Abstract
Background
Recent observational studies show that patients with multivessel coronary disease have a long-term survival advantage with coronary artery bypass grafting (CABG) compared to percutaneous coronary intervention (PCI). Important non-fatal outcomes may also affect optimal treatment recommendation.
Methods
Coronary artery bypass grafting was compared to percutaneous catheter intervention by using a composite of death, myocardial infarction (MI ) or stroke. Medicare patients undergoing revascularization for stable multi-vessel coronary disease from 2004 through 2008 were identified in national registries. Short-term clinical information from the registries was linked to Medicare data to obtain long-term follow-up out to 4 years from the time of the procedure. Propensity scoring with inverse probability weighting was used to adjust for baseline risk factors.
Results
There were 86,244 CABG and 103,549 PCI patients. The mean age was 74 with a median 2.67 years follow-up. At 4-years the propensity-adjusted adjusted cumulative incidence of MI was 3.2% in CABG compared with 6.6% in PCI (RR = 0.49, 95% CI 0.45 to 0.53). At 4 years, cumulative incidence of stroke was 4.5% in CABG compared with 3.1% in PCI patients (RR = 1.43, 95% CI 1.31 to 1.54). This difference was primarily due to the higher 30-day stroke rate for CABG (1.55% vs 0.37%). For the composite of death, MI, or stroke, the 4-year adjusted cumulative incidence was 21.6% for CABG and 26.7% for PCI (RR = 0.81, 95% CI 0.78 to 0.83).
Conclusion
The 4-year composite event rate of death, MI and stroke favored CABG while the risk of stroke alone favored PCI.
Keywords: Coronary artery bypass grafting, CABG Coronary stents, PCI Statistics, propensity matching
INTRODUCTION
The optimal revascularization strategy for patients with stable multi-vessel coronary artery disease remains controversial. The decision to recommend either coronary artery bypass grafting (CABG) or percutaneous catheter intervention (PCI) is ideally driven by a comparison of the short and long term impact on death and important non-fatal outcomes such as myocardial infarction (MI) and stroke.
While randomized controlled trials (RCT) have assessed the efficacy of CABG vs PCI, they typically enroll selected patient populations and reflect procedural results from specialized centers. Thus, these studies may not reflect the effectiveness of treatment in general community practice. In contrast, observational studies must contend with the potential for unmeasured confounders and selection bias, but they have the advantage of analyzing large populations of “real-world” patients.
Using extensive detailed clinical information found in the Society of Thoracic Surgeons (STS) National Database and the American College of Cardiology Foundation (ACCF) National Cardiovascular Data Registry (NCDR), we recently carried out a comparison of the Comparative Effectiveness of Revascularization Strategies (ASCERT) on survival outcomes in over 180,000 Medicare patients undergoing revascularization [1]. The study showed that Medicare patients with multivessel coronary disease who underwent CABG had a long term survival advantage relative to PCI.
While long term survival is a critical outcome for patients, other non-fatal endpoints are also vitally important. The development of post-procedural myocardial infarction (MI) can lead to significant cardiac disability that compromises function and quality of life. Likewise, neurologic compromise can be a devastating complication of an otherwise successful procedure. It is therefore critically important to explore these outcomes in each treatment arm.
Accordingly, as the next phase of the ASCERT study, our objective was to compare a composite of fatal and non-fatal outcomes among a Medicare cohort undergoing multi-vessel revascularization from 2004 through 2008. We also analyzed MI and stroke individually. These endpoints were examined overall and among important patient subgroups.
PATIENTS AND METHODS
A detailed description of the ASCERT inclusion criteria, patient characteristics, and patient selection has been published previously [1]. Briefly, we included patients aged 65 years or older who underwent non-emergent isolated CABG or PCI for multi-vessel coronary disease between January 1, 2004 and December 31, 2007. The main exclusions were patients with left main disease, prior cardiac surgery, PCI within 6 months, MI within 7 days, emergency procedure, shock within 24 hours, or pre-procedural intra-aortic balloon pump. We linked Medicare inpatient claims to PCI records from NCDR and CABG records from the STS database [2]. Eligibility for the final analysis dataset was then based on a combination of Medicare and registry data elements.
Pre-specified endpoints included stroke, re-hospitalization for myocardial infarction (MI), and a composite of death, stroke, or re-hospitalization for MI. Peri-procedural strokes occurring during the index hospitalization were identified from STS and NCDR registry records, whereas strokes after discharge were identified from Medicare inpatient claims with International Classification of Disease – Ninth Revision Clinical Modification diagnostic codes. Re-hospitalization for MI was identified from Medicare inpatient claims with ICD-9-CM diagnostic codes. Deaths were identified using the Medicare denominator file. All patients were followed from the time of their index revascularization procedure until December 31, 2008.
Propensity scores with inverse probability weighting (IPW) were used to adjust for differences between the two treatment groups [3]. The propensity score represents a patient’s probability of receiving CABG versus PCI as a function of hospital and patient characteristics. Propensity scores were estimated using a non-parsimonious logistic regression model which included 4 hospital-level covariates and 22 patient-level covariates. The ability of the propensity model to balance the two treatment groups was assessed by comparing hospital and patient characteristics across the two treatment groups before and after weighting. After propensity weighting, the observed differences in covariates were small and in all cases were less than 3% of the estimated standard deviation.
Follow-up of all endpoints was considered to be administratively censored on December 31, 2008. For analyzing the composite endpoint, unadjusted event rates were estimated using the Kaplan-Meier method and propensity-adjusted event rates were estimated using the propensity-weighted Breslow estimator. For analyzing stroke and MI, time to first event was analyzed using the cumulative incidence method to address the competing risk of mortality. For each endpoint, risk ratios comparing cumulative event rates for PCI versus CABG at 1 and 4 years were calculated. Pointwise 95% confidence intervals for these ratios were generated using the bootstrap. Comparisons of CABG with PCI were performed in the overall population and in pre-specified subgroups.
Variables for analysis were highly complete in both treatment arms for the majority of variables. Exceptions were ejection fraction (missing 21% in PCI, 4% in CABG) and GFR (missing 25% in PCI, 1% in CABG). For our primary analysis, missing values of continuous risk factors were imputed by stratifying on treatment group and combinations of other related risk factors. Categorical variables had <1% missing data and were imputed to the most common category.
RESULTS
Study Population
After application of exclusion criteria, there were 103,549 patients undergoing PCI and 86,244 patients undergoing CABG in the 644 sites participating in both the STS Database and the CathPCI Registry. In PCI patients 78% had drug eluting stents, 16% had bare metal stents and 6% had PCI without stents. Follow-up ranged from 1 to 5 years (average overall 2.72, CABG 2.82, PCI 2.63; median overall 2.67, CABG 2.83, PCI 2.53).
Before IPW adjustment, those undergoing PCI were older on average, more often women, and had more prior myocardial infarction, unstable angina and urgent procedures compared to CABG patients. PCI patients had more 2 vessel disease and CABG patients had more 3 vessel disease. There were more CABG patients with heart failure, diabetes, hypertension, chronic lung disease, cerebrovascular disease, smoking history and peripheral arterial disease. Ejection fraction was higher in PCI patients than in CABG patients. After IPW adjustment, all clinical covariates were well balanced.
Unadjusted Results
In the population prior to risk adjustment one year results demonstrated an MI rate of 1.18% for CABG and 2.58% for PCI, with a risk ratio (RR) of 0.46, 95% CI 0.42 to 0.49. The stroke rates were 2.43% and 1.21%, respectively (RR 2.01, 95% CI 1.86 to 2.15). The composite cumulative risk was 9.11% for CABG and 9.43% for PCI (RR 0.95, 95% CI 0.92 to 0.98).
Four year cumulative results in the unadjusted population showed an MI rate of 2.95% for CABG and 6.60% for PCI, with a risk ratio (RR) of 0.45, 95% CI 0.42 to 0.47. The stroke rates were 4.46% and 3.31%, respectively (RR 1.35, 95% CI 1.27 to 1.42). The composite cumulative risk was 20.95% for CABG and 26.95% for PCI (RR 0.78, 95% CI 0.76 to 0.79).
Adjusted Results
Myocardial Infarction Analysis
Propensity-adjusted cumulative MI event curves are displayed in Figure 1. At one year there was considerable difference in the IPW-adjusted AMI risk between the groups (1.2% and 2.6% for CABG and PCI, respectively; RR 0.48, 95% CI 0.42 to 0.53). The adjusted 4-year MI risk was 3.2% with CABG and 6.6% with PCI (risk ratio 0.49, 95% CI 0.45 to 0.53).
Figure 1.
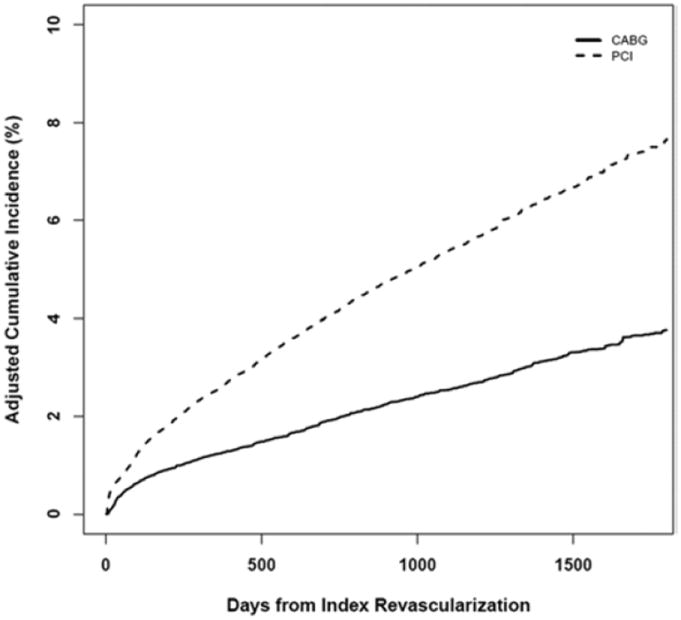
Cumulative Incidence of Myocardial Infarction
Stroke Analysis
Propensity-adjusted cumulative stroke event curves are displayed in Figure 2. There was a pronounced difference in the IPW-adjusted stroke risk between the groups at one year (2.5% and 1.2% for CABG and PCI, respectively; RR 2.12, 95% CI 1.90 to 2.34). The adjusted 4-year risk with CABG was 4.5% and with PCI was 3.2% (RR 1.43, 95% CI 1.31 to 1.54).
Figure 2.
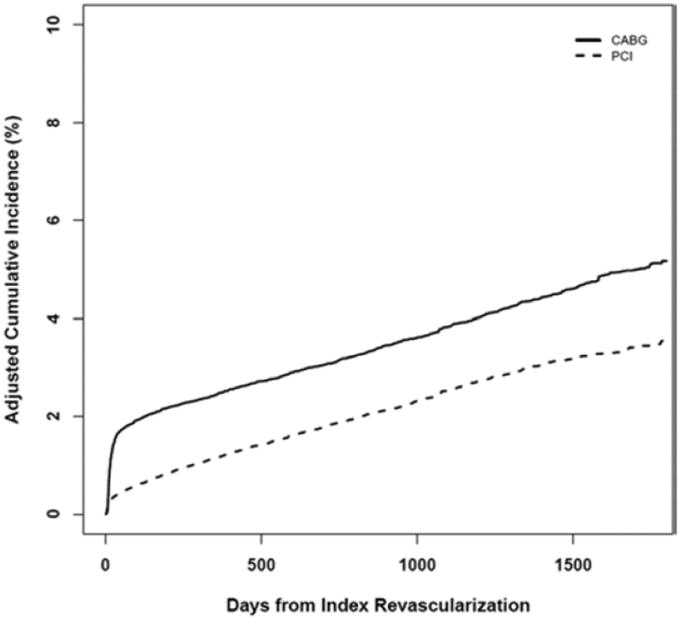
Cumulative Incidence of Stroke
Composite Analysis
Propensity-adjusted composite endpoint event curves are displayed in Figure 3. At one year there was little difference in the IPW-adjusted composite cumulative risk between the groups (9.1% and 9.4% for CABG and PCI, respectively; RR 0.97, 95% CI 0.92 to 1.01). The adjusted 4-year risk with CABG was 21.6% and with PCI was 26.7% (RR 0.81, 95% CI 0.78 to 0.83).
Figure 3.
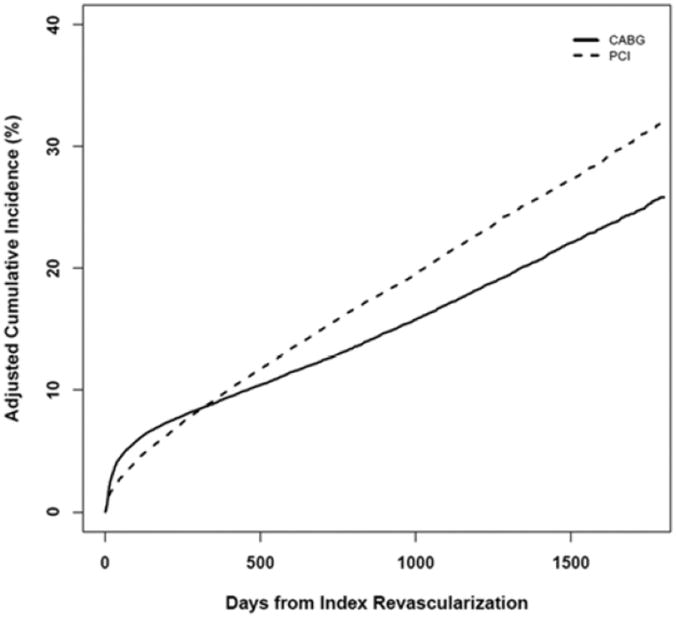
Cumulative Incidence of Composite
Four year risk ratios favored CABG across subgroups including gender, age, diabetes, BMI, chronic lung disease, ejection fraction, GFR, a high risk group and a low risk group, and by quintile of propensity for CABG. Figure 4 shows a forest plot for various clinical subgroups at 4 years following the index procedure.
Figure 4.
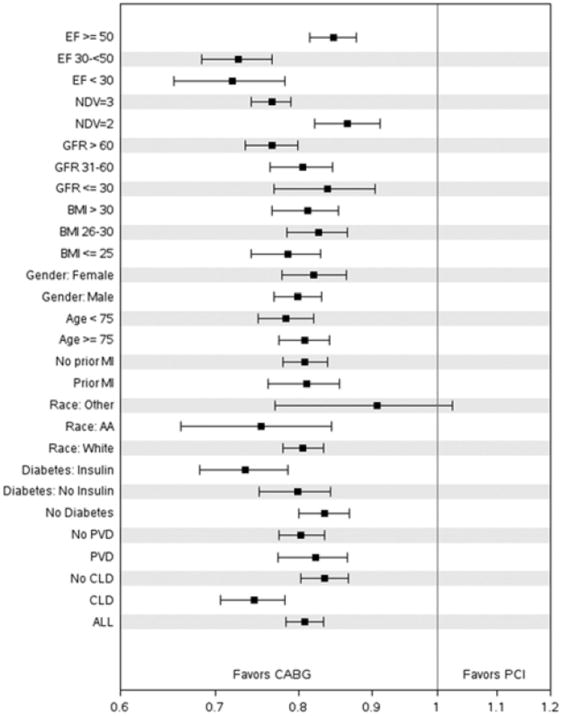
Forest Plot of Clinical Subgroups. Abbreviations :
AA = Afro-American
BMI = body mass index
CLD = chronic lung disease
EF = ejection fraction
GFR = glomerular filtration rate
NDV = number of diseased vessels
PVD = peripheral vascular disease
Sensitivity Analyses
Sensitivity analyses using alternative analytic methods yielded similar estimates. Depending on the choice of method, the estimated 4-year risk of the composite endpoint ranged from 26.6% to 26.8% for PCI and from 21.6% to 21.9% for CABG. The estimated hazard ratio (HR) for CABG versus PCI in each Cox model was similar to the 4-year risk-ratio estimated in the primary analysis (HR from covariate-adjusted Cox model = 0.78; HR from covariate-adjusted Cox model with stratification by hospital = 0.76; 4-year risk-ratio from primary IPW analysis = 0.80).
COMMENT
The ASCERT study has been developed from an unique collaboration between major professional organizations. STS and ACCF maintain mature, independently audited, nationally recognized registries having high participation rates and extensive clinical information on millions of patients having undergone coronary revascularization. Information from the STS and ACCF registries provides detailed peri-procedural clinical data with the ability to risk-adjust and identify important clinical subgroups, while the link to CMS data provides long-term follow-up information. Use of these large registries has made it possible to conduct the largest observational study comparing CABG with PCI. Broad enrollment in the clinical registries creates a study population that is truly national in scope. Furthermore, a “real-world” experience is assured by the fact that participating centers enter all patients undergoing revascularization into the registries. Using these resources, we found that the 4-year composite event rate of death, MI and stroke favored CABG. The individual risk of MI also favored CABG while the risk of stroke alone favored PCI.
While ASCERT does have unique strengths, it is fully recognized that comparative effectiveness research produced from this infrastructure must necessarily be retrospective and observational. As with all observational studies, the results of ASCERT must be evaluated with due consideration of the potential for selection bias and unmeasured confounders. Exhaustive statistical techniques have been applied to mitigate the influence of such factors, but one can never be completely certain of their true impact on observational study results. An analysis of propensity scores by quintiles further examines the potential for selection bias.
It seems most appropriate to consider results of both RCT and observational studies in making treatment decisions. The full analysis may be incomplete without considering both. Perhaps it is appropriate to regard each study type as a scissor blade - one blade is insufficient without the other. In this spirit, the ASCERT findings are best interpreted in the context of other studies.
Myocardial Infarction
Previous studies have shown that the occurrence of MI following PCI is either significantly higher [4-6] or approximately equal [7, 8] to the MI occurrence after CABG. The reports demonstrating higher PCI rates have follow-up greater than 18 months, thereby reflecting the long-term outcomes found in ASCERT. Also consistent with these studies is our finding that the MI event curves progressively diverge with time. This may be due to the higher number of PCI reinterventions coupled with periprocedural infarctions associated with those repeat procedures [9, 10]. Late stent thrombosis may also account for this finding. As with the evaluation of stroke, an assessment of the medical regimen is important since higher MI rates might be due to suboptimal medical treatment rather than procedural complications.
Placing our results in context with previous findings, it does appear that PCI is associated with significantly higher long-term myocardial infarction rates compared to CABG.
Stroke
Early findings in the SYNTAX trial demonstrated a higher stroke rate in CABG patients [11], but four-year[12] and five-year[13] results show no significant difference in CABG and PCI strokes[14]. Observational studies have also found similar risk-adjusted stroke rates for CABG and PCI at 3 years [6]. Two randomized trials showed no difference in neurologic outcome at 6 months, 1 year, and 5 years [15-17]. In a review of 23 RCTs, CABG was found to have a higher periprocedural stroke rate than PCI [7]. A recent randomized study of diabetic patients showed a significantly higher stroke rate in CABG patients compared to PCI patients after 5 years [18]. In an observational study with follow-up at 1 year, there was no significant difference in CABG and PCI stroke rates for patients with 3-vessel disease, but in patients with 2-vessel disease there was a significantly higher stroke rate with CABG [8].
Our findings demonstrate a higher periprocedural CABG stroke rate. In contrast to the SYNTAX findings, however, we did not observe a convergence of the event curves over time. Figure 2 shows that after the periprocedural period the curves are generally parallel, indicating similar long-term stroke rates for CABG and PCI.
The statistical balance achieved through propensity scoring should minimize the impact of patient covariates, so there is some indication that the difference in stroke rate is due to the procedure rather than comorbidity. Interpretation of these results, however, is challenging even if adequate risk adjustment is carried out. As demonstrated in the SYNTAX trial, an evaluation of long-term results requires an assessment of secondary prevention medical therapy[19].
Survival
The ASCERT survival results have been presented elsewhere [1]. At 4 years the CABG mortality was 16.4% compared to 20.8% for PCI. The CABG/PCI risk ratio was 0.79 (CI 0.76 to 0.82) at 4 years. These results are consistent with several recent observational studies [6, 8, 20-26] and at least 2 contemporary randomized trials [5, 13, 15]. Survival results of the ASCERT diabetic population were similar to the results reported in a recent randomized study of diabetics [18]. A pooled analysis of 7,812 patients in 10 RCT demonstrated a CABG survival advantage in patients over 65 years of age (HR = 0.82; CI 0.70 to 0.97).[27]
Certainly in the stent era there are small randomized trials [28] showing no survival advantage of CABG compared to PCI, but the majority of larger studies are consistent with the survival results found in ASCERT.
Composite
Composites are often used in relatively small studies to obtain improved statistical power by combining individual outcomes into a single measure. The large study herein has ample statistical power, but it nevertheless seems helpful to analyze a composite in order to obtain a metric that consolidates the impact of individual outcomes.
This composite has been used in three recent reports. In the SYNTAX trial results at 5 years [13] and the Asian Revascularization Registry experience at 5 years [29], there was no significant CABG-PCI difference in this composite. Other studies reported a statistically significant advantage for CABG, particularly in the diabetic population [8, 18].
In the ASCERT composite analysis, we found an adjusted and unadjusted advantage for CABG over PCI. The average age of the ASCERT population is higher than that of SYNTAX and the Asian Registry, which may contribute to the difference in composite findings of ASCERT compared to those studies. The IPW-adjusted risk ratios (CABG/PCI) for each element of the composite are 0.79 for survival, 0.49 for MI, and 1.43 for stroke. In the composite IPW-adjusted analysis, the risk ratio was 0.81 at 4 years, indicating a 19% advantage for the CABG cohort.
Limitations
The use of mature clinical registries provides a massive amount of valuable clinical information, but the databases do have some limitations. While harmonization of definitions has been addressed by both STS and ACCF, there are inherent specialty differences that preclude total consistency. This leads to occasional small variations in definitions between the two registries. Missing data are infrequent, but higher rates of missing data are seen in some data elements such as glomerular filtration rate and ejection fraction. Standard statistical approaches have been used to address the influence of missing data.
Matching clinical data with CMS claims data was carried out using probabilistic matching. This may be less reliable than using unique patient identifiers, but the probabilistic approach has high matching accuracy rates [30].
In addition, this study exclusively uses a Medicare population, so it may be inadvisable to extrapolate ASCERT findings to patients less than 65 years of age.
Conclusions
In the largest study comparing CABG and PCI outcomes, 4 years following revascularization there is a PCI advantage for stroke and a CABG advantage for MI. A composite of stroke, MI, and death demonstrates a 19% advantage for CABG compared to PCI. This information is largely consistent with previous findings and should be shared in a “heart team” approach with full engagement of patients, surgeons, and cardiologists focused on a collegial search for optimal treatment.
Acknowledgments
The ASCERT Study is supported by Award Number RC2HL101489 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of The National Heart, Lung, and Blood Institute or the National Institutes of Health, The Society of Thoracic Surgeons or The American College of Cardiology Foundation. This award was issued under the American Recovery and Reinvestment Act of 2009.
Footnotes
Meeting Presentation : 49th Annual Meeting of The Society of Thoracic Surgeons J. Maxwell Chamberlain Memorial Paper Los Angeles January 2013
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’Brien SM, Peterson ED, Kolm P, et al. Comparative Effectiveness of Revascularization Strategies. N Engl J Med. 2012 Mar 27;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs JP, Edwards FH, Shahian DM, Prager RL, Wright CD, Puskas JD, et al. Successful linking of the Society of Thoracic Surgeons database to social security data to examine survival after cardiac operations. Ann Thorac Surg. 2011 Jul;92(1):32–7. doi: 10.1016/j.athoracsur.2011.02.029. discussion 38-9. [DOI] [PubMed] [Google Scholar]
- 3.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004 Jul;75(1):45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Hannan EL, Wu C, Walford G, Culliford AT, Gold JP, Smith CR, et al. Drug-Eluting Stents vs. Coronary-Artery Bypass Grafting in Multivessel Coronary Disease. N Engl J Med. 2008 Jan 24;358(4):331–41. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 5.Kappetein AP, Feldman TE, Mack MJ, Morice MC, Holmes DR, Stahle E, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011 Sep;32(17):2125–34. doi: 10.1093/eurheartj/ehr213. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zheng Z, Xu B, Zhang S, Li W, Gao R, et al. Comparison of drug-eluting stents and coronary artery bypass surgery for the treatment of multivessel coronary disease: three-year follow-up results from a single institution. Circulation. 2009 Apr 21;119(15):2040–50. doi: 10.1161/CIRCULATIONAHA.108.819730. [DOI] [PubMed] [Google Scholar]
- 7.Bravata DM, Gienger AL, McDonald KM, Sundaram V, Perez MV, Varghese R, et al. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med. 2007 Nov 20;147(10):703–16. doi: 10.7326/0003-4819-147-10-200711200-00185. [DOI] [PubMed] [Google Scholar]
- 8.Javaid A, Steinberg DH, Buch AN, Corso PJ, Boyce SW, Pinto Slottow TL, et al. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention with drug-eluting stents for patients with multivessel coronary artery disease. Circulation. 2007 Sep 11;116(11 Suppl):I200–6. doi: 10.1161/CIRCULATIONAHA.106.681148. [DOI] [PubMed] [Google Scholar]
- 9.Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005 Mar 1;111(8):1027–32. doi: 10.1161/01.CIR.0000156328.28485.AD. [DOI] [PubMed] [Google Scholar]
- 10.Taggart DP. Coronary Artery Bypass Grafting is Still the Best Treatment for Multivessel and Left Main Disease, But Patients Need to Know. Ann Thorac Surg. 2006 Dec 1;82(6):1966–75. doi: 10.1016/j.athoracsur.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Serruys PW, Morice M-C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med. 2009 Mar 5;360(10):961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 12.SYNTAX at four years : Death rates diverge but no change in advice. 2011 [cited 2011 10/19/2011]; Available from: http://www.theheart.org/article/1293047.do.
- 13.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013 Feb 23;381(9867):629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 14.Mack MJ, Head SJ, Holmes DR, Jr, Stahle E, Feldman TE, Colombo A, et al. Analysis of Stroke Occurring in the SYNTAX Trial Comparing Coronary Artery Bypass Surgery and Percutaneous Coronary Intervention in the Treatment of Complex Coronary Artery Disease. JACC Cardiovasc Interv. 2013 Apr;6(4):344–54. doi: 10.1016/j.jcin.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Booth J, Clayton T, Pepper J, Nugara F, Flather M, Sigwart U, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS) Circulation. 2008 Jul 22;118(4):381–8. doi: 10.1161/CIRCULATIONAHA.107.739144. [DOI] [PubMed] [Google Scholar]
- 16.Hlatky MA, Bacon C, Boothroyd D, Mahanna E, Reves JG, Newman MF, et al. Cognitive function 5 years after randomization to coronary angioplasty or coronary artery bypass graft surgery. Circulation. 1997 Nov 4;96(9 Suppl):II-11–4. discussion II-15. [PubMed] [Google Scholar]
- 17.Wahrborg P, Booth JE, Clayton T, Nugara F, Pepper J, Weintraub WS, et al. Neuropsychological outcome after percutaneous coronary intervention or coronary artery bypass grafting: results from the Stent or Surgery (SoS) Trial. Circulation. 2004 Nov 30;110(22):3411–7. doi: 10.1161/01.CIR.0000148366.80443.2B. [DOI] [PubMed] [Google Scholar]
- 18.Farkouh ME, Domanski M, Fuster V. Revascularization strategies in patients with diabetes. N Engl J Med. 2013 Apr 11;368(15):1455–6. doi: 10.1056/NEJMc1301244. [DOI] [PubMed] [Google Scholar]
- 19.Smith PK. Treatment Selection for Coronary Artery Disease: The Collision of a Belief System with Evidence. Ann Thorac Surg. 2009 May 1;87(5):1328–31. doi: 10.1016/j.athoracsur.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation. 2004 May 18;109(19):2290–5. doi: 10.1161/01.CIR.0000126826.58526.14. [DOI] [PubMed] [Google Scholar]
- 21.Hannan EL, Racz M, Holmes DR, Walford G, Sharma S, Katz S, et al. Comparison of Coronary Artery Stenting Outcomes in the Eras Before and After the Introduction of Drug-Eluting Stents. Circulation. 2008 Apr 22;117(16):2071–78. doi: 10.1161/CIRCULATIONAHA.107.725531. [DOI] [PubMed] [Google Scholar]
- 22.Hannan EL, Racz MJ, Walford G, Jones RH, Ryan TJ, Bennett E, et al. Long-Term Outcomes of Coronary-Artery Bypass Grafting versus Stent Implantation. N Engl J Med. 2005 May 26;352(21):2174–83. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 23.Malenka DJ, Leavitt BJ, Hearne MJ, Robb JF, Baribeau YR, Ryan TJ, et al. Comparing long-term survival of patients with multivessel coronary disease after CABG or PCI: analysis of BARI-like patients in northern New England. Circulation. 2005 Aug 30;112(9 Suppl):I371–6. doi: 10.1161/CIRCULATIONAHA.104.526392. [DOI] [PubMed] [Google Scholar]
- 24.Smith PK, Califf RM, Tuttle RH, Shaw LK, Lee KL, Delong ER, et al. Selection of Surgical or Percutaneous Coronary Intervention Provides Differential Longevity Benefit. Ann Thorac Surg. 2006 Oct 1;82(4):1420–29. doi: 10.1016/j.athoracsur.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 25.van Domburg RT, Takkenberg JJM, Noordzij LJ, Saia F, van Herwerden LA, Serruys PWJC, et al. Late Outcome After Stenting or Coronary Artery Bypass Surgery for the Treatment of Multivessel Disease: A Single-Center Matched-Propensity Controlled Cohort Study. Ann Thorac Surg. 2005 May 1;79(5):1563–69. doi: 10.1016/j.athoracsur.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Hlatky MA, Boothroyd DB, Baker L, Kazi DS, Solomon MD, Chang TI, et al. Comparative Effectiveness of Multivessel Coronary Bypass Surgery and Multivessel Percutaneous Coronary Intervention: A Cohort Study. Ann Intern Med. 2013 Apr 23; doi: 10.7326/0003-4819-158-10-201305210-00639. [cited; 2013/04/24:[Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=23609014. [DOI] [PMC free article] [PubMed]
- 27.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009 Apr 4;373(9670):1190–7. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez AE, Baldi J, Fernandez Pereira C, Navia J, Rodriguez Alemparte M, Delacasa A, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II) J Am Coll Cardiol. 2005 Aug 16;46(4):582–8. doi: 10.1016/j.jacc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 29.Park DW, Kim YH, Song HG, Ahn JM, Oh J, Kim WJ, et al. Long-term comparison of drug-eluting stents and coronary artery bypass grafting for multivessel coronary revascularization: 5-year outcomes from the Asan Medical Center-Multivessel Revascularization Registry. J Am Coll Cardiol. 2011 Jan 11;57(2):128–37. doi: 10.1016/j.jacc.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Douglas PS, Brennan JM, Anstrom KJ, Sedrakyan A, Eisenstein EL, Haque G, et al. Clinical effectiveness of coronary stents in elderly persons: results from 262,700 Medicare patients in the American College of Cardiology-National Cardiovascular Data Registry. J Am Coll Cardiol. 2009 May 5;53(18):1629–41. doi: 10.1016/j.jacc.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


