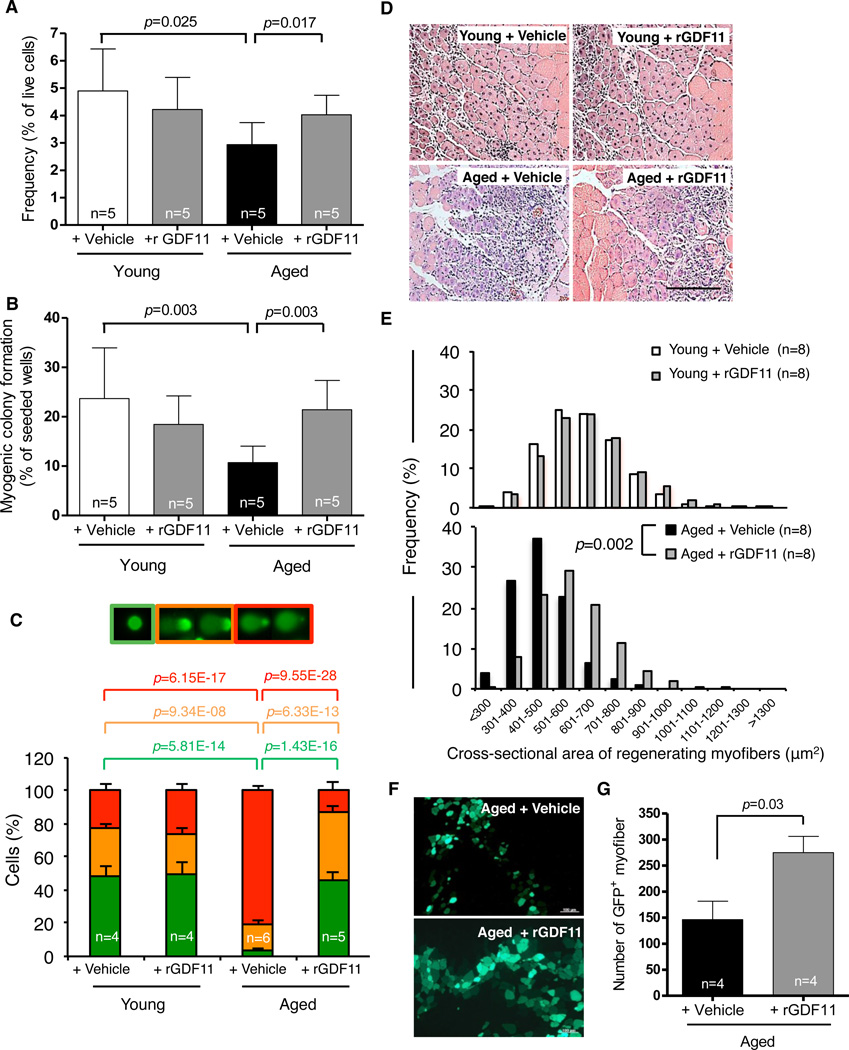
Our prior studies showed that restoration of more youthful levels of systemic GDF11 could reverse age-related cardiac hypertrophy (13). To determine whether supplementation of GDF11 from the young partner might similarly underlie changes in skeletal muscle in heterochronic parabionts, we treated aged mice with daily intraperitoneal injection of recombinant GDF11 (rGDF11, 0.1mg/kg) to increase systemic GDF11 levels. After 4 weeks, satellite cell frequency, determined by flow cytometry (Fig. 2A), and function (Fig. 2B) increased in the muscles of rGDF11-treated mice, while other myofiber-associated mononuclear cell populations were unaffected (Fig. S8). Aged mice treated with rGDF11 also showed increased numbers of satellite cells with intact DNA (Fig. 2C), as compared to cells from aged mice receiving vehicle alone for the same length of time. Moreover, the percent of freshly isolated satellite cells with severely damaged DNA was reduced 4-fold upon treatment with rGDF11 (red bars, Fig. 2C). In contrast to results obtained in aged mice, young mice treated with an identical regimen of rGDF11 injections showed no changes in satellite cell frequency, myogenic colony formation, or DNA damage (Fig. 2A–C).
Figure 2. Rejuvenation of muscle stem cells by rGDF11 supplementation.
(A, B). Frequency (A) and myogenic colony formation (B) of satellite cells from vehicle- or rGDF11-treated mice. (C). Quantification of DNA damage assays using freshly sorted satellite cells from vehicle- or rGDF11-treated mice, scored as in Fig. 1B. (D, E). H&E staining (D) and frequency distribution of myofiber size (E) in regenerating TA muscles 7 days after cryoinjury in vehicle- or rGDF11-treated young and aged mice. Scale bar = 100µm. (F). Representative images of transverse cryosections of TA muscles 2 weeks after transplantation. (G) Quantification of transplant data as maximal number of GFP+ myofibers found in each engrafted muscle. Graphs represent mean ± SD (in A–C, G). p-values were calculated by Mann-Whitney analysis (A–C), Wilcoxon Exact analysis (E), or Student’s t-test (G). “n=” indicates number of mice used for each analysis.
We next evaluated the effect of rGDF11 supplementation on the in vivo regenerative activity of satellite cells by injuring a cohort of rGDF11-treated and control mice. Treatment was initiated 28 days before cryoinjury to the tibialis anterior muscle and continued for 7 days thereafter. Remarkably, supplementation of rGDF11 in aged mice restored more youthful profiles of myofiber caliber in regenerating muscle (Fig. 2D, E), and increased the mean size of regenerating myofibers in these animals to 92% of the size of regenerating fibers in young control mice (Fig. S9A). However, rGDF11 supplementation for 5 weeks did not alter the myofiber caliber of uninjured young or aged muscles (Fig. S9B, C). rGDF11 supplementation in aged mice also enhanced the regenerative capacity of satellite cells in a transplantation model, in which equal numbers of GFP-marked satellite cells were injected into the injured muscles of aged animals treated with rGDF11 or vehicle alone for 4 weeks prior and 2 weeks following transplantation. Recipients treated with rGDF11 showed almost twice as many engrafted (GFP+) fibers as vehicle-treated recipients (Fig. 2F, G). Newly regenerated fibers in rGDF11-treated recipients were also larger in caliber (Fig. S10), consistent with the effects of rGDF11 on endogenous repair of muscle injury in aged mice (Fig. 2D, E).
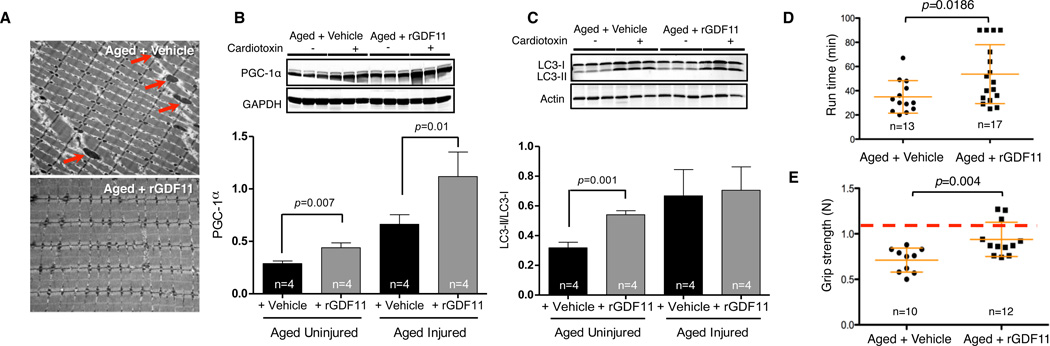
We next interrogated the mechanistic basis for rGDF11’s effects on aged muscle. Although we saw no alterations in gross anatomy, body weight, fat mass, or muscle mass (Fig. S11), immunofluorescence analysis demonstrated increases in the size of neuromuscular junctions following rGDF11 treatment (Fig S12). In addition, electron microscopy of uninjured muscle revealed striking improvements of myofibrillar and mitochondrial morphology in aged mice treated with rGDF11 (Fig. 3A). Treated muscles showed reduction of atypical and swollen mitochondria, reduced accumulation of vacuoles, and restoration of regular sarcomeric and interfibrillar mitochondrial patterning (Fig. 3A). Consistent with these ultrastructural improvements, levels of Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis, were increased in the muscle of aged rGDF11-treated mice (Fig. 3B), suggesting that GDF11 may affect mitochondrial dynamics of fission and fusion to generate new mitochondria. Consistent with this notion, in vitro treatment of differentiating cultures of aged satellite cells with rGDF11 yielded increased numbers of multi-nucleated myotubes exhibiting greater mitochondrial content and enhanced mitochondrial function (Fig. S13). Finally, we observed increased basal levels of autophagosome (macroautophagy) markers (assessed as the ratio of autophagic intermediates LC3-II over LC3-I), in the uninjured skeletal muscle of rGDF11-treated aged animals (Fig. 3C). Collectively, these data suggest enhanced autophagy/mitophagy and mitochondrial biogenesis as likely explanations for the cellular remodeling of muscle fibers in rGDF11-treated aged mice.
Figure 3. Improved muscle physiology and physical function after rGDF11 supplementation.
(A). Electron micrographs of transverse sections of TA muscle from vehicle- or rGDF11-treated aged mice (representative of n=4 per group). Arrows indicate swollen mitochondria. (B, C). Western blot of PGC-1α (B) and LC3 forms I and II (C) in TA muscle extracts from cardiotoxin-injured or uninjured vehicle- or rGDF11-treated aged mice. Three animals are shown for each experimental group. Densitometric quantification of Western data are provided below each blot, normalized to GAPDH (B) or Actin (C). (D, E). Scatter plots of exercise endurance (D, maximum treadmill runtime in a 90 minute window) or forelimb grip-strength (E) of vehicle- or rGDF11-treated aged mice. Grip-strength is plotted as maximum force (Newton, N) exerted in triplicate trials. Red line represents the maximum grip-strength of 33-39 week-old young male mice. Data are presented for individual mice (black symbols) overlaid with mean ± SD (orange lines). p-values were calculated by Mann-Whitney analysis. “n=” indicates number of mice used for each analysis.
We next questioned whether improvements in muscle ultrastructure and mitochondrial turnover in rGDF11-treated aged mice might translate into improved physical function in exercise endurance and grip strength analyses. Aged mice treated with rGDF11 showed increased average exercise endurance (35min. vs. 57min.), despite variation in the responses of individual animals (Fig. 3D). rGDF11-treated animals also exhibited improved clearance of systemic lactate (Fig. S14A) and lower levels of glucose (Fig. S14B) after 40 minutes of strenuous running, providing additional evidence indirectly supporting improved mitochondrial function in aged rGDF11-treated animals. Finally, in accord with rGDF11-stimulated remodeling of myofiber ultrastructure, rGDF11-treated animals exhibited increased average grip strength (Figs. 3E and S15) in a standardized testing platform (14).
Our studies reported here establish GDF11 as a novel humoral regulator of youthful regenerative potential and demonstrate that restoration of aged satellite cell function by this factor is coincident with reversal of accumulated DNA damage. These data suggest that genome toxicity may constrain stem cell function in aged muscle, as reported previously for the hematopoietic system (15). However, accumulating evidence indicates that DNA strand breaks may arise in stem cells not only as a result of genomic insult, but also as a conserved and necessary event for nuclear reprogramming to allow for cell differentiation (16). Transcriptome profiling of young and aged muscle stem cells indicates that some muscle differentiation genes are upregulated in aged satellite cells ((6) and Fig. S16), despite the fact that aged cells show compromised regenerative capacity ((7–9, 11) and Fig. 2). In addition, we failed to detect age-related alterations in expression of DNA damage recognition and repair enzymes by immunofluorescence analysis (Figure S17). These data raise the possibility that aged satellite cells display an apparent increase in DNA damage because they are arrested at an early stage of myogenesis, in which differentiation-associated DNA strand breaks have been induced but not resolved. Extending this logic, systemic “rejuvenation” by parabiosis or rGDF11 treatment may release these cells from this age-induced differentiation block. This notion is consistent with our data demonstrating (i) increased detection of DNA damage and an increase in activated cleaved-Caspase3 in aged satellite cells ((Figs. S2 and S18) and (16)), (ii) the resolution of these changes upon restoration of myogenic activity in “rejuvenated” muscle (Figs. 1, 2), and (iii) the ability of in vitro treatment with rGDF11 to increase myogenic cell number and promote myotube formation in cultures of aged satellite cells (Figs. S19 and S13A). Given that rGDF11-stimulated rejuvenation of aged satellite cells coincides with remodeling of the aged satellite cell niche (Figs. 2, 3 and S10), we speculate that increasing GDF11 levels in aged mice may act both directly and indirectly to restore satellite cell regenerative function, stimulating intrinsic changes in satellite cell differentiation capacity (Fig. S13A) and producing a more “promyogenic” niche that extrinsically supports endogenous regeneration and transplant-associated myogenic engraftment (Figs. 2 and 3).
GDF11 belongs to a conserved family of growth factors that regulate diverse cellular processes (17). Genetic deficiency of GDF11 in mice causes profound developmental abnormalities, including agenesis of the kidneys, and perinatal lethality (12, 18, 19). The mature form of GDF11 shares ~90% sequence identity with MSTN, known for its potent negative influence on skeletal muscle mass (12), and binds the same receptors (ACVR1B/ALK4, ACVR1A/ALK5 and ACVR1C/ALK7) (20). A prior study reported relatively low levels of ALK4/5 expression by postnatal (P12) satellite cells and failed to detect a difference in proliferation of these cells upon exposure to MSTN (21). We found that in vitro exposure of aged satellite cells to rGDF11, but not recombinant MSTN or TGF-β1, produced dose-responsive increases in cell proliferation (Fig S19) and differentiation (Fig. S13A), suggesting that GDF11, in contrast to MSTN, can act directly on satellite cells to alter their function. Interestingly, growth promotion may be the primordial role of MSTN/GDF11, as invertebrates possess only a single ortholog of the MSTN/GDF11 family, and downregulation of this gene results in retarded growth (22). In any event, the unique combination of rGDF11’s pro-myogenic effects in skeletal muscle, anti-hypertrophic effects in the heart (13), and beneficial effects on neurogenesis and neuronal function (23) in aged mice, should encourage further investigation of its therapeutic potential for a variety of age-related diseases and suggests that GDF11 should be regarded as a new molecular regulator of mammalian aging with potentially broad-reaching applications.
Supplementary Material
Acknowledgements
We thank the HSCI/HSCRB Flow Cytometry Core, Harvard’s Center for Biological Imaging, and C. Dall’Osso and B. Hunter for technical assistance. This work was funded in part by grants from the American Heart Association to FSL (postdoctoral fellowship), NIH (R01 AR42238) to LJG, Glenn Foundation for Medical Research, Harvard Stem Cell Institute, and NIH (1R01 AG033053, 1DP2 OD004345, and 5U01 HL100402) to AJW, NIH (R01 AG032977 1R01 AG040019) to RTL and Project Ascelegen to AJW and RTL. AJW is an Early Career Scientist of the Howard Hughes Medical Institute. Some of the authors have a pending patent application related to this work.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Supplementary Materials
Materials and Methods
Figs. S1 – S19
References (24–25)
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang YC, Sinha M, Cerletti M, Dall'osso C, Wagers AJ. Skeletal Muscle Stem Cells: Effects of Aging and Metabolism on Muscle Regenerative Function. Cold Spring Harb Symp Quant Biol. 2011 Sep 29; doi: 10.1101/sqb.2011.76.010652. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood RI, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004 Nov 12;119:543. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008 Jul 11;134:37. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012 May 4;10:515. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012 Oct 18;490:355. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 8.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014 Mar;20:255. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010 Apr;24:679. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 11.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433:760. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 12.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013 May 9;153:828. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakim CH, Li D, Duan D. Monitoring murine skeletal muscle function for muscle gene therapy. Methods Mol Biol. 2011;709:75. doi: 10.1007/978-1-61737-982-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007 Jun 7;447:725. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 16.Larsen BD, et al. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci U S A. 2010 Mar 2;107:4230. doi: 10.1073/pnas.0913089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel J SW, Groppe JC, Mueller TD. Intricacies of BMP receptor assembly. Cytokine & Growth Factor Reviews. 2009;20:367. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 18.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999 Jul;22:260. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, et al. Growth differentiation factor 11 signaling controls retinoic acid activity for axial vertebral development. Dev Biol. 2010 Nov 1;347:195. doi: 10.1016/j.ydbio.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WH, Lander J, Lyons AD, Matzuk KM, Calof MM. AL GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- 21.Amthor H, et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009 May 5;106:7479. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Santis C, et al. Growing backwards: an inverted role for the shrimp ortholog of vertebrate myostatin and GDF11. J Exp Biol. 2011 Aug 15;214:2671. doi: 10.1242/jeb.056374. [DOI] [PubMed] [Google Scholar]
- 23.Katsimpardi L, et al. Cerebrovascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. 2014 doi: 10.1126/science.1251141. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012 Jan 6;10:96. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004 Mar;26:249. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.