Abstract
Ankylosing spondylitis (AS) is a chronic systemic arthritic disease that leads to significant disability and loss of quality of life in the ~0.5% of the worldwide human population it affects. There is currently no cure for AS and mechanisms underlying its pathogenesis remain unclear. AS is highly genetic, with over 70% of the genetic risk being associated with the presence of HLA-B27 and endoplasmic reticulum aminopeptidase-1 (ERAP1) alleles. Furthermore, gene-gene interactions between HLA-B27 and ERAP1 AS risk alleles have recently been confirmed. Here, we demonstrate that various ERAP1 alleles can differentially mediate surface expression of antigens presented by HLA-B27 on human cells. Specifically, for all peptides tested, we found that an ERAP1 variant containing high AS risk SNPs reduced the amount of the peptide presented by HLA-B27, relative to low AS risk ERAP1 variants. These results were further validated using peptide catalysis assays in vitro, suggesting that high AS risk alleles have an enhanced catalytic activity that more rapidly destroys many HLA-B27-destined peptides, a result that correlated with decreased HLA-B27 presentation of the same peptides. These findings suggest that one mechanism underlying AS pathogenesis may involve an altered ability for AS patients harboring both HLA-B27 and high AS risk ERAP1 alleles to correctly display a variety of peptides to the adaptive arm of the immune system, potentially exposing such individuals to higher AS risk due to abnormal display of pathogen or self derived peptides by the adaptive immune system.
Keywords: ankylosing spondylitis, autoimmunity, antigen presentation, HLA-B27, endoplasmic reticulum aminopeptidase-1, inflammation, antigenic epitope
Ankylosing Spondylitis (AS) is a progressive, chronic, inflammatory autoimmune disease, affecting 5 in 1000 people (0.5%) worldwide (1–3). The hallmark of AS is painful inflammation of the axial skeleton, spine, and sacroiliac joints, followed by structural damage and secondary ossification in various regions, resulting in severe disability to AS patients. AS has a strong genetic component, with nearly 90% of the risk for AS being attributable to hereditary factors (3). As seen with many other autoimmune diseases, HLA-B27 positivity is strongly correlated with AS development, with 90% of AS patients carrying this HLA allele. However, the presence of HLA-B27 by itself is not causative, as only between one and five percent of individuals possessing HLA-B27 alleles develop AS (1). As the HLA-B27 MHC class I allele is responsible for only 40–50% of all genetic susceptibility to AS, other genes are also involved in the pathogenesis of AS (1–3). To identify these genes, several genome-wide association studies (GWAS) to identify other genetic risk loci that are associated with the development of AS have been carried out. Several large scale GWAS in various populations identified ERAP1 as the gene that contributed the most risk for development of AS, as well as identifying other candidate genes such as IL-23R, ANTXR2, IL1R2, PTGER4, TBKBP1, CARD9, RUNX3, LTBR-TNFRSF1a, IL12B, EDIL3 and HAPLN1 (1, 2, 4). More recently, specific variants of ERAP1 have been identified to be responsible for ~26% of the total genetic risk of AS, with HLA-B27 and ERAP1 together comprising ~70% of the overall genetic risk for development of AS (3, 5, 6). In HLA-B27+ individuals, several ERAP1 SNPs, including rs30187 (K528R) and rs27044 (Q730E), are significantly associated with AS (Table I) (2, 3, 5–7). In the HLA-B27+ population, individuals that are homozygous for ERAP1_528K have been found to have the highest predisposition for AS. Furthermore, the most recent AS GWAS have confirmed epistasis between HLA-B27 and ERAP1 genes, revealing these ERAP1 alleles to be associated with AS risk only in HLA-B27+ individuals, and not in HLA-B27− individuals (2). This epistasis suggests that ERAP1 is directly interacting with HLA-B27 in the pathogenesis of AS, in contrast to other AS susceptibility loci (e.g. IL23R, IL12B) that were shown to contribute risk to AS independently of HLA-B27 status (2).
Table I.
ERAP1 alleles associated with an altered risk of Ankylosing Spondylitis
| SNP# | P value association with AS |
Allele | 349 | 528 | 575 | 725 | 730 |
|---|---|---|---|---|---|---|---|
| ERAP1 High | M | K | D | R | Q | ||
| ERAP1 Low | V | R | N | Q | E | ||
| rs2287987 | 1.6×10^−4 | M349V | V | K | D | R | Q |
| rs30187 | 3.0×10^−6 | K528R | M | R | D | R | Q |
| rs10050860 | 1.1×10^−4 | D575N | M | K | N | R | Q |
| rs17482078 | 2.3×10^−4 | R725Q | M | K | D | Q | Q |
| rs27044 | 1.0×10^−6 | Q730E | M | K | D | R | E |
| HeLa (endogenous) |
M/M (atg/atg) |
K/R (aag/agg) |
D/D (gac/gac) |
R/R (cga/cga) |
Q/E (caa/gaa) |
Construction of plasmids, encoding for various ERAP1 alleles was performed as described in Materials and Methods. P value for association with AS is derived from recent GWAS studies (1–4), predominantly study described in (3) on UK patients. Amino acids positions, associated with high risk of AS are shown in grey. Endogenous ERAP1 alleles in our HeLa-Kb-B27/47 resemble ERAP1_High, however, have heterogeneity at positions 528 and 730.
HLA-B27 and ERAP1 are both central members of the antigen presentation pathway. The initial step in the processing of intracellular proteins occurs in the cytosol. Here, proteins are cleaved by the proteasome into 2–25 amino acid length peptides (8). A small fraction (less than 1% (9)) of these peptides (those that are not completely degraded by the cytoplasmic proteases) are actively transported by the transporter associated with antigen processing (TAP) into the endoplasmic reticulum (ER) where ERAP1 and other ER-localized peptidases trim these peptides at their N-termini to either generate peptides of optimal size for loading onto MHC class I molecules, or alternatively degrade peptides to a size so small (below 8–9 amino acids) that they can no longer be efficiently loaded onto MHC class I molecules (10–13).
ERAP1 is a central component of the antigen presentation pathway, and has been shown to be necessary for the generation of certain MHC class I presented peptides that can effect the ability to respond to specific diseases such as Toxoplasma gondi, and cancers such as cervical cancer (14, 15). The recently discovered genetic epistasis between HLA-B27 and ERAP1 in AS, and their cooperative role in antigen presentation lends strength to the theory that AS is caused by aberrant antigen presentation. If aberrant antigen presentation is a factor in the pathogenesis of AS, one possibility is that in AS there is are novel peptides presented in AS patients relative to non-AS patients, which may lead to autoimmunity. The theory of “arthogenic peptides” was strengthened by the observation that rats transgenic for human HLA-B27 develop arthritis only in the presence of pathogens, but not in specific-pathogen-free environments (16). Additionally these HLA-B27 transgenic rats require T-cells to develop arthritis (17). These observations lead to the idea that arthogenic peptides present in intracellular bacteria were being presented on MHC class I, and potentially by mimicking endogenous epitopes, lead to activation of auto-reactive T-cells, However, CD8+ T-cells were not necessary for the development of the arthritic phenotype (18), and recent work has suggested that Th17 cells may be involved in the inflammatory process, because CD4+ KIR3DL2+ T-cells are directly stimulated by HLA-B27 homodimers present on the surface of HLA-B27+ antigen presenting cells (19).
Of the five ERAP1 SNPs originally linked to the AS by GWAS, all five have been shown to affect the biochemical function of ERAP1. M349V, K528R, R725Q and Q730E variants of ERAP1 directly affect trimming properties of ERAP1. For example, the Q730E and K528R variants have been shown to affect the activity and specificity of ERAP1 trimming (20) and K528R has been shown to affect ERAP1 preferences for substrate length (21). The 725Q variant was shown to reduce peptide trimming (2), and in the additional presence of 349V, 528R, or 730Q the reduction of function in ERAP1 trimming caused by 725Q was further exagerated (22). The D575N polymorphism is thought to play an important role in folding of ERAP1, and has been shown to directly affect peptide trimming by ERAP1 in the presence of the 349V polymorphism (22, 23).
In the following study, we have investigated the specific role that AS-associated variants of ERAP1 have upon HLA-B27 antigen presentation. We have determined that the presence of a specific ERAP1 allele associated with an increased risk for development of AS (referred to here as ERAP1_High) mediates reduced presentation of both pathogen-derived and host-derived antigens onto HLA-B27 in cultured cells, relative to a low AS risk ERAP1 variant (ERAP1_Low). These effects are consistent with in vitro biochemical assays for peptide catalysis, which independently validated that the ERAP1_High variant has higher catalytic activity and is able to destroy HLA-B27 epitopes more efficiently than the ERAP1_Low variant. Together, these findings suggest that a mechanism for AS induction may be due to global alterations in presentation of HLA-B27 and HLA-B27 destined-peptides by the presence of high-risk ERAP1 alleles, resulting in AS.
Materials and Methods
Cell-based antigen presentation assay (transfection and flow cytometry staining)
Construction of plasmids, encoding the respective ERAP1 alleles (summarized in Table I) was performed as previously described in pTracer-CMV-RFP, where the GFP/Zeo fusion protein was replaced with red fluorescent protein ORF (20, 24). Specific primers were designed to create point nucleotide mutations, resulting in corresponding ERAP1 amino acid substitutions, as shown in Table I. pTracer-CMV-RFP plasmids, encoding different ERAP1 alleles (Table I) were generated using a QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Integrity of ERAP1 genes, cloned into pTracer-CMV-RFP was confirmed by sequencing of the entire length of the ERAP1 ORF using primers listed in Supplemental Table I. An HA-tag (YPYDVPDYA) was introduced into the C-terminus of each ERAP1 allele constructed.
HeLa cells, stably expressing H-2Kb, human HLA-B27 and Herpesvirus protein ICP47 (TAP1 blocker), (referred to as HeLa-Kb-B27/47) were described previously (25). To construct plasmids expressing HLA-B27 restricted peptides (and their N-terminal precursors, Supplemental Table II), nucleotide sequences corresponding to specific peptides were subcloned into pTracer-CMV (Invitrogen, Carlsbad, CA). All peptides were designed to contain the ER-signal sequence MRYMILGLLALAAVCSA (from the Ad2E3gp19K protein) upstream of the peptide sequence (24).
HeLa-Kb-B27/47 cells (3×105 cells per well in 6-well plates) were transiently co-transfected with the indicated plasmids expressing specific HLA-B27 specific peptides (1µg of pTracer-CMV-GFP-peptide) and ERAP1-expressing plasmids (1µg) for 48 hours using HeLa specific TransIT HeLaMonster transfection kit (Mirus Bio LLC, Madison, WI). Transfection efficiency of 5–10% of GFP+/RFP+ was routinely achieved as verified by flow cytometry (Supplemental Figure 3). Surface expression of HLA-B27 in transfected (GFP+/RFP+) cells was subsequently measured using flow cytometry, as previously described (20). Briefly, at 48 hours post transfection, cells were incubated with ME1, an HLA-B27-specific monoclonal antibody (from murine hybridoma supernatant) for 30 minutes on ice, rinsed and subsequently stained with the secondary antibody Cy5-AffiniPure Goat Anti-Mouse IgG (from Jackson Immunoresearch Laboratories; West Grove, PA)) for 30 min on ice (1:200 dilution). Samples were then analyzed on a BD LSR II, or Influx instruments and analyzed using FlowJo software (Tree Star, San Carlos, CA, USA).
Ad5-[E1-]-ICP (utilized in some antigen presentation experiments) was kindly provided by David. C. Johnson from Oregon Health and Science University (Portland, OR). For some experiments we have used ME1, HC10 and W6/32 monoclonal antibodies (all from murine hybridoma supernatant) to quantify levels of MHC class I antigen presentation as well as levels of HLA-B27 free heavy chain (FHC) formation in HeLa-Kb-B27/47 cells during transfection with different ERAP1 alleles and peptides. ME1 recognizes intact HLA-B27 (and some other HLA-B alleles) complexes (7, 26), HC10 recognizes FHC of HLA-B27 (and other HLA class I molecules) (7, 26, 27), W6/32 recognizes all intact HLA-B, -A, –C, -E, -G complexes (27).
Recombinant enzyme expression and purification
ERAP1 alleles were purified from 293F cells after transfection with a plasmid (pTracer-CMV-RFP) carrying the appropriate allelic sequence (ERAP1_High and ERAP1_Low) as described (20). Active enzyme was purified to homogeneity, by lysing the cells using a Potter-Elvehjem tissue homogenizer, and using affinity (anti-HA) and size-exclusion (S200) chromatography. Enzymes were stored in aliquots at −80° C in the presence of 10% glycerol and subjected to a single freeze-thaw cycle. This approach was found to retain full enzymatic activity during storage. All comparisons between alleles were made with enzyme preparations that were performed in parallel.
ERAP1 enzymatic assays
All enzymatic assays were performed in 50mM Hepes pH 7.0, 150mM NaCl as previously described (20, 28). Leucine 4-methyl-coumaryl-7-amide (L-AMC) assays were performed as previously described (20, 29). 100µM SIINFEKL peptide, shown previously to be resistant to ERAP1 hydrolysis and to stabilize the enzyme, was present in L-AMC assays (12, 20). Peptide VRYQSKNYY was purchased from JPT Peptide Technologies (Berlin, Germany) and purified by RP-HPLC to more than 95% purity. For peptide digestion assays, 50microM peptide was mixed with indicated concentrations of ERAP1 alleles and incubated at 25° C for 20 minutes. The reaction was stopped by the addition of trifluoroacetic acid to a final concentration of 0.1% (for HPLC analysis) or formic acid to a final concentration of 1% (for LC/MS analysis). Digestion products were analyzed by reversed-phase HPLC (C-18 chromolith™ column, Merck) using a linear acetonitrile gradient for elution. Chromatographic peaks were identified by running peptide controls and by mass spectrometry. Reaction rates were calculated by comparing the relative peak area of the product peak to the substrate peak measured by absorbance at 222nm and quantified by running peptide controls.
Quantitative RT-PCR
To determine ERAP1 mRNA levels in transfected and non-transfected HeLa-Kb-B27/47 cells, RNA was harvested using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) at 24 hours post transfection with pTracer-CMV-RFP (with or without ERAP1 alleles) per the manufacturer’s protocol. Following RNA isolation, reverse transcription was performed on 1 µg of total RNA using SuperScript III (Invitrogen, Grand Island, NY) reverse transcriptase (RT) and random hexamers / oligo dT primers per manufacturer’s protocol. RT reactions were diluted to a total volume of 60 µl, and 2 µl was used as the template in the subsequent PCR reactions. Primers were designed using Primer Bank web-based software (http://pga.mgh.harvard.edu/primerbank/):
ERAP1-For1 5’-CCCCTCAAATGGTCCCTTGC
ERAP1-Rev1 5’-GAGATGCTTCAGTGCTCTGAC
ERAP1-For2 5’-GCAAACCTTACCACGCTGAC
ERAP1-Rev2 5’-GGTTCTTCCGATAGCCTCTCTC
GAHDH-For 5’- GGGTGTGAACCATGAGAAGTATGAC
GAPDH-Rev 5’- GCCATCCACAGTCTTCTGGGT
Quantitative PCR (qPCR) was carried out on an ABI 7900HT Fast Real-Time PCR System using SYBR Green PCR Mastermix (Applied Biosystems) in a 15 µl reaction. PCRs were subjected to the following procedure: 95.0°C for 10 min followed by 40 cycles of 95.0°C for 15 sec followed by 60.0°C for 1 min. The comparative Ct method was used to determine relative gene expression using GAPDH to standardize expression levels across all samples. Relative transcription changes were calculated based on comparing experimental levels of a respective transcript to those quantified in samples derived from mock-transfected cells.
Western blotting
HeLa-Kb-B27/47 cells were transfected with pTracer-CMV-RFP-ERAP1_High (or _Low). At 24 hours post transfection, protein was harvested in lysis buffer (20mM Tris-HCl, pH 7.4, 1mM EDTA, 150mM NaCl) containing 1% Triton X-100 with protease inhibitors. Lysed samples were then centrifuged at maximum speed (16,000×g) for 15 minutes at 4°C, after which the protein concentration of the supernatant was determined using the BCA method (30). Equivalent concentrations of protein samples (5–15 µg) were electrophoretically separated in 7% polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were then probed with the mouse-anti-hTubulin monoclonal primary antibody (Sigma-Aldrich, St. Louis, MO) at 1:30000 dilution for 20 minutes and primary polyclonal rabbit-anti-hERAP1 (Proteintech, Chicago, IL) at 1:1000 dilution for 2–16 hours or rabbit anti-HA-tag (YPYDVPDYA) antibody (Rockland, Gilbertsville, PA) at 1:2000 dilution for 2–16 hours (to measure exogenous, plasmid derived levels of ERAP1). Following incubations, membranes were probed with fluorescent antibodies (IRDYE800 conjugated goat anti-mouse IgG (Rockland) and Alexa Fluor 680 conjugated goat anti-rabbit IgG (Invitrogen, Grand Island, NY)) for 45 minutes at 1:5000 dilution. Optimally exposed membranes were scanned, and indicated protein bands were quantified using Licor’s Odyssey scanner (models 2.1 or 3.0) (31). For data analysis, the fluorescence of ERAP1 proteins was normalized to Tubulin levels.
Cytokine and chemokine analysis
A human-based 27-plex multiplex-based assay was used to determine the indicated cytokine/chemokine concentrations in the media collected from cultured HeLa-Kb-B27/47 cells transfected with ERAP1 alleles. Media was collected at 48 hours post transfection. Assay was performed according to the manufacturer’s instructions (Bio-Rad, Hercules, CA) via Luminex 100 technology (Luminex, Austin, TX) as previously described (32).
Statistical analysis
For antigen presentation studies, we have performed 4–10 independent experiments (biological replicates). Statistical analysis was completed using one sample t-test [(mean ratio – 1)/SE)] to determine any possible differences in antigen presentation mediated by different ERAP1 alleles (ERAP1_Low as compared to ERAP1_High) for each respective peptide analyzed. Furthermore, a two-tailed Student t-test was used to compare values between two experimental groups in other assays (qRTPCR, Western blotting, biochemical assays for peptide catalysis, p < 0.05). Graphs in this paper are presented as Mean of the average ± SEM, unless otherwise specified. GraphPad Prism software was utilized for statistical analysis.
Results
Validation of an HLA-B27 surface rescue system for use in AS studies
In this study, we have focused our investigation upon recombinant ERAP1 alleles containing a combination of amino acid substitutions associated with high and low AS risk, and refer to these as the ERAP1_High and ERAP1_Low alleles, respectively (Table I). Since there are at least five AS-linked SNPs (3, 33–36) and the possible number of haplotypes is at least 32, we chose to focus our study on the two extreme haplotypes, even though these specific haplotypes do not represent all possible haplotypes found within the human population. This approach also evaluates possible synergism between SNPs, although it cannot deconvolute them. Importantly, the ERAP1_High allele (Genebank accession # NP_057526.3) is present in a significant number of humans (37, 38).
We next set out to evaluate the effect that the presence of various ERAP1 allelic variants has on HLA-B27 antigen presentation in intact cells by using a previously described HeLa-Kb-B27/47 surface rescue system (Figure 1) (20, 25, 39). HeLa-Kb-B27/47 cells are HeLa cells that have been stably transduced with murine Kb, human HLA-B27, and the herpes simplex virus TAP-blocker ICP47 (40). Using this system, only peptides containing an appropriate ER-localization signal can be substrates for ERAP-mediated loading onto HLA-B27 molecules. Because this ER-localization signal cleaves peptides between two alanines, each peptide that did not already contain an N-terminal alanine, had one added as part of the ER-localization signal. This is not expected to effect ERAP1 trimming rates substantially because ERAP1 efficiently cleaves N-terminal alanines generating the correct substrates (10). Of all HLA-B27 subtypes, the B*2705 allele has one of the most significant associations with AS (41–44), and therefore this B27 subtype was chosen for use in this system.
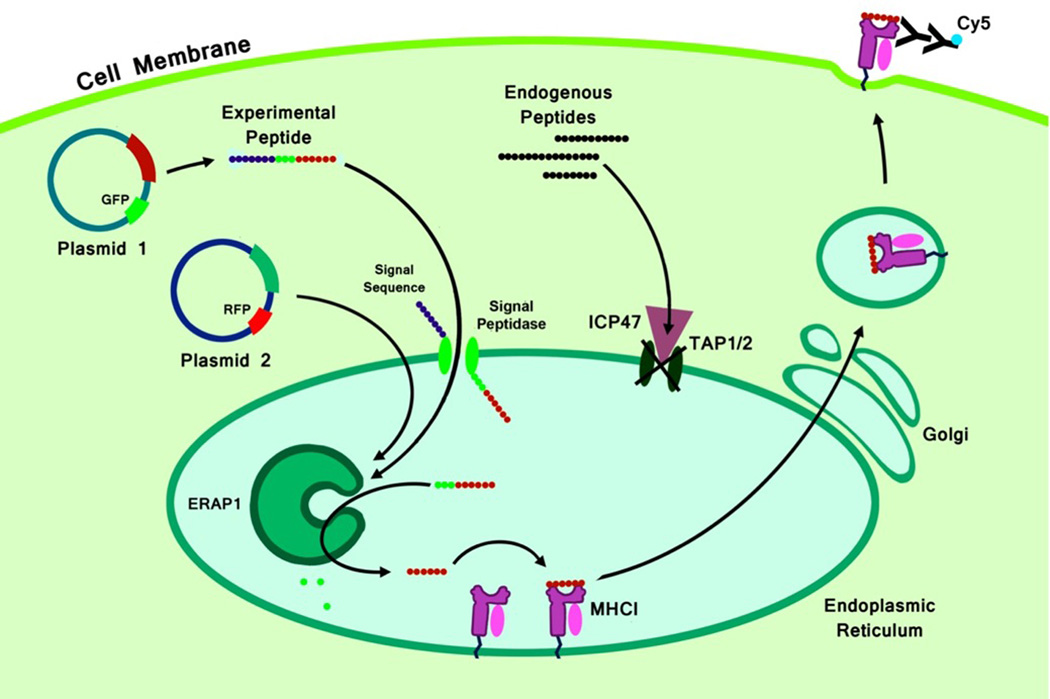
Figure 1. An HLA-B27 surface rescue system: model of action.
The initial critical step in the processing of intracellular proteins occurs in the cytosol. Proteins are cleaved by the proteasome into 2–25 aa peptides (8). A fraction (9) of these peptides (those which were not degraded by the cytoplasmic proteases) is actively transported into the ER by TAP and trimmed at the N-terminus by aminopeptidase ERAP1 (13, 24, 56, 57) to generate peptides of optimal size for loading into MHC class I (8–10 aa). The MHC class I heavy chain first assembles with β2-microglobulin; this heterodimer is then assembled with 8–10 aa peptide (58). Upon successful assembly, “mature” MHC I/peptide complexes are transported to the cell surface (57). We have used HeLa cells stably expressing human HLA-B*2705 and TAP1/2 blocker (ICP47 protein), HeLa-Kb-B27/47, to test the effect of ERAP1 allelic variants on HLA-B27 antigen presentation in intact cells (20, 25, 39). We used transient transfection with pTracer-CMV-GFP-peptide and pTracer-CMV-RFP-ERAP1 (one of the alleles). When transfected plasmid-derived peptide is delivered into ER (by means of ER-signal sequence from Ad2 E3gp19K protein, bypassing TAP blockage), surface HLA-B27 (or other MHC class I) can be increased proportionally to the amounts of “mature” epitope available. By delivering N-extended HLA-B27-specific peptide precursors to the ER and measuring surface expression of HLA-B27, we can semi-quantitatively determine how much of the precursor was converted into “mature” HLA-B27 epitope. Conversely, if a “mature” peptide is delivered, we can measure the rate of destruction of the peptide.
HeLa-Kb-B27/47 cells have reduced surface levels of HLA-B27, as compared to HeLa-Kb-B27 cells, as the presence of ICP-47 prevents transport of peptides into the ER. Without peptides being delivered to the ER, empty MHC class I molecules are retained in the ER and not subject to surface display (25, 39). Utilizing HeLa-Kb-B27/47 and HeLa-Kb-B27 cells and adenovirus vectors transducing ICP47, we further confirmed that nearly 80% of all HLA-B27 molecules are retained in HeLa-Kb-B27/47 cells as compared to HeLa-Kb-B27 cells. This amount appears to be the maximal level of inhibition attainable, as the additional use of the ICP47 expressing adenovirus minimally decreased HLA-B27 surface expression in HeLa-Kb-B27/47 cells (Supplemental Figure 1). We have also analyzed the amino acid composition of the endogenous ERAP1 alleles present in the genome of HeLa-Kb-B27/47 cells, this analysis revealed heterogeneity at positions 528 and 730, but otherwise the endogenous ERAP1 alleles contain an identical amino acid sequence to the ERAP1_High allele (Table I).
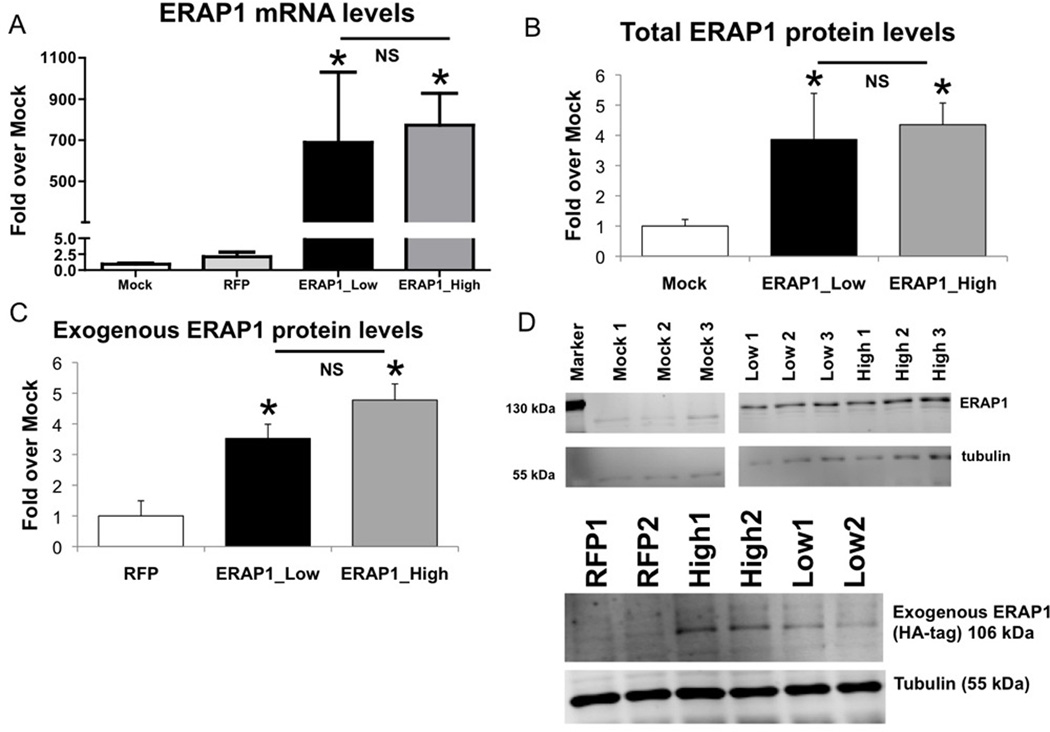
To further validate the system, we demonstrated that transfection of HeLa-Kb-B27/47 with an ERAP1 expressing plasmid (pTracer-CMV-RFP-ERAP1) results in a significant increase in detectable ERAP1 transcription and expression within the cells (Figure 2A). Levels of ERAP1 transcription were measured by qRT-PCR, revealing a 500–1000 fold induction of ERAP1 mRNA levels in pTracer-CMV-RFP-ERAP1 transfected cells, while a minor ~2 fold induction of ERAP1 transcription occurred upon transfection with the control vector (Figure 2A). We also found a significant ~3–4 fold induction of ERAP1 protein expression in HeLa-Kb-B27/47 cells, transfected with ERAP1_High (or Low) alleles, as compared to mock-transfected cells (Figure 2B, D).
Figure 2. Transfection of HeLa-Kb-B27/47 cells with ERAP1 alleles results in significant increases of ERAP1 mRNA and protein levels, increases which are not different between ERAP1 alleles.
HeLa-Kb-B27/47 cells were transiently transfected with plasmids expressing ERAP1_Low or ERAP1_High alleles (or no ERAP1). RNA and protein samples were harvested at 24 hours post transfection as described in Materials and Methods. Statistical analysis was completed using two-tailed Student’s t-test, * - represent significant difference in ERAP1 levels as compared to control (mock or empty pTracer-RFP transfection), p<0.05; n=2 independent experiments performed. (A) Levels of ERAP1 mRNA are shown for each transfection group. About 700-fold induction in ERAP1 mRNA levels were noted for ERAP1_Low and ERAP1_High transfected cells, as compared to mock transfected cells. (B) Total ERAP1 levels were measured by Western blot. Significant ~3–4 fold induction in total ERAP1 protein levels were noted for ERAP1_Low and ERAP1_High transfected cells, as compared to mock transfected cells. (C) Exogenous ERAP1 levels were measured by Western blot with anti-HA-tag antibody. Significant exogenous ERAP1 levels were found in cells transfected with ERAP1_Low and ERAP1_High. (D) Representative Western blots for total ERAP1 (top) and exogenous ERAP1 (bottom) are shown. ERAP1 bands (106 kDa) and tubulin bands (55 kDa) are depicted.
ERAP1 alleles were designed to contain an HA-tag sequence at the C-termini (YPYDVPDYA). By using an HA-tagged antibody, we were able to measure the levels of exogenous ERAP1 expression derived from transfection in the HeLa-Kb-B27/47 cells (Figure 2C, D). Importantly, we did not detect any significant differences in levels of ERAP1 transcription/expression when these cells were transfected with either the ERAP1_High or ERAP1_Low variant expressing plasmids, indicating that potential differences observed in the antigen presentation assay (see below) cannot be attributed to differences in ERAP1 allele specific levels of transcription, expression and/or stability.
Finally, we observed a minimal increase in TNFα release, representing a minimal inflammatory response, in HeLa-Kb-B27/47 cells treated with the transfection reagent (mock transfection as compared to naïve, 48 hours post transfection, Supplemental Figure 2), as well as moderate increases in cytokine/chemokine release in response to dsDNA (pTracer-CMV-RFP or pTracer-CMV-RFP-ERAP1 transfection). However, no additional increases in cytokine/chemokine levels were induced by ERAP1 expression (Supplemental Figure 2).
An ERAP1 allele associated with increased risk of AS reduces surface expression of HLA-B27 regardless of HLA-B27 peptide utilized
HLA-B27 restricted epitopes have been empirically found to be predominantly nonamers with an invariant arginine at position 2 (41, 45). When signal-sequence containing versions of these peptides are delivered into the ER of HeLa-Kb-B27/47 cells, surface HLA-B27 (or other MHC class I) levels are increased proportionately to the amounts of “mature” epitope available for HLA-B27 loading. To capitalize upon this system, we constructed plasmids that co-express RFP along with individual AS-associated ERAP1 alleles, each respectively containing up to five AS-correlated SNPs (Table I) (3, 33–36).
To determine if HLA-B27 surface presentation was significantly altered due to the presence of ERAP1 allelic variants, we co-transfected Hela-Kb-B27/47 cells with plasmids expressing various ERAP1 variants, together with plasmids expressing various signal sequence containing HLA-B27-restricted peptides. By measuring surface expression of HLA-B27 by flow cytometry we can semi-quantitatively compare how ERAP1 variants affect specific peptide loading onto HLA-B27 molecules (Figure 1). We chose to investigate a variety of HLA-B27 restricted peptides in our studies to determine if pathogen derived, or autologous peptides, (that have both been previously shown to correlate with AS) may show ERAP1 allele specific differences when processed for HLA-B27 surface expression. (Supplemental Table II) (41, 43, 46–52). Transfection efficiencies were found to be high, with double positive cells averaging ~7.5% of the cells in each experiment (Supplemental Figure 3).
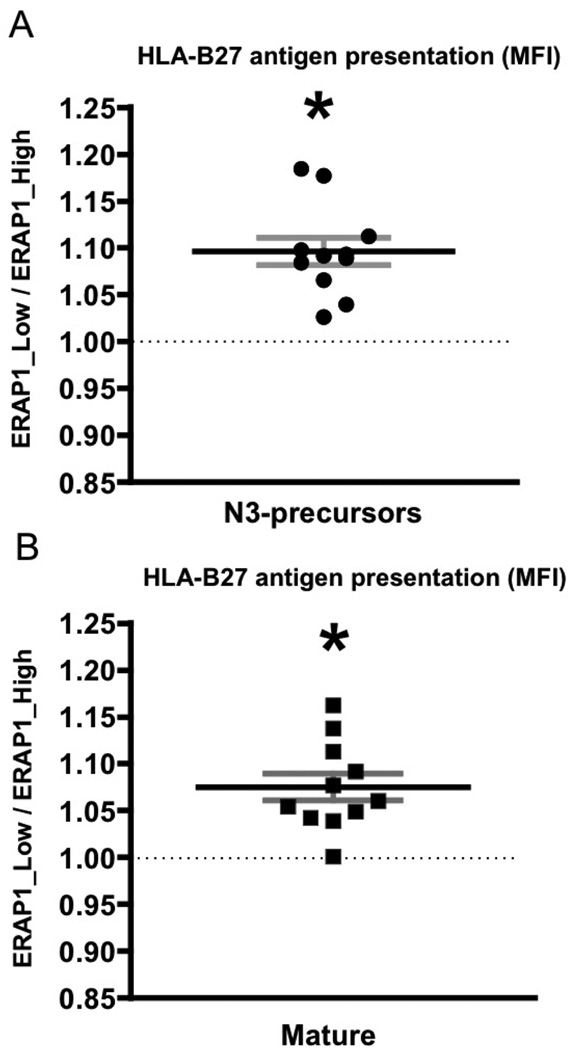
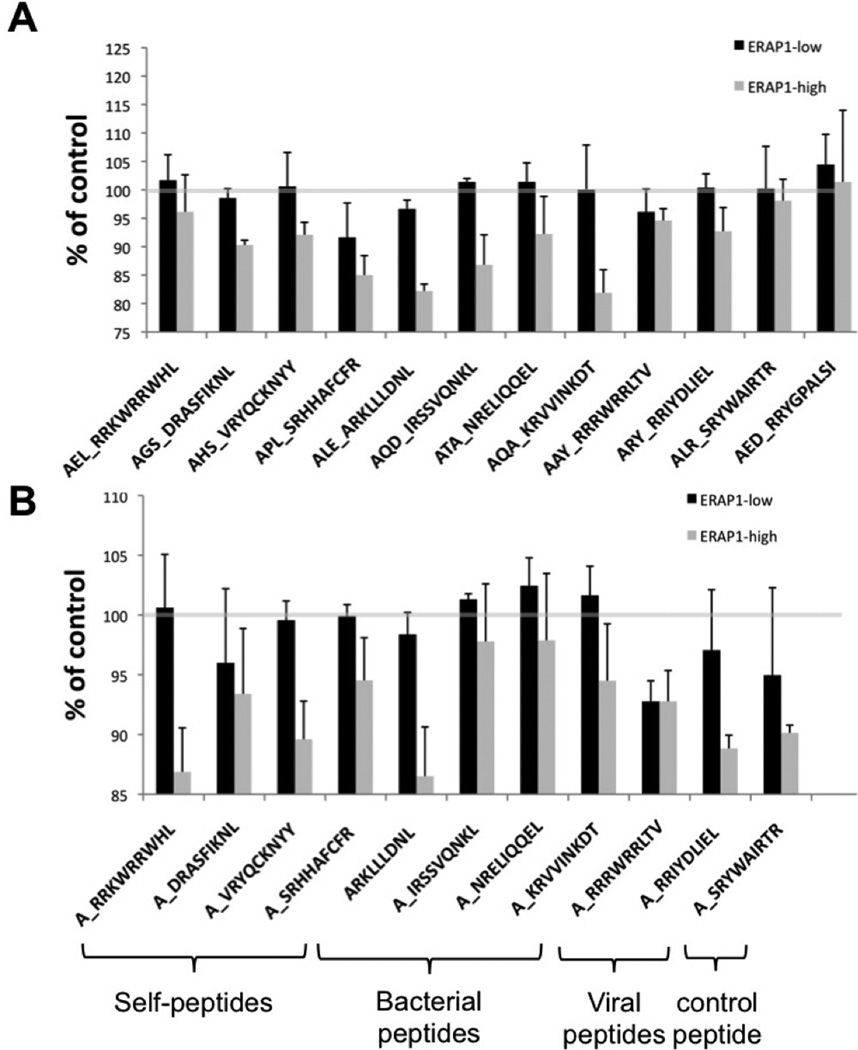
In sum, we found that relative to the ERAP1_Low variant, the presence of ERAP1_High alleles generally reduced HLA-B27 antigen presentation on the cell surface, (as measured by HLA-B27 mean fluorescent intensity (MFI)), regardless of which peptide was present for ER trimming (Figure 3A, B). Increased HLA-B27 surface expression (and by inference, antigen presentation) mediated by the presence of the ERAP1_Low allele was in fact independently observed for over 20 unique peptides tested in our studies, including both epitope precursors and mature epitope versions of each of these respective peptides (Figure 3A, B). Consistent with this, we further noted that this same phenomenon was observed when both N-extended (by 3 amino acids) peptides (Figure 4A) or the “mature” versions of these same respective peptides (Figure 4B) were expressed in the HeLa-Kb-B27/47 cells. Although all peptides tested followed the same general trend, we noted that transfections with AGS_DRASFIKNL, ALE_ARKLLLDNL, APL_SRHHAFCFR, AQD_IRSSQNKL and AQA_KRVVINKDT and mature ARKLLLDNL showed the most dramatic differences in surface expression of HLA-B27, differences that were also dependent upon whether the ERAP1_High or ERAP1_Low alleles were present in the HeLa-Kb-B27/47 cells, (Figures 3 and 4).
Figure 3. ERAP1_High reduces HLA-B27 antigen presentation in intact cells as compared to ERAP1_Low for vast majority of HLA-B27-restricted peptides.
HeLa-Kb-B27/47 cells were transiently transfected with plasmids expressing HLA-B27-specific peptides (with ER-signal sequence) with GFP and either empty vector (control) or the indicated ERAP1 allele with RFP. At 48 hours post transfection surface HLA-B27 surface expression on double-transfected (GFP+RFP+) was analyzed by flow cytometry using the ME-1 antibody. Peptides were transfected either as (A) N3-extended precursors or (B) “mature” epitopes. Ratio in mean fluorescent intensity (MFI) between ERAP1_Low and ERAP1_High variants is depicted. Combined graph is shown (n=11). Bars represent mean ± SEM. Statistical analysis was completed using one sample t-test [(mean ratio – 1)/SE)]; * - represent significant differences in HLA-B27 surface expression mediated by ERAP1 polymorphism (i.e. fold ratio ERAP1_Low/ERAP1_High is significantly different from 1, dashed line), p<0.05.
Figure 4. ERAP1_High reduces HLA-B27 antigen presentation in intact cells as compared to ERAP1_Low when peptide precursors or mature B27 peptides are delivered.
HeLa-Kb-B27/47 cells were transiently transfected with plasmids expressing HLA-B27-specific peptides (with ER-signal sequence) with GFP and either empty vector (control) or the indicated ERAP1 allele with RFP. At 48 hours post transfection surface HLA-B27 surface expression on double-transfected (GFP+RFP+) was analyzed by flow cytometry. Peptides were transfected as N3-extended precursors (A) or as “mature” B27 specific epitopes (B), extended with Alanine on N-terminus for all peptides except ARKLLLDNL, which by coincidence already had N-terminal alanine. Mean fluorescent intensity (MFI), normalized to control (empty RFP vector, grey line, representing the level of endogenous antigen presentation) is shown. Several (5–10) independent transfection experiments were performed for every peptide / ERAP1 combination. Combined graph is shown. Bars represent mean ± SEM.
Specifically, pathogen derived peptides previously associated with AS pathogenesis (i.e.: Chlamydia trachomatis derived peptide: ALE_ARKLLLDNL or Yersinia enterocolitica derived peptides: AQD_IRSSVQNKL, AQA_KRVVINKDT) and peptides derived from self proteins previously noted in AS patients (AHS_VRYQCKNYY, AGS_DRASFIKNL, APL_SRHHAFCFR), and a control peptide (AED_RRYGPALSI) all showed similar ERAP1 dependent effects upon HLA-B27 surface expression. These results confirm that the presence of the ERAP1_Low allele correlates with increased surface expression of HLA-B27 antigens, regardless of the source (bacterial or autologous) or peptide composition of the antigen.
The ERAP1_High variant more efficiently degrades B27-specific epitopes below 9 amino acids in length, as compared to the ERAP1_Low variant
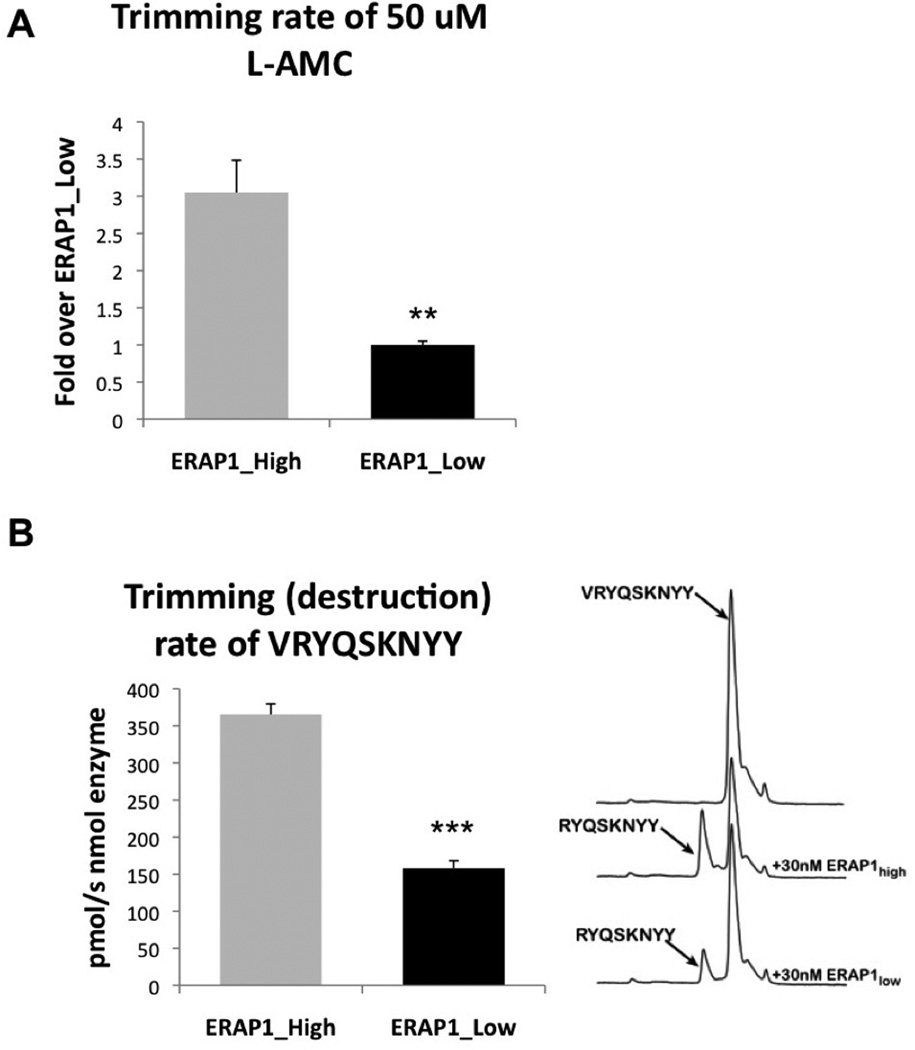
We have recently reported that ERAP1 variants differing by single SNPs can have significant differences in activity towards hydrolysis of the fluorogenic substrate L-AMC and 10-mer peptides (20). Given the known ability of ERAP1 to destroy antigenic epitopes, one possible unifying interpretation for the results described above is that the ERAP1_High variant is relatively more efficient at degrading HLA-B27 destined epitopes, decreasing the overall abundance of “loadable” HLA-B27-specific 9 amino acid long peptides, and thereby resulting in decreased levels of HLA-B27 surface expression. To investigate potential mechanistic differences between ERAP1 alleles ability to degrade antigenic epitopes, we purified the recombinant proteins and analyzed their in vitro enzymatic activity. The ERAP1_High variant had a significantly (at least 3-fold) increased rate of hydrolysis of the L-AMC substrate, a result that suggests that the ERAP1_High variant is more catalytically active relative to the low AS risk ERAP1 allele (Figure 5A). Given the lower levels of HLA-B27 surface expression in cells transfected with ERAP1_High, we also tested if the recombinant enzyme could destroy an HLA-B27 epitope in vitro. The purified ERAP1_High variant efficiently trimmed the B27-specific epitope VRYQSKNYY, at a significantly higher (p<0.0001) rate compared to the ERAP1_Low variant, further suggesting that alleles containing these amino acid changes may be more efficient in destroying antigenic epitopes (Figure 5B).
Figure 5. ERAP1_High allele has significantly higher ability to hydrolyze L-AMC substrate and significantly higher ability to degrade B27-specific epitopes below 9 amino acids in length, as compared to ERAP1_Low.
(A) Trimming of the model fluorigenic substrate L-AMC by purified ERAP1_Low and ERAP1_High alleles was performed. Statistical analysis was completed using two-tailed Student’s t-test (** - indicates p<0.001). Two experiments performed, representative experiment shown. (B) Purified ERAP1_Low and ERAP1_High alleles (30 nM) were mixed with purified peptide VRYQSKNYY (50 µM) and N-terminal (V) trimming was measured by HPLC. HPLC traces and bar graph, showing intact and “degraded” peptides are shown. Statistical analysis was completed using two-tailed Student’s t-test. *** - Indicate that ERAP1_High enzyme is able to trim VRYQSKNYY peptide with significantly higher efficiency as compared to ERAP1_Low, p<0.0001. Four independent experiments performed.
ERAP1 allele effects on surface expression of HLA complexes
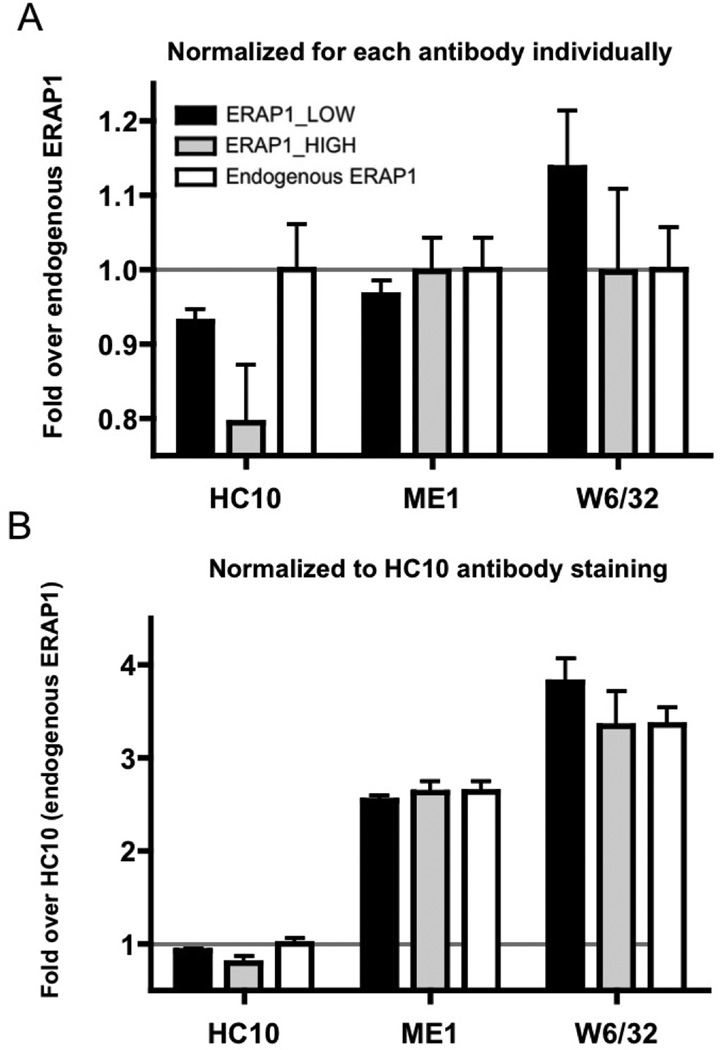
To study possible differential effects that expression of ERAP1 alleles has on intact HLA complexes, as well HLA class I free heavy chain (FHC) complex presence at the cell surface, HeLa-Kb-B27/47 cells were transfected with either the ERAP1_Low or ERAP1_High expression plasmids together with a control peptide (AED_RRYGPALSI), and at 48 hours post-transfection the cells were stained with ME1, HC10 or W6/32 antibodies. ME1 recognizes intact HLA-B27 complexes on the cell surface (7, 26), HC10 recognizes HLA class I molecules not bound to peptide, including FHC and HLA-B27 homodimers, on the cell surface (7, 26, 27), while W6/32 recognizes all intact HLA-B, -A, –C, –E, –G complexes on the cell surface (27).
As expected, staining with the W6/32 antibody was the most robust, with the majority of HLAs present at the surface of the transfected cells being HLA-B27 (Figure 6A, B). We also detected a significant amount of FHC formation in our system (HC10 staining), comprising up to 30% of all surface HLAs being detected regardless of whether the ERAP1_Low or the ERAP1_High alleles were being expressed in the transfected cells, (as well when only endogenous ERAP1 was present in control transfections), a result suggesting that overall rates of HLA-B27 FHC formation do not seem to be influenced by the presence of different ERAP1 alleles (Figure 6B). It should be noted that we did not detect a significant difference in the amount of surface HLA-B27 (detected by ME-1) using the B27-specific control peptide (sequence: AED_RRYGPALSI), in pTracer-CMV-GFP, transfected cells, (Figure 6), but did observe a difference in the surface HLA-B27 for the other peptides tested (Figure 3).
Figure 6. Despite FHC detection, no significant role of ERAP1 allelic polymorphism on FHC formation was identified in HeLa-Kb-B27/47 cells.
HeLa-Kb-B27/47 cells were transiently transfected with B27-specific control peptide (sequence: AED_RRYGPALSI, in pTracer-CMV-GFP) and one of ERAP1-expressing plasmids (pTracer-CMV-RFP-ERAP1 or empty vector control, pTRacer-CMV-RFP) for 48 hours. Surface expression (MFI) of intact HLA-B, -A, -C, -E, -G was measured by staining with W6/32 antibody; surface expression of HLA-B27 was measured by staining with ME1 antibody; surface expression of FHC of HLA class I was measured by HC10 antibody. (A) MFI was normalized for each antibody individually (as fold over endogenous ERAP1), (B) MFI was normalized for HC10 antibody staining in endogenous ERAP1 setting.
Interestingly, although the differences were not significant, W6/32 staining trends to be higher in ERAP1_Low compared to ERAP1_High and the control plasmid transfected cells, while ME1 staining appears lower or unchanged for ERAP1_Low compared to ERAP1_High transfected cells. This may indirect suggest that ERAP1_Low variants are tailoring peptides more efficiently for presentation by non-B27 HLA molecules as compared to ERAP1_High variants.
Finally, as shown on Supplemental Figure 2 we did not detect any significant differences in levels of pro-inflammatory cytokines or chemokines being released by cells transfected with plasmids expressing control (empty RFP), the ERAP1_Low, or the ERAP1_High variants, suggesting that slight differences in FHC formation and antigen presentation observed in Figures 6A and 6B do not result in significant inflammatory responses in this system.
Discussion
In this study we investigated how in intact human cells, the over-expression of AS-specific ERAP1 alleles alters HLA-B*2705 subtype (41–43) surface expression in isolation, and upon presentation of HLA-B27 specific peptides. We found that regardless of the source of the HLA-B27-specific peptide (e.g. self-derived antigens, pathogen-derived antigen, and non-AS associated antigens (Supplemental Table II)), an ERAP1 allele associated with increased risk of AS consistently reduced overall HLA-B27 surface expression levels (and by inference, antigen presentation) in intact cells as compared to cells expressing a low risk AS allele. Experiments utilizing both N3-extended peptide precursors and their shorter, or “mature” epitopes revealed the same overall results in our antigen presentation system, further verifying that the presence of high AS risk ERAP1 alleles results in globally reduced surface levels of HLA-B27 (and HLA-B27 antigen presentation) in intact cells regardless of antigenic peptide source or sequence. Enzymatic studies using purified ERAP1 proteins in vitro also demonstrated that high AS risk ERAP1 alleles trim HLA-B27 epitopes more rapidly, and to a length that is below the length suitable for optimal loading onto the β2m/HLA-B27 complex, as compared to the enzymatic properties of low AS risk ERAP1 alleles. Overall, the results are consistent with a model suggesting that the presence of high AS risk ERAP1 alleles may result in destruction of antigenic epitopes destined for HLA-B27 loading, and also result in reduced surface expression of HLA-B27 loaded peptide complexes.
These results are in agreement with a recent study by Evans et.al. in which the lower risk AS alleles ERAP1_528R and ERAP1_725Q appeared to have slower rates for enzymatic trimming of a model fluorigenic peptide, as compared to the reference allele (2). Moreover, our results and proposed model is in direct agreement with a recent study by Garcia-Medel et.al., where the authors found that ERAP1 alleles associated with low AS risk have diminished enzymatic activity and less efficient peptide trimming as compared to the high AS risk ERAP1 alleles, when the HLA-B*27:04-bound peptidome is analyzed (53).
Our data shed light on the role that high AS risk ERAP1 alleles may have in the induction and pathogenesis of AS (37, 38). Because this model only allows for testing of a limited number of peptides, it cannot entirely rule out the arthogenic peptide hypothesis for causation of AS, however, it does place limitations on the possible characteristics of such peptides should they be casusative for AS. We show that all peptides we tested to date are degraded at a much faster rate by ERAP1_High than ERAP1_Low variants in vitro, a result consistent with the decreases in overall peptide presentation noted in intact human cells in vivo. This strongly suggests that if an arthogenic peptide exists, it is one that is not easily degraded by ERAP1 (ie: to a size to small to load onto MHC class I).
It has also been suggested that the mere presence of HLA-B27 homodimers on the cell surface may be activating Th17 CD4+ T-cells by activating the KIR3DL2 receptor (19). This hypothesis is consistent with observations that arthritis in HLA-B27+ rats requires T-cells, but not CD8+ T-cells (17, 18). Our data suggests that If ERAP1_High variants degrade peptides at a higher rate than ERAP_Low variants, that cells overexpressing the ERAP1_High variant may have more B27 homodimers present on their surface, as compared to cells over-expressing the ERAP1_Low variant. However, surface staining for MHC class I using the HC10 antibody (detects MHC I molecules not associated with peptide) did not show a significant increase in surface staining in the ERAP1_High variant transfected cellsv (Figure 6). Thus, if Th17 cells are the mechanism behind AS, our data suggests that ERAP1_High is not contributing to this phenomenon by increasing the amount of surface HLA-B27 homodimers, but through a different mechanism. It has been observed that ERAP1 causes the shedding of certain cytokine receptors on the cell surface and it is possible that different ERAP1 alleles differentially effect the shedding of an important receptor on cells expressing B27-homodimers that then effects the way that KIR3DL2-positive cells respond to them (19, 55). Future studies will be required to test these hypotheses.
Finally, our study raises the possibility that the SNPs that are low risk for AS, are contributing a protective effect by increasing the amount of non-B27 HLA molecules presented on the cell surface. Though the results were not significant, Figure 6 demonstrates an increased trend in W6/32 staining in ERAP_Low variant expressing cells, and a decreased ME1 staining of these cells. This trend suggests that ERAP_Low may favor peptide trimming of non-B27 HLA alleles. This could increase the amount of non-B27 HLA Class I on the surface of cells, which could possibly confer a protective effect, potentially by altering the ratio of B27 to non-B27 present on the cell surface and thereby altering interactions with T cells or NK cells that are sensitive to surface expression levels of HLA molecules.
In summary, this report demonstrates that AS associated ERAP1 alleles differentially mediate levels of HLA-B27 surface expression in cells processing a variety of HLA-B27 destined epitopes. High AS risk ERAP1 alleles substantially reduce HLA-B27 surface expression and antigen presentation of all tested peptides relative to low AS risk ERAP1 alleles. These findings suggest that the simultaneous presence of high AS risk ERAP1 alleles and HLA-B27 may increase the chances for exacerbations of inflammatory responses induced by lack of adequate levels of HLA-B27 antigen complex surface expression. These alterations may also increased risk for abnormal interactions with the innate and adaptive arms of the immune response, with numerous consequences inclusive of an increased chance for development of auto-reactive T-cell clones. These possible mechanisms for ERAP1 dependent pathogenesis of AS will require further investigation.
Supplementary Material
Acknowledgements
We wish to thank Dr Louis King at Michigan State University, and the Michigan State University Flow cytometry facility for assistance in performing flow cytometry runs; David. C. Johnson and Todd W. Wisner from Oregon Health and Science University (Portland, OR) for providing Ad5-[E1-]-ICP47. A.A. was supported by the National Institutes of Health grant 5R01AR056981-02, the MSU Foundation as well the Osteopathic Heritage Foundation. E.S. acknowledges support from the Greek Secretariat for Science and Technology “Aristeia” action.
Footnotes
Conflicts of Interest: none.
References
- 1.Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, Jin R, Zhou X, Bradbury LA, Appleton LH, Davis JC, Diekman L, Doan T, Dowling A, Duan R, Duncan EL, Farrar C, Hadler J, Harvey D, Karaderi T, Mogg R, Pomeroy E, Pryce K, Taylor J, Savage L, Deloukas P, Kumanduri V, Peltonen L, Ring SM, Whittaker P, Glazov E, Thomas GP, Maksymowych WP, Inman RD, Ward MM, Stone MA, Weisman MH, Wordsworth BP, Brown MA. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, Opperman U, Dilthey A, Pirinen M, Stone MA, Appleton L, Moutsianis L, Leslie S, Wordsworth T, Kenna TJ, Karaderi T, Thomas GP, Ward MM, Weisman MH, Farrar C, Bradbury LA, Danoy P, Inman RD, Maksymowych W, Gladman D, Rahman P, Morgan A, Marzo-Ortega H, Bowness P, Gaffney K, Gaston JS, Smith M, Bruges-Armas J, Couto AR, Sorrentino R, Paladini F, Ferreira MA, Xu H, Liu Y, Jiang L, Lopez-Larrea C, Diaz-Pena R, Lopez-Vazquez A, Zayats T, Band G, Bellenguez C, Blackburn H, Blackwell JM, Bramon E, Bumpstead SJ, Casas JP, Corvin A, Craddock N, Deloukas P, Dronov S, Duncanson A, Edkins S, Freeman C, Gillman M, Gray E, Gwilliam R, Hammond N, Hunt SE, Jankowski J, Jayakumar A, Langford C, Liddle J, Markus HS, Mathew CG, McCann OT, McCarthy MI, Palmer CN, Peltonen L, Plomin R, Potter SC, Rautanen A, Ravindrarajah R, Ricketts M, Samani N, Sawcer SJ, Strange A, Trembath RC, Viswanathan AC, Waller M, Weston P, Whittaker P, Widaa S, Wood NW, McVean G, Reveille JD, Wordsworth BP, Brown MA, Donnelly P. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MA. Breakthroughs in genetic studies of ankylosing spondylitis. Rheumatology (Oxford) 2008;47:132–137. doi: 10.1093/rheumatology/kem269. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Bei JX, Shen M, Li Q, Liao Z, Zhang Y, Lv Q, Wei Q, Low HQ, Guo YM, Cao S, Yang M, Hu Z, Xu M, Wang X, Wei Y, Li L, Li C, Li T, Huang J, Pan Y, Jin O, Wu Y, Wu J, Guo Z, He P, Hu S, Wu H, Song H, Zhan F, Liu S, Gao G, Liu Z, Li Y, Xiao C, Li J, Ye Z, He W, Liu D, Shen L, Huang A, Tao Y, Pan X, Yu B, Tai ES, Zeng YX, Ren EC, Shen Y, Liu J, Gu J. A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat Genet. 2012;44:73–77. doi: 10.1038/ng.1005. [DOI] [PubMed] [Google Scholar]
- 5.Tsui FW, Haroon N, Reveille JD, Rahman P, Chiu B, Tsui HW, Inman RD. Association of an ERAP1 ERAP2 haplotype with familial ankylosing spondylitis. Annals of the rheumatic diseases. 2010;69:733–736. doi: 10.1136/ard.2008.103804. [DOI] [PubMed] [Google Scholar]
- 6.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haroon N, Tsui FW, Uchanska-Ziegler B, Ziegler A, Inman RD. Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Annals of the rheumatic diseases. 2012;71:589–595. doi: 10.1136/annrheumdis-2011-200347. [DOI] [PubMed] [Google Scholar]
- 8.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 9.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nature immunology. 2004;5:670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 10.Evnouchidou I, Momburg F, Papakyriakou A, Chroni A, Leondiadis L, Chang SC, Goldberg AL, Stratikos E. The internal sequence of the peptide-substrate determines its N-terminus trimming by ERAP1. PLoS One. 2008;3:e3658. doi: 10.1371/journal.pone.0003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Endert PM, Tampe R, Meyer TH, Tisch R, Bach JF, McDevitt HO. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity. 1994;1:491–500. doi: 10.1016/1074-7613(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TT, Chang SC, Evnouchidou I, York IA, Zikos C, Rock KL, Goldberg AL, Stratikos E, Stern LJ. Structural basis for antigenic peptide precursor processing by the endoplasmic reticulum aminopeptidase ERAP1. Nat Struct Mol Biol. 2011;18:604–613. doi: 10.1038/nsmb.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang SC, Momburg F, Bhutani N, Goldberg AL. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci U S A. 2005;102:17107–17112. doi: 10.1073/pnas.0500721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta AM, Jordanova ES, Corver WE, Wezel Tvan, Uh HW, Kenter GG, Fleuren GJan. Single nucleotide polymorphisms in antigen processing machinery component ERAP1 significantly associate with clinical outcome in cervical carcinoma. Genes, chromosomes & cancer. 2009;48:410–418. doi: 10.1002/gcc.20648. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nature immunology. 2008;9:937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khare SD, Luthra HS, David CS. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: a model of human spondyloarthropathies. J Exp Med. 1995;182:1153–1158. doi: 10.1084/jem.182.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breban M, Fernandez-Sueiro JL, Richardson JA, Hadavand RR, Maika SD, Hammer RE, Taurog JD. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol. 1996;156:794–803. [PubMed] [Google Scholar]
- 18.May E, Dorris ML, Satumtira N, Iqbal I, Rehman MI, Lightfoot E, Taurog JD. CD8 alpha beta T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003;170:1099–1105. doi: 10.4049/jimmunol.170.2.1099. [DOI] [PubMed] [Google Scholar]
- 19.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evnouchidou I, Kamal RP, Seregin SS, Goto Y, Tsujimoto M, Hattori A, Voulgari PV, Drosos AA, Amalfitano A, York IA, Stratikos E. Coding single nucleotide polymorphisms of endoplasmic reticulum aminopeptidase 1 can affect antigenic peptide generation in vitro by influencing basic enzymatic properties of the enzyme. J Immunol. 2011;186:1909–1913. doi: 10.4049/jimmunol.1003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochan G, Krojer T, Harvey D, Fischer R, Chen L, Vollmar M, Delft Fvon, Kavanagh KL, Brown MA, Bowness P, Wordsworth P, Kessler BM, Oppermann U. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc Natl Acad Sci U S A. 2011;108:7745–7750. doi: 10.1073/pnas.1101262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves E, Edwards CJ, Elliott T, James E. Naturally Occurring ERAP1 Haplotypes Encode Functionally Distinct Alleles with Fine Substrate Specificity. J Immunol. 2013 doi: 10.4049/jimmunol.1300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratikos E, Stern LJ. Antigenic peptide trimming by ER aminopeptidases-Insights from structural studies. Molecular immunology. 2013;55:212–219. doi: 10.1016/j.molimm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nature immunology. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 25.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nature immunology. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 26.Boyle LH, Goodall JC, Opat SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) T lymphocytes. J Immunol. 2001;167:2619–2624. doi: 10.4049/jimmunol.167.5.2619. [DOI] [PubMed] [Google Scholar]
- 27.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 28.Georgiadou D, Hearn A, Evnouchidou I, Chroni A, Leondiadis L, York IA, Rock KL, Stratikos E. Placental leucine aminopeptidase efficiently generates mature antigenic peptides in vitro but in patterns distinct from endoplasmic reticulum aminopeptidase 1. J Immunol. 2010;185:1584–1592. doi: 10.4049/jimmunol.0902502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zervoudi E, Papakyriakou A, Georgiadou D, Evnouchidou I, Gajda A, Poreba M, Salvesen GS, Drag M, Hattori A, Swevers L, Vourloumis D, Stratikos E. Probing the S1 specificity pocket of the aminopeptidases that generate antigenic peptides. Biochem J. 2011;435:411–420. doi: 10.1042/BJ20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seregin SS, Aldhamen YA, Appledorn DM, Aylsworth CF, Godbehere S, Liu CJ, Quiroga D, Amalfitano A. TRIF Is a Critical Negative Regulator of TLR Agonist Mediated Activation of Dendritic Cells In Vivo. PLoS One. 2011;6:e22064. doi: 10.1371/journal.pone.0022064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parameswaran N, Pao CS, Leonhard KS, Kang DS, Kratz M, Ley SC, Benovic JL. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–34170. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 32.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, Wei J, Bujold M, Nance W, Godbehere S, Amalfitano A. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, Clair DSt, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdo’ttir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi CB, Kim TH, Jun JB, Lee HS, Shim SC, Lee B, Pope A, Uddin M, Rahman P, Inman RD. ARTS1 polymorphisms are associated with ankylosing spondylitis in Koreans. Annals of the rheumatic diseases. 2010;69:582–584. doi: 10.1136/ard.2008.105296. [DOI] [PubMed] [Google Scholar]
- 35.Harvey D, Pointon JJ, Evans DM, Karaderi T, Farrar C, Appleton LH, Sturrock RD, Stone MA, Oppermann U, Brown MA, Wordsworth BP. Investigating the genetic association between ERAP1 and ankylosing spondylitis. Hum Mol Genet. 2009;18:4204–4212. doi: 10.1093/hmg/ddp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maksymowych WP, Inman RD, Gladman DD, Reeve JP, Pope A, Rahman P. Association of a specific ERAP1/ARTS1 haplotype with disease susceptibility in ankylosing spondylitis. Arthritis Rheum. 2009;60:1317–1323. doi: 10.1002/art.24467. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Yao L, Meng T, Xu W. The association between seven ERAP1 polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis involving 8,530 cases and 12,449 controls. Rheumatol Int. 2012;32:909–914. doi: 10.1007/s00296-010-1712-y. [DOI] [PubMed] [Google Scholar]
- 38.Lee YH, Choi SJ, Ji JD, Song GG. Associations between ERAP1 polymorphisms and ankylosing spondylitis susceptibility: a meta-analysis. Inflamm Res. 2011;60:999–1003. doi: 10.1007/s00011-011-0374-x. [DOI] [PubMed] [Google Scholar]
- 39.York IA, Grant EP, Dahl AM, Rock KL. A mutant cell with a novel defect in MHC class I quality control. J Immunol. 2005;174:6839–6846. doi: 10.4049/jimmunol.174.11.6839. [DOI] [PubMed] [Google Scholar]
- 40.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 41.Lopez de Castro JA, Alvarez I, Marcilla M, Paradela A, Ramos M, Sesma L, Vazquez M. HLA-B27: a registry of constitutive peptide ligands. Tissue antigens. 2004;63:424–445. doi: 10.1111/j.0001-2815.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- 42.Atagunduz P, Appel H, Kuon W, Wu P, Thiel A, Kloetzel PM, Sieper J. HLA-B27-restricted CD8+ T cell response to cartilage-derived self peptides in ankylosing spondylitis. Arthritis Rheum. 2005;52:892–901. doi: 10.1002/art.20948. [DOI] [PubMed] [Google Scholar]
- 43.Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Jiang L, Cai Q, Danoy P, Barnardo MC, Brown MA, Xu H. Predominant association of HLA-B*2704 with ankylosing spondylitis in Chinese Han patients. Tissue antigens. 2010;75:61–64. doi: 10.1111/j.1399-0039.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 45.Scofield RH, Kurien B, Gross T, Warren WL, Harley JB. HLA-B27 binding of peptide from its own sequence and similar peptides from bacteria: implications for spondyloarthropathies. Lancet. 1995;345:1542–1544. doi: 10.1016/s0140-6736(95)91089-1. [DOI] [PubMed] [Google Scholar]
- 46.Cheuk E, Chamberlain JW. Strong memory CD8+ T cell responses against immunodominant and three new subdominant HLA-B27-restricted influenza A CTL epitopes following secondary infection of HLA-B27 transgenic mice. Cell Immunol. 2005;234:110–123. doi: 10.1016/j.cellimm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–5723. [PubMed] [Google Scholar]
- 48.Brooks JM, Murray RJ, Thomas WA, Kurilla MG, Rickinson AB. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993;178:879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appel H, Kuon W, Kuhne M, Wu P, Kuhlmann S, Kollnberger S, Thiel A, Bowness P, Sieper J. Use of HLA-B27 tetramers to identify low-frequency antigen-specific T cells in Chlamydia-triggered reactive arthritis. Arthritis Res Ther. 2004;6:R521–R534. doi: 10.1186/ar1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuon W, Holzhutter HG, Appel H, Grolms M, Kollnberger S, Traeder A, Henklein P, Weiss E, Thiel A, Lauster R, Bowness P, Radbruch A, Kloetzel PM, Sieper J. Identification of HLA-B27-restricted peptides from the Chlamydia trachomatis proteome with possible relevance to HLA-B27-associated diseases. J Immunol. 2001;167:4738–4746. doi: 10.4049/jimmunol.167.8.4738. [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Zhu P, Peng J, Li K, Du J, Gu J, Ou Y. Identification of disease-associated proteins by proteomic approach in ankylosing spondylitis. Biochem Biophys Res Commun. 2007;357:531–536. doi: 10.1016/j.bbrc.2007.03.179. [DOI] [PubMed] [Google Scholar]
- 52.Kuon W, Kuhne M, Busch DH, Atagunduz P, Seipel M, Wu P, Morawietz L, Fernahl G, Appel H, Weiss EH, Krenn V, Sieper J. Identification of novel human aggrecan T cell epitopes in HLA-B27 transgenic mice associated with spondyloarthropathy. J Immunol. 2004;173:4859–4866. doi: 10.4049/jimmunol.173.8.4859. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Medel N, Sanz-Bravo A, Nguyen DVan, Galocha B, Gomez-Molina P, Martin-Esteban A, Alvarez-Navarro C, Castro JALopez de. Functional interaction of the ankylosing spondylitis associated endoplasmic reticulum aminopeptidase 1 polymorphism and HLA-B27 in vivo. Mol Cell Proteomics. 2012 doi: 10.1074/mcp.M112.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hearn A, York IA, Rock KL. The specificity of trimming of MHC class I-presented peptides in the endoplasmic reticulum. J Immunol. 2009;183:5526–5536. doi: 10.4049/jimmunol.0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui X, Hawari F, Alsaaty S, Lawrence M, Combs CA, Geng W, Rouhani FN, Miskinis D, Levine SJ. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest. 2002;110:515–526. doi: 10.1172/JCI13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saveanu L, Carroll O, Lindo V, Val MDel, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, Endert PMvan. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nature immunology. 2005;6:689–697. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- 57.Jensen PE. Recent advances in antigen processing and presentation. Nature immunology. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 58.Rufer E, Leonhardt RM, Knittler MR. Molecular architecture of the TAP-associated MHC class I peptide-loading complex. J Immunol. 2007;179:5717–5727. doi: 10.4049/jimmunol.179.9.5717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.