Abstract
Achondroplasia is the most common form of skeletal dysplasia, resulting in disproportionate short stature, and affects over 250,000 people worldwide. Individuals with achondroplasia demonstrate a number of well-recognized anatomical features that impact on growth and development, with a complex array of medical issues that are best managed through a multidisciplinary team approach. The complexity of this presentation, whereby individual impairments may impact upon multiple activity and participation areas, requires consideration and discussion under a broad framework to gain a more thorough understanding of the experience of this condition for individuals with achondroplasia. This paper examines the general literature and research evidence on the medical and health aspects of individuals with achondroplasia and presents a pictorial model of achondroplasia based on The International Classification of Functioning, Disability, and Health (ICF). An expanded model of the ICF will be used to review and present the current literature pertaining to the musculoskeletal, neurological, cardiorespiratory, and ear, nose, and throat impairments and complications across the lifespan, with discussion on the impact of these impairments upon activity and participation performance. Further research is required to fully identify factors influencing participation and to help develop strategies to address these factors.
Keywords: achondroplasia, complications, management, ICF model
Introduction
Achondroplasia is the most common form of nonlethal skeletal dysplasia, affecting more than 250,000 people worldwide.1,2 It is caused by a mutation in the gene that codes for fibroblast growth factor receptor 3 (FGFR3) and is transmitted as an autosomal dominant trait.1,3–5 The estimated prevalence is currently 0.36 to 0.6 per 10,000 live births (1/27,780–1/16,670 live births).6 The defining characteristics of achondroplasia are short stature with disproportionately shorter proximal limb bones, narrow trunk, and macrocephaly.1,7,8 There is contraction at the base of the skull with a prominent forehead and flattened midface region and short, broad hands with a trident appearance of the fingers.3,8,9
Disproportionate growth between endochondral bone and the underlying organs leads to a number of orthopedic, neurological, respiratory, ear, nose, and throat (ENT), and dental issues for individuals with achondroplasia.8–10 Whilst serious complications (such as sudden death due to severe compression of the spinal cord at the foramen magnum) impact on only 5%–10% of children,8,10 early monitoring and judicious medical and surgical interventions are important for reducing morbidity and mortality rates across the population.1,8,10–12 Knowledge regarding the type and timing of medical complications has led to international consensus for clinical management guidelines, documented originally in 1995 by the American Academy of Pediatrics, Committee on Genetics in the “Health supervision for children with achondroplasia” (HSCA) guidelines3 and revised by Trotter et al8 in 2005. These guidelines provide recommendations for examination and anticipatory management by multidisciplinary teams across the lifespan, with the aim of reducing and promptly treating complications. While it is likely that appropriate and coordinated management in childhood could reduce or minimize medical complications for adults with achondroplasia, there remain considerable gaps in knowledge regarding medical and social experiences of adults with forms of skeletal dysplasia, including achondroplasia.13,14
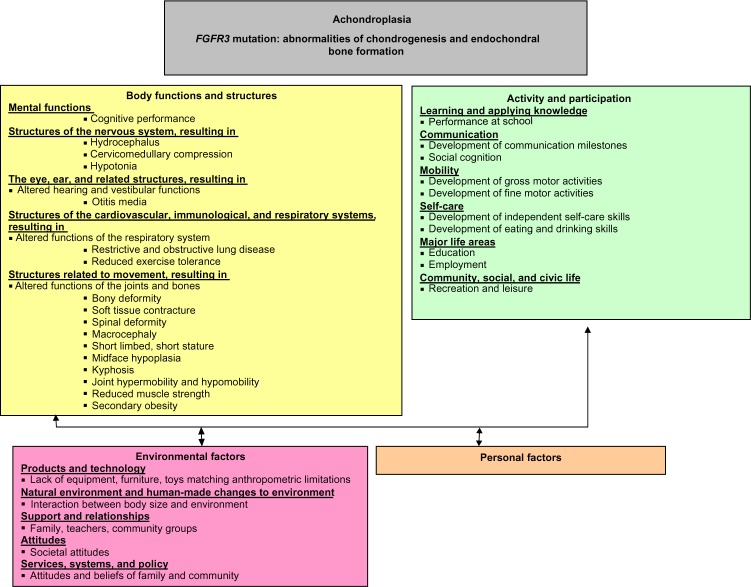
Expression of the defective FGFR3 gene in achondroplasia results in impairments of multiple body structures and functions. These impairments impact, both singularly and in combination, upon later development and performance. The complexity of this presentation, whereby individual impairments may impact upon multiple activity and participation areas, requires consideration under a broad framework to gain a thorough understanding of the complications experienced by individuals with achondroplasia. The International Classification of Functioning, Disability, and Health (ICF) published by the World Health Organization (WHO),15 is currently considered the international gold standard for describing and measuring functioning, disability, and health. Haga16 considered this framework when he developed the initial disablement model of achondroplasia. It is clear in Haga16 that considering the interplay of individual factors across the ICF framework is beneficial for clinical reasoning, guidance of assessment, and in discussing prognostic factors with families. We propose an expanded model (Figure 1) that provides more detail regarding the dynamic linkages between body structures and functions, and related activity capacity/limitations and participation performance/restrictions. This expanded model will be used as the framework for the assessment and interpretation of the literature reported upon in this review.
Figure 1.
ICF model of achondroplasia.
Abbreviations: FGFR3, fibroblast growth factor receptor 3; ICF, the International Classification of Functioning, Disability, and Health.
Musculoskeletal impairments and complications
As seen in Figure 1, there are a number of changes related to body structures and functions in individuals with achondroplasia, including the musculoskeletal, neurological, cardiorespiratory, and ENT systems that impact upon overall participation. These will be discussed in more detail in each of the following sections. The head of the infant with achondroplasia is larger than in other infants,1 potentially due to either a communicating hydrocephalus and/or dilated ventricles, which may be related to intracranial venous hypertension.17 Close monitoring of head circumference (HC) using achondroplasia-specific growth charts, and periodic head ultrasound in the first year of life is essential, so that children who demonstrate a rapid increase in head size, or symptoms of increased pressure, such as irritability or a bulging fontanelle, can be referred to a pediatric neurologist or neurosurgeon for examination and consideration of ventricular shunting.8 Macrocephaly has also been implicated in the development of a fixed, thoracolumbar kyphosis, which has led to the recommendation of restricted early sitting in this population.18–23
Children with achondroplasia demonstrate rhizomelic limb shortening with disproportionate intra-limb segmental ratios.24 Rhizomelia also alters the limb-to-trunk growth ratio. Disproportionate shortening of the upper limbs creates a situation where children are unable to reach the top of their head, the middle of their back, or the intergluteal region.25 The disproportionate limb-to-trunk ratio is greater during infancy and fingertip contact in an overhead reach is generally established by around 6 years of age. Individuals with achondroplasia also typically demonstrate a mixed pattern of joint mobility, including both joint contracture and/or joint hypermobility at characteristic joints. Infants and children consistently demonstrate limited elbow and hip extension ranges.26 Both children and adults with achondroplasia uniformly display hip flexion contractures that have been postulated to contribute to the well-recognized lumbosacral lordosis and may be a contributing factor to the back pain and muscle fatigue reported by individuals with achondroplasia.27 Hypermobility is typically observed in the knees and fingers of children with achondroplasia.1,16 This disproportionate limb shortening, joint contracture, and joint hypermobility were identified as possible factors contributing to the delayed development of independent self-care skills such as dressing and toileting identified by Ireland et al.28,29 These authors recommended that review by a physiotherapist or occupational therapist prior to school commencement could allow for targeted environmental modifications and equipment prescription to increase independence across self-care and mobility for the school-aged child with achondroplasia.
Another musculoskeletal change noted in the older child and adult is tibial bowing, and/or genu varum.1,7,8,30 Kopits25 noted that tibial bowing was observed by parents in 40% of children with achondroplasia as they began to stand, with a more rapid progression of the deformity occurring at 3–4 years and 6–7 years. Hunter et al10 reported that while approximately 10% of children demonstrate marked tibial bowing by 5 years, progression of this deformity through childhood leads to over 40% of adults being affected. Tibial bowing has been associated with recurrent periods of leg pain and discomfort. Chronic knee pain and changes in bony alignment can contribute to delays in weight-bearing, an altered gait pattern, and the potential for adult onset osteoarthritis associated with uneven weight distribution across the knee and ankle joints.1,9,31 For those individuals demonstrating pain, and/or an altered gait pattern, corrective surgery such as tibial osteotomies, fibula epiphysiodesis, or continuous distraction epiphysiodesis may be utilized.30,32,33 Hunter et al10 reported that 22% of individuals in their multicenter review had had osteotomies, most commonly between 12 and 20 years of age.
Thoracolumbar kyphosis is a major secondary musculoskeletal impairment thought to arise from the combined impact of other common primary musculoskeletal impairments, such as macrocephaly, hypotonia, and joint hypermobility. Flexible kyphotic curves have been reported in over 90% of infants with achondroplasia, although the majority of these curves spontaneously improve once ambulation commences. Progression to a fixed kyphotic deformity occurs in 15%–30% of adults.19,34,35 Progressive disruption of the vertebral epiphyseal ring that begins during childhood, combined with decreased growth in the anterior vertebral sections can create a progressive thoracolumbar kyphosis in adolescents, which is further exacerbated by age-related degenerative changes in the facet joints during adulthood.36 This curve is then thought to contribute to adult onset spinal stenosis.19,36,37 The kyphosis is thought to be exacerbated by early sitting where the effects of trunk hypotonia, increased head weight, and increased ligamentous laxity combine with the effect of gravity to force the child into a slumped sitting position, which can contribute to permanent spinal damage by increasing the anterior wedging of the vertebra and subsequent narrowing of the spinal canal.4,25,27,35,36,38,39 This knowledge has led to the recommendation that sitting be restricted until the infant with achondroplasia is able to independently achieve this transition. Furthermore, parents of infants with achondroplasia should avoid pieces of equipment that reinforce this characteristic kyphosis such as baby slings or umbrella strollers.35 When a kyphotic deformity is identified after commencement of independent ambulation, management may include education about positioning for nonfixed deformities, bracing,18,39,40 and/or operative correction, usually for progressive curves or anterior vertebral wedging associated with a fixed angular kyphosis.36,41 Pauli et al35 developed an algorithm for the monitoring and treatment of kyphosis in children, which they proposed could eliminate the neurological risks of angular kyphosis. However, the management of spinal kyphosis in achondroplasia is not consistent and requires further investigation to determine the optimal type and timing of management strategies.
Obesity is common in individuals with achondroplasia and is recognized as contributing to common medical problems for this group, including obstructive sleep apnea, genu varus, spinal stenosis, and hyperlordosis.42–44 Several authors have also identified a higher rate of cardiovascular disease-related death in adults with achondroplasia when compared with adults of average stature.11,45 While obesity is the most commonly recognized weight issue associated with this population, Hoover-Fong et al42 stressed the importance of screening for and recognizing early failure to gain weight in infants and young children with achondroplasia, particularly if the infant presents with a number of more serious medical complications, including restrictive lung disease and obstructive sleep apnea. Nutritional counseling (such as smaller meal portion sizes), should be implemented early to help reduce the later effects of obesity in adult life. The inclusion of appropriately selected forms of exercise is also necessary for maintenance of a healthy weight range and increased social inclusion. Current recommendations are that children with achondroplasia stay within one standard deviation of the average height–weight curve on the achondroplasia-specific height–weight charts.3,8,9,42–44,46 Appropriate weight for height is also thought to reduce back and knee pain and reduce the chance of knee joint injury.
Neurological impairments and complications
Cervical cord compression at the cervicomedullary junction is frequently observed radiographically in children with achondroplasia, but symptomatic cord compression is less common. Significant compression at the foramen magnum can lead to severe neurological complications, including sleep apnea, disordered respiration, myelopathy, hydrocephalus, and sudden infant death.21,47,48 There continues to be debate on the optimal assessment and type and timing of intervention for this problem. Some groups advocate for routine investigation of cervicomedullary compression by magnetic resonance imaging (MRI) or computer tomography (CT) studies in the infant with achondroplasia.8,49 These groups believe that the decision to intervene should not be based on traditional signs of neurological compression such as hypotonia, poor head control, and feeding issues, since these are common in children with achondroplasia.50 In contrast, other clinical groups postulate that problems related to cervicomedullary compression will resolve spontaneously during childhood without specific intervention as the foramen magnum grows relative to the size of the spinal cord.1,22 They recommend undertaking investigation and intervention only if clear clinical evidence is present. The role of sleep studies and MRI cerebrospinal fluid flow studies remains unclear in the overall assessment of whether a child with achondroplasia might require decompressive surgery.
A proportion of children with achondroplasia will go on to require cervicomedullary decompression (CMD). Review of the literature identifies that 6.7%–13.3% of infants and children required CMD within the first 2 years,12,51 rising to 6.8%–28% by 4 years.10,52 The varying rates of CMD are likely to be a result of the different assessment and management approaches outlined in the previous paragraph.
Although the optimal methods of screening for cervicomedullary compression continue to be debated, there is consensus that education regarding safe early positioning and handling is beneficial in reducing the risks associated with cervicomedullary compression within this cohort. Careful handling around the head and neck area in the young infant with achondroplasia and the avoidance of baby equipment such as umbrella strollers and carrying slings, which may allow sudden, uncontrolled head movements that could create sudden compression at the brainstem level, is now considered best clinical practice in this population group.8
Hydrocephalus, observed as ventriculomegaly and excessive extra-axial fluid, is another common complication resulting from disproportionate growth between the endochondral bone and surrounding structures. Restriction at the craniocervical junction can lead to increased venous pressure and altered cerebrospinal fluid dynamics, creating an increase in total head size.1 Frequent monitoring of the occipital-frontal circumference (OFC) is recommended during the first 12 months using specialized head circumference growth charts for children with achondroplasia.51,53 Referral to a neurosurgeon or neurologist is recommended if the OFC is above the 95th percentile and/or there has been a rapid change in head size on the achondroplasia-specific head circumference charts associated with symptoms of increased pressure, and/or there have been other clinical signs or symptoms of symptomatic hydrocephalus.1,8,9
Hypotonia is frequently observed in the infant and young child with achondroplasia. The precise cause remains unclear. Yang et al54 found histological changes in the spinal cord of two infants with achondroplasia, and suggested that hyperextension type injuries with spinal cord damage might occur during delivery. A second hypothesis is that impairments in the craniocervical junction anatomy, in particular the narrow foramen magnum, constrict long tracts of the spinal cord, resulting in impaired signaling.21–23 Reynolds et al20 found no correlation between the severity of hypotonia in infants with achondroplasia and the size of the foramen magnum. They hypothesized that the altered FGFR3 gene, expressed in the brain and nerve cells, caused a primary “brain-based” hypotonia. This hypotonia is thought to be a contributing factor to the delays reported in gross motor development for this group.28,55,56
Neurological symptoms associated with lumbar spinal stenosis are present in a significant proportion of children and adults with achondroplasia. A number of factors are thought to contribute to spinal stenosis, including reduced size of the spinal canal, disc protrusions, spondyloarthritic spurs, kyphotic wedging, excessive lumbar lordosis related to hip flexion contractures, and vertebral malalignment or instability.27,37,57,58 Early reports of neurological symptoms associated with spinal stenosis suggested that this was predominantly an adult onset condition.58 However, Hunter et al10 noted that while issues related to spinal stenosis increased with age (with approximately 80% of individuals having clinical signs and symptoms by 60 years), nearly 10% of children had neurological signs, including claudication and hyperreflexia, by 10 years of age. They highlighted that these symptoms can lead to significant limitations in physical functioning and quality of life for these individuals. As early as 1978, Siebens et al27 stressed the importance of reducing spinal canal disproportion through restriction of premature sitting, hip stretching whilst stabilizing the lumbar spine, and general education. As stated earlier, early best practice recommendations for this population now include caregiver education regarding avoidance of sitting to minimize potential complications and preserve function later in life. Surgery such as a decompressive laminectomy may be used to treat spinal canal stenosis in individuals with achondroplasia, although King et al51 noted that evaluation of both spinal stenosis and instability is necessary prior to surgical intervention.
Cardiorespiratory impairments and complications
Respiratory signs and symptoms occur frequently in individuals with achondroplasia,59 with a number of contributing mechanisms proposed: i) reduced chest circumference with altered mechanical function, ii) upper airway obstruction, and iii) cervicomedullary compression,54,59,60 or iv) a significant combination of these factors. Upper airway obstruction is common in children with achondroplasia; with 10%–85% of patients requiring treatment for issues related to obstructive sleep apnea and chronic respiratory insufficiency.1,60,61 In 1993, Waters et al60 further stressed that airway obstruction should be considered a significant part of achondroplasia rather than an infrequent complication and that full polysomnography (overnight sleep studies) are required to clearly demonstrate and define the abnormalities. Tasker et al62 observed a discrete group of children with persistent upper airway obstruction despite adenotonsillectomy, and reasoned that this was due to progressive hydrocephalus linked to foramen magnum stenosis. Management of respiratory and sleep disorders in this specific group required surgical management of the hydrocephalus combined with nocturnal positive airway pressure. More recently, Bagley et al50 supported the finding that compression of the respiratory centers by musculoskeletal impairments at the craniocervical junction can reduce the central respiratory drive, and also that compression of the lower motor neurons innervating the respiratory muscles may result in weak respiratory efforts.50
Tasker et al62 identified a final group of children, who presented with progressive respiratory problems with chronic cardiorespiratory failure and a complex clinical presentation involving gastroesophageal reflux, pulmonary small airway pathology, and both obstructive and central sleep apneas. These children required several different forms of treatment, including foramen magnum decompression. Respiratory compromise can contribute to daytime respiratory problems, sleep disturbances and fatigue, and may influence development in this group. As such, the HSCA guidelines recommend regular examination of sleep apnea via polysomnography to identify potential central nervous system causes that may need immediate surgical management.8
Exercise intolerance and exercise-induced fatigue is a common concern for children with achondroplasia. Takken et al63 found that exercise capacity for children with achondroplasia was significantly reduced when compared with reference values for the general population and that children with achondroplasia demonstrated reduced muscle strength in almost all muscle groups. They hypothesized that these changes may have been caused by a decrease in muscle mass, reduced neuromuscular coordination, or altered biomechanics. Exercise-induced fatigue may influence a number of participation areas, including self-care performance and leisure pursuits.
ENT impairments and complications
Persistent or recurrent otitis media is common in children with achondroplasia and may cause hearing impairment.8,10,64 Hunter et al10 identified that over 25% of children reported chronic recurrent otitis media and Tunkel et al65 observed that at least 25% also presented with hearing loss. Hunter et al10 linked chronic recurrent otitis media to midface hypoplasia, shortened Eustachian tubes, small pharynx, and relative enlargement of the tonsils and adenoids. Other factors suggested include impaired nasal airflow and temporal bone abnormalities.66 Consensus among clinicians is that recurrent otitis media in children with achondroplasia should be treated aggressively with adenotonsillectomy and insertion of ventilation tubes (grommets) to prevent conductive hearing loss.1 A recent study by Ireland et al67 found that insertion of grommets occurred frequently in children with achondroplasia <5 years of age, with over 50% of children undergoing this procedure over an 11-year period.
When poorly managed, the problem appears to persist into adulthood. McDonald et al68 evaluated the peripheral auditory system in 18 adults with achondroplasia and found that 72% reported hearing loss at the time of assessment. This was supported by Tunkel et al,65 who found that in a sample of 73 individuals with achondroplasia, 54% of the adults failed a hearing screening test. Both studies concluded that there is significant risk of long-term conductive hearing loss in this population and recommended that all patients with achondroplasia receive an early audiological evaluation to allow for the detection of hearing loss.
Hearing loss related to otitis media is thought to be a major contributor to the speech delays and articulation problems noted in approximately one in five children with achondroplasia.10 This has significant impacts on communication development, and may influence learning and school performance.28,56 The high prevalence of otitis media and hearing loss in this population has led to the formal recommendation for routine early hearing tests and referral for speech and language review for infants and young children with achondroplasia as part of general surveillance.3,8,9
Impact upon activity and participation
While much of the available literature evaluates the genetic basis of achondroplasia and the management of impairments in body structures and functions, there is comparatively less information regarding the influence that these factors have on activity and participation domains. As seen in Figure 1, the complex interplay of the characteristic impairments of body structure can contribute directly and cyclically to activity limitations and participation restrictions for individuals with achondroplasia, including communication, mobility, and self-care.
The challenges posed by the combination of impairments in body structure and function can directly impact upon the major areas of participation for children, including mobility, self-care, education, and performance at school. Children with achondroplasia have a specific profile of developmental sequences, which differs from typical development, but is relatively consistent within the group. The developmental profile is influenced by both the musculoskeletal impairments characteristic of achondroplasia such as macrocephaly, short stature, and rhizomelic limb shortening, as well as positioning and handling restrictions necessary to reduce the risk of secondary injuries, eg, restricted sitting to avoid development of a fixed thoracolumbar kyphosis. Recognition that children with achondroplasia have a specific developmental profile has led to the development of milestone reference tables.28,55,56,69 Furthermore, a recent study by Ireland et al29 noted a delay in the development of independence in functional self-care, mobility, and social cognition tasks for children with achondroplasia, with a need for caregiver assistance extending beyond 7 years. Delays in the development of gross motor, communication, and self-care skills may directly influence performance at school. Brinkmann et al70 postulated that the altered body schema seen in short stature could lead to impaired social interaction with parents and other adults. This is supported by Ireland et al29 who noted that many families reported an increased preference to remain in close proximity to their child during social situations because of the child’s short stature. The combination of short stature, physical mobility challenges, potential delays in language development and difficulty accessing appropriate equipment, may impact significantly on the child’s experiences within the educational setting, particularly during the formative school years. The knowledge of these challenges supports the need for children with achondroplasia to be reviewed by an occupational therapist and/or physiotherapist before school commencement to assist in school facility modification, equipment prescription, and problem solving. As there are currently no effective medical interventions to counteract the primary anatomical impairments seen in achondroplasia, improving overall participation will continue to be dependent on targeted assessment of limitations, and provision of environmental adaptations or equipment prescription.
Impact during adulthood
Thompson et al14 reviewed the literature and research evidence on medical, health, and social aspects of life for adults with skeletal dysplasia including achondroplasia, and found substantial gaps in knowledge regarding medical and social aspects, recommending further robust research in this area. The medical and social complications associated with achondroplasia may impact on performance in areas such as employment. Roizen et al71 compared education and occupation for 20 adults with achondroplasia with their same-sex siblings and found differences in occupation level despite comparable years of formal education for women, suggesting that other factors may be important. While Hunter et al10 noted a trend towards the earlier diagnosis of achondroplasia, they went on to observe that a significant number of adults develop major physical limitations and pain, which impacts on their quality of life. It is possible that the onset of age-related medical issues (eg, spinal stenosis and leg pain), is exacerbated by physical access challenges related to short stature (eg, stairs) and is a contributing factor to differences in employment level. Further research is required to identify barriers to activity interactions for this population group, including more emphasis on those environmental factors that hinder or support participation performance.
New treatment options for achondroplasia
Recently, there have been large strides taken in terms of finding a medical (drug) treatment to alleviate some of the medical complications observed in achondroplasia. One medication that is currently undergoing early Phase II clinical trials is C-natriuretic peptide (CNP), which antagonizes the downstream effects of the aberrant FGFR3 signal, and has been shown to normalize bone growth in mouse models of achondroplasia.72 In addition to this, other medications and antibodies are also being developed that may also aid in increasing bone growth and decreasing complications in this condition.
Conclusion
Children and adults with achondroplasia are impacted by a variety of medical issues created by the unique complexities associated with a form of disproportionate short stature. The pictorial presentation of achondroplasia based on the ICF that is presented in this paper highlights the need for ongoing commitment to coordinated multidisciplinary care to ensure that families receive timely service provision from medical subspecialists and therapists skilled in the management of this population. Further research on the changes in medical issues presenting across the lifespan for individuals with achondroplasia is needed to further drive health- and community-based services needs and assist with directing appropriate and timely service provision.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The authors alone are responsible for the content and writing of this paper.
References
- 1.Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370(9582):162–172. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- 2.Baujat G, Legeai-Mallet L, Finidori G, Cormier-Daire V, Le Merrer M. Achondroplasia. Best Pract Res Clin Rheumatol. 2008;22(1):3–18. doi: 10.1016/j.berh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Committee on Genetics Health supervision for children with achondroplasia. Pediatrics. 1995;95(3):443–451. [PubMed] [Google Scholar]
- 4.Scott CI., Jr Achondroplastic and hypochondroplastic dwarfism. Clin Orthop Relat Res. 1976;(114):18–30. [PubMed] [Google Scholar]
- 5.Weinstein SL, Buckwalter JA, editors. Turek’s Orthopaedics: Principles and Their Application. 5th ed. Philadelphia, PA: JB Lippincott & Co; 1994. [Google Scholar]
- 6.Waller DK, Correa A, Vo TM, et al. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am J Med Genet A. 2008;146A(18):2385–2389. doi: 10.1002/ajmg.a.32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter EM, Davis JG, Raggio CL. Advances in understanding etiology of achondroplasia and review of management. Curr Opin Pediatr. 2007;19(1):32–37. doi: 10.1097/MOP.0b013e328013e3d9. [DOI] [PubMed] [Google Scholar]
- 8.Trotter TL, Hall JG, American Academy of Pediatrics Committee on Genetics Health supervision for children with achondroplasia. Pediatrics. 2005;116(3):771–783. doi: 10.1542/peds.2005-1440. [DOI] [PubMed] [Google Scholar]
- 9.Wright MJ, Irving MD. Clinical management of achondroplasia. Arch Dis Child. 2012;97(2):129–134. doi: 10.1136/adc.2010.189092. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AG, Bankier A, Rogers JG, Sillence D, Scott CI., Jr Medical complications of achondroplasia: a multicentre patient review. J Med Genet. 1998;35(9):705–712. doi: 10.1136/jmg.35.9.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht JT, Francomano CA, Horton WA, Annegers JF. Mortality in achondroplasia. Am J Hum Genet. 1987;41(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- 12.Pauli RM, Horton VK, Glinski LP, Reiser CA. Prospective assessment of risks for cervicomedullary-junction compression in infants with achondroplasia. Am J Hum Genet. 1995;56(3):732–744. [PMC free article] [PubMed] [Google Scholar]
- 13.Savarirayan R, Rimoin DL. The skeletal dysplasias. Best Pract Res Clin Endocrinol Metab. 2002;16(3):547–560. doi: 10.1053/beem.2002.0210. [DOI] [PubMed] [Google Scholar]
- 14.Thompson S, Shakespeare T, Wright MJ. Medical and social aspects of the life course for adults with a skeletal dysplasia: a review of current knowledge. Disabil Rehabil. 2008;30(1):1–12. doi: 10.1080/09638280701192857. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . International Classification of Functioning, Disability and Health: Children and Youth Version. Geneva, Switzerland: World Health Organization Press; 2007. [Google Scholar]
- 16.Haga N. Management of disabilities associated with achondroplasia. J Orthop Sci. 2004;9(1):103–107. doi: 10.1007/s00776-003-0729-4. [DOI] [PubMed] [Google Scholar]
- 17.Steinbok P, Hall J, Flodmark O. Hydrocephalus in achondroplasia: the possible role of intracranial venous hypertension. J Neurosurg. 1989;71(1):42–48. doi: 10.3171/jns.1989.71.1.0042. [DOI] [PubMed] [Google Scholar]
- 18.Borkhuu B, Nagaraju DK, Chan G, Holmes L, Jr, Mackenzie WG. Factors related to progression of thoracolumbar kyphosis in children with achondroplasia: a retrospective cohort study of forty-eight children treated in a comprehensive orthopaedic center. Spine (Phila Pa 1976) 2009;34(16):1699–1705. doi: 10.1097/BRS.0b013e3181ac8f9d. [DOI] [PubMed] [Google Scholar]
- 19.Kopits SE. Thoracolumbar kyphosis and lumbosacral hyperlordosis in achondroplastic children. Basic Life Sci. 1988;48:241–255. doi: 10.1007/978-1-4684-8712-1_34. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds KK, Modaff P, Pauli RM. Absence of correlation between infantile hypotonia and foramen magnum size in achondroplasia. Am J Med Genet. 2001;101(1):40–45. doi: 10.1002/ajmg.1307. [DOI] [PubMed] [Google Scholar]
- 21.Rimoin DL. Cervicomedullary junction compression in infants with achondroplasia: when to perform neurosurgical decompression. Am J Hum Genet. 1995;56(4):824–827. [PMC free article] [PubMed] [Google Scholar]
- 22.Wassman ER, Jr, Rimoin DL. Cervicomedullary compression with achondroplasia. J Pediatr. 1988;113(2):411. doi: 10.1016/s0022-3476(88)80295-6. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Nakamura S, Tajima M, Kageyama N. Neurological manifestations of pediatric achondroplasia. J Neurosurg. 1981;54(1):49–57. doi: 10.3171/jns.1981.54.1.0049. [DOI] [PubMed] [Google Scholar]
- 24.Nehme AM, Riseborough EJ, Tredwell SJ. Skeletal growth and development of the achondroplastic dwarf. Clin Orthop Relat Res. 1976;(116):8–23. [PubMed] [Google Scholar]
- 25.Kopits SE. Orthopedic aspects of achondroplasia in children. Basic Life Sci. 1988;48:189–197. doi: 10.1007/978-1-4684-8712-1_28. [DOI] [PubMed] [Google Scholar]
- 26.Kitoh H, Kitakoji T, Kurita K, Katoh M, Takamine Y. Deformities of the elbow in achondroplasia. J Bone Joint Surg Br. 2002;84(5):680–683. doi: 10.1302/0301-620x.84b5.13107. [DOI] [PubMed] [Google Scholar]
- 27.Siebens AA, Hungerford DS, Kirby NA. Curves of the achondroplastic spine: a new hypothesis. Johns Hopkins Med J. 1978;142(6):205–210. [PubMed] [Google Scholar]
- 28.Ireland PJ, Donaghey S, McGill J, et al. Development in children with achondroplasia: a prospective clinical cohort study. Dev Med Child Neurol. 2012;54(6):532–537. doi: 10.1111/j.1469-8749.2012.04234.x. [DOI] [PubMed] [Google Scholar]
- 29.Ireland PJ, McGill J, Zankl A, et al. Functional performance in young Australian children with achondroplasia. Dev Med Child Neurol. 2011;53(10):944–950. doi: 10.1111/j.1469-8749.2011.04050.x. [DOI] [PubMed] [Google Scholar]
- 30.Stanley G, McLoughlin S, Beals RK. Observations on the cause of bowlegs in achondroplasia. J Pediatr Orthop. 2002;22(1):112–116. [PubMed] [Google Scholar]
- 31.Lee ST, Song HR, Mahajan R, Makwana V, Suh SW, Lee SH. Development of genu varum in achondroplasia: relation to fibular overgrowth. J Bone Joint Surg Br. 2007;89(1):57–61. doi: 10.1302/0301-620X.89B1.18223. [DOI] [PubMed] [Google Scholar]
- 32.Beals RK, Stanley G. Surgical correction of bowlegs in achondroplasia. J Pediatr Orthop B. 2005;14(4):245–249. doi: 10.1097/01202412-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kaissi AA, Farr S, Ganger R, Hofstaetter JG, Klaushofer K, Grill F. Treatment of varus deformities of the lower limbs in patients with achondroplasia and hypochondroplasia. Open Orthop J. 2013;7:33–39. doi: 10.2174/1874325001307010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonstein JE. Treatment of kyphosis and lumbar stenosis in achondroplasia. Basic Life Sci. 1988;48:283–292. doi: 10.1007/978-1-4684-8712-1_38. [DOI] [PubMed] [Google Scholar]
- 35.Pauli RM, Breed A, Horton VK, Glinski LP, Reiser CA. Prevention of fixed, angular kyphosis in achondroplasia. J Pediatr Orthop. 1997;17(6):726–733. [PubMed] [Google Scholar]
- 36.Misra SN, Morgan HW. Thoracolumbar spinal deformity in achondroplasia. Neurosurg Focus. 2003;14(1):e4. doi: 10.3171/foc.2003.14.1.5. [DOI] [PubMed] [Google Scholar]
- 37.Bailey JA., 2nd Orthopaedic aspects of achondroplasia. J Bone Joint Surg Am. 1970;52(7):1285–1301. [PubMed] [Google Scholar]
- 38.Hall JG. The natural history of achondroplasia. Basic Life Sci. 1988;48:3–9. doi: 10.1007/978-1-4684-8712-1_1. [DOI] [PubMed] [Google Scholar]
- 39.Siebens AA, Hungerford DS, Kirby NA. Achondroplasia: effectiveness of an orthosis in reducing deformity of the spine. Arch Phys Med Rehabil. 1987;68(6):384–388. [PubMed] [Google Scholar]
- 40.Hall JG. Kyphosis in achondroplasia: probably preventable. J Pediatr. 1988;112(1):166–167. doi: 10.1016/s0022-3476(88)80157-4. [DOI] [PubMed] [Google Scholar]
- 41.Siebens AA, Kirby N, Hungerford D. Orthotic correction of sitting abnormality in achondroplastic children. Basic Life Sci. 1988;48:313–317. doi: 10.1007/978-1-4684-8712-1_42. [DOI] [PubMed] [Google Scholar]
- 42.Hoover-Fong JE, McGready J, Schulze KJ, Barnes H, Scott CI. Weight for age charts for children with achondroplasia. Am J Med Genet A. 2007;143A(19):2227–2235. doi: 10.1002/ajmg.a.31873. [DOI] [PubMed] [Google Scholar]
- 43.Hoover-Fong JE, Schulze KJ, McGready J, Barnes H, Scott CI. Age-appropriate body mass index in children with achondroplasia: interpretation in relation to indexes of height. Am J Clin Nutr. 2008;88(2):364–371. doi: 10.1093/ajcn/88.2.364. [DOI] [PubMed] [Google Scholar]
- 44.Hunter AG, Hecht JT, Scott CI., Jr Standard weight for height curves in achondroplasia. Am J Med Genet. 1996;62(3):255–261. doi: 10.1002/(SICI)1096-8628(19960329)62:3<255::AID-AJMG10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Wynn J, King TM, Gambello MJ, Waller DK, Hecht JT. Mortality in achondroplasia study: a 42-year follow-up. Am J Med Genet A. 2007;143A(21):2502–2511. doi: 10.1002/ajmg.a.31919. [DOI] [PubMed] [Google Scholar]
- 46.Hecht JT, Hood OJ, Schwartz RJ, Hennessey JC, Bernhardt BA, Horton WA. Obesity in achondroplasia. Am J Med Genet. 1988;31(3):597–602. doi: 10.1002/ajmg.1320310314. [DOI] [PubMed] [Google Scholar]
- 47.Pauli RM, Scott CI, Wassman ER, Jr, et al. Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr. 1984;104(3):342–348. doi: 10.1016/s0022-3476(84)81092-6. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Rosenbaum AE, Reid CS, Zinreich SJ, Pyeritz RE. Pediatric patients with achondroplasia: CT evaluation of the craniocervical junction. Radiology. 1987;164(2):515–519. doi: 10.1148/radiology.164.2.3602395. [DOI] [PubMed] [Google Scholar]
- 49.Keiper GL, Jr, Koch B, Crone KR. Achondroplasia and cervicomedullary compression: prospective evaluation and surgical treatment. Pediatr Neurosurg. 1999;31(2):78–83. doi: 10.1159/000028838. [DOI] [PubMed] [Google Scholar]
- 50.Bagley CA, Pindrik JA, Bookland MJ, Camara-Quintana JQ, Carson BS. Cervicomedullary decompression for foramen magnum stenosis in achondroplasia. J Neurosurg. 2006;104(Suppl 3):166–172. doi: 10.3171/ped.2006.104.3.166. [DOI] [PubMed] [Google Scholar]
- 51.King JA, Vachhrajani S, Drake JM, Rutka JT. Neurosurgical implications of achondroplasia. J Neurosurg Pediatr. 2009;4(4):297–306. doi: 10.3171/2009.3.PEDS08344. [DOI] [PubMed] [Google Scholar]
- 52.Ho NC, Guarnieri M, Brant LJ, et al. Living with achondroplasia: quality of life evaluation following cervico-medullary decompression. Am J Med Genet A. 2004;131(2):163–167. doi: 10.1002/ajmg.a.30342. [DOI] [PubMed] [Google Scholar]
- 53.Horton WA, Rotter JI, Rimoin DL, Scott CI, Hall JG. Standard growth curves for achondroplasia. J Pediatr. 1978;93(3):435–438. doi: 10.1016/s0022-3476(78)81152-4. [DOI] [PubMed] [Google Scholar]
- 54.Yang SS, Corbett DP, Brough AJ, Heidelberger KP, Bernstein J. Upper cervical myelopathy in achondroplasia. Am J Clin Pathol. 1977;68(1):68–72. doi: 10.1093/ajcp/68.1.68. [DOI] [PubMed] [Google Scholar]
- 55.Fowler ES, Glinski LP, Reiser CA, Horton VK, Pauli RM. Biophysical bases for delayed and aberrant motor development in young children with achondroplasia. J Dev Behav Pediatr. 1997;18(3):143–150. doi: 10.1097/00004703-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Ireland PJ, Johnson S, Donaghey S, et al. Developmental milestones in infants and young Australasian children with achondroplasia. J Dev Behav Pediatr. 2010;31(1):41–47. doi: 10.1097/DBP.0b013e3181c72052. [DOI] [PubMed] [Google Scholar]
- 57.Lutter LD, Langer LO. Neurological symptoms in achondroplastic dwarfs – surgical treatment. J Bone Joint Surg Am. 1977;59(1):87–92. [PubMed] [Google Scholar]
- 58.Lutter LD, Longstein JE, Winter RB, Langer LO. Anatomy of the achondroplastic lumbar canal. Clin Orthop Relat Res. 1977;(126):139–142. [PubMed] [Google Scholar]
- 59.Stokes DC, Phillips JA, Leonard CO, et al. Respiratory complications of achondroplasia. J Pediatr. 1983;102(4):534–541. doi: 10.1016/s0022-3476(83)80180-2. [DOI] [PubMed] [Google Scholar]
- 60.Waters KA, Everett F, Sillence D, Fagan E, Sullivan CE. Breathing abnormalities in sleep in achondroplasia. Arch Dis Child. 1993;69(2):191–196. doi: 10.1136/adc.69.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afsharpaiman S, Sillence DO, Sheikhvatan M, Ault JE, Waters K. Respiratory events and obstructive sleep apnea in children with achondroplasia: investigation and treatment outcomes. Sleep Breath. 2011;15(4):755–761. doi: 10.1007/s11325-010-0432-6. [DOI] [PubMed] [Google Scholar]
- 62.Tasker RC, Dundas I, Laverty A, Fletcher M, Lane R, Stocks J. Distinct patterns of respiratory difficulty in young children with achondroplasia: a clinical, sleep, and lung function study. Arch Dis Child. 1998;79(2):99–108. doi: 10.1136/adc.79.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takken T, van Bergen MW, Sakkers RJ, Helders PJ, Engelbert RH. Cardiopulmonary exercise capacity, muscle strength, and physical activity in children and adolescents with achondroplasia. J Pediatr. 2007;150(1):26–30. doi: 10.1016/j.jpeds.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 64.Collins WO, Choi SS. Otolaryngologic manifestations of achondroplasia. Arch Otolaryngol Head Neck Surg. 2007;133(3):237–244. doi: 10.1001/archotol.133.3.237. [DOI] [PubMed] [Google Scholar]
- 65.Tunkel D, Kerbavaz R, Smith B, Alade Y, Hoover-Fong J. Hearing loss in skeletal dysplasia patients; 10th Biennial Meeting of the International Skeletal Dysplasia Society; June 23–26, 2011; Palm Cove, Australia. [Google Scholar]
- 66.Gordon N. The neurological complications of achondroplasia. Brain Dev. 2000;22(1):3–7. doi: 10.1016/s0387-7604(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 67.Ireland PJ, Johnson S, Donaghey S, et al. Medical management of children with achondroplasia: evaluation of an Australasian cohort aged 0–5 years. J Paediatr Child Health. 2012;48(5):443–449. doi: 10.1111/j.1440-1754.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 68.McDonald JM, Seipp WS, Gordon EM, Heroy J. Audiologic findings in achondroplasia. Basic Life Sci. 1988;48:143–147. doi: 10.1007/978-1-4684-8712-1_19. [DOI] [PubMed] [Google Scholar]
- 69.Todorov AB, Scott CI, Jr, Warren AE, Leeper JD. Developmental screening tests in achondroplastic children. Am J Med Genet. 1981;9(1):19–23. doi: 10.1002/ajmg.1320090105. [DOI] [PubMed] [Google Scholar]
- 70.Brinkmann G, Schlitt H, Zorowka P, Spranger J. Cognitive skills in achondroplasia. Am J Med Genet. 1993;47(5):800–804. doi: 10.1002/ajmg.1320470540. [DOI] [PubMed] [Google Scholar]
- 71.Roizen N, Ekwo E, Gosselink C. Comparison of education and occupation of adults with achondroplasia with same-sex sibs. Am J Med Genet. 1990;35(2):257–260. doi: 10.1002/ajmg.1320350222. [DOI] [PubMed] [Google Scholar]
- 72.Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91(6):1108–1114. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]