Abstract
Objective
To compare the risk of giving birth to large for gestational age infants in women with and without preeclampsia, after adjustment for obesity and glucose intolerance.
Study Design
Prospective cohort study of pregnant women with and without preeclampsia who delivered infants between 1998 and 2006 at Massachusetts General Hospital.
Results
The risk of LGA was similar in women with and without preeclampsia (OR 0.81 95% CI 0.59–1.14). After adjustment for body mass index, glucose intolerance, and other factors, the risk of LGA was significantly lower in women with preeclampsia compared to those without preeclampsia (OR 0.69 95% CI 0.49–0.96). Stratified analysis in groups with a higher risk of LGA revealed that preeclampsia has a similar effect on the risk of LGA regardless of maternal obesity, glucose intolerance, parity, and race
Conclusion
Preeclampsia appears to be characterized by reduced, and not increased, fetal growth.
Keywords: fetal growth, gestational diabetes, large for gestational age, preeclampsia
INTRODUCTION
Preeclampsia is a systemic syndrome unique to pregnancy, characterized clinically by new onset hypertension and proteinuria after 20 weeks gestation.1 An important initiating event in the pathogenesis of preeclampsia is thought to be abnormal placentation with shallow invasion of the placental cytotrophoblast and consequent compromised placental perfusion.2–5 This altered placentation is thought to lead to placental ischemia and hypoxia, triggering the release of circulating factors which lead to systemic endothelial dysfunction.6 If placental ischemia is a critical pathogenic factor in preeclampsia, one would expect this disorder to be associated with reduced fetal growth. Consistent with this, the risk of fetal growth restriction and small size for gestational age among infants born to women with preeclampsia is reported to be 2–5 times the risk among infants born to women without preeclampsia.7–9 Recently, however, several epidemiologic studies have reported an association between large for gestational age (LGA) infants and preeclampsia.10–13 These studies challenge experimental and human data suggesting that placental hypoperfusion and ischemia are important to the pathogenesis of preeclampsia.5, 14–16 In fact, in light of these reports, some have suggested that preeclampsia may be two diseases with two different biological processes, one which results in reduced fetal growth, and one which results in increased fetal growth.12 Clarity on this matter is needed to guide clinicians caring for women with preeclampsia, and importantly directing future clinical and experimental research in preeclampsia.
Obesity and glucose intolerance are some of the most common risk factors for both preeclampsia and large for gestational age infants.17–22 Infant birth weight is strongly associated with maternal pre-pregnancy body mass index, independent of gestational weight gain,23 and among obese women the risk of preeclampsia is elevated by 2–3 fold.24–25 Similarly, maternal glucose intolerance is linearly associated with large for gestational age infants and outcomes related to infant size, including birth injury and cesarean delivery.26 Glucose intolerance also increases the risk for preeclampsia; in fact, treatment of gestational diabetes mellitus with diet or insulin decreases the risk of preeclampsia by half.17, 27–28 While some studies reporting an excess risk of large for gestational age infants in preeclampsia excluded women with pre-gestational diabetes mellitus from their analyses, none adjusted for body mass index or controlled for more subtle variation in glucose intolerance.10–13 In addition, other factors such as smoking, which is directly associated with small for gestational age infants and less frequent in women with preeclampsia,29–30 may have falsely inflated the rate of large for gestational age infants in preeclampsia (i.e. not smoking increases the risk for both preeclampsia and LGA).
The Massachusetts General Hospital (MGH) birth database includes detailed information on all women who enrolled in prenatal care at MGH and affiliated health centers between 1998 and 2006. We hypothesized that the reported excess risk of large for gestational age infants in preeclampsia could be accounted for by confounding by obesity, glucose intolerance, and other factors associated with preeclampsia and fetal growth. We sought to test this hypothesis in our cohort by comparing the risk of delivering a large for gestational age infant in women with and without preeclampsia after adjustment for possible confounding factors.
MATERIALS AND METHODS
Subjects and Data Collection
We performed a study of pregnancies in the Massachusetts General Hospital obstetric service birth database between September 1, 1998 and December 31, 2006. This database contains clinical information on all women who enroll in prenatal care at Massachusetts General Hospital or one of its affiliated health care centers. The Massachusetts General Hospital obstetrics service provides community-based obstetrics care for women from the metropolitan Boston area and high-risk obstetrics care for women referred from throughout New England. This cohort represents a population of women from varied ethnic and socioeconomic backgrounds with 38% of patients being ethnic minorities. Clinical information such as medical histories, prenatal blood pressures, and delivery information are entered into the database prospectively.
For this study we included all singleton pregnancies in women who enrolled in prenatal care during the study period (1998–2006, n=22,980). The database contains information downloaded from the obstetrical electronic medical record that was entered prospectively during the incident pregnancy. These data include maternal age, body mass index, race, and smoking status at the first prenatal visit. They also include and information on blood pressure, results of urine dipstick testing throughout pregnancy and the results of a 50 gram, 1 hour, oral glucose loading test (GLT) performed routinely at 24–28 weeks gestation to screen for gestational diabetes mellitus and from a 100 gram, 3 hour, oral glucose tolerance test performed if the glucose loading test result was abnormal (≥140 mg/dl).31 Finally the database contains data from the delivery including infant birth weight, and maternal and infant complications. We excluded pregnancies with missing blood pressure data from the first prenatal visit (n=3124), missing height or weight data (n=2135) from the first prenatal visit, or missing birth weight data from delivery (n=90). We also excluded women who did not have a recorded glucose loading test result, as they may have had pre-gestational diabetes (n=166). This left 17,465 pregnancies for analysis.
Ascertainment of Exposures and Outcomes
The diagnosis of preeclampsia was based on blood pressures and spot urine protein measurements made at prenatal visits. In women who were normotensive at their first prenatal visit (blood pressure <140/90), gestational hypertension was defined as blood pressure greater than or equal to 140/90 after 20 weeks gestation. In women who were hypertensive at their first prenatal visit (blood pressure greater than or equal to 140/90) gestational hypertension was defined by the presence of a rise in systolic blood pressure greater than 30 mm Hg or a rise in diastolic blood pressure greater than 15 mm Hg after 20 weeks gestation. Cases of preeclampsia were women with gestational hypertension and greater than 2+ proteinuria after 20 weeks gestation (n=386) or gestational hypertension and greater than 1+ proteinuria after 20 weeks gestation with confirmation of the diagnosis in the electronic delivery record (n=102). Obesity was defined as body mass index ≥30 kg/m2 at the first prenatal visit. Nulliparity was defined as never having had a previous live birth >20 weeks gestation. Gestational diabetes was defined as greater than or equal to 2 abnormal values on a glucose tolerance test by Carpenter-Coustan criteria.31 Large for gestational age was defined as the 90th percentile for completed week of gestation based on national standards from Oken et al.32 Small for gestational age was defined as the 10th percentile for completed week of gestational age based on the same national standards.
Statistical Analysis
Characteristics of women with and without preeclampsia were compared using Student’s t-tests and chi-squared tests as appropriate. Univariate and multivariate logistic regression models were used to compare the odds of delivering a large for gestational age infant in women with and without preeclampsia both before and after stratification for obesity, gestational diabetes, and parity. Multivariate logistic regression models included variables associated with preeclampsia and size for gestational age. Statistical analyses were conducted using STATA 11 (Stata Corporation, College Station, TX).
RESULTS
At the first prenatal visit, women who subsequently developed preeclampsia (n=474, 2.8%) had similar ages and gestational ages, but higher average body mass index and blood pressure compared with those who did not go on to develop preeclampsia (Table 1). Women who subsequently developed preeclampsia were more likely to be obese, nulliparous, and Hispanic than women who did not subsequently develop preeclampsia. Women who subsequently developed preeclampsia were less likely to have smoked, but this did not reach statistical significance. Women who developed preeclampsia had higher glucose loading test results and a higher rate of gestational diabetes mellitus. Infants of women with preeclampsia were delivered earlier, and likely as a consequence, had a smaller mean birth weight and were admitted to the neonatal intensive care unit more often than infants born to mothers without preeclampsia. Pregnancies complicated by preeclampsia resulted in delivery of small for gestational age infants more often than in pregnancies not complicated by preeclampsia (15.9% vs. 7.3%; Table 1). Large for gestational age infants were slightly less common in pregnancies complicated by preeclampsia compared with those that were not, but this did not reach statistical significance. (8.4% vs 10.1%, p=0.230; Table 1).
Table 1.
Characteristics of Subjects with and without Preeclampsia
| Control | Preeclampsia | p-value | |
|---|---|---|---|
| N=16991 | N=474 | ||
| First Prenatal Visit | |||
| Gestational Age (weeks) | 12.8 (4.9) | 12.3 (3.9) | 0.286 |
| Age (years) | 30.9 (5.7) | 31.3 (5.9) | 0.169 |
| BMI (kg/m2) | 25.3 (5.2) | 28.4 (6.1) | <0.0001* |
| %Obese | 15.4% | 33.1% | <0.001* |
| % Nulliparous | 48.7% | 65.2% | <0.001* |
| % Caucasian | 62.8% | 59.7% | 0.167 |
| % Hispanic | 19.9% | 25.7%% | 0.002* |
| Systolic Blood Pressure (mmHg) | 111 (11.1) | 116 (10.5) | <0.0001* |
| Diastolic Blood Pressure (mmHg) | 68.5 (8.4) | 72.6 (8.2) | <0.0001* |
| % Current Smoker | 6.2% | 4.4% | 0.120 |
| Third Trimester | |||
| GLT Result (mg/dl) | 115 (28.6) | 124 (30.2) | <0.0001* |
| % GDM | 3.5% | 7.4% | 0.001* |
| Gestational Age (weeks) | 39.5 (1.6) | 38.1 (2.4) | <0.0001* |
| Birth Weight (g) | 3429 (519) | 3108 (743) | <0.0001* |
| % LGA | 10.1% | 8.4% | <0.230 |
| % SGA | 7.3% | 15.0% | <0.001* |
| % NICU Admission | 2.7% | 12.0% | <0.001* |
BMI=body mass index; GLT result=glucose loading test result, blood glucose 1 hour after the ingestion of a 50g oral glucose load; % LGA=percent of women delivering a large for gestational age infant (>90th percentile); % SGA=percent of women delivering a small for gestational age infant (<10th percentile); %NICU Admission=percent of women delivering an infant who was admitted to the neonatal intensive care unit
As detailed in Table 2, women who delivered large for gestational age infants were less likely to be nulliparous and more likely to be Caucasian. At the first prenatal visit women who subsequently delivered a large for gestational age infant were older and had higher blood pressures. Women who delivered a large for gestational age infant had higher glucose loading test results and a higher incidence of gestational diabetes mellitus as defined by Carpenter-Coustan criteria.31
Table 2.
Characteristics of Women Delivering Infants of Appropriate, Large, and Small Size for Gestational Age
| AGA | LGA | P-value | SGA | P-value | |
|---|---|---|---|---|---|
| N=14397 | N=1760 | N=1308 | |||
| First Prenatal Visit | |||||
| Gestational Age | 12.7 (4.9) | 12.5 (4.6) | 0.046* | 13.3 (5.7) | <0.001* |
| Age | 30.9 (5.7) | 31.9 (5.3) | <0.001* | 29.9 (6.2) | <0.001* |
| BMI | 25.3 (5.2) | 27.1 (5.8) | <0.001* | 24.7 (5.2) | <0.001* |
| %Obese | 15.0% | 24.8% | <0.001* | 13.8% | 0.247 |
| % Nulliparous | 49.7% | 35.3% | <0.001* | 63.1% | <0.001* |
| % White race | 61.6% | 71.3% | <0.001* | 50.1% | <0.001* |
| % Hispanic | 20.0% | 16.8% | 0.001* | 24.8% | <0.001% |
| Current Smoker | 5.8% | 5.1% | 0.216 | 11.0% | <0.001* |
| SBP at PNV | 111 (11.0) | 113 (11.1) | <0.001* | 110 (12.2) | 0.199 |
| DBP at PNV | 68.4 (8.3) | 70.1 (8.3) | <0.001* | 68.1 (9.0) | 0.130 |
| Third Trimester | |||||
| GLT Result | 115 (28.6) | 122 (29.7) | <0.001* | 110 (25.9) | <0.001* |
| %GDM | 3.4% | 6.5% | <0.001* | 1.8% | 0.004* |
BMI=body mass index; GLT result=glucose loading test result, blood glucose 1 hour after the ingestion of a 50g oral glucose load.
In univariate analyses, the risk of delivering a large for gestational age infant was not significantly different in women with and without preeclampsia (OR 0.81 95% CI 0.59–1.14; Table 3). However, after adjustment for body mass index, the risk of LGA was significantly lower in women with preeclampsia compared to women without preeclampsia (Table 3). After adjustment for glucose loading test result, a measure of glucose tolerance, and nulliparity, the risk of LGA in women with preeclampsia remained about 30% lower in women with preeclampsia. In a multivariate analysis after adjustment for nulliparity, body mass index, glucose loading test result, current smoking, diastolic blood pressure at baseline, and non-white race, the odds ratio of delivering a large for gestational age infant in women with preeclampsia remained significantly lower when compared to women without preeclampsia (Table 3). When only women who were normotensive (BP<140/90) at the first prenatal visit were considered, the risk of LGA in women with preeclampsia were considered, these results were not changed (multivariate OR 0.65, 95% CI 0.46–0.91) When only women at term (delivery ≥ 37 weeks of gestation) were considered, the risk of LGA in women with preeclampsia was slightly lower than in women without preeclampsia, but this difference did not reach statistical significance (multivariate OR 0.84, 95% CI 0.59–1.18).
Table 3.
Risk of Abnormal Growth in Women with and without Preeclampsia
| % LGA | %SGA | |
|---|---|---|
| Control | 10.1% | 7.3% |
| Preeclampsia | 8.4% | 15.0% |
| Adjustment | OR (95% CI) for LGA | OR (95% CI) for SGA |
| Univariate | 0.81 (0.59, 1.14) | 2.24 (1.73, 2.91)* |
| BMI | 0.67 (0.48, 0.93)* | 2.50 (1.93, 3.25)* |
| BMI, GLT | 0.63 (0.45, 0.88)* | 2.64 (2.03, 3.44)* |
| BMI, GLT, Nulliparity | 0.70 (0.50, 0.98)* | 2.38 (1.83, 3.10)* |
| BMI, GLT, DBP at baseline, | 0.69 (0.49, 0.96)* | 2.46 (1.88, 3.21)* |
| Race, Smoking, Nulliparity, Age |
OR=odds ratio, CI=confidence interval, BMI=body mass index; GLT result=glucose loading test result, blood glucose 1 hour after the ingestion of a 50g oral glucose load; % LGA=percent of women delivering a large for gestational age infant (>90th percentile); % SGA=percent of women delivering a small for gestational age infant(<10th percentile); DBP=diastolic blood pressure.
Significant at the p<0.05 level.
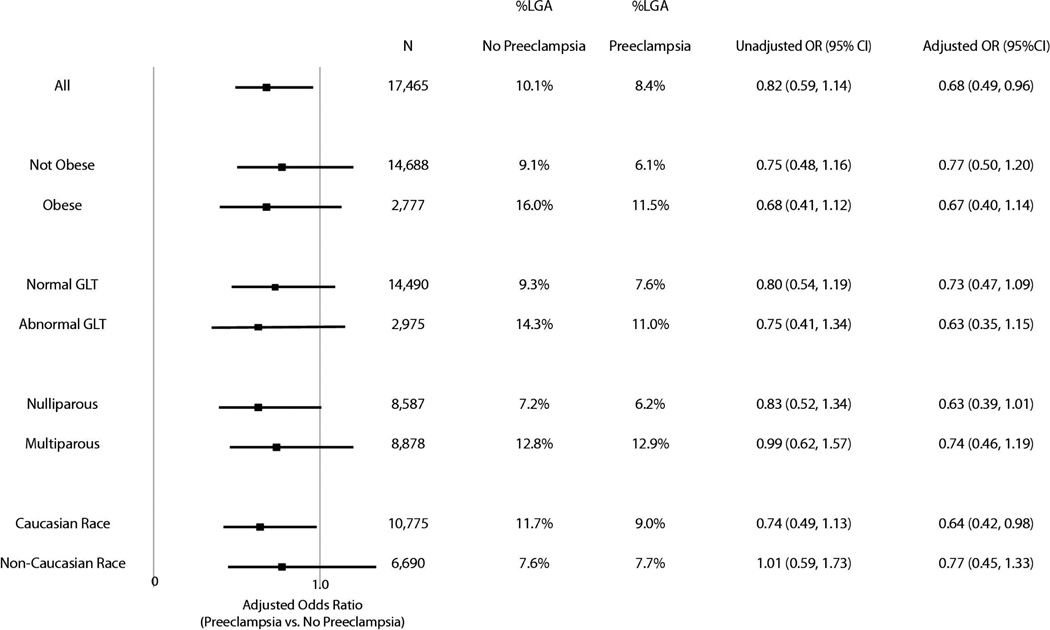
Stratified analysis revealed that preeclampsia had a similar effect on the risk of LGA in obese and non-obese women, nulliparous and multiparous women, women with and without an abnormal glucose loading test result, and white and non-white women. (Figure 1). After exclusion of obese women, women with abnormal glucose loading test results (≥140 mg/dl), and multiparous women, the risk of delivering an LGA infant among women without preeclampsia was 6.1% while the risk of delivering an LGA infant among women with preeclampsia was 5.1% (Adjusted OR 0.77 95% CI 0.39–1.52.)
Figure 1. Risk of LGA by Obesity, GLT Result, Parity, and Race.
Black squares represent the adjusted odds ratio in a given subgroup while lines depict the 95% confidence interval for the adjusted OR. The unadjusted risk of LGA in each subgroup as well as the adjusted OR and 95% confidence interval are shown in the table to the right. GLT=Result from the 50g, 1 hour, glucose loading test. ≥140 mg/dL was considered abnormal.
COMMENT
Among a large cohort of women receiving prenatal care at a large New England hospital, we found no evidence of risk of large for gestational age infants in preeclampsia. In fact, the risk of delivering a large for gestational age infant was significantly decreased among women with preeclampsia after adjustment for possible confounding factors including obesity, glucose intolerance, smoking, race, and nulliparity. We found no evidence that any subtype of preeclampsia was associated with a greater risk for large for gestational age infants. Although our data conflicts with data from previous studies which reported an increased risk of large for gestational age in preeclampsia,10–13 our results are consistent with previous experimental and human data which suggest that preeclampsia is characterized by abnormal placentation, placental ischemia, and subsequent reduced fetal growth.2–5, 9, 14, 16
Obesity is a known risk factor for both preeclampsia and the delivery of large for gestational age infants; our data confirm this relationship.18–21 The relationship between body mass index and the risk of delivering a large for gestational age infant is continuous and consistent across all levels of body mass index, making it essential to adjust for this factor to determine the true effect of preeclampsia on the risk of delivering a large for gestational age infant. While most of the studies reporting an excess risk of large for gestational age infants did not adjust for body mass index, one study which found an excess risk for large for gestational age infants in preeclampsia did adjust for body weight greater than 200 lbs.13 This simple stratification was likely inadequate both because of the continuous relationship between body mass index and the risk of large for gestational age and because many women classified as obese weigh less than 200 lbs. While we did not find an increased risk of large for gestational age infants born to women with preeclampsia even before adjustment for obesity, based on the decrease in the odds ratio after adjustment for body mass index in our own study and the magnitude of the increased risk of large for gestational age infants in preeclampsia found in other studies (odds ratios of 1.4–1.9),10–13 the excess risk reported in these studies might likely have been abolished after adjustment for body mass index. Interestingly, the risk of delivering a large for gestational age infant appeared to be higher in obese women with preeclampsia than in non-obese women without preeclampsia (see Figure 1). This demonstrates how in prior studies, without adjustment for obesity, the risk of large for gestational age infants may have appeared greater in women with preeclampsia than in women without the disease. However, our multivariate analysis shows that preeclampsia had a negative effect of similar magnitude on the risk of delivering a large for gestational age infant in both obese and non-obese women.
Independent of body mass index, glucose intolerance is also associated with an elevated risk of both large for gestational age infants and preeclampsia.17, 19, 26, 28 Maternal hyperglycemia is thought to cause infant hyperinsulinemia, which leads to fat accumulation in the infant (Pedersen hypothesis).33 The mechanism by which glucose intolerance increases the risk of preeclampsia is unclear, but given that preeclampsia is a disease of the endothelium, glucose intolerance may increase the susceptibility of the endothelium to injury as it does in type 2 diabetes mellitus.34 Likely because gestational diabetes mellitus and preeclampsia have opposite effects on fetal growth, in our study women with both conditions had a similar risk of LGA to that of women without either disease. Previous studies finding an excess risk of LGA in women with preeclampsia have excluded women with diabetes mellitus from the study population, but did not adjust for glucose intolerance, an exposure we now know is linked to fetal weight in a linear fashion.10–13 Thus, confounding by both obesity and glucose intolerance likely contributed to the higher proportion of large for gestational age infants born to mothers with preeclampsia in other study populations. Of note, clinicians at the Massachusetts General Hospital and affiliated health centers use the National Diabetes Data Group31 criteria to diagnose gestational diabetes mellitus, not the Carpenter-Coustan criteria,31 as was used in this study. Treatment of GDM may lower rates of LGA and preeclampsia,26–28 thus reducing strength of an apparent association between these two conditions. Thus, past studies may have found a stronger association between PE and LGA if criteria for GDM treatment differed from that in our cohort. We used the glucose loading test result as a continuous measure of glucose tolerance in multivariate models, rather than the diagnosis of gestational diabetes, in an attempt to account for the linear relationship between glucose tolerance and infant birth weight regardless of gestational diabetes diagnosis.
Strengths of our study include the use of clinical data including blood pressure, urine protein measurements, body mass index, and glucose loading test results. In previous studies, the use of hospital discharge or registry data may have led to misclassification of preeclampsia cases, which might have falsely inflated the risk of large for gestational age infants. Furthermore, the detailed information which we collected on each subject in the cohort allowed us to adjust for possible confounders. Limitations of the study include the fact that in classifying women, we only used blood pressures measured at prenatal visits, rather than also when women were hospitalized. Therefore we may have missed some cases of preeclampsia. However, if we had relied on the clinician's diagnosis on the delivery record we would have found an even lower risk of large for gestational age infants in pregnancies complicated by preeclampsia (unpublished observations). Thus, we are likely to have underestimated the magnitude of decreased risk for large for gestational age infants in women with preeclampsia. Finally, this study was based at a single large tertiary care hospital; thus the results may not be generalizable to all pregnant women.
In conclusion, among a large, diverse cohort of pregnant women, we found a decreased risk of large for gestational age infants among women with preeclampsia. Previous studies in which an excess risk of large for gestational age infants in preeclampsia was reported may not have adequately adjusted for obesity, glucose tolerance, and other possible confounders associated with both preeclampsia and large for gestational age infants.10–13 This may have led to a falsely inflated risk of large for gestational age infants in pregnancies complicated by preeclampsia. In preeclamptic, nulliparous, non-obese women, without abnormal glucose testing, the risk of delivering a large for gestational infant was very low, around 5%. Our data imply that had these women not had preeclampsia, their infants would have been even larger. The residual risk of large for gestational age infants in preeclampsia is likely due to both unmeasured and unknown factors including genetic factors which lead to larger birth weight. Consistent with the prevailing paradigm for its pathogenesis, preeclampsia in our cohort appears to be an entity characterized by reduced fetal growth.15 Future studies should determine whether this holds true in other populations.
ACKNOWLEDGEMENTS
C.E.P is a Howard Hughes Medical Institute Medical Research Training Fellow. Dr. Levine receives salary support from the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. S.A.K is an investigator of the Howard Hughes Medical Institute. We thank Zahra Kanji for assistance with the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 2.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol. 1994;83:378–386. [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Lang JM, Cohen A, Lieberman E. Risk factors for small-for-gestational-age birth in a preterm population. Am J Obstet Gynecol. 1992;166:1374–1378. doi: 10.1016/0002-9378(92)91607-c. [DOI] [PubMed] [Google Scholar]
- 8.Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol. 1993;169:1112–1118. doi: 10.1016/0002-9378(93)90265-k. [DOI] [PubMed] [Google Scholar]
- 9.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–955. [PubMed] [Google Scholar]
- 10.Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003;101:575–583. doi: 10.1016/s0029-7844(02)03071-5. [DOI] [PubMed] [Google Scholar]
- 11.Eskild A, Romundstad PR, Vatten LJ. Placental weight and birthweight: does the association differ between pregnancies with and without preeclampsia? Am J Obstet Gynecol. 2009;201:595 e1–595 e5. doi: 10.1016/j.ajog.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 13.Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for age. Am J Obstet Gynecol. 2000;183:148–155. doi: 10.1067/mob.2000.105735. [DOI] [PubMed] [Google Scholar]
- 14.Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 15.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 16.Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffe GM, Esterlitz JR, Levine RJ, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1998;179:1032–1037. doi: 10.1016/s0002-9378(98)70210-8. [DOI] [PubMed] [Google Scholar]
- 18.Rowan JA, Gao W, Hague WM, Mcintyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 21.Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- 22.Gross T, Sokol RJ, King KC. Obesity in pregnancy: risks and outcome. Obstet Gynecol. 1980;56:446–450. [PubMed] [Google Scholar]
- 23.Green JR, Schumacher LB, Pawson IG, Partridge JC, Kretchmer N. Influence of maternal body habitus and glucose tolerance on birth weight. Obstet Gynecol. 1991;78:235–240. [PubMed] [Google Scholar]
- 24.Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 25.Robinson HE, O'connell CM, Joseph KS, Mcleod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357–1364. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 26.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 27.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yogev, Chen, Hod, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol. 2010;202:255 e1–255 e7. doi: 10.1016/j.ajog.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.England LJ, Levine RJ, Qian C, et al. Smoking before pregnancy and risk of gestational hypertension and preeclampsia. Am J Obstet Gynecol. 2002;186:1035–1040. doi: 10.1067/mob.2002.122404. [DOI] [PubMed] [Google Scholar]
- 30.Xiong X, Wang FL, Davidge ST, et al. Maternal smoking and preeclampsia. J Reprod Med. 2000;45:727–732. [PubMed] [Google Scholar]
- 31.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 32.Oken E, Kleinman KP, Rich-edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]