Abstract
This review is aimed to focus on NSCLC as an emerging and promising model for active immunotherapy and the challenges for its inclusion in the current clinical scenario. Cancer vaccines for NSCLC have been focused as a therapeutic option based on the identification of a tumor hallmark and the active immunization with the related molecules that triggers cellular and/or humoral responses that consequently destroy or delay the rate of malignant progression. This therapeutic intervention in an established disease state has been aimed to impact into prolonging patient´s survival with ethically accepted quality of life. Understanding of relationship between structure and function in cancer vaccines is essential to interpret their opportunities to impact into prolonging survival and increasing quality of life in cancer patients. It is widely accepted that the failure of the cancer vaccines in the NSCLC scenario is related with its introduction in the advanced disease stages and poor performance status of the patients due to the combination of the tumor induced immunosuppression with the immune senescence. Despite first, second and emerging third line of onco-specific treatments the life expectancy for NSCLC patients diagnosed at advanced stages is surrounding the 12 months of median survival and in facts the today real circumstances are extremely demanding for the success inclusion of cancer vaccines as therapeutic choice in the clinical scenario. The kinetics of the active immunizations encompasses a sequential cascade of clinical endpoints: starting by the activation of the immune system, followed by the antitumor response and finalizing with the consequential impact on patients’ overall survival. Today this cascade of clinical endpoints is the backbone for active immunization assessment and moreover the concept of cancer vaccines, applied in the NSCLC setting, is just evolving as a complex therapeutic strategy, in which the opportunities for cancer vaccines start from the selection of the target cancer hallmark, followed by the vaccine formulation and its platforms for immune potentiating, also cover the successful insertion in the standard of care, the chronic administration beyond progression disease, the personalization based on predictors of response and the potential combination with other targeted therapies.
Keywords: NSCLC, cancer vaccine, cancer hallmarks.
INTRODUCTION
Cancer vaccine concept have been encompasses a broad spectrum of platforms intended to trigger or increase an adaptive immune response against a malignant tumor [1]. Thus cancer vaccines are aimed as active immunotherapeutic interventions mainly in an established disease state in which the malignancy is expressing all its functional capabilities or hallmarks [2].
Understanding of relationship between structure and function in cancer vaccines is essential to interpret their opportunities to impact into prolonging survival and increasing quality of life in cancer patients. The structure of cancer vaccines is referred to a tumor associated (or specific) antigen shaped as peptides, recombinant vectors, whole tumor cells or antigen presenting cells linked or supported by an adequate immunopotentiating platform, which should be inserted in a context of therapeutic maneuvers. The expected function of cancer vaccines is basically to circumvent the immunologic tolerance in the tumor microenvironment [3]inducing a lowest rate of tumor progression and increasing the host survival. So, cancer vaccines function is expected to be relevant at all levels of biological organization of the malignancy host, from the tumor microenvironment up to the whole organism. Then, to success with active immunotherapy strategy two items need to be considered: the role of target antigens in tumor biology (cancer hallmark related) and the immunogenic capacity of vaccine composition [4]. Once selected the right antigen and the most adequate platform for immune potentiating, the battle field for cancer vaccines is the immunosuppressive microenvironment induced by the malignant machinery; the corner between structure and function in cancer vaccines is accurately the interaction between the tumors hallmarks and the host immune response.
Prostate cancer has been proposed as a prototype for vaccine therapy scenario [5]. Besides the increasing spectrum of therapeutic tools for the disease control at the advanced disease stage, meaning new androgen deprivation modalities such as Abiraterone, [6] advanced chemotherapeutic agents such as cabazitaxel [7] and small tyrosine kinase inhibitors such as cabozantinib, [8] a cancer vaccine appear in the prostate carcinoma treatments alternative. Evidences supporting that Sipuleucel-T, a vaccine based on dendritic cells pulsed with prostatic acid phosphatase linked to granulocyte–macrophage colony-stimulating factor, prolonged survival in minimal or non-symptomatic mCRPC patients and its FDA approval (April, 2010), [9] constitute the proof of concept of cancer vaccines application in the scenario of a solid tumor with long term disease course, characterized by well described tumor associated antigens, a defined biomarker (serum PSA) [10] and a good physicians tool to follow up the patients therapeutic response (Halabi nomogram) [11].
But the reality of NSCLC is very different. Non- small cell lung cancer (NSCLC) is the first cause of cancer associated death worldwide, [12] the 60- 80% of patients are diagnosed at the advanced stages (IIIb/IV) in which their life expectancy is not too far from 12 months median survival even second and emerging third line of onco-specific treatments [13]. In this particular case the active immune intervention using cancer vaccines is strongly challenged by the tumor induced immunologic tolerance. There are several evidences of a marked infiltration of different types of immune cells in the NSCLC microenvironment, and the distribution, tissue localization, and cell types are significantly associated with progression and patient’s survival [14]. The clinical relevance of the microenvironmental immunological milieu in this malignacy is highlighted by the facts that higher FOXP3+/CD8+ ratio in tumor sites is an independent factor for poor response to platinum-based neoadjuvant chemotherapy in a setting of advanced stages patients [15].
This review is aimed to focus on NSCLC as an emerging and promising model for active immunotherapy and the challenges for its inclusion in the current clinical scenario, [16] considering the increasing body of evidences of the interaction between inflammatory cells and tumor cells, facilitating the lung carcinogenesis and tumor hallmarks expression (angiogenesis and tumor cell migration among others), in which antigen-specific antitumor responses are overtly present. In this context, the clinical relevance of cancer vaccines should be predicted pointing out the emerging determinants of its structure and function relationships in the particular situation of NSCLC malignancy hallmarks. The kinetics of the active immunizations encompasses a sequential cascade of clinical endpoints: starting by the activation of the immune system, followed by the antitumor response and finalizing with the consequential impact on patients’ overall survival [17]. Today this cascade of clinical endpoints is the backbone for active immunization assessment and moreover the concept of cancer vaccines, applied in the NSCLC setting, is just evolving as a complex therapeutic strategy, in which the opportunities for cancer vaccines start from the selection of the target cancer hallmark, followed by the vaccine formulation and its platforms for immune potentiating, also encompass the successful insertion in the standard of care, the chronic administration beyond progression disease, the personalization based on predictors of response and the potential combination with other targeted therapies.
NSCLC BIOLOGY AND TARGET ANTIGENS FOR CANCER VACCINES
As an inherently condition of all malignant processes, NSCLC carcinogenesis is resulting from the interaction between the epithelial mucosa genome and the lung microenvironment [18]. In this context, the intrinsic cell capacity for DNA repair, the nutrients accessibility, the angiogenesis state and the hypoxia levels, seems to be the common context of selective pressures for the cascade of mutations during tumor progression and coming out of the malignant cells phenotypes. Particularly, the continuing exposure of DNA to metabolically activated carcinogens, throughout the exposition to air contaminants or cigarette- smoke, resulting in the formation of DNA adducts and consequential genetic instability. The rate of DNA injure, taking place day after day over many years, is fully consistent with multiple genetic changes in lung cancer and is associated with increasingly severe histopathological phenotypes [19].
The importance of the target antigens to tumor biology is considered relevant for the success of active immunotherapy. Indeed, the selection of vaccine antigens must be focused mainly on those molecules significantly associated with the hallmarks of cancer, also called oncoantigens; those specific or tumor associated antigens that are highly correlated with the phenotypic consolidation of the malignancy and tumor progression [20, 21].
The EGFR network induce a broad range of biological effects by controlling the cellular outcome, through a multiple ligands activation-dependent regulatory loops, which fall into time dependants temporal domains; early loops comprise protein modifications mechanisms, while delayed loops involve transcription regulation mechanisms; both became aberrant and without feedback control in malignant cells [22]. During development and progression of lung neoplasms the EGFR is overexpressed in the following proportions: 62% of all NSCLC tumors, 89% of squamous cell tumors, 41% of adenocarcinomas and 80% of bronchoalveolar tumors [23]. In the case of adenocarcinoma in the peripheral compartment of the lung (terminal bronquiole and alveoli) in never smokers, the EGFR overexpression is associated with a lower methylation index of the p53 cancer suppressor gene through high proportion of transition mutations (ie, purine for purine or pyrimidine for pyrimidine) [24]. The degree of EGFR expression has been reported as a predictive factor of response to biological therapy in NSCLC patients [25].
There are two classes of anti-EGFR agents that have shown clinical activity in NSCLC. These are either monoclonal antibodies directed at the extracellular domain of the EGFR and inhibiting the binding of natural ligands to the receptor, or low molecular weight tyrosine kinase inhibitors (TKIs) that inhibit the tyrosine kinase activity of EGFR, generally by competing reversibly with ATP for the ATP binding site [26]. Targeting the EGFR with a cancer vaccine is a newly strategy currently in clinical scrutiny using the active immune deprivation of EGF ligand as important tumor growth factor (CIMAvax EGF) [27, 28].
MUC1 is over-expressed in almost all NSCLC patients, being associated with bad prognosis. The role in tumor biology of the Nglycosylated MUC1C (MUC1 C-terminal transmembrane subunit) seems to be associated to a functionally important extracellular bridge mediated by galectin 3 between this recognized mucin antigen and tyrosine kinases receptors, such as the EGFR [29]. MUC1 looks to be related not only with promoting cell growth and survival but also with T cell proliferation inhibition and accordingly, immunosuppression [30].
In this context, other molecules became aberrant expressed in the cascade of events associated with the sustained proliferation and it´s negative regulation, apoptosis evasion and the limitless replicative potential. Some of them are already being targeted with cancer vaccines candidates in NSCLC. Tumor suppressor gene P53 receives stress signals from excessive DNA damage and suboptimal nutrients triggering DNA repairing mechanism or apoptosis [31]. Loss of P53 function by mutations are frequent events in human oncogenesis, which leads to persistent aberrant expression in about 60% of NSCLC, correlated with poor ChT response and lowest overall SV of the patients [32]. This conduced to exploration of a mutant p53 peptide pulsed dendritic cell vaccine in a phase II clinical trial with stage III NSCLC patients [33].
The specialized DNA polymerase, named Telomerase, which adds telomere repeat segments to the end of telomeric DNA is nearly absent in nonimmortalized cells but expressed at functionally significant levels in 90% of human tumors [34]. Telomerase- based vaccine GV1001 is starting the clinical scrutiny, [35] supported also by the facts that its presence in sputum samples has been identified as a potential specific marker for early detection of lung cancer [36].
The activation of Cdk1-cyclin B1 triggers the mitosis progression and its translocation from the cytoplasm to the nucleus. Positive feedback loops regulate its biological activity and spatial localization, ensuring a rapid, complete, robust, and irreversible transition from interphase to mitosis [37]. There is reported the aberrant expression of these regulators of the G2 ⁄M cell cycle checkpoint in many neoplasms, including NSCLC and significant association between increased cyclin B1 expression and reduced patients survival in stage I/ II NSCLC has been described [38]. Furthermore, Cyclin B1 Peptide-Pulsed Autologous Dendritic Cell Vaccine is early of clinical evaluation in resectable NSCLC patients [39].
The RAS genes, including H-RAS, K-RAS and N-RAS, encode a family of proteins regulating cell growth, differentiation and apoptosis. Mutations in the K-RAS gene, mainly in codons 12 and 13, have been found in 20-30% of NSCLC tumor samples and occur most commonly, but not exclusively, in adenocarcinoma histology and in heavy smokers. Active immune strategies aimed at interfering with the Ras-Raf-MEK pathway in NSCLC are also incipient exploring the use of a Ras peptide based vaccine [40].
The continuous cascade of molecular events starting from at least a cell clone and characterized by unregulated cellular proliferation and clonal heterogeneity, is resulting in a characteristic NSCLC malignant phenotype. Based only on histology NSCLC comprises i.e. for adenocarcinoma, squamous-cell carcinoma and large-cell carcinoma subsets, while, based on the molecular subsets expressed in the resultant malignant phenotypes, NSCLC drive a broad spectrum of diseases subsets: EGFR, HER2, KRAS, ALK, BRAF, PIK3CA, AKT1, ROS1, NRAS, MAP2K1 and the recently identified KIF5B-RET fusion subset, each one with dissimilar pattern of incidence in a population. The personalization of cancer vaccines therapy is a pending chapter in this background, and the selection of the right vaccine for the right patient is becoming as challenge [41].
The design of cancer vaccines attempt to harness the specificity and resistance potentials of the human immune system. With the aim to stimulate the immune system to recognize, attack and destroy tumor cells the personalization treatment is intrinsic for MHC context dependent antigens vaccines (peptides based vaccines) and for cell based cancer vaccines. In the context of the adaptive immune response one foremost challenge for clinical researchers is to classify the patient’s population according the pre-existing immune response in potential responders or non- responders to the target antigen.
It is widely accepted that the failure of the cancer vaccines in the NSCLC scenario is related with its introduction in the advanced disease stages and poor performance status of the patients due to the combination of the tumor induced immunosuppression with the immune senescence [42].
The facts that NSCLC occur in subjects who have never smoked and have no evident cause for chronic inflammatory changes in the lung parenchyma, suggest that the interactions between the broncho-alveolar compartment and the in situ immune regulators are involved in facilitating tumor occurrence, growth, and spread [43]. It is progressively ostensible that crosstalk between cancer cells and cells of tumor stroma is involved in the acquired capability for invasive growth and metastases. Tumor stroma has been described as pivotal in the established hostile immune environment through the secretion of immunosuppressive factors and the recruitment of suppressive immune cells of myeloid and lymphoid origin, while the tumor cells directly contributing to immune resistance through the secretion of immunosuppressive mediators, such as transforming growth factorβ (TGFβ) or natural killer (NK) cell receptor decoys, and through the expression of ligands for immune checkpoint (or co-inhibitory) receptors, such as programmed cell death ligand 1 (PDL1). The distribution, tissue localization, and cell types infiltrating lung tumor microenvironment are significantly associated with the disease progression and patient’s survival [44]. For example dendritic cell defects increases immature forms that may play an immunosuppressive role and facilitate cancer migration, invasion, and epithelial-to-mesenchymal transition (Schneider et al. 2011). Also, genetic variations in the transforming growth factor-beta pathway are considered as predictors of survival in advanced non-small cell lung cancer [45] and higher FOXP3+/CD8+ ratio in tumor sites is described as an independent factor for poor response to platinum-based neoadjuvant chemotherapy in a setting of advanced stages patients [46].
Summarizing, a variety of cellular immune abnormalities, antigen processing and presentation machinery defects, cytokine alterations and also individual conditions, increase the challenge for NSCLC therapeutic vaccines. Vaccines candidates should activate the immune system and elicit a protective antitumor response even when chronic inflammation, the initiator of malignancy, is prevalent in tissues, modulate the tumor microenvironment and circumvent the dominant tolerance to tumor antigens to induce a tumor rejection.
CANCER VACCINES DESIGNED TO FACE UP THE IMMUNOSUPPRESSIVE NSCLC MICROENVIRONMENT
Overcoming the immunosuppressive microenvironment with cancer vaccines in NSCLC, became an active immunotherapeutic intervention in an established malignant disease, which structurally imply the precise antigen selection, supported by at least two immune potentiating platforms; the correct immune system activation, and the accurate therapeutic maneuvers, aimed for induce the lowest rate of tumor progression and increase the host survival with ethical acceptable quality of life.
Hopeful, certain immunomodulating agents, including a monoclonal antibody directed against CTLA-4 (ipilimumab), PD-1 and PD-L1 blocking antibodies and talactoferrin, a dendritic cell activator, are just in scrutiny and have shown clinical activity in NSCLC patients, highlighting the space of the immunotherapy as valid choice in this malignant condition [47, 48].
The well-known Tumor Associated Antigens MAGE, MUC1, NGc GM3ganglioside, CEA and NY-ESO-1 are overtly in the resultant malignant phenotype of NSCLC, supporting an initial generation of tumor antigen-specific cancer vaccines presently under pressure for achieve highest level of clinical evidences. Vaccines as MAGE-A3, MUC1 (Emepepimut-S, TG4010) and Racotumumab (Anti-idiotipic Mab NGc GM3 specific) are currently ongoing phase III proof of efficacy clinical trials, whilst CEA based cancer vaccines are just in ongoing phase I or II studies [49]. Other vaccines as NY-ESO- 1 plasmid DNA Cancer Vaccine have been explored in a phase I clinical trial [50].
Cell-based cancer vaccines (CDX-1401, L-Vax, Dribble, MRC-5, tergenpumatucel-L, and Belagenpumatucel-L) became as a second generation of active immunization approaches that becoming the scrutiny for clinically applicability in NSCLC and Belagenpumatucel-L or Lucanix (allogeneic cells TGF-b2 antisense gene modification) seem to be the more advanced competitors with ongoing phase III clinical trials [51].
As shown, the strategies improved for overcome the tumor immunosuppressive machinery in lung cancer vaccines are supported on the primary platform comprising peptides (RAS peptides, Epemimut-S, TG4010 from MUC-1 peptides and MAGE-12 peptides), proteins (CIMAvax EGF and NY-ESO-1), neo-antigen anti-idiotypes (racotumumab), recombinant vectors (MRC-5), whole tumor cells (Dribble, L-Vax, HyperAcute, tergenpumatucel-L and Belagenpumatucel-L) or antigen presenting cells (p53, Cyclin B1, CDX-1401) [39, 52, 53].
The above primary structure of the immunization strategy is commonly linked or supported by an adequate secondary platform for the correct APC presentation and immune potentiation. It is the case of the autologous EGF antigen coupled with non-self-molecule from Neisseria meningitides, emulsified with an oil adjuvant (CIMAvax EGF), or the case of a fusion protein between the NY-ESO-1 tumor antigen and a fully human monoclonal antibody specific for the dendritic cell receptor DEC-205 (CDX-1401 vaccine). Other examples are the Emepepimut-S vaccine that targets the exposed core peptide of MUC1 by a liposomal formulation and racotumumab anti-idiotipic vaccine, which act as a surrogate of NeuGM3 generating specific autologous antibodies [54, 55].
Once selected the right antigen and the most adequate platform for immune potentiating, those primary and secondary platforms, are not in condition to be winners in the battle field of the immunosuppressive microenvironment induced by the malignant machinery, and should be additionally inserted in a context of therapeutic maneuvers such as the priming and boosting strategies, the pre-treatment with a single low dose of cyclophosphamide (CIMAvax EGF, Epemimut-S), the chemotherapy induced lymphopenia and combinations with GM-CSF, IL-2, resiquimod and BCG, among others, for improve a tertiary platform for immune potentiation (Table 1) [56, 57].
Table 1.
Examples of NSCLC Vaccines Formulations with the Therapeutic Maneuvers Explored for Immune Potentiating.
| Cancer Vaccine | Immune Potentiating Platform | Priming & Boosting or Others Strategies for Immune Potentiating | Evidences of Immunogenicity | Level of Evidence | Reference |
|---|---|---|---|---|---|
| MAGE-12 peptide –based vaccine | Montanide ISA 51 | CTX | T cells secreting IFNg and CTL immune response | Phase II clinical trial | J. Immunology 2005 |
| CIMAvax EGF | EGF/P64k chemically linked Montanide ISA 51 adjuvant |
CTX 72 hours before first immunization. Single injection site/Vaccine after first line ChT Four induction doses/ Monthly re-immunizations |
High antibodies titters correlate with lower EGF serum concentrations | Phase II clinical trial | JCO 2008 |
| Racotumumab | Alum | Five induction doses at 2-week intervals and monthly re-immunizations | High IgM and IgG specific response against NeuGcGM3, able to react with lung carcinoma tissues sections. Preliminary data suggests a correlation between the induction of antibodies and increased survival times of vaccinated patients. |
Clinical exploratory study | Frontiers in Oncology, 2012 |
| Belagenpumatucel-L | TGF-β2 antisense gene modified irradiated allogeneic tumor-cell vaccine | intradermal injections of the vaccine every 4-8 weeks | Strong correlation between the achievement of a combination of cellular and humoral immune responses and a significant increase in overall survival in the subjects vaccinated | Phase II clinical trial | Clin Oncol 27:15s, 2009 (suppl; abstr 3013) |
NSCLC vaccines should be able to elicit both potent CD4+ and CD8+ T-cells to achieve an antitumor response. The most useful reaction of the immune system against tumors is to kill the abnormal cells using CTLs. But a broad spectrum of mechanism of action, from cellular to humoral effector, is present in more advances vaccines candidates, mainly depending on targeted antigen. Success of CIMAvax EGF vaccine, which is oriented to castrate the autologous EGF from the serum to subtract this growth factor from the tumor microenvironment, is obtained by inducing high titers of sequestering antibodies. In this case the cellular response seems to be not relevant for the vaccine functionality. Using CIMAvax EGF higher anti-EGF antibodies titers correlate with lowest serum EGF and longer patient’s survival. The serums from patients with longer survival block the EGFR phosphorylation [58].
Other vaccines are most pointing the induction of CTL response to be effective. MAGE-A3-vaccine, based on a fusion protein containing the MAGE-A3 antigen and protein D, use a second-generation adjuvant system AS02B, a saponin based adjuvant containing monophosphoryl lipid A. This vaccine induces both humoral and cellular immune response, but appears to be effective in patients expressing HLA-A1 allele, only present in the 20% of patients, by the induction of specific CTL response. The use of the adjuvant have been proved to be a prerequisite for cellular response to L-BLP25 vaccine (Stimuvax), based on MUC1 lipopeptide (25 aminoacids), use a liposomal delivery system and monophosphoryl lipid A, added to enhance the immune response. Vaccination is assumed to provoke internalization mediated by liposomal structure inducing CD4+ and CD8+ T cells, and also B cell response. Induced antibodies mediate antibody dependent cellular cytotoxicity (ADCC) against tumor [59].
Up to date the clinical insertions of cancer vaccines as therapeutic interventions in NSCLC start in the more advanced disease settings in which the malignancy is already established and acquired all its functional capabilities, while the scenario in which the tumor is surgically removed is fewer explored.
It is important to point out that in this setting the immunosupresive machinery of the malignancy is extremely developed. Additionally cancer condition is most common in elderly persons in which the immune response could be modified by the natural immunosenescence mechanisms. This situation is very controversial and should be carefully considered to design the immunotherapeutic intervention.
CANCER VACCINES MEDICAL POSITIONING IN THE CURRENT NSCLC CLINICAL SCENARIO
Cancer vaccines for NSCLC have been focused as a therapeutic option based on the identification of a tumor hallmark and the active immunization with the related molecules that triggers cellular and/or humoral responses that consequently destroy or delay the rate of malignant progression. This therapeutic intervention in an established disease state has been aimed to impact into prolonging patient´s survival with ethically accepted quality of life.
Currently MAGE-A3, Stimuvax, Lucanix, TG4010, Racotumumab and CIMAvax EGF are just in advanced proof of efficacy phase III clinical trials in NSCLC. While MAGE-A3 improve a trend to benefit the disease free interval in the post-resection of early disease stages pIB to II (HR 0.73; p= 0.109), the advanced disease setting remain the most clinically explored. In this disease setting TG4010 showed a trend to benefit PFS at 6 months with rates of 44% for vaccinated versus 35% for controls (p= 0.13) and in terms of trends for benefit patient’s overall survival Stimuvax show a HR 0.74 with p = 0.112, Lucanix achieve a clinical response of 15% and better survival in the high-dose group, Racotumumab achieve numerical differences of 9.93 months for Vaccinated group vs. 4.53 months for Control group and CIMAvax EGF achieve 3.5 month of benefit for the subset of 60 years old and younger patients (Table 2).
Table 2.
Cancer Vaccines Currently in Advanced Proof of Efficacy Phase III Clinical Trials in NSCLC.
| Vaccine | Level of Evidence | Therapeutic Context |
Effect on PFS | Effect on SV | Ongoing Phase III Trial | Reference |
|---|---|---|---|---|---|---|
| MAGE-A3 (Full protein AS02B or AS15) |
Phase II Placebo controlled clinical trial (n = 182) |
Post-resection of early-stage pIB to II NSCLC | Improved DFI (HR 0.73; P = 0.109) | No evidenced | Resected stages pIB to IIIA NCT00480025 (MAGRIT) Ongoing—target 2270 Primary endpoint: DFS |
Vansteenkiste J, Zielinski M, Dahabre J et al. JCO 2007; 25 (Suppl 18): 398S. (Abstr). |
| Emepepimut-S or Stimuvax (L-BLP25/ MUC1-peptide Liposomal formulation) | Phase II Placebo controlled clinical trial (n = 171) |
Post-chemoradiotherapy for unresectable stage III NSCLC | No evidenced | Improved OS (HR 0.74; P = 0.112) |
Unresectable stage III NCT00409188 (START) Recruited—N = 1464 Primary endpoint OS |
Butts C, Murray N, Maksymiuk A et al. JCO 2005; 23: 6674–6681. |
| Lucanix (Belagenpumatucel-L Allogeneic cells TGF-b2 antisense gene modification) | Phase II clinical trial (open dose comparison) | Advanced Stages II to IV NSCLC added to ChT and Maintenance after ChT | No evidenced | Clinical response 15% Better survival in high-dose group |
Advanced stage III/IV NCT00676507 (STOP) Ongoing—target 700 Primary endpoint OS |
Nemunaitis J, Dillman RO, Schwarzenberger PO et al. JCO 2006; 24: 4721–4730. |
| TG4010 (MUC1-peptide IL-2 co-expressing viral vector) |
Phase II Placebo controlled clinical trial (n = 148) |
Stages IIIB and IV | PFS at 6 months 44% versus 35% (P = 0.13) |
No evidenced | Advanced stage IIIB/IV NCT01383148 Planned—target 1000 Primary endpoint OS |
Quoix E, Ramlau R, Westeel V et al. Lancet Oncol 2011; 12: 1125–1133. |
| Racotumumab | Compassionate clinical study (n = 71) |
Stages IIIB and IV | No evidenced | Vaccinated 9.93 months vs. Controls 4.53 months | Advanced stage IIIA (non-resectable), IIIB/IV NCT01460472 Planned—target 1082 Primary endpoint OS |
VÁzquez AM, et al. Frontiers in Oncology 2012 2(10)150 1-6. |
| CIMAvax EGF (hu-recEGF/ P64k/ Montanide ISA 51 adjuvant) |
Phase II Placebo controlled clinical trial (n = 80) |
Stages IIIB and IV Switch maintenance after first line platinum- based ChT Use beyond progression Chronic use |
No evidenced | 3.5 month for 60 years old and younger | Advanced stage IIIB/IV NCT00516685 Ongoing—target 230 Primary endpoint OS |
Neninger Vinageras E, De La Torre A, Osorio Rodriguez M et al. JCO 2008; 26: 1452–1458. |
Despite first, second and emerging third line of onco-specific treatments the life expectancy for NSCLC patients diagnosed at advanced stages is surrounding the 12 months of median survival and in facts the today real circumstances are extremely demanding for the success inclusion of cancer vaccines as therapeutic choice in the clinical scenario [60].
As corollary of the described development, an increasing interest in utilizing the multiple new agents that show activity in NSCLC and have a tolerable side-effect profile, to maintain response to initial therapy after treatment with platinum-based doublets is rising. However considerable controversy, maintenance therapy has been considered a suitable treatment alternative [61] and becoming a plausible therapeutic space for cancer vaccines, in the two proposed modalities; continuous maintenance or switch maintenance. In both cases the goal is to delay the second line therapy without treatment holidays for the malignancy.
The incorporation of the active vaccination in the current standard of care based generally in chemotherapeutic agents is yet poorly exploit in the clinical setting. There is accumulating evidence that conventional therapies may profit the antitumor effects from the participation of the immune system by several mechanisms such as the immunogenic tumour-cell death [62].
In a phase I/II clinical trial, 20 patients diagnosed with advanced NSCLC, received a schedule of two vaccinations of CIMAvax EGF before the platinum based first line chemotherapy and subsequent monthly vaccinations were improved once concluded the chemotherapy cycles. This small study evidenced that anti-EGF antibody titers were not affected and correlate with a reduction of serum EGF concentration, while the patients overall survival also correlate with the highest anti-EGF antibody titers without modification of the safety profile [63].
The therapeutic vaccine TG4010 was added to NSCLC first-line chemotherapy and used until documentation of progression disease in a phase IIB, open-label, controlled trial, recruiting 148 chemotherapy-naive patients with diseases stages IIIB or IV. This study show that 43.2% of patients who receiving the combination therapy were progression free, compared with 35.1% of the chemotherapy-alone group at 6 months of follow up. These results assessed the addition of a therapeutic cancer vaccine to first-line chemotherapy in advanced NSCLC as another choice for insertion in the current standard of care [57].
The emergency during the last five years of a methodological framework to enhance the clinical success of cancer immunotherapy is strong-minded the therapeutic insertion of cancer vaccines in NSCLC. From this perspective, the kinetics of active immunizations is understood as a sequential cascade of clinical endpoints. Starting by the initiation of vaccinations that leads to activation of the immune system which results in a cellular immune response developed in days to weeks; followed by the antitumor response that becomes evident weeks to months after first immunization and finalizing with the consequential impact on patients’ overall survival after several months of treatment. The immunotherapists and oncologists communities are recognizing this cascade of clinical endpoints as a backbone for active immunization assessment and each one of these three clinical ends points implies their own specific challenges and opportunities [18].
Connected with the circumstances of the currently clinical scenario, the exactly position of cancer vaccines in the advanced NSCLC setting is foremost challenging the current criteria for clinical evaluation of tumor response, because the possibility of pseudo- progression induced by immunotherapy, due to the elicited antitumor CD4+ and CD8+ T-cells infiltration, justifying the forthcoming immune related criteria of tumor progression and therefore the immunization beyond disease progression [64].
CIMAvax EGF have been explored after first line therapy and administered beyond diseases progression in a referred phase II, open, controlled trial in 80 patients after first line platinum based treatment with promising results [27]. The metanalysis of 58 patients who were vaccinated monthly for more than one or two years evidenced that long term vaccination was feasible and safe, without evidences of cumulative toxicity or immune response exhaustion [65]. The results of the planned interim analysis with the first 226 patients recruited in the ongoing randomized phase III trial, vaccinating the patients who respond to first line chemotherapy with at least stable disease, in the modality of switch maintenance, beyond progression disease and until change their performance status as per physician criteria, confirm previous results in benefit the patient’s overall survival by intent to treat analysis (manuscript in preparation).
So, chronic vaccination improved beyond progression disease in advanced stages; seems to be one of the transitions points for positioning cancer vaccines in the current and imminent NSCLC therapeutic scenario [66].
Regarding the insertion of cancer vaccines in the standard of care for advanced NSCLC is important to highlight the appearance of an algorithm proposed for choose a first line chemotherapy alternative. In this algorithm the presence of positive EGFR mutations or ALK fusions, the clinical conditions of the patients (performance status) and the tumor histological type (Non squamous or squamous), are driving the selection of erlotinib, crizotinib, bevacizumab combined or not with platinum/pemetrexed, platinum/gemcitabine doublets or single- agent chemotherapy [60].
Besides, another transition points for the inclusion of cancer vaccines in the current NSCLC therapeutic context is the opportunity to personalize this immunotherapy, based on biomolecules that will be predictive of the potential efficacy for a predetermined subset of patients. Up to now during active immunization some patients become not responders due to their specific pattern of immune response. For instance, the case of CIMAvax EGF in which those patients who reach the super responder condition, based on their Anti- EGF antibodies titers, achieve significant benefit in overall survival, [67] but so far there is not predictable this subset of patients before start immunizations. On the other hand, due to the intrinsic relation with the vaccine mechanism of action, intended to induce a humoral immunity that sequestering the circulating EGF and its possible effect on patients’ outcome, [58] today the correlation between the basal serum EGF concentrations and the patients survival after vaccine treatment, is been exhaustive evaluated for determine its value as a predictor of the CIMAvax EGF response.
Today is widely accepted that the blockade of a single hallmark is not enough to achieve clinical noteworthy benefit on patient´s survival. Because the complexity and redundancy of the molecular pathways that promote tumour growth and maintenance, the generation of potent anti-tumour immune responses requires the blockade of multiple steps. An additional transition points for the successful inclusion of cancer vaccines in the current NSCLC clinical scenario is the combination with other targeted therapies or the called “combinatorial immunotherapy”. This concept supports the criteria that optimizing immunotherapy requires treatments that affect multiple aspects of the immune response [68].
A small trial combining autologous granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting tumour cell vaccines with CTLA4 blockade found increased inflammatory infiltrates and tumour regression, suggesting that vaccine-induced anti-tumour T cells were present within the tumour but anergized owing to CTLA4 co-inhibition [69].
The combinatorial approach was explored in prostate cancer preclinical models using anti-CTLA-4 mAb in combination with rV-CEA-TRICOM cancer vaccine to augment antigen-specific T-cell responses and an independent prognostic effect of CTLA-4 overexpression in radically resected NSCLC has been recently described pointing out the potentials of this checkpoint modulation in this malignancy [70, 71].
The clinical development of combinatorial approaches has been moved forward after the FDA approval of antiCTLA4 therapy, quickly followed by reports of encouraging preliminary clinical data for antiPD1 therapies [47, 48]. Immune checkpoints are a tiny fraction of the receptors and ligands that have been defined by genetic and biological analyses to inhibit specific types of immune responses at various levels and its immunomodulatory manipulation may results in enhancing efficacy of therapeutic vaccinations. Thus, the blockade of immune checkpoints promises broad and diverse opportunities to enhance antitumour immunity with the potential durable clinical responses [72].
Moreover combinatorial immunotherapies such as bispecific T cell engagers (BiTEs) and immunotoxins Fc-fusion proteins are entering clinical testing in acute lymphoblastic leukemia and breast cancer patients respectively; opening a new era for cancer active immunotherapy [73, 74].
Targeted therapies and immunotherapy offer a number of possible synergies in treatment when used together; however, these combinations should be more intensive studied and dose optimization, timing and sequence will required rationally design in future clinical trials with the aim of maximize anti-tumour efficacy whereas minimizing every possible immunosuppressive adverse reactions.
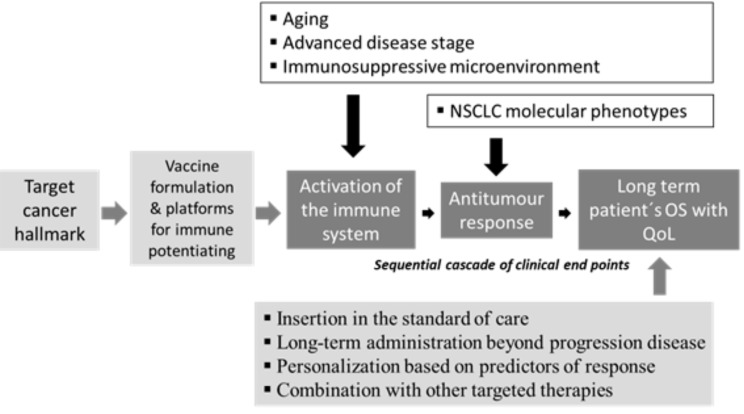
The critical obstacles for cancer immunotherapy have been defined [75], so, for the medical positioning of the cancer vaccines in the current NSCLC clinical scenario is important to understand that this concept is evolving into a complex therapeutic strategy. (Fig. 1). For enlargement and support the use of therapeutic vaccines in NSCLC is essential to consider the following:
Fig. (1).
Challenges and opportunities for cancer vaccines inclusion in the current NSCLC clinical scenario. The cascade of clinical endpoints as backbone for active immunization assessment and each one of the three clinical ends points (white letter filled in dark grey), are challenged by the aging of the majority of patients, the advanced disease stage at diagnosis, the immunosuppressive microenvironment improved by the malignancy and by the broad spectrum of NSCLC molecular phenotypes (black letter non filled). The opportunities start from the selection of the target cancer hallmark, followed by the vaccine formulation and its platforms for immune potentiating, also cover the successful insertion in the standard of care, the long-term administration beyond progression disease, the personalization based on predictors of response and the potential combination with other targeted therapies (black letter filled in light grey).
Insertion in the standard of care. During past two decades the onco-specific treatments advanced from the unique option of platinum doublets to the routine use of first-line, second-line, and even third-line treatment.
Chronic vaccination beyond progression disease. First; therapeutic vaccines has evidenced a safety profile during long term administration. Second; immunotherapies may induce tumor pseudo- progression, challenging current criteria for clinical evaluation of tumor response and allowing the forthcoming immune related criteria of tumor progression.
Personalization based on predictors of response. Targeted drugs such as erlotinib and crizotinib are inserted in the therapeutic context of NSCLC using specific biomolecules indicatives of therapeutic efficacy for selected subset of patients.
Combination with other targeted therapies. The blockade of a single hallmark is not enough to achieve clinical noteworthy benefit on patient´s survival. Combinations may achieve a synergism, strength of antitumor effects and also avoid or overcome the tumor mechanism of resistance.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
ABBREVIATIONS
- APC
= Antigen Presenting Cells
- ChT
= Chemotherapy
- CTX
= Cyclophosphamide
- ECOG
= Eastern Cooperative Oncology Group
- EGF
= Epidermal Growth Factor
- EGFR
= Epidermal Growth Factor Receptor
- NSCLC
= Non- Small Cell Lung Cancer
- mCRPC
= Metastatic Castration Resistant Prostate Cancer
- P64k
= P64k carrier protein from Neisseria meningitides
- PS
= Performance status
- RCT
= Randomised controlled trial
- TKI
= Tirosine kinases inhibitors
REFERENCES
- 1.Dalgleish A G, Whelan M A. Cancer vaccines as a therapeutic modality: the long trek. Cancer Immunol Immunother. 2006;55:1025–1032. doi: 10.1007/s00262-006-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich G A, Gabrilovich D, Sotomayor E M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman R L, Sher A, Seder R A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake C G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. COU-AA-301 Investigators. Abiraterone and Increased Survival in Metastatic Prostate Cancer. JEM. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO. TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration resistant prostate cancer progressing after docetaxel treatment a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 8. Smith DC, Smith M R, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark A L, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PNJr, Lin CC, Srinivas S, Sella A, Schöffski P, Scheffold C, Weitzman AL, Hussain M. Cabozantinib in Patients With Advanced Prostate Cancer Results of a Phase II Randomized Discontinuation Trial. J. Clin. Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantoff P W, Higano C S, Shore N D, Berger E R, Small E J, Penson D F, Redfern C H, Ferrari A C, Dreicer R, Sims R B, Xu Y, Frohlich M W, Schellhammer P F. IMPACT Study Investigators.Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 10.Bok RA, Small EJ. Bloodborne biomolecular markers in prostate cancer development and progression. Nat Rev Cancer. 2002;2:918–926. doi: 10.1038/nrc951. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46:517–525. doi: 10.1016/j.ejca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century A period analysis. Lancet. 2002;360:1131–1135. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 14.Bremnes R R, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, Stenvold H, Camps C, Busund LT. The Role of Tumor-Infiltrating Immune Cells and Chronic Inflammation at the Tumor Site on Cancer Development Progression and Prognosis.Emphasis on Non-small Cell Lung Cancer. J Thorac Oncol. 2011;6:824–833. doi: 10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–56. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd F A, Douillard JY, Blumenschein G R. Immunotherapy for Non-small Cell Lung Cancer Novel Approaches to Improve Patient Outcome. J Thorac Oncol. 2011;6:1763–1773. doi: 10.1097/JTO.0b013e31822e28fc. [DOI] [PubMed] [Google Scholar]
- 17.Hoos A, Britten C M. The immuno-oncology framework Enabling a new era of cancer therapy. OncoImmunology. 2012;1:334–339. doi: 10.4161/onci.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong KM, Sekido Y, Gazdar AF, Minna JD. l Molecular biology of lung cancer clinical implications. Thorax. 2003;58L:892–900. doi: 10.1136/thorax.58.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blankenstein T, Coulie P G, Gilboa E, Jaffee EM. l The determinants of tumour immunogenicity. Nat. Rev. Cancer. 2012;12L:307–313. doi: 10.1038/nrc3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKinnon A C, Kopatz J, Sethi T. The molecular and cellular biology of lung cancer identifying novel therapeutic strategies. Br Med Bull. 2010;95:47–61. doi: 10.1093/bmb/ldq023. [DOI] [PubMed] [Google Scholar]
- 21.Toyooka S, Mitsudomi T, Soh J, Aokage K, Yamane M, Oto T, Kiura K, Miyoshi S. Molecular oncology of lung cancer. Gen. Thorac. Cardiovasc. Surg. 2011;59:527–537. doi: 10.1007/s11748-010-0743-3. [DOI] [PubMed] [Google Scholar]
- 22.Avraham R, Yarden Y. Feedback regulation of EGFR signalling decision making by early and delayed loops. Nat. Cancer Mol. Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch FR, Varella-Garcia M, Bunn PAJr, Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J. Clin Oncol. 2003;21:3798–807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian J, Govindan R. Molecular genetics of lung cancer in people who have never smoked. Lancet Oncol. 2008;9:676–682. doi: 10.1016/S1470-2045(08)70174-8. [DOI] [PubMed] [Google Scholar]
- 25.Dancey JE. Predictive factors for epidermal growth factor receptor inhibitors the bull’s-eye hits the arrow. Cancer Cell. 2004;5:411–415. doi: 10.1016/s1535-6108(04)00122-9. [DOI] [PubMed] [Google Scholar]
- 26.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 27.Neninger E, de la Torre A, Osorio M, Catalá M, Bravo I, Mendoza M, Abreu D, Acosta S, Rives R, del Castillo C, González M, Viada C, García B, Crombet T, González G, Lage A. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced NSCLC. J. Clin Oncol. 2008;26:1452–1458. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 28.Hall RD, Gray JE, Chiappori AA. Beyond the standard of care a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control. 2013;20:22–31. doi: 10.1177/107327481302000105. [DOI] [PubMed] [Google Scholar]
- 29.Kufe D W. Mucins in cancer function prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding C, Wang L, Marroquin J, Yan J. Targeting of antigens to B cells augments antigen specific T-cell responses and breaks immune tolerance to tumor-associated antigen MUC1. Blood. 2008;112:2817–2825. doi: 10.1182/blood-2008-05-157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiewe T. The p53 family in differentiation and tumorigenesis. Nat Rev Cancer. 2007;7:165–168. doi: 10.1038/nrc2072. [DOI] [PubMed] [Google Scholar]
- 32.Gajra A, Tatum A H, Newman N, Gamble G P, Lichtenstein S. Rooney, M. T. Graziano S L. The predictive value of neuroendocrine markers and p53 for response to chemotherapy and survival in patients with advanced non-small cell lung cáncer. Lung Cancer. 2002;36:159–165. doi: 10.1016/s0169-5002(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 33.http://clinicaltrials.ov/show/ NCT00019929. Phase II Trial of Individualized Mutant p53 Peptide-Pulsed Cultured Autologous Dendritic Cells in the Adjuvant Treatment of Patients with Locally Advanced Non-Small Cell Lung Cancer after Standard Therapy. ClinicalTrials.gov processed this data on. 2013;September 11 [Google Scholar]
- 34.Aras G, Kanmaz D, Urer N, Purisa S, Kadakal F, Yentürk E, Tuncay E. Immunohistochemical expression of telomerase in patients with non small cell lung cancer prediction of metastasis and prognostic significance. Anticancer Res. 2013;33:2643–50. [PubMed] [Google Scholar]
- 35.Brunsvig P F, Kyte JA, Kersten C, Sundstrùm S, Mùller M, Nyakas M, Hansen G L, Gaudernack G, Aamdal S. Telomerase Peptide Vaccination in NSCLC A Phase II Trial in Stage III Patients Vaccinated after Chemoradiotherapy and an 8-Year Update on a Phase I/II Trial. Clin. Cancer Res. 2011;17:6847–6857. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 36.Sen S, Reddy V G, Guleria R, Jain S K, Kapila K, Singh N. Telomerase-a potential molecular marker of lung and cervical cancer. Clin Chem Lab Med. 2002;40:994–1001. doi: 10.1515/CCLM.2002.173. [DOI] [PubMed] [Google Scholar]
- 37.Santos S D M, Wollman R, Meyer T, Ferrell JE. Spatial Positive Feedback at the Onset of Mitosis. Cell. 2012;149:1500–1513. doi: 10.1016/j.cell.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper WA, Kohonen-Corish MRJ, McCaughan B, Kennedy C, Sutherland RL, Lee CS. Expression and prognostic significance of cyclin B1 and cyclin A in non-small cell lung cancer. Histopathology. 2009;55:28–36. doi: 10.1111/j.1365-2559.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 39.Vella LA, Yu M, Fuhrmann SR, El-Amine M, Epperson DE, Finn OJ. Healthy individuals have T cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. PNAS. 2009;106:14010–14015. doi: 10.1073/pnas.0903225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyera R G, Korna S, Mickeb P, Beckera K, Hubera C, Wolfel T, Buhl R. An open-label prospective phase I/II study evaluating the immunogenicity and safety of a ras peptide vaccine plus GM-CSF in patients with non-small cell lung cancer. Lung Cancer. 2007;58:88–94. doi: 10.1016/j.lungcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nat Rev Drug Discovery. 2012;11:201–214. doi: 10.1038/nrd3651. [DOI] [PubMed] [Google Scholar]
- 42.Dalgleish AG. Therapeutic cancer vaccines Why so few randomised phase III studies reflect the initial optimism of phase II studies. Vaccine. 2011;29:8501– 8505. doi: 10.1016/j.vaccine.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian J, Govindan R. Lung cancer in never smokers a review. J Clin Oncol. 2007;25:561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 44.Kadota K, Nitadori JI, Adusumilli PS. Prognostic value of the immune microenvironment in lung adenocarcinoma. Oncoimmunology. 2013;2:e24036. doi: 10.4161/onci.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin M, Stewart D J, Spitz M R, Hildebrandt M A, Lu C, Lin J, Gu J, Huang M, Lippman SM, Wu X. Genetic variations in the transforming growth factor-beta pathway as predictors of survival in advanced non-small cell lung cancer. Carcinogenesis. 2011;32:1050–1056. doi: 10.1093/carcin/bgr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, Okabe K, Matsumoto T, Sugi K, Ueoka H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Tomasini P, Khobta N, Greillier L, Barlesi F. Ipilimumab: its potential in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2012;4:43–50. doi: 10.1177/1758834011431718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brahmer J R, Tykodi SS, Chow L Q, Hwu W J, Topalian S L, Hwu P, Drake C G, Camacho L H, Kauh J, Odunsi K, Pitot H C, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay T M, Alaparthy S, Grosso J F, Korman A J, Parker S M, Agrawal S, Goldberg S M, Pardoll D M, Gupta A, Wigginton J M. Safety and Activ-ity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brahmer J R. Harnessing the immune system for the treatment of non-small-cell lung cancer. J. Clin. Oncol. 2013;10:1021–1028. doi: 10.1200/JCO.2012.45.8703. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida N, Abe H, Ohkuri T, Wakita D, Sato M, Noguchi D, Miyamoto M, Morikawa T, Kondo S, Ikeda H, Nishimura T. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens and T cell infiltration in non-small cell lung carcinoma and their prognostic significance. Inter. J. Oncol. 2006;28:1089–1098. [PubMed] [Google Scholar]
- 51.Nemunaitis J, Murray N. Immune-Modulating Vaccines in Non-small Cell Lung Cancer. J. Thorac. Oncol. 2006;1:756–761. [PubMed] [Google Scholar]
- 52.Rodríguez P C, Rodríguez G, González G, Lage A. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small cell lung cancer therapy. MEDICC Rev. 2010;12:17–23. doi: 10.37757/MR2010.V12.N1.4. [DOI] [PubMed] [Google Scholar]
- 53.Riedmann E M. CDX-1401 combined with TLR agonist: positive phase 1 results. Hum. Vaccin. Immunother. 2012;8:1742. [PubMed] [Google Scholar]
- 54.Bender A, Karbach J, Neumann A, Jäger D, Al-Batran SE, Atmaca A, Weidmann E, Biskamp M, Gnjatic S, Pan L, Hoffman E, Old LJ, Knuth A, Jäger E. LUD 00-009 Phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immunity. 2007;7:16–24. [PMC free article] [PubMed] [Google Scholar]
- 55.Vázquez A M, Hernández A M, Macías A, Montero E, Gómez D E, Alonso D F, Gabri M R, Gómez R E. Racotumomab an anti-idiotype vaccine related to N glycolyl containing gangliosides-preclinical and clinical data. Frontiers in Oncology. 2012;2:150 1–6. doi: 10.3389/fonc.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corrales-Rodriguez L, Blais N, Soulieres D. Emepepimut-S for non-small cell lung cancer. Expert Opin Biol Ther. 2011;11:1091–1097. doi: 10.1517/14712598.2011.592490. [DOI] [PubMed] [Google Scholar]
- 57.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton J, Stoelben E, Braun D, Debieuvre D, Lena H, Buyse M, Chenard M, Acres B, Lacoste G, Bastien B, Tavernaro A, Bizouarne N, Bonnefoy J, Limacher J. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–33. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 58.García B, Neninger E, de la Torre A, Leonard I, Martínez R, Viada C, González G, Mazorra Z, Lage A, Crombet T. Effective Inhibition of the Epidermal Growth Factor/Epidermal Growth Factor Receptor Binding by Anti-Epidermal Growth Factor antibodies is Related to Better Survival in Advanced Non-Small-Cell Lung Cancer Patients Treated with the Epidermal Growth Factor Cancer Vaccine. Clin Cancer Res. 2008;14:840–846. doi: 10.1158/1078-0432.CCR-07-1050. [DOI] [PubMed] [Google Scholar]
- 59.Atanackovic D, Altorki N K, Cao Y, Ritter E, Ferrara C A, Ritter G, Hoffman E W, Bokemeyer C, Old L J, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. PNAS. 2008;105:1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldstraw P, Ball D, Jett J R, Le Chevalier T, Lim E, Nicholson A G, Shepherd F A. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 61.Coate L E, Shepherd F A. Maintenance therapy in advanced non-small cell lung cancer evolution tolerability and outcomes. Ther. Adv. Med. Oncol. 2011;3:139–157. doi: 10.1177/1758834011399306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunology. 2012;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 63.Neninger E, Verdecia B G, Crombet T, Viada C, Pereda S, Leonard I, Mazorra Z, Fleites G, González M, Wilkinson B, González G., Lage A. Combining an EGF based cancer vaccine with chemotherapy in advanced non small cell lung cancer. J Immunother . 2009; 32:92–99. doi: 10.1097/CJI.0b013e31818fe167. [DOI] [PubMed] [Google Scholar]
- 64.Hoos A. Evolution of end points for cancer immunotherapy trials. Ann Oncol. 2012;23 8:47–52. doi: 10.1093/annonc/mds263. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez G, Crombet T, Lage A. Chronic Vaccination with a Therapeutic EGF-Based Cancer Vaccine: A Review of Patients Receiving Long Lasting Treatment. Curr. Cancer Drug Targets. 2011;11:103–110. doi: 10.2174/156800911793743583. [DOI] [PubMed] [Google Scholar]
- 66.Lage A, Crombet T. Control of Advanced Cancer: The Road to Chronicity. Int. J. Environ. Res. Public Health. 2011;8:683–697. doi: 10.3390/ijerph8030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez P C, Neninger E, García B, Popa X, Viada C, Luaces P, González G, Lage A, Montero E, Crombet T. Safety, immunogenicity and preliminary efficacy of multiple-site vaccination with an Epidermal Growth Factor (EGF) based cancer vaccine in advanced non small cell lung cancer (NSCLC) patients. J. Immune Based Ther. Vaccines. 2011;24:9–7. doi: 10.1186/1476-8518-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodi F S, Butler M, Oble D A, Seiden M V, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch M T, Ramaiya N, Chen T C, Neuberg D, Allison J P, Mihm M C, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulley J L, Madan R A, Arlen P M. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25 S:B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salvi S, Fontana V, Boccardo S, Merlo D F, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello M G, Mora M, Ratto G B, Grossi F, Truini M, Pistillo M P. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;9:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardoll D M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y, Huang H, Wei G. Novel agents and biomarkers for acute lymphoid leukemia. J. Hematol. Oncol. 2013;18:6–40. doi: 10.1186/1756-8722-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichert J M. Antibodies to watch in 2013: Mid-year update. MAbs. 2013;4:513–517. doi: 10.4161/mabs.24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox B A, Schendel D J, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, Atkins MB, Bartunkova J, Bergmann L, Berinstein N, Bonorino CC, Borden E, Bramson JL, Britten CM, Cao X, Carson WE, Chang AE, Characiejus D, Choudhury AR, Coukos G. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214–227. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]