Heart failure and functional recovery
Heart failure is a leading cause of healthcare expenditures, hospitalization, and mortality in developed countries, and its burden is growing globally.1 With the aging of the population and increasing prevalence of chronic diseases, including hypertension, diabetes and obesity, the current heart failure epidemic is guaranteed to significantly worsen in the near future. Thus, new disease-modifying treatments for heart failure are urgently needed and represent an area of intense investigation.2
Multiple studies in humans and animals have shown that the functionality of myocardial tissue of a failing heart can be restored. First, in ischemic heart failure due to severe coronary artery stenosis, revascularization therapy is known to improve heart function in a proportion of patients.3, 4 Second, the mechanical unloading of the heart by left ventricular assist device (LVAD) is associated with improvements in cardiac function. In a recent report of 80 patients with heart failure who underwent implantation of a continuous-flow LVAD, the ejection fraction increased by more than 50% in about one-third of the patients, with corresponding improvements in LV end-systolic and end-diastolic volumes and decreases in LV mass at 6-months post LVAD unloading.5 In addition, normalization of echocardiographic parameters has completely obviated the need for continuing LVAD support or cardiac transplantation in several patients.6, 7 Importantly, the positive effects of mechanical unloading were noted in patients with both ischemic and non-ischemic heart failure,5 suggesting that dysfunctional but potentially salvageable segments of myocardium exist in the failing heart regardless of etiology. Third, in patients with broken heart syndrome (also known as Takotsubo cardiomyopathy), characterized by a rapid and severe loss of cardiac contractility secondary to emotional stress, myocardial function normalized spontaneously, again arguing for the reversibility of heart failure.8 In summary, while maladaptive changes observed in failing hearts were initially considered to be terminal, the accumulating body of evidence argues strongly for the reversibility of cardiac dysfunction due to multiple and distinct etiologies, raising the exciting possibility of curing the failing heart.
Myocardial adaptations to ischemia – a continuum
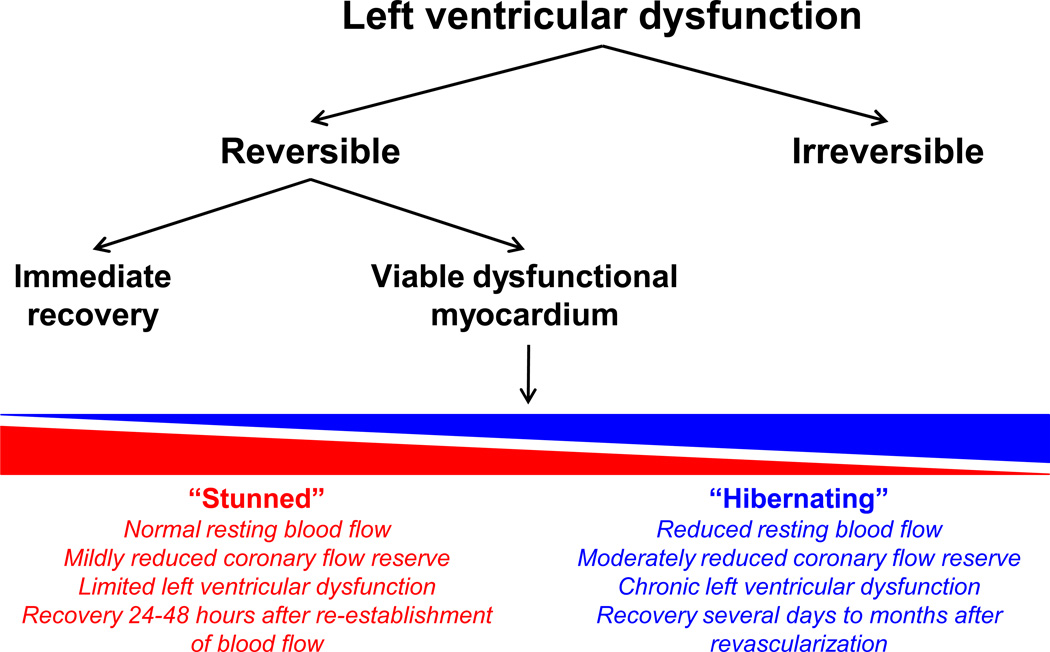
Initially, it was believed that myocardial ischemia resulted in either irreversible myocardial necrosis (i.e., myocardial infarction) or complete and rapid recovery of myocyte function (i.e., typical angina). However, it is now clear that ischemia produces a continuum of myocardial adaptive responses (Figure 1). Several animal models have shown that resting contractile dysfunction is dependent on the physiological significance of the coronary stenosis, which can progress from a state associated with normal resting flow to a state with regional reductions in resting flow.9–11 The degree of stenosis severity determines many of the intrinsic molecular adaptations of the myocardium, and this continuum of adaptations may be partly responsible for the variable time course and extent of reversibility of cardiac function after revascularization.12
Figure 1.
Schematic representation of myocardial responses to left ventricular dysfunction.
For example, myocardial stunning is a brief, fully reversible depression of cardiac function, usually of less than 24–48 hours in duration. Myocardial stunning mostly occurs after a single brief episode of ischemia and is associated with normal resting myocardial blood flow. Heyndrickx et al.13 demonstrated myocardial stunning by subjecting the hearts of conscious dogs to no-flow ischemia for 5 or 15 minutes, and showing depression in mechanical function for 3 to over 24 hours after reperfusion, respectively, but with subsequent complete recovery. Myocardial stunning can occur in several clinical settings, such as exercise in the presence of coronary stenosis and variant angina.14
On the contrary, myocardial hibernation develops in response to repetitive ischemia or worsening chronic coronary stenosis.15 It is characterized by a series of adaptations in the setting of reduced regional resting flow to preserve myocardial viability at the expense of ventricular function. Myocardial hibernation may also result from repetitive stunning due to repeated ischemia,9 and it is now widely believed that myocardial stunning and hibernation are part of a continuous disease spectrum. Although myocardial stunning and hibernation are both reversible, myocardial stunning is usually fully reversible within 1–2 days while hibernating myocardium may require several days to months to recover its function.16 Hibernating myocardium may occur in unstable and chronic stable angina, myocardial infarction, and heart failure, and has important clinical implications for prognosis as functional recovery may take from several days to months after the re-establishment of coronary flow.17, 18 If myocardial hibernation is not reversed by improving blood flow or reducing myocardial oxygen demand, then it is associated with cellular damage, recurrent myocardial ischemia and infarction, heart failure, and ultimately, death.19 The term “hibernating myocardium” has historically been used to describe myocardial dysfunction secondary to ischemia, and it does not encompass the dysfunctional myocardium in a non-ischemic failing heart.
While stunned and hibernating myocardium are commonly used to describe hypokinetic myocardium in humans and animal models, both of these terms have largely been used in the context of myocardial ischemia, whereas viable dysfunctional (VD) myocardium remains an important therapeutic target in both non-ischemic as well as ischemic heart failure patients. VD myocardium in a human failing heart may be hypokinetic or akinetic, but nevertheless retains intact cellular membranes, glucose uptake and metabolism, and an increase in contractility in response to low-dose dobutamine infusion, providing the rationale for evaluating patients with suspected VD myocardium using dobutamine echocardiography. Alternatively, the non-viable myocardium, or scar tissue, is non-contractile, metabolically hypo- or inactive, and non-responsive to dobutamine stimulation.20 This distinction is important in the clinical setting because VD myocardium would be expected to improve with revascularization, while truly infarcted tissue would not. Since the goal of this manuscript is to delineate the molecular features of the potentially salvageable myocardium in a failing heart, we will use the broad term “VD myocardium”, which would include both hibernating ischemic and dysfunctional non-ischemic myocardium.
Development of VD myocardium in the ischemic heart
VD myocardium has been extensively studied in patients with severe coronary stenosis, and thus its pathophysiology has been linked to myocardial ischemia. Two hypotheses were put forward to explain myocardial dysfunction. Initially, the “smart heart” hypothesis was proposed by Rahimtoola et al.,18 who suggested that prolonged subacute or chronic ischemia due to limited coronary blood flow causes the heart to reduce its energy/oxygen utilization by suppressing myocardial metabolism and contractile force. Thus, the “smart heart” establishes a new equilibrium between cardiac energy supply and demand to maintain its viability in the setting of hypoperfusion. However, the reduction in coronary flow in the ischemic heart central to this hypothesis remains controversial, with many studies reporting no change in baseline perfusion of the dysfunctional myocardium.21–23 Subsequently, another hypothesis argued that in the setting of limited coronary flow reserve, a transient elevation in myocardial metabolic demand due to exercise or adrenergic stimulation causes short but frequent episodes of ischemia and myocardial stunning. While each individual stunning episode is fully reversible within 24 hours, multiple bouts of ischemia will lead to sustained depression of contractile function.24 This “repetitive stunning” hypothesis has been validated in large animal models of sublethal ischemia-reperfusion, which recapitulate the pathology of the human heart, although the suppression of myocardial function may be deliberate and adaptive in accordance with the “smart heart” hypothesis. Unfortunately, the mechanism by which the myocardium loses its functionality in non-ischemic heart failure has not been well-characterized and remains an important, but understudied, area of research.
Structural changes in VD myocardium
In the setting of decreased myocardial blood flow, the heart undergoes structural remodeling, involving changes in the morphology and protein content of the myocardium, to maintain contractility. As a result of this remodeling, the VD myocardium may take months to fully recover its function once blood flow is restored.25 A large number of structural changes have been described in VD cardiomyocytes in both humans and animal models of ischemic cardiomyopathy. Biopsies of dysfunctional myocardium obtained during coronary bypass surgery in humans revealed replacement of sarcomeres in the perinuclear area by deposits of glycogen and mitochondria,26–28 There was also a profound reduction in, and disorganization of, the sarcoplasmic reticulum (SR) and abnormalities in the size and shape of mitochondria.26, 29 Based on these morphological changes, VD ischemic cardiomyocytes were proposed to dedifferentiate to resemble fetal cells, a hypothesis that was supported by identification of embryonic/fetal gene isoforms in adult VD myocardium, including α-smooth muscle actin, titin, desmin and cardiotin.30–32
Two mechanisms were proposed to mediate the return of the VD myocyte to an embryonic/fetal morphology: myocardial ischemia and mechanical stretch. The first mechanism appears to be less likely, as similar changes in cardiomyocyte morphology and signs of dedifferentiation were detected in other models of cardiac dysfunction, including chronic atrial fibrillation,33 infarct border zone,34 and in explanted hearts from patients with heart failure.35 Moreover, many changes that are prominent in the viable region of the dysfunctional myocardium were also detected in the remote, non-ischemic zones, arguing for a heart-wide mechanism.27, 36, 37
The stretch hypothesis was supported by studies in vitro and in a pig animal model of chronic coronary stenosis. Co-culturing of adult rabbit ventricular myocytes with fibroblasts was previously shown to induce a pattern of dedifferentiation similar to that of VD myocardium,38 and thus was used as a model to elucidate the molecular mechanisms for this phenomenon.39 The authors reported redistribution of adhesion molecules such as β-1 integrin, N-cadherin, desmoplakin and vinculin, from the intercalated disc area at the distal end of cardiomyocytes to the lateral membranes of these cells, thus increasing the tensile force experienced by cardiac myocytes. The myocytes were cultured under normoxic conditions, ruling out the role of ischemia in dedifferentiation.39 While the model of cardiomyocyte-fibroblast co-culture may not fully recapitulate all of the ultrastructural changes that occur during chronic dysfunction of the myocardium, its findings are nevertheless supported by studies in pigs, in which cardiomyocyte dedifferentiation was noted after two weeks of coronary stenosis.40 Importantly, in addition to re-expression of fetal α-smooth muscle actin, there was a significant loss of desmoplakin and desmin from the intercalated disks on immunofluorescent staining, with no change in the total content of these proteins present in cells. Interestingly, the changes in adhesion molecules were not restricted to the dysfunctional ischemic myocardium, but were also observed in the well-perfused remote zones of the heart, similar to previous reports of myofibrillar loss in both dysfunctional and normal myocardium.40
Whether or not mechanical stretch plays a role in the development of non-ischemic heart failure is still not known. However, a gene array analysis of recovered and non-recovered myocardium after LVAD removal or cardiac transplantation revealed striking differences in the expression of β-integrins, the transmembrane sensors of stretch signals.41–43 Thus, the mechanical stretch may also play a role in the suppression of cardiac function in non-ischemic hearts, although the detailed mechanism remains to be elucidated.
Molecular mechanisms of VD myocardium
While significant research has been devoted to understanding the structural and functional changes that occur in VD myocardium, little progress was made in elucidating the molecular mechanisms governing suppression and recovery of myocardial function due to both ischemic and non-ischemic cardiomyopathy.
The studies of ischemic VD myocardium in human patients were conducted by comparing the molecular profiles of dysfunctional ischemic myocardium and non-ischemic remote zones. Additionally, some investigators compared ischemic VD myocardium to control non-myopathic donor hearts. The majority of animal studies on ischemic VD myocardium were conducted in swine and canine models, in which myocardial dysfunction was induced either by various protocols of repetitive stenosis and reperfusion, or by surgically narrowing coronary arteries for chronic reduction in cardiac perfusion.44 These models display suppression of myocardial contractile function and molecular changes in cardiomyocytes reminiscent of the VD ischemic myocardium in human patients.
For evaluation of VD myocardium in non-ischemic heart failure patients, myocardial samples were taken from patients during the implantation of LVAD and following removal of the device upon recovery of cardiac function. In addition, a series of studies examined the myocardium of patients who received LVAD as a bridge to transplantation, although many of these studies failed to record the extent of functional recovery of the failing heart and correlate the molecular changes to the restoration of cardiac viability.
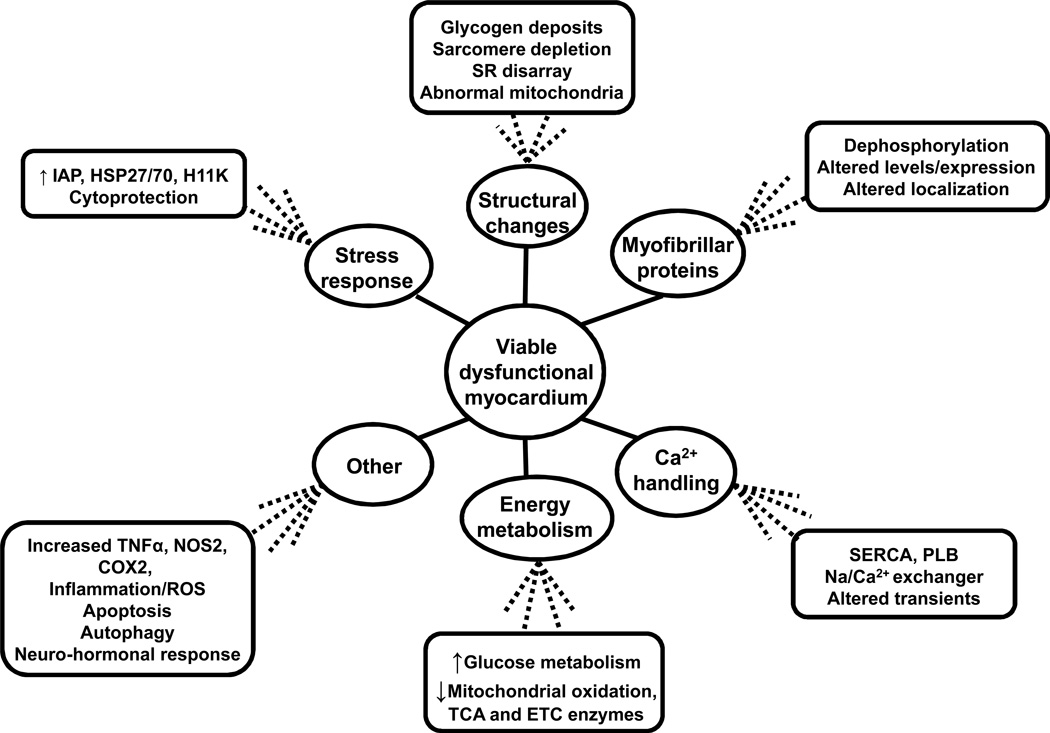
The molecular changes that occur in VD myocardium due to ischemic etiology include alterations in contractile proteins, depression of energy metabolism and mitochondrial function, disruption of calcium signaling and sensitivity, and induction of a distinct subset of cardioprotective genes44, 45 (Figure 2). Similar pathways are affected in non-ischemic failing hearts, and mechanical unloading was shown to normalize their function.46 Unfortunately, it remains unknown which of these pathways are primarily responsible for the depression of myocardial contractility in heart failure, and which are altered non-specifically, secondary to neurohormonal axis activation, increased workload of the heart, or other processes.
Figure 2.
Summary of molecular changes in viable dysfunctional myocardium.
Myofibrillar proteins
Myocardial cells contain several myofibrils, long chains of individual sarcomeres composed of alternating thick and thin myofilaments that coordinate cardiac contraction and relaxation.47 The reduction in contractility of ischemic VD myocardium may be purely related to the structural changes associated with long-term coronary perfusion-consumption mismatch, or may represent an adaptive mechanism which minimizes energetically-expensive myofibrillar cycling. A number of changes in structural and signaling proteins in myofilaments of VD myocardium have been described, all consistent with suppressed contractility.12, 27, 40, 48 In a model of limited coronary flow for 24 hours in a pig heart, a reduction in myofibrillar volume density was observed, but returned to normal after 7 days of reperfusion,49 supporting the reversibility of the VD myocardial phenotype. A recent unbiased proteomic analysis of dysfunctional and healthy remote heart tissue from a pig model with chronic coronary artery stenosis identified several contractile proteins differentially expressed in VD myocardium.12 Among those were thin filament proteins including troponin, tropomyosin, myosin light chains, and myosin heavy chain β. Additionally, Elsasser et al. found significant loss of myofilaments in 38 patients with identified VD myocardium, characterized by reductions in the protein and mRNA content of actin, α-actin, myosin, desmin, and titin.28 Consistent with depressed contractility, a reduction in phosphorylation of myosin regulatory light chain 2 (MLC2) and cardiac troponin I (TnI) was observed in a novel mouse model of myocardial dysfunction in which chronic ischemia was induced by VEGF sequestration.48 Since phosphorylation of MLC2 is critical for cardiac muscle contraction,50 dephosphorylation of this protein may be a potential mechanism that significantly limits contractility in the VD heart. These findings suggest that structural disassembly and altered regulation of myofilament proteins contribute to cardiac dysfunction in VD myocardium.
In non-ischemic human failing hearts, profound changes in myofibrillar proteins also took place in the setting of LVAD support.43 In a study comparing hearts that recovered their function with LVAD to those that failed to show improvement, sarcomeric proteins β-actin, α-tropomyosin, α1-filamin A and α1-actinin were increased in the recovered group. On the other hand, troponin T, α2-actinin and syntrophin levels were lower in the recovered hearts compared to the non-recovered ones.41, 42 At the protein level, there was a significant increase in myosin heavy chain, troponins C and T, sarcomeric actin and other cytoskeletal proteins in hearts whose function was restored with mechanical unloading,51 suggestive of their suppression in VD myocytes. Finally, LVAD support was associated with changes in post-translational modifications of contractile proteins, including reduced PKA-dependent phosphorylation of troponin I.52 In summary, VD myocardium shows a wide range of changes in myofibrillar proteins, and restoration of cardiac function is associated with reorganization of the contractile apparatus. However, it is difficult to compare the changes that occur in myofibrils of ischemic and non-ischemic failing cardiomyocytes, and it remains to be determined whether interventions targeting myofibrillar dynamics will be effective in treating myocardial dysfunction.
Calcium-handling proteins
Maintenance of calcium handling is essential for cardiomyocyte contraction. Upon depolarization of the cardiomyocyte cell membrane by an action potential, L-type calcium channels are activated and the resulting calcium influx leads to calcium-induced calcium release from the SR, raising the cytosolic calcium concentration. This cytosolic calcium binds to and activates cross-bridge attachments in myofilaments, leading to muscle contraction. Disruption of proteins mediating calcium flux is associated with reduced contractile force.47 A study of regional myocardial short-term ischemia in pigs showed a decrease in overall myocardial calcium responsiveness of VD segments, suggestive of an excitation-contraction coupling defect.53 Follow-up investigations revealed significant reductions in mRNA and protein levels of sarcoplasmic reticulum Ca2+-ATPase (SERCA), responsible for calcium uptake into the SR during the relaxation phase.54 SERCA2a activity was also reduced in heart homogenates from patients with documented VD myocardium, compared to control non-myopathic hearts.55 This finding may be explained by de-phosphorylation of SERCA inhibitor phospholamban (PLB), which would block SERCA2 action through physical interaction with this transporter.55 Expression and activity of calcium-handling proteins was also altered in non-ischemic cardiomyopathy, and reversed with LVAD-mediated unloading. Thus, LVAD support increased SERCA2a protein and mRNA levels in the failing hearts regardless of heart failure etiology.56
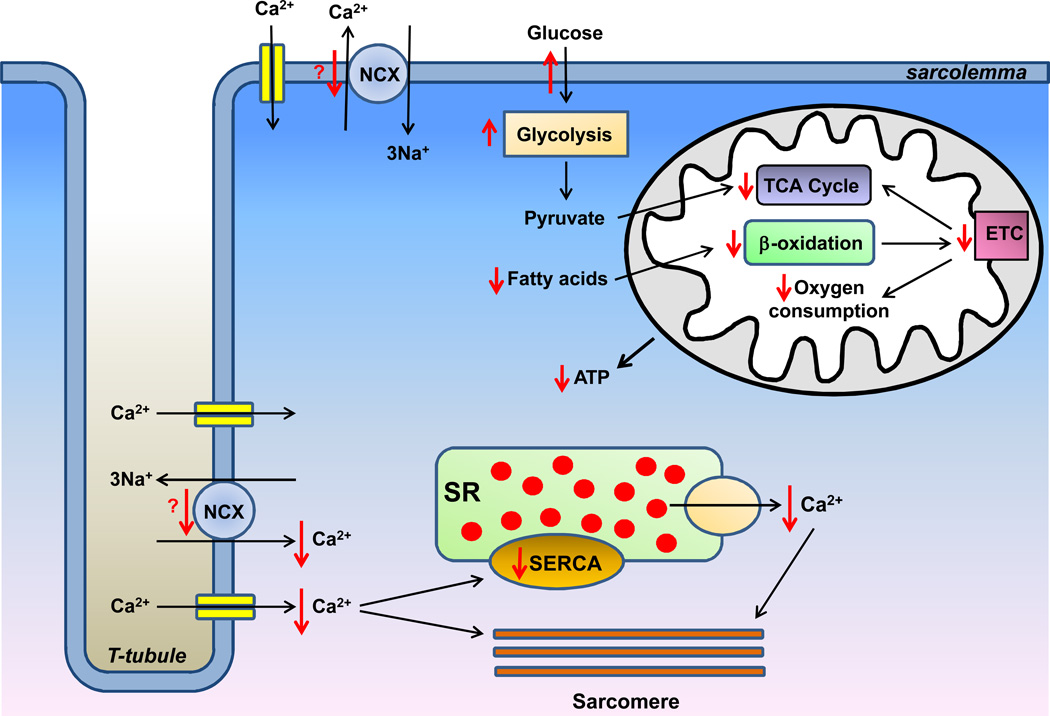
The functional characterization of calcium homeostasis in VD myocardium is scarce, but nevertheless supports the notion of repressed calcium flux (Figure 3). Both Ca2+ influx and free intracellular Ca2+ concentration were reduced in ischemic VD myocardium, consistent with an excitation-contraction coupling defect.57 In non-ischemic hearts supported with LVAD, there was a similar depletion of intracellular Ca2+ stores, which was improved by mechanical unloading.58 Defects in other parameters of calcium transients were also reversed in the recovered non-ischemic hearts, including normalization of the force-frequency relationship, faster time-to-peak and decay of the Ca2+ transient, and shortening of the action potential.58–61 Consistently, myocyte force generation was significantly improved following mechanical unloading.62, 63 In summary, while the exact changes in the expression and activity of calcium-handling proteins remain to be resolved, it appears that calcium homeostasis is severely disrupted in VD myocardium, and may be partially responsible for the reduction in contractile force, while the recovery of myocardial function correlates with normalization of calcium handling in cardiomyocytes.
Figure 3.
Overview of changes in calcium-handling proteins and energy metabolism in viable dysfunctional myocardium. See text for details. SR, sarcoplasmic reticulum. SERCA, sarcoplasmic reticulum Ca2+-ATPase. NCX, sodium-calcium exchanger. TCA, tricarboxylic acid cycle. ETC, electron transport chain. Orange dots represent calcium. Red arrows represent changes observed in viable dysfunctional myocardium.
Energy metabolism
The majority of studies on energy metabolic remodeling in VD myocardium are limited to the ischemic cardiomyopathy. VD ischemic myocardium displays a profound remodeling of energy metabolism, which is proposed to be an adaptive response to coronary hypo-perfusion. The major changes that occur in VD myocardium include suppression of oxygen consumption and mitochondrial function with a concomitant upregulation of glucose uptake and utilization (Figure 3).
Several studies reported a reduction in mitochondrial oxygen consumption,64, 65 and reduced respiratory control index in the VD myocardium in a pig model with chronic coronary stenosis.12, 65 Similarly, a study of human patient biopsies revealed reduced oxygen consumption and oxidative phosphorylation capacity in chronically-ischemic myocardium.66 These changes were paralleled by a coordinated downregulation of many mitochondrial enzymes and subunits of mitochondrial electron transport chain (ETC) proteins.65 VD myocardium in a swine model with chronic coronary stenosis showed reduced expression and activity of several subunits of the pyruvate dehydrogenase complex, enzymes of the tricarboxylic acid (TCA) cycle, fatty acid oxidation, mitochondrial ETC complexes and ATP synthase, consistent with global and coordinated downregulation of mitochondrial oxidative capacity.67 The results of a recent metabolomic analysis of ischemic VD pig myocardium were supportive of these data, showing a reduction in TCA cycle metabolites α-ketoglutarate, succinate, fumarate and malate.68 Moreover, the intermediates of lipid metabolism, carnitine, acetylcarnitine and palmitoylcarnitine, were also reduced in VD myocardium.68 Thus, mitochondrial oxidative phosphorylation is consistently suppressed in VD myocardium of humans and animals.
Contrary to the suppression of mitochondrial respiration, glucose uptake and utilization were significantly enhanced. This was demonstrated non-invasively in human patients undergoing positron emission tomography (PET) scan with fluorine-18 labeled deoxyglucose (18F-FDG) tracer.69 Insulin was able to stimulate myocardial glucose uptake in chronic VD myocardium, demonstrating that insulin control over glucose uptake is preserved. However, after stimulation by glucose or insulin, 18F-FDG uptake myocardial uptake was similar in both VD and normal myocardium, suggesting that although fasting glucose uptake is enhanced in VD myocardium, the maximal stimulated glucose uptake is not increased.10, 70, 71 Elevations in fasting 18F-FDG uptake may be explained by upregulation of glucose transporter 1 (GLUT1), found in humans, pigs72 and in a novel mouse model of VD myocardium through VEGF sequestration.48 This increase in GLUT1 expression in VD cardiomyocytes may be mediated by the HIF-1α signaling pathway.72 Additionally, a study in pigs with chronic coronary artery constriction demonstrated increased expression of mitogen-activated protein kinase (MAPK) p38 which correlated with increased membrane localization of the GLUT4 transporter,73 although the causal relationship between the two events, as well as the mechanism by which MAPK is induced in VD myocardium were not established.
The switch of preferred substrate from fatty acids to glucose in VD myocardium provides the rationale for using radionuclide myocardial perfusion imaging with glucose analogs, such as 18F-FDG, to differentiate VD myocardium from non-viable myocardium. 18F-FDG PET is an established, non-invasive method of simultaneously imaging myocardial perfusion and metabolism through the use of positron-emitting isotopes.74 Regional reperfusion is assessed by using an isotope that remains in the vascular space, such as nitrogen-13 ammonia or rubidium-82, to demonstrate blood flow distribution.75 Enhanced FDG uptake in regions of decreased blood flow (“PET mismatch”) represents VD myocardium, while a reduction in both FDG uptake and blood flow (“PET match”) represents primarily necrotic myocardium. Therefore, PET imaging can differentiate among normal, VD, and necrotic myocardium. Compared to dobumatine echocardiography, 18F-FDG PET has improved sensitivity and negative predictive value in detecting VD myocardium.76 The detection of VD myocardium by 18F-FDG PET is important in the management of patients with heart failure, as regions designated as VD myocardium by PET have an 80–85% chance of functional improvement following coronary revascularization, while regions designated as necrotic myocardium have only a 20% chance of improvement following revascularization.77–79
Several radiolabeled FA compounds are also under clinical investigation for use in myocardial viability imaging. FAs used in myocardial viability imaging are commonly labeled with the single-photon emitter, 123I, or more rarely with the position-emitting radionuclide, 11C, and require structural modification to prolong myocardial clearance of the tracer. For example, 123I-iodophenylpentadecanoic acid (IPPA) is a radiolabelled straight chain FA with a phenyl group substituted at the ω-carbon, which stabilizes the iodine on the molecule to inhibit β-oxidation at its terminal end.80 Under non-ischemic conditions, IPPA is rapidly metabolized, while under ischemic conditions, myocardial fatty acid metabolism is suppressed, resulting in longer myocardial retention of IPPA and a “redistribution pattern” on serial single-photon emission computer tomography (SPECT) imaging.81, 82 In a prospective, multicenter trial to evaluate the use of 123I-IPPA SPECT for the detection of VD myocardium, 119 patients underwent 123I-IPPA SPECT imaging and blood-pool radionuclide angiography before CABG.83 Radionuclide angiography was also repeated 6–8 weeks after CABG. Although the number of IPPA-viable myocardial segments was the most significant predictor of improved ejection fraction, the overall sensitivity and specificity of IPPA was low (48% and 79%, respectively). Methyl-branched analogs of IPPA, such as 123I-(p-iodopheynl)-3-(R,S)-methylpentadecanoic acid (BMIPP), demonstrate superior protection from β-oxidation.84, 85 This modification slows down the rapid dynamics of FA metabolism to allow for more accurate SPECT imaging. BMIPP has been well studied in patients with ischemic heart disease, and is currently the only radiolabelled FA available for clinical use in some parts of the world, particularly Japan.
In addition to radiolabelled FA compounds, 11C-acetate is a radiolabelled 2-carbon compound that is rapidly metabolized to 11C-acetyl-CoA in the mitochondria, where it enters the TCA cycle and undergoes oxidative phosphorylation to yield 11CO2 and H2O.86 The myocardial uptake rate of 11C-acetete is proportional to myocardial blood flow while the rate of clearance of 11C-acetate from the myocyte reflects the rate of myocardial oxygen consumption (MVO2).87 Therefore, the uptake and turnover of 11C-acetate as assessed by PET can potentially be used as a single-tracer technique to yield quantitative data on both flow and metabolism, respectively. Despite significant progress in our understanding of energy metabolism in VD myocardium, several important questions remain unanswered. First, the debate exists about the adaptive vs. maladaptive role for metabolic remodeling in the ischemic VD hearts. Recently, Stride et al66 described mitochondria isolated from VD human hearts as “dysfunctional,” citing diminished mitochondrial oxidative capacity, increased reactive oxygen species (ROS) production, and reduced antioxidant defenses. Others have characterized the suppression of mitochondrial function as an adaptive mechanism, based on preservation of multiple energetic indices despite the overall reduction in metabolic rate.48, 64, 67, 68, 88 These include preserved phosphocreatine (PCr)-to-ATP ratio,67, 68, 88, maintenance of the ATP-to-ADP ratio,88 and a slower rate of ATP depletion,88 suggestive of energetic efficiency of the VD heart. Finally, despite reduced mitochondrial function and lower O2 consumption at rest, VD myocardium was able to increase its metabolism and utilize diverse energy substrates in response to an increased workload without the development of ischemia.64, 65, 89, 90 Thus, the cellular energetics remains robust in VD myocardium despite the suppression of mitochondrial oxidative phosphorylation, which may be an adaptive response that prevents a supply-demand imbalance in the setting of limited coronary flow reserve.
The question of how suppression of energy metabolism is achieved during the transition towards myocardial dysfunction in heart failure still remains unanswered, with no molecule or pathway emerging as a possible master regulator of energy metabolism in VD myocytes. Hu et al showed that in addition to VD segments, energy homeostasis is also suppressed in remote zones with no evidence of ischemia or reduction in contractility,88 suggesting the involvement of a circulating humoral factor. However, Stride et al66 detected significant differences in mitochondrial oxidative phosphorylation between functional and dysfunctional regions of the same human heart. Thus, the question remains unresolved and additional mechanistic studies are needed.
Cytoprotective and stress-response pathways
Fully-differentiated cardiomyocytes have reduced capacity for regeneration, and death of myocytes is linked to various forms of cardiac pathology. Thus, the ability of cardiomyocytes to survive cytotoxic insults is indispensable for maintenance of the heart’s health. Most of the early studies examining survival pathways in ischemic VD cardiomyocytes focused on the role of heat shock protein 70 (HSP70) in the heart,54, 91, 92 while newer studies employed unbiased genomic and proteomic approaches to identify additional stress-response and cytoprotective genes in VD myocardium.93 These genes included a powerful suppressor of caspases, inhibitor of apoptosis (IAP), stress-response genes HSP27 and HSP70, as well as a novel growth-promoting H11 kinase (H11K). The findings were further validated in human heart failure with PET-confirmed VD cardiac segments. Both, mRNA and protein levels of IAP, HSP70 and H11K were significantly upregulated in VD human myocardium, compared to the remote normal tissue.72
While the role of IAP and HSP27/HSP70 in preservation of cell viability was previously established,94–96 the function of H11K in VD myocardium remains unknown. H11K was linked to various forms of cancer,97, 98 and cardiac-specific overexpression of H11K in the mouse resulted in significant hypertrophy, with increased cardiac cell diameter and volume.99 It has been suggested that H11K may promote myocyte viability through an increase in glucose uptake. Consistently, a 3-fold increase in GLUT1 expression and accumulation of glycogen were found in the hearts of H11K-transgenic mice, mimicking the changes observed in VD myocardium.100 Yet, the causative role for the H11K pathway in induction and maintenance of VD myocardium has not been definitively established.
Other mechanisms
Differences in several additional pathways and molecules have been noted in VD myocardium, but little progress has been made in elucidating their role in a failing heart. For example, the levels of the pro-inflammatory cytokine tumor necrosis factor-α (TNFα) were elevated by more than 13-fold in human ischemic VD myocardium.101 Moreover, high TNFα levels were found in non-ischemic failing hearts prior to LVAD implantation, and a reduction in TNFα was observed after mechanical unloading.102 Importantly, accumulation of TNFα in the heart was previously shown to suppress contractility and damage cardiac function.103
Additionally, the levels of inducible nitric oxide synthase (iNOS, also known as NOS2) which generates an important modulator of heart function, nitric oxide (NO), were significantly upregulated in two independent studies of human patients with ischemic VD myocardium.101, 104 NOS2 expression has also co-localized with that of cyclooxygenase-2 (COX2), the enzyme which generates superoxide anion.104 Since the reaction of superoxide with NO produces a very strong oxidant peroxynitrite, physical proximity of the two enzymes may significantly boost oxidative stress and damage cardiomyocyte structural components. Consistently, elevated levels of nitrotyrosine, a marker of peroxynitrite formation, were found in VD sections of the heart and co-localized with COX2 and NOS2 expression.104 Yet, the role of peroxynitrite in the pathology of VD myocardium remains to be determined.
The regional adaptations of VD myocardium also include myocyte loss via an apoptotic mechanism. In swine with chronic LAD occlusion, Lim et al. demonstrated that VD myocardium was associated with myocyte apoptosis, leading to compensatory regional cellular hypertrophy independent of replacement fibrosis.105 This may represent a common mechanism through which repetitive ischemia and elevations in left ventricular filling pressure lead to a similar molecular phenotype in VD myocardium independent of changes in coronary perfusion.106, 107 Although clinical studies regarding the importance of myocyte apoptosis in VD myocardium have yielded mixed conclusions, these differences may be explained by the low overall frequency of apoptosis and time-dependent variations in the magnitude of apoptosis during the development of VD myocardium.108, 109
Autophagy was also upregulated in VD myocardium after the third cycle of repetitive ischemia-reperfusion bouts in pigs. Importantly, induction of autophagy correlated temporally with a decrease in apoptosis, and thus was suggested to play a protective role in the VD heart.110 In a mouse model of chronic ischemia through VEGF sequestration, autophagy was also identified as a key prosurvival mechanism.111
Finally, the neurohormonal response was altered in VD myocardium, as partial sympathetic denervation of VD myocytes and changes in the expression of α- and β-adrenergic receptors were reported in both ischemic and non-ischemic heart failure.112–114
Therapies for VD myocardium
Patients with VD myocardium demonstrate time-dependent functional deterioration due to progressive structural degeneration and worsening left ventricular function. Revascularization, either by percutaneous transluminal angioplasty or CABG, has traditionally been demonstrated to reverse depressed LV contractility, improve LV function, and reduce long-term mortality in patients with VD myocardium.115 However, revascularization may be delayed in patients with poor status or certain comorbidities, and can be complicated by the incidence of morbidity or restenosis. Furthermore, the recent results of three prospective randomized trials, the Surgical Treatment for Ischemic Heart Failure (STICH) trial, Heart Failure Revascularization Trial (HEART), and PET And Recovery following Revascularization (PARR-2) trial, found no mortality benefit from revascularization compared to optimal medical therapy and from viability testing, although each of these trials had significant methodological limitations.116–118 These findings highlight the need for additional or alternative pharmacological interventions in VD myocardium.119
Several medical therapeutics currently used in the treatment of heart failure have also been demonstrated to be beneficial in the treatment of VD myocardium. In ischemic heart failure, beta-blockers may improve VD myocardial function by reducing myocardial oxygen consumption and slowing the heart rate, leading to increased diastolic filling time. The clinical benefits of carvedilol on ventricular function in VD myocardium were examined in the randomized controlled Carvedilol Hibernation Reversible Ischemia Trial: Marker of Success (CHRISTMAS) study, in which 397 patients with chronic heart failure caused by ischemic LV systolic dysfunction were randomized to carvedilol or placebo for 6 months and designated as hibernators or non-hibernators after echocardiographic assessment and myocardial perfusion imaging at rest and during exercise.120 Although carvediolol increased LVEF compared to placebo, no significant difference was observed between hibernators and non-hibernators with regard to mean increase in LVEF. However, a linear relationship was observed between the increase in LVEF with carvediolol and the volume of myocardium affected by hibernation or hibernation/ischemia, suggesting that the beneficial effects of carvedilol may be mediated in part through improved function of VD myocardium. In addition to its vasodilatory effects and suppression of cardiac contractility, carvedilol is known to affect multiple intracellular pathways that are disrupted in failing cardiomyocytes.121 Thus, the reversibility of heart failure with carvedilol is a proof-of-principle that normalization of heart function in the setting of VD myocardium may be achieved without surgical intervention.
Angiotensin I converting enzyme (ACE) inhibitors have also been demonstrated to attenuate VD myocardium. During acute coronary artery occlusion, angiotensin I converting enzyme (ACE) is elevated, leading to increased production of the potent vasoconstrictor and positive inotropic agent, angiotensin II, and increased breakdown of bradykinin.122 Thus, increased ACE activity during coronary artery occlusion aggravates myocardial stunning, leading to VD myocardium. Several investigators have reported attenuation of myocardial stunning and improved recovery of contractile function by different ACE inhibitors administered before occlusion or immediately before reperfusion.123, 124 Ehring et al. studied the role of bradykinin in the beneficial effects of ramiprillat in open-chest dogs subjected to occlusion of the left circumflex coronary artery with subsequent reperfusion.125 The effect of ramiprilat on the recovery of post-ischemic wall thickening was shown to be through the bradykinin-mediated synthesis of prostacyclins, demonstrating the cardio-protective effects of ACE inhibitors on VD myocardial function.
Cardiac resynchronization therapy (CRT) is used for selected patients with advanced heart failure, improving heart failure symptoms, exercise capacity, and LV function.126 However, the effects of CRT vary significantly among individuals, and the observation that 20–30% of patients do not respond to CRT has resulted in a search for accurate predictors of response to this treatment.127 In a study of 51 patients with ischemic heart failure and LV dyssynchrony undergoing CRT, the response to CRT was directly related to the extent of viable myocardium and inversely related to the extent of scar tissue.128 Therefore, evaluation for VD myocardium and scar tissue may be considered in the patient selection process for CRT.
Several novel approaches are currently being sought in the treatment of VD myocardium. Neovascularization may be achieved through growth factors such as endothelial nitric oxide synthase (eNOS), fibroblast growth factor (FGF), and angiopoietin-1. eNOS is involved in the regulation of vascular tone and homeostasis by activating endothelial proliferation and inhibiting smooth muscle cell proliferation.129, 130 In a swine model of VD myocardium, regional retroinfusion of eNOS cDNA induced neovascularization via capillary proliferation and collateral vessel growth, resulting in improved functional reserve of VD myocardium.131 In addition, cardiomyocytes produce several different isoforms of FGF, and FGF-5 is a proto-oncogene known to promote angiogenesis in VD myocardium.132 Intracoronary injection of an adenoviral construct overexpressing FGF-5 has also been demonstrated to improve the function of VD myocardium in swine by stimulating myocyte number and hypertrophy.133, 134 Finally, angiopoietin-1, first identified as the ligand for endothelial-specific TIE-2 tyrosine receptor kinase, promotes endothelial cell sprouting and survival.135 In a swine model of chronic myocardial ischemia, injection of human angiopoietin-1 into the free wall of the compromised ventricle induced arteriogenesis with enhanced regional perfusion of VD myocardium.136 These studies suggest that neovascularization through therapeutic angiogenesis may have the potential to complement traditional revascularization techniques in the future.
In addition to neovascularization, stem cell therapy has been proposed as a novel therapeutic option for patients with VD myocardium. Mesenchymal stem cells (MSCs) have been shown to repair several injured tissues, and improve LV function in animal models of myocardial infarction.137, 138 In a swine model of chronic VD myocardium, intracoronary administration of mesenchymal stem cells improved function of the VD myocardium independent of coronary flow by stimulating myocyte proliferation and reducing cellular hypertrophy.139 Such a regression of cellular hypertrophy coupled with MSC-mediated myocyte proliferation may potentially restore many of the contractile and metabolic alterations in VD myocardium. Recent clinical trials, such as the Transendocardial Autologous Cells in Ischemic Heart Failure Trial (TAC-HFT) and Cardiopoietic stem Cell therapy in heart failURE (C-CURE), have suggested that stem cell therapy may be a safe and effective option for the future treatment of heart failure, and potentially, VD myocardium.140, 141
Limitations and unresolved questions
The existence of VD myocardium has been convincingly shown in humans and in an array of animal models. As a result, molecular changes that take place in VD myocardium, including alterations in cardiomyocyte architecture, contractile myofibrils, calcium signaling, energy metabolism and cytoprotective pathways, have been described. Many of these changes are similar in heart failure due to ischemic and non-ischemic etiologies, suggesting that common mechanisms may be responsible for the depression in myocardial function. Reversal of several of these molecular pathways with mechanical unloading and correlation with improvements in cardiac function suggests that they may be amenable to pharmacologic intervention and should be evaluated as potential targets for the treatment of heart failure. Yet, the development of specific therapies is hindered by the lack of mechanistic studies that delineate primary causative factors in the development of myocardial dysfunction with preserved myocyte viability. Moreover, technical differences in inducing VD myocardium in large animal models are likely to be responsible for discordance in many reported findings. However, despite these challenges in studying and targeting the changes occurring during the myocardial transition into dysfunction, the last decade has brought significant advances in our understanding of the molecular underpinnings of the VD heart. In the future, a systems biology approach to understanding VD myocardium would allow for an unbiased, integrative analysis of all the pathways impaired in this pathology, as opposed to focusing on the contributions of single molecular mechanisms.
Conclusions
VD myocardium is a well-established phenomenon. A large number of molecular changes have been reported for VD myocardium in humans, large animals and mouse models. These include disruption of myocyte architecture, characterized by loss of myofibrils, glycogen accumulation, and changes in organelle morphology; altered expression and/or post-translational modifications of contractile proteins; alterations in calcium signaling and excitation-contraction coupling; suppression of mitochondrial oxidative phosphorylation with preservation of high energy phosphates and ATP/ADP ratio; as well as induction of cytoprotective genes. Altogether, a VD myocyte appears to require less energy to maintain its viability, likely as a protective response that counteracts the disrupting effects of perfusion mismatch in the setting of reduced coronary reserve. These abnormalities span both ischemic and non-ischemic heart failure etiologies. Neovascularization, stem cell therapy, and advancements in surgical revascularization are promising areas of research that provide hope for improved clinical outcomes in patients with VD myocardium. Gaining further insights into the molecular mechanisms responsible for the changes in VD myocardium, and subsequently targeting specific therapies individualized to such abnormalities, will allow for the development of additional cardioprotective treatments aimed at the restoration of myocardial functionality in patients with heart failure.
Acknowledgments
Sources of Funding
Dr. Ardehali is supported by the National Institutes of Health Grants (K02 HL107448, R01 HL104181, and 1P01 HL108795). Dr. Butler is supported by grants from National Institutes of Health, European Union, Health Resource Services Administration, Food and Drug Administration.
Disclosures
Dr. Ardehali is a Consultant for Takeda and Cubist Pharmaceuticals. Dr. Butler is a Consultant for Amgen, Bayer, Celladon, Gambro, GE Healthcare, Janssen, Medtronic, Novartis, Ono, Relypsa, Trevena, and has stock Options with Stemedica. Dr. Gheorghiade has relationship with the following companies: Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma AG, Cardiorentis Ltd, CorThera, Cytokinetics, CytoPherx, Inc, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Intersection Medical, INC, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Ono Parmaceuticals USA, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT,Takeda Pharmaceuticals North America, Inc and Trevena Therapeutics; and has received signficant (> $10,000) support from Bayer Schering Pharma AG, DebioPharm S.A., Medtronic,Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT and Takeda Pharmaceuticals North America, Inc.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norton C, Georgiopoulou VV, Kalogeropoulos AP, Butler J. Epidemiology and cost of advanced heart failure. Prog Cardiovasc Dis. 2011;54:78–85. doi: 10.1016/j.pcad.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Fath-Ordoubadi F, Pagano D, Marinho NV, Keogh BE, Bonser RS, Camici PG. Coronary revascularization in the treatment of moderate and severe postischemic left ventricular dysfunction. Am J Cardiol. 1998;82:26–31. doi: 10.1016/s0002-9149(98)00241-0. [DOI] [PubMed] [Google Scholar]
- 4.vom Dahl J, Eitzman DT, al-Aouar ZR, Kanter HL, Hicks RJ, Deeb GM, Kirsh MM, Schwaiger M. Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation. 1994;90:2356–2366. doi: 10.1161/01.cir.90.5.2356. [DOI] [PubMed] [Google Scholar]
- 5.Drakos SG, Wever-Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: Insights into cardiac recovery. J Am Coll Cardiol. 2013;61:1985–1994. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandel M, Weng Y, Siniawski H, Potapov E, Krabatsch T, Lehmkuhl HB, Drews T, Knosalla C, Hetzer R. Pre-explant stability of unloading-promoted cardiac improvement predicts outcome after weaning from ventricular assist devices. Circulation. 2012;126:S9–S19. doi: 10.1161/CIRCULATIONAHA.111.084640. [DOI] [PubMed] [Google Scholar]
- 7.Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, Lehmkuhl HB, Knosalla C, Hetzer R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: Criteria for weaning from ventricular assist devices. Eur Heart J. 2011;32:1148–1160. doi: 10.1093/eurheartj/ehq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 9.Shivalkar B, Flameng W, Szilard M, Pislaru S, Borgers M, Vanhaecke J. Repeated stunning precedes myocardial hibernation in progressive multiple coronary artery obstruction. J Am Coll Cardiol. 1999;34:2126–2136. doi: 10.1016/s0735-1097(99)00467-2. [DOI] [PubMed] [Google Scholar]
- 10.Fallavollita JA, Canty JM., Jr Differential 18f-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: Evidence for chronic stunning in pigs. Circulation. 1999;99:2798–2805. doi: 10.1161/01.cir.99.21.2798. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF. Persistent stunning induces myocardial hibernation and protection: Flow/function and metabolic mechanisms. Circ Res. 2003;92:1233–1239. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 12.Page BJ, Young RF, Suzuki G, Fallavollita JA, Canty JM., Jr The physiological significance of a coronary stenosis differentially affects contractility and mitochondrial function in viable chronically dysfunctional myocardium. Basic Res Cardiol. 2013;108:354. doi: 10.1007/s00395-013-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyndrickx GR, Millard RW, McRitchie RJ, Maroko PR, Vatner SF. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975;56:978–985. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber BL, Wijns W, Vanoverschelde JL, Heyndrickx GR, De Bruyne B, Bartunek J, Melin JA. Myocardial perfusion and oxygen consumption in reperfused noninfarcted dysfunctional myocardium after unstable angina: Direct evidence for myocardial stunning in humans. J Am Coll Cardiol. 1999;34:1939–1946. doi: 10.1016/s0735-1097(99)00451-9. [DOI] [PubMed] [Google Scholar]
- 15.Rahimtoola SH. The hibernating myocardium in ischaemia and congestive heart failure. Eur Heart J. 1993;14(Suppl A):22–26. doi: 10.1093/eurheartj/14.suppl_a.22. [DOI] [PubMed] [Google Scholar]
- 16.Marban E. Myocardial stunning and hibernation. The physiology behind the colloquialisms. Circulation. 1991;83:681–688. doi: 10.1161/01.cir.83.2.681. [DOI] [PubMed] [Google Scholar]
- 17.Diamond GA, Forrester JS, deLuz PL, Wyatt HL, Swan HJ. Post-extrasystolic potentiation of ischemic myocardium by atrial stimulation. Am Heart J. 1978;95:204–209. doi: 10.1016/0002-8703(78)90464-7. [DOI] [PubMed] [Google Scholar]
- 18.Rahimtoola SH. A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation. 1985;72:V123–V135. [PubMed] [Google Scholar]
- 19.Knight C, Fox K. The vicious circle of ischemic left ventricular dysfunction. Am J Cardiol. 1995;75:10E–15E. doi: 10.1016/s0002-9149(99)80442-1. [DOI] [PubMed] [Google Scholar]
- 20.Schinkel AF, Poldermans D, Elhendy A, Bax JJ. Assessment of myocardial viability in patients with heart failure. J Nucl Med. 2007;48:1135–1146. doi: 10.2967/jnumed.106.038851. [DOI] [PubMed] [Google Scholar]
- 21.Vanoverschelde JL, Wijns W, Borgers M, Heyndrickx G, Depre C, Flameng W, Melin JA. Chronic myocardial hibernation in humans. From bedside to bench. Circulation. 1997;95:1961–1971. doi: 10.1161/01.cir.95.7.1961. [DOI] [PubMed] [Google Scholar]
- 22.Camici PG, Wijns W, Borgers M, De Silva R, Ferrari R, Knuuti J, Lammertsma AA, Liedtke AJ, Paternostro G, Vatner SF. Pathophysiological mechanisms of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium) Circulation. 1997;96:3205–3214. doi: 10.1161/01.cir.96.9.3205. [DOI] [PubMed] [Google Scholar]
- 23.Canty JM, Jr, Fallavollita JA. Resting myocardial flow in hibernating myocardium: Validating animal models of human pathophysiology. Am J Physiol. 1999;277:H417–422. doi: 10.1152/ajpheart.1999.277.1.H417. [DOI] [PubMed] [Google Scholar]
- 24.Vanoverschelde JL, Wijns W, Depre C, Essamri B, Heyndrickx GR, Borgers M, Bol A, Melin JA. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513–1523. doi: 10.1161/01.cir.87.5.1513. [DOI] [PubMed] [Google Scholar]
- 25.Ross J., Jr Myocardial perfusion-contraction matching. Implications for coronary heart disease and hibernation. Circulation. 1991;83:1076–1083. doi: 10.1161/01.cir.83.3.1076. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz ER, Schoendube FA, Kostin S, Schmiedtke N, Schulz G, Buell U, Messmer BJ, Morrison J, Hanrath P, vom Dahl J. Prolonged myocardial hibernation exacerbates cardiomyocyte degeneration and impairs recovery of function after revascularization. J Am Coll Cardiol. 1998;31:1018–1026. doi: 10.1016/s0735-1097(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 27.Sherman AJ, Klocke FJ, Decker RS, Decker ML, Kozlowski KA, Harris KR, Hedjbeli S, Yaroshenko Y, Nakamura S, Parker MA, Checchia PA, Evans DB. Myofibrillar disruption in hypocontractile myocardium showing perfusion-contraction matches and mismatches. Am J Physiol Heart Circ Physiol. 2000;278:H1320–H1334. doi: 10.1152/ajpheart.2000.278.4.H1320. [DOI] [PubMed] [Google Scholar]
- 28.Elsasser A, Schlepper M, Klovekorn WP, Cai WJ, Zimmermann R, Muller KD, Strasser R, Kostin S, Gagel C, Munkel B, Schaper W, Schaper J. Hibernating myocardium: An incomplete adaptation to ischemia. Circulation. 1997;96:2920–2931. doi: 10.1161/01.cir.96.9.2920. [DOI] [PubMed] [Google Scholar]
- 29.Laky D, Parascan L. Hibernating myocardium, morphological studies on intraoperatory myocardial biopsies and on chronic ischemia experimental model. Rom J Morphol Embryol. 2007;48:407–413. [PubMed] [Google Scholar]
- 30.Driesen RB, Verheyen FK, Debie W, Blaauw E, Babiker FA, Cornelussen RN, Ausma J, Lenders MH, Borgers M, Chaponnier C, Ramaekers FC. Re-expression of alpha skeletal actin as a marker for dedifferentiation in cardiac pathologies. J Cell Mol Med. 2009;13:896–908. doi: 10.1111/j.1582-4934.2008.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ausma J, Schaart G, Thone F, Shivalkar B, Flameng W, Depre C, Vanoverschelde JL, Ramaekers F, Borgers M. Chronic ischemic viable myocardium in man - aspects of dedifferentiation. Cardiovasc Pathol. 1995;4:29–37. doi: 10.1016/1054-8807(94)00028-p. [DOI] [PubMed] [Google Scholar]
- 32.Ausma J, Thone F, Dispersyn GD, Flameng W, Vanoverschelde JL, Ramaekers FC, Borgers M. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem. 1998;186:159–168. [PubMed] [Google Scholar]
- 33.Thijssen VLJL, Ausma J, Liu GS, Allessie MA, van Eys GJJM, Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol. 2000;9:17–28. doi: 10.1016/s1054-8807(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 34.Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. American Journal of Pathology. 1996;148:141–149. [PMC free article] [PubMed] [Google Scholar]
- 35.Mubagwa K, Kaplan P, Shivalkar B, Miserez M, Leunens V, Borgers M, Flameng W. Calcium uptake by the sarcoplasmic reticulum, high energy content and histological changes in ischemic cardiomyopathy. Cardiovascular Research. 1998;37:515–523. doi: 10.1016/s0008-6363(97)00279-4. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM., Jr Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res. 2002;91:970–977. doi: 10.1161/01.res.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- 37.Gunning MG, Kaprielian RR, Pepper J, Pennell DJ, Sheppard MN, Severs NJ, Fox KM, Underwood SR. The histology of viable and hibernating myocardium in relation to imaging characteristics. J Am Coll Cardiol. 2002;39:428–435. doi: 10.1016/s0735-1097(01)01766-1. [DOI] [PubMed] [Google Scholar]
- 38.Dispersyn GD, Geuens E, Ver Donck L, Ramaekers FC, Borgers M. Adult rabbit cardiomyocytes undergo hibernation-like dedifferentiation when co-cultured with cardiac fibroblasts. Cardiovasc Res. 2001;51:230–240. doi: 10.1016/s0008-6363(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 39.Driesen RB, Verheyen FK, Dispersyn GD, Thone F, Lenders MH, Ramaekers FC, Borgers M. Structural adaptation in adult rabbit ventricular myocytes: Influence of dynamic physical interaction with fibroblasts. Cell Biochem Biophys. 2006;44:119–128. doi: 10.1385/CBB:44:1:119. [DOI] [PubMed] [Google Scholar]
- 40.Thijssen VL, Borgers M, Lenders MH, Ramaekers FC, Suzuki G, Palka B, Fallavollita JA, Thomas SA, Canty JM., Jr Temporal and spatial variations in structural protein expression during the progression from stunned to hibernating myocardium. Circulation. 2004;110:3313–3321. doi: 10.1161/01.CIR.0000147826.13480.99. [DOI] [PubMed] [Google Scholar]
- 41.Hall JL, Birks EJ, Grindle S, Cullen ME, Barton PJ, Rider JE, Lee S, Harwalker S, Mariash A, Adhikari N, Charles NJ, Felkin LE, Polster S, George RS, Miller LW, Yacoub MH. Molecular signature of recovery following combination left ventricular assist device (lvad) support and pharmacologic therapy. Eur Heart J. 2007;28:613–627. doi: 10.1093/eurheartj/ehl365. [DOI] [PubMed] [Google Scholar]
- 42.Birks EJ, Hall JL, Barton PJ, Grindle S, Latif N, Hardy JP, Rider JE, Banner NR, Khaghani A, Miller LW, Yacoub MH. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112:I57–I64. doi: 10.1161/CIRCULATIONAHA.104.526137. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigue-Way A, Burkhoff D, Geesaman BJ, Golden S, Xu J, Pollman MJ, Donoghue M, Jeyaseelan R, Houser S, Breitbart RE, Marks A, Acton S. Sarcomeric genes involved in reverse remodeling of the heart during left ventricular assist device support. J Heart Lung Transplant. 2005;24:73–80. doi: 10.1016/j.healun.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Canty JM, Jr, Fallavollita JA. Lessons from experimental models of hibernating myocardium. Coron Artery Dis. 2001;12:371–380. doi: 10.1097/00019501-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Heusch G, Schulz R. The biology of myocardial hibernation. Trends Cardiovasc Med. 2000;10:108–114. doi: 10.1016/s1050-1738(00)00058-x. [DOI] [PubMed] [Google Scholar]
- 46.Birks EJ. Molecular changes after left ventricular assist device support for heart failure. Circ Res. 2013;113:777–791. doi: 10.1161/CIRCRESAHA.113.301413. [DOI] [PubMed] [Google Scholar]
- 47.Lilly LS. Pathophysiology of heart disease : A collaborative project of medical students and faculty. Baltimore, MD: Wolters Kluwer/Lippincott Williams & Wilkins; 2011. Harvard Medical School. [Google Scholar]
- 48.Mayr M, May D, Gordon O, Madhu B, Gilon D, Yin X, Xing Q, Drozdov I, Ainali C, Tsoka S, Xu Q, Griffiths J, Horrevoets A, Keshet E. Metabolic homeostasis is maintained in myocardial hibernation by adaptive changes in the transcriptome and proteome. J Mol Cell Cardiol. 2011;50:982–990. doi: 10.1016/j.yjmcc.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Chen L, Fallon JT, Ma L, Li L, Bow L, Knibbs D, McKay R, Gillam LD, Waters DD. Functional and structural alterations with 24-hour myocardial hibernation and recovery after reperfusion. A pig model of myocardial hibernation. Circulation. 1996;94:507–516. doi: 10.1161/01.cir.94.3.507. [DOI] [PubMed] [Google Scholar]
- 50.Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287:H2712–H2718. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- 51.Latif N, Yacoub MH, George R, Barton PJ, Birks EJ. Changes in sarcomeric and non-sarcomeric cytoskeletal proteins and focal adhesion molecules during clinical myocardial recovery after left ventricular assist device support. J Heart Lung Transplant. 2007;26:230–235. doi: 10.1016/j.healun.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Milting H, Scholz C, Arusoglu L, Freitag M, Cebulla R, Jaquet K, Korfer R, D VL, Kassner A, Brodde OE, Kogler H, El Banayosy A, Pieske B. Selective upregulation of beta1-adrenergic receptors and dephosphorylation of troponin i in end-stage heart failure patients supported by ventricular assist devices. J Mol Cell Cardiol. 2006;41:441–450. doi: 10.1016/j.yjmcc.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Heusch G, Rose J, Skyschally A, Post H, Schulz R. Calcium responsiveness in regional myocardial short-term hibernation and stunning in the in situ porcine heart. Inotropic responses to postextrasystolic potentiation and intracoronary calcium. Circulation. 1996;93:1556–1566. doi: 10.1161/01.cir.93.8.1556. [DOI] [PubMed] [Google Scholar]
- 54.Fallavollita JA, Jacob S, Young RF, Canty JM., Jr Regional alterations in sr ca(2+)-atpase, phospholamban, and hsp-70 expression in chronic hibernating myocardium. Am J Physiol. 1999;277:H1418–H1428. doi: 10.1152/ajpheart.1999.277.4.H1418. [DOI] [PubMed] [Google Scholar]
- 55.Nef HM, Mollmann H, Skwara W, Bolck B, Schwinger RH, Hamm C, Kostin S, Schaper J, Elsasser A. Reduced sarcoplasmic reticulum ca2+ -atpase activity and dephosphorylated phospholamban contribute to contractile dysfunction in human hibernating myocardium. Mol Cell Biochem. 2006;282:53–63. doi: 10.1007/s11010-006-1171-7. [DOI] [PubMed] [Google Scholar]
- 56.Heerdt PM, Klotz S, Burkhoff D. Cardiomyopathic etiology and serca2a reverse remodeling during mechanical support of the failing human heart. Anesth Analg. 2006;102:32–37. doi: 10.1213/01.ane.0000183642.09435.ad. [DOI] [PubMed] [Google Scholar]
- 57.Bito V, Heinzel FR, Weidemann F, Dommke C, van der Velden J, Verbeken E, Claus P, Bijnens B, De Scheerder I, Stienen GJ, Sutherland GR, Sipido KR. Cellular mechanisms of contractile dysfunction in hibernating myocardium. Circ Res. 2004;94:794–801. doi: 10.1161/01.RES.0000124934.84048.DF. [DOI] [PubMed] [Google Scholar]
- 58.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 59.Terracciano CM, Harding SE, Adamson D, Koban M, Tansley P, Birks EJ, Barton PJ, Yacoub MH. Changes in sarcolemmal ca entry and sarcoplasmic reticulum ca content in ventricular myocytes from patients with end-stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J. 2003;24:1329–1339. doi: 10.1016/s0195-668x(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 60.Terracciano CM, Koban MU, Soppa GK, Siedlecka U, Lee J, Stagg MA, Yacoub MH. The role of the cardiac na+/ca2+ exchanger in reverse remodeling: Relevance for lvad-recovery. Ann N Y Acad Sci. 2007;1099:349–360. doi: 10.1196/annals.1387.061. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhary KW, Rossman EI, Piacentino V, 3rd, Kenessey A, Weber C, Gaughan JP, Ojamaa K, Klein I, Bers DM, Houser SR, Margulies KB. Altered myocardial ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol. 2004;44:837–845. doi: 10.1016/j.jacc.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 62.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004;110:982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 63.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: A review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fallavollita JA, Malm BJ, Canty JM., Jr Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res. 2003;92:48–55. doi: 10.1161/01.res.0000049104.57549.03. [DOI] [PubMed] [Google Scholar]
- 65.Kelly RF, Cabrera JA, Ziemba EA, Crampton M, Anderson LB, McFalls EO, Ward HB. Continued depression of maximal oxygen consumption and mitochondrial proteomic expression despite successful coronary artery bypass grafting in a swine model of hibernation. J Thorac Cardiovasc Surg. 2011;141:261–268. doi: 10.1016/j.jtcvs.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 66.Stride N, Larsen S, Hey-Mogensen M, Hansen CN, Prats C, Steinbruchel D, Kober L, Dela F. Impaired mitochondrial function in chronically ischemic human heart. Am J Physiol Heart Circ Physiol. 2013;304:H1407–H1414. doi: 10.1152/ajpheart.00991.2012. [DOI] [PubMed] [Google Scholar]
- 67.Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina L, Patel MS, Blumenthal KM, Fallavollita JA, Canty JM., Jr Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res. 2008;102:103–112. doi: 10.1161/CIRCRESAHA.107.155895. [DOI] [PubMed] [Google Scholar]
- 68.Bravo C, Kudej RK, Yuan C, Yoon S, Ge H, Park JY, Tian B, Stanley WC, Vatner SF, Vatner DE, Yan L. Metabolomic analysis of two different models of delayed preconditioning. J Mol Cell Cardiol. 2013;55:19–26. doi: 10.1016/j.yjmcc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Schindler TH, Prior JO, Sayre J, Dahlbom M, Huang SC, Schelbert HR. Blood flow, flow reserve, and glucose utilization in viable and nonviable myocardium in patients with ischemic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2013;40:532–541. doi: 10.1007/s00259-012-2311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maki M, Luotolahti M, Nuutila P, Iida H, Voipio-Pulkki LM, Ruotsalainen U, Haaparanta M, Solin O, Hartiala J, Harkonen R, Knuuti J. Glucose uptake in the chronically dysfunctional but viable myocardium. Circulation. 1996;93:1658–1666. doi: 10.1161/01.cir.93.9.1658. [DOI] [PubMed] [Google Scholar]
- 71.Fallavollita JA, Perry BJ, Canty JM., Jr 18f-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95:1900–1909. doi: 10.1161/01.cir.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 72.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, Dreyfus GD, Gaussin V, Pennell DJ, Vatner DE, Camici PG, Vatner SF. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res. 2004;95:433–440. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 73.McFalls EO, Hou M, Bache RJ, Best A, Marx D, Sikora J, Ward HB. Activation of p38 mapk and increased glucose transport in chronic hibernating swine myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1328–H1334. doi: 10.1152/ajpheart.01188.2003. [DOI] [PubMed] [Google Scholar]
- 74.Schelbert HR. Metabolic imaging to assess myocardial viability. J Nucl Med. 1994;35:8S–14S. [PubMed] [Google Scholar]
- 75.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using n-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 76.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26:147–186. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]
- 77.Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, Schelbert H. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 78.Marwick TH, MacIntyre WJ, Lafont A, Nemec JJ, Salcedo EE. Metabolic responses of hibernating and infarcted myocardium to revascularization. A follow-up study of regional perfusion, function, and metabolism. Circulation. 1992;85:1347–1353. doi: 10.1161/01.cir.85.4.1347. [DOI] [PubMed] [Google Scholar]
- 79.Tamaki N, Kawamoto M, Tadamura E, Magata Y, Yonekura Y, Nohara R, Sasayama S, Nishimura K, Ban T, Konishi J. Prediction of reversible ischemia after revascularization. Perfusion and metabolic studies with positron emission tomography. Circulation. 1995;91:1697–1705. doi: 10.1161/01.cir.91.6.1697. [DOI] [PubMed] [Google Scholar]
- 80.Machulla HJ, Marsmann M, Dutschka K. Biochemical concept and synthesis of a radioiodinated phenylfatty acid for in vivo metabolic studies of the myocardium. European journal of nuclear medicine. 1980;5:171–173. doi: 10.1007/BF00252480. [DOI] [PubMed] [Google Scholar]
- 81.Reske SN, Biersack HJ, Lackner K, Machulla HJ, Knopp R, Hahn N, Winkler C. Assessment of regional myocardial uptake and metabolism of omega-(p-123i-phenyl) pentadecanoic acid with serial single-photon emission tomography. Nuklearmedizin. Nuclear medicine. 1982;21:249–253. [PubMed] [Google Scholar]
- 82.Yang JY, Ruiz M, Calnon DA, Watson DD, Beller GA, Glover DK. Assessment of myocardial viability using 123i-labeled iodophenylpentadecanoic acid at sustained low flow or after acute infarction and reperfusion. J Nucl Med. 1999;40:821–828. [PubMed] [Google Scholar]
- 83.Verani MS, Taillefer R, Iskandrian AE, Mahmarian JJ, He ZX, Orlandi C. 123i-ippa spect for the prediction of enhanced left ventricular function after coronary bypass graft surgery. Multicenter ippa viability trial investigators. 123i-iodophenylpentadecanoic acid. J Nucl Med. 2000;41:1299–1307. [PubMed] [Google Scholar]
- 84.Tamaki N, Tadamura E, Kawamoto M, Magata Y, Yonekura Y, Fujibayashi Y, Nohara R, Sasayama S, Konishi J. Decreased uptake of iodinated branched fatty acid analog indicates metabolic alterations in ischemic myocardium. J Nucl Med. 1995;36:1974–1980. [PubMed] [Google Scholar]
- 85.Knapp FF, Jr, Goodman MM, Callahan AP, Kirsch G. Radioiodinated 15-(p-iodophenyl)-3,3-dimethylpentadecanoic acid: A useful new agent to evaluate myocardial fatty acid uptake. J Nucl Med. 1986;27:521–531. [PubMed] [Google Scholar]
- 86.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. The clinical use of pet with (11)c-acetate. American journal of nuclear medicine and molecular imaging. 2012;2:33–47. [PMC free article] [PubMed] [Google Scholar]
- 87.Brown MA, Myears DW, Bergmann SR. Validity of estimates of myocardial oxidative metabolism with carbon-11 acetate and positron emission tomography despite altered patterns of substrate utilization. J Nucl Med. 1989;30:187–193. [PubMed] [Google Scholar]
- 88.Hu Q, Suzuki G, Young RF, Page BJ, Fallavollita JA, Canty JM., Jr Reductions in mitochondrial o(2) consumption and preservation of high-energy phosphate levels after simulated ischemia in chronic hibernating myocardium. Am J Physiol Heart Circ Physiol. 2009;297:H223–H232. doi: 10.1152/ajpheart.00992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiggers H, Norrelund H, Nielsen SS, Andersen NH, Nielsen-Kudsk JE, Christiansen JS, Nielsen TT, Moller N, Botker HE. Influence of insulin and free fatty acids on contractile function in patients with chronically stunned and hibernating myocardium. Am J Physiol Heart Circ Physiol. 2005;289:H938–H946. doi: 10.1152/ajpheart.00150.2005. [DOI] [PubMed] [Google Scholar]
- 90.McFalls EO, Kelly RF, Hu Q, Mansoor A, Lee J, Kuskowski M, Sikora J, Ward HB, Zhang J. The energetic state within hibernating myocardium is normal during dobutamine despite inhibition of atp-dependent potassium channel opening with glibenclamide. Am J Physiol Heart Circ Physiol. 2007;293:H2945–H2951. doi: 10.1152/ajpheart.00012.2007. [DOI] [PubMed] [Google Scholar]
- 91.Thomas SA, Fallavollita JA, Lee TC, Feng J, Canty JM., Jr Absence of troponin i degradation or altered sarcoplasmic reticulum uptake protein expression after reversible ischemia in swine. Circ Res. 1999;85:446–456. doi: 10.1161/01.res.85.5.446. [DOI] [PubMed] [Google Scholar]
- 92.Meissner A, Luss I, Rolf N, Boknik P, Kirchhefer U, Kehm V, Knapp J, Linck B, Luss H, Muller FU, Weber T, Schmitz W, Van Aken H, Neumann J. The early response genes c-jun and hsp-70 are induced in regional cardiac stunning in conscious mammals. J Thorac Cardiovasc Surg. 2000;119:820–825. doi: 10.1016/S0022-5223(00)70019-5. [DOI] [PubMed] [Google Scholar]
- 93.Depre C, Tomlinson JE, Kudej RK, Gaussin V, Thompson E, Kim SJ, Vatner DE, Topper JN, Vatner SF. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. Proc Natl Acad Sci U S A. 2001;98:9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holly TA, Drincic A, Byun Y, Nakamura S, Harris K, Klocke FJ, Cryns VL. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 95.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 96.Radford NB, Fina M, Benjamin IJ, Moreadith RW, Graves KH, Zhao P, Gavva S, Wiethoff A, Sherry AD, Malloy CR, Williams RS. Cardioprotective effects of 70-kda heat shock protein in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:2339–2342. doi: 10.1073/pnas.93.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith CC, Yu YX, Kulka M, Aurelian L. A novel human gene similar to the protein kinase (pk) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (icp10) codes for a serine-threonine pk and is expressed in melanoma cells. J Biol Chem. 2000;275:25690–25699. doi: 10.1074/jbc.M002140200. [DOI] [PubMed] [Google Scholar]
- 98.Charpentier AH, Bednarek AK, Daniel RL, Hawkins KA, Laflin KJ, Gaddis S, MacLeod MC, Aldaz CM. Effects of estrogen on global gene expression: Identification of novel targets of estrogen action. Cancer Res. 2000;60:5977–5983. [PubMed] [Google Scholar]
- 99.Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu X, Kudej RK, Wagner T, Sadoshima J, Vatner SF. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res. 2002;91:1007–1014. doi: 10.1161/01.res.0000044380.54893.4b. [DOI] [PubMed] [Google Scholar]
- 100.Wang L, Zajac A, Hedhli N, Depre C. Increased expression of h11 kinase stimulates glycogen synthesis in the heart. Mol Cell Biochem. 2004;265:71–78. doi: 10.1023/b:mcbi.0000044311.58653.54. [DOI] [PubMed] [Google Scholar]
- 101.Kalra DK, Zhu X, Ramchandani MK, Lawrie G, Reardon MJ, Lee-Jackson D, Winters WL, Sivasubramanian N, Mann DL, Zoghbi WA. Increased myocardial gene expression of tumor necrosis factor-alpha and nitric oxide synthase-2: A potential mechanism for depressed myocardial function in hibernating myocardium in humans. Circulation. 2002;105:1537–1540. doi: 10.1161/01.cir.0000013846.72805.7e. [DOI] [PubMed] [Google Scholar]
- 102.Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon GP. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support : A potential mechanism for cardiac recovery. Circulation. 1999;100:1189–1193. doi: 10.1161/01.cir.100.11.1189. [DOI] [PubMed] [Google Scholar]
- 103.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 104.Baker CS, Dutka DP, Pagano D, Rimoldi O, Pitt M, Hall RJ, Polak JM, Bonser RS, Camici PG. Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol. 2002;97:409–415. doi: 10.1007/s003950200050. [DOI] [PubMed] [Google Scholar]
- 105.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation. 1999;100:2380–2386. doi: 10.1161/01.cir.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 106.Canty JM, Jr, Fallavollita JA. Hibernating myocardium. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2005;12:104–119. doi: 10.1016/j.nuclcard.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Canty JM, Jr, Suzuki G. Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol. 2012;52:822–831. doi: 10.1016/j.yjmcc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dispersyn GD, Ausma J, Thone F, Flameng W, Vanoverschelde JL, Allessie MA, Ramaekers FC, Borgers M. Cardiomyocyte remodelling during myocardial hibernation and atrial fibrillation: Prelude to apoptosis. Cardiovasc Res. 1999;43:947–957. doi: 10.1016/s0008-6363(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 109.Dispersyn GD, Borgers M, Flameng W. Apoptosis in chronic hibernating myocardium: Sleeping to death? Cardiovascular Research. 2000;45:696–703. doi: 10.1016/s0008-6363(99)00277-1. [DOI] [PubMed] [Google Scholar]
- 110.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.May D, Gilon D, Djonov V, Itin A, Lazarus A, Gordon O, Rosenberger C, Keshet E. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci U S A. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandez SF, Ovchinnikov V, Canty JM, Jr, Fallavollita JA. Hibernating myocardium results in partial sympathetic denervation and nerve sprouting. Am J Physiol Heart Circ Physiol. 2013;304:H318–H327. doi: 10.1152/ajpheart.00810.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shan K, Bick RJ, Poindexter BJ, Nagueh SF, Shimoni S, Verani MS, Keng F, Reardon MJ, Letsou GV, Howell JF, Zoghbi WA. Altered adrenergic receptor density in myocardial hibernation in humans: A possible mechanism of depressed myocardial function. Circulation. 2000;102:2599–2606. doi: 10.1161/01.cir.102.21.2599. [DOI] [PubMed] [Google Scholar]
- 114.Iyer VS, Canty JM., Jr Regional desensitization of beta-adrenergic receptor signaling in swine with chronic hibernating myocardium. Circ Res. 2005;97:789–795. doi: 10.1161/01.RES.0000184675.80217.9e. [DOI] [PubMed] [Google Scholar]
- 115.Di Carli MF, Asgarzadie F, Schelbert HR, Brunken RC, Laks H, Phelps ME, Maddahi J. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436–3444. doi: 10.1161/01.cir.92.12.3436. [DOI] [PubMed] [Google Scholar]
- 116.Cleland JG, Calvert M, Freemantle N, Arrow Y, Ball SG, Bonser RS, Chattopadhyay S, Norell MS, Pennell DJ, Senior R. The heart failure revascularisation trial (heart) Eur J Heart Fail. 2011;13:227–233. doi: 10.1093/eurjhf/hfq230. [DOI] [PubMed] [Google Scholar]
- 117.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, Gulenchyn KY, Garrard L, deKemp R, Guo A, Ruddy TD, Benard F, Lamy A, Iwanochko RM. Investigators P-. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (parr-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL, Investigators S. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heusch G, Schulz R. Characterization of hibernating and stunned myocardium. Eur Heart J. 1997;18(Suppl D):D102–D110. doi: 10.1093/eurheartj/18.suppl_d.102. [DOI] [PubMed] [Google Scholar]
- 120.Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, Mule JD, Vered Z, Lahiri A. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (christmas trial): Randomised controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 121.Cheng J, Kamiya K, Kodama I. Carvedilol: Molecular and cellular basis for its multifaceted therapeutic potential. Cardiovasc Drug Rev. 2001;19:152–171. doi: 10.1111/j.1527-3466.2001.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 122.Yang HY, Erdos EG, Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin i and inactivates bradykinin. Biochim Biophys Acta. 1970;214:374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- 123.Westlin W, Mullane K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation. 1988;77:I30–I39. [PubMed] [Google Scholar]
- 124.Przyklenk K, Kloner RA. Angiotensin converting enzyme inhibitors improve contractile function of stunned myocardium by different mechanisms of action. Am Heart J. 1991;121:1319–1330. doi: 10.1016/0002-8703(91)90134-4. [DOI] [PubMed] [Google Scholar]
- 125.Ehring T, Baumgart D, Krajcar M, Hummelgen M, Kompa S, Heusch G. Attenuation of myocardial stunning by the ace inhibitor ramiprilat through a signal cascade of bradykinin and prostaglandins but not nitric oxide. Circulation. 1994;90:1368–1385. doi: 10.1161/01.cir.90.3.1368. [DOI] [PubMed] [Google Scholar]
- 126.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 127.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 128.Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Bax JJ. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33–41. doi: 10.1093/eurheartj/ehl379. [DOI] [PubMed] [Google Scholar]
- 129.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kupatt C, Hinkel R, von Bruhl ML, Pohl T, Horstkotte J, Raake P, El Aouni C, Thein E, Dimmeler S, Feron O, Boekstegers P. Endothelial nitric oxide synthase overexpression provides a functionally relevant angiogenic switch in hibernating pig myocardium. J Am Coll Cardiol. 2007;49:1575–1584. doi: 10.1016/j.jacc.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 132.Vatner SF. Fgf induces hypertrophy and angiogenesis in hibernating myocardium. Circ Res. 2005;96:705–707. doi: 10.1161/01.RES.0000164184.63158.6c. [DOI] [PubMed] [Google Scholar]
- 133.Lynch P, Lee TC, Fallavollita JA, Canty JM, Jr, Suzuki G. Intracoronary administration of advfgf-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation. 2007;116:I71–I76. doi: 10.1161/CIRCULATIONAHA.106.681866. [DOI] [PubMed] [Google Scholar]
- 134.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of fgf-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res. 2005;96:767–775. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- 135.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 136.Shim WS, Li W, Zhang L, Li S, Ong HC, Song IC, Bapna A, Ge R, Lim YT, Chuah SC, Sim EK, Wong P. Angiopoietin-1 promotes functional neovascularization that relieves ischemia by improving regional reperfusion in a swine chronic myocardial ischemia model. Journal of biomedical science. 2006;13:579–591. doi: 10.1007/s11373-006-9082-x. [DOI] [PubMed] [Google Scholar]
- 137.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 138.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous mesenchymal stem cells mobilize ckit+ and cd133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 140.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA : the journal of the American Medical Association. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: The c-cure (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]